Simple Summary
The present systematic review aimed to gain deeper insight into the epidemiology, clinical presentation, and histopathology of HPV-related benign and malignant lesions of the oral mucosa in pediatric patients to improve the multidisciplinary preventive and therapeutic management of oral and general healthcare. The emerging role of HPV in oral carcinogenesis in pediatric subjects, along with benign oral mucosal lesions and asymptomatic infections, brings HPV vaccination to the forefront specifically for this age group.
Abstract
The present systematic review aimed to assess the prevalence of oral HPV-related lesions, categorized as benign (verruca vulgaris “VV”, squamous cell papilloma “SP”, condyloma acuminata “CA”, and focal epithelial hyperplasia “FEH”) and malignant (oral squamous cell carcinoma “OSCC”), in descending order of occurrence in pediatric subjects (≤18 years of age). The secondary objectives were to evaluate the frequency and types of oral lesions described in relation to HPV genotypes and the HPV vaccine type (if any). The study protocol, compliant with the PRISMA statement, was registered at PROSPERO (CRD42022352268). Data from 60 studies, of which quality was assessed using the ROBINS-I tool, were independently extracted and synthesized. Along with seven poorly described benign HPV-related oral lesions that could not be categorized, a total of 146 HPV-related oral lesions, namely 47.26% (n = 69) VV, SP, and CA, 51.37% (n = 75) FEH, and 1.37% (n = 2) OSSC, were diagnosed in 153 pediatric subjects (M:F ratio = 1:1.4) with a mean age of lesion onset of 8.46 years. The viral genotypes detected were HPV-13 (30.61%), -6 (20.41%), -11 (16.33%), HPV-2 (12.24%), -32 (10.20%), -57 (6.12%), and -16 (4.08%). No HPV vaccination was reported in any case. Further studies should be conducted to evaluate the prevalence of HPV-related benign and malignant lesions and the potential role of HPV and associated vaccination in oral carcinogenesis in pediatric subjects.
1. Introduction
Human papillomavirus (HPV) is associated with benign and malignant diseases of various locations, mainly affecting the skin and genital and oral mucosa [1]. In detail, the benign HPV-related lesions of the oral mucosa recognized by the World Health Organization are squamous cell papilloma (SP), condyloma acuminatum (CA), verruca vulgaris (VV), and focal epithelial hyperplasia (FEH) [2].
Of the approximately 100 different HPV genotypes that have been identified, 25 are generally associated with oral lesions, including HPV-1, -2, -3, -4, -6, -7, -10, -11, -13, -16, -18, -31, -32, -33, -35, -40, -45, -52, -55, -57, -58, -59, -69, -72, and -73 [3], with low-risk HPV-6 and 11 being the most commonly detected [2].
In particular, HPV-6 and HPV-11 are frequently found in squamous cell papillomas [3], HPV-6 and HPV-11 in condyloma acuminata, HPV-2 followed by HPV-57 [3], -4, and -40 [2] in vulvar warts, and HPV-13 and -32 in FEH [3].
HPV is also considered an independent risk factor for benign and malignant tumors [1], and an increased incidence of HPV-positive oropharyngeal cancers has been recorded in the last decades [4]. Although the role of HPV in oral squamous cell carcinoma (OSCC) has not been fully elucidated [4], to date, HPV-related oral carcinomas, in which HPV-16 and HPV-18 are the most commonly associated genotypes [5,6], are estimated to account for a smaller proportion (3%) [7] than oral carcinomas attributable to other risk factors, such as tobacco and alcohol [6,8].
However, while benign and malignant oral HPV-related lesions in adults have been extensively described in the literature, available data on the pediatric population (≤18 years of age) remain sparse, heterogeneous, and characterized by low evidence, as they are mainly case reports and series.
Moreover, the emerging role of HPV in oral carcinogenesis in pediatric subjects [9], along with benign lesions and asymptomatic oral mucosal infections, brings to the forefront HPV vaccination specifically for this age group. Indeed, three types of HPV vaccines are currently available: Gardasil (Quadrivalent; Merck & Co., Kenilworth, NJ, USA) against HPV-6, -11, -16, and -18 genotypes, Gardasil9 (Nonavalent; Merck & Co, Kenilworth, NJ, USA) against HPV-6, -11, -16, -18, -31, -33, -45, -52, and -58 genotypes, and Cervarix (Bivalent; GSK, Brentford, UK) against HPV-16 and -18 [10].
The Centers for Disease Control and Prevention have developed new recommendations for routine HPV vaccination at ages 9 to 12 years to maximize vaccine effectiveness and increase the number of cancers prevented [11].
In light of these considerations, the present systematic review aimed to assess the prevalence of oral HPV-related lesions in pediatric subjects (≤18 years of age), categorize them as benign and malignant, and rank them in descending order of occurrence. The secondary objectives were to evaluate the frequency and type of oral lesions described in relation to HPV genotypes and the HPV vaccine type (if any).
2. Materials and Methods
2.1. Study Protocol
The study protocol currently used was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [12] prior to the literature search and data analysis and focused the research questions on the prevalence, clinical presentation, and histopathology of oral HPV-related benign and malignant lesions in pediatric subjects [13].
The formulation of the search question and strategy and criteria for study selection were developed using the PECO model [14].
The study question focused on the following:
- P—pediatric subjects (≤18 years of age)
- E—human papillomavirus (HPV) infection
- C—none or subjects with and without an HPV vaccine and the type
- O—oral lesions.
2.2. Search Strategy
Case reports, case series, and observational studies in English on oral HPV-related lesions in pediatric subjects (≤18 years of age) were searched electronically in the Scopus, MED-LINE/PubMed, and BioMed Central databases, without filters, by two independent reviewers (F.D.S., M.P.D.P.) until 11 August 2022, using the following appropriate keywords combined with Boolean operators:
- 1.
- Human papillomavirus OR HPV
AND
- 2.
- Oral lesions OR oral manifestations OR oral signs
AND
- 3.
- Children OR pediatric OR child OR infant OR adolescent OR teenager OR young.
2.3. Study Selection and Eligibility Criteria
The collected citations were recorded, duplicates were eliminated, and the titles and abstracts obtained were independently screened by two reviewers (F.D.S., M.P.D.P.). The full texts of potentially relevant papers and ambiguous abstracts were reviewed independently by the same authors (F.D.S., M.P.D.P.), who resolved disagreements through discussion and consensus and, if necessary, with the involvement of a third reviewer (G.P.).
An additional manual search was also performed on the reference lists of articles under consideration; relevant titles and abstracts were screened, and full texts were reviewed as described above. If full texts were missing, study authors were contacted.
Inclusion criteria were as follows: case reports, case series, cross-sectional, case-control, prospective, and retrospective studies accepted and published in English between 1970 and 11 August 2022, without limitations of sample size or gender, describing HPV-related oral lesions confirmed via histopathologic analysis (with or without PCR confirmation), excluding FEH cases diagnosed clinically only.
Exclusion criteria were as follows: in vitro and preclinical in vivo studies, systematic and narrative reviews, conference papers, oral communications, and books/chapters; participants ≥ 18 years of age and HIV-positive subjects; normal oral mucosal variations, likely preexisting, and self-reported oral mucosal lesions.
2.4. Data Extraction and Collection
Two reviewers (M.P.D.P., F.D.S.) extracted data independently, and a third reviewer (G.P.) was consulted in cases of discrepancies. A standardized data extraction form was used, developed under models proposed for intervention reviews of RCTs and non-RCTs [13].
The following data meeting eligibility criteria were collected from each study included in this systematic review:
- ▪
- First author, year, journal, funding, study quality;
- ▪
- Design and number of studies reported; sample size, gender ratio, mean age, comorbidities of the population investigated; HPV vaccine (if any; targeted HPV genotypes);
- ▪
- Total number or prevalence of pediatric subjects with oral HPV-related lesions diagnosed through a clinical examination confirmed based on histopathologic analysis (except for FEH) with/without PCR confirmation;
- ▪
- Macroscopic and microscopic features of oral lesions categorized as benign and malignant oral lesions [15];
- ▪
- Definitive diagnosis, diagnostic procedures, treatment, and progression of the oral lesions;
- ▪
- HPV genotypes associated with oral lesions.
2.5. Data Synthesis
A narrative synthesis was performed that focused on the reported characteristics of the pediatric population investigated, HPV exposure and related vaccination (if any), and oral outcomes.
Data from the included studies were qualitatively synthesized through descriptive statistical analysis using the Statistical Package for the Social Sciences (SPSS, version 25.0, Armonk, NY, USA)/Microsoft Excel Software 2019, Microsoft Corporation, Redmond, WA, USA):
- ➢
- Estimating the prevalence of HPV-related lesions in pediatric subjects diagnosed via clinical examination and confirmed based on histopathologic analysis;
- ➢
- Categorizing oral HPV-related lesions into benign, potentially malignant, and malignant lesions and ranking them in descending order of occurrence;
- ➢
- Evaluating the frequency of oral lesions categorized as benign, potentially malignant, and malignant in pediatric subjects
- ➢
- Calculating the frequency of HPV genotype detected in oral lesions;
- ➢
- Comparing the frequency and type of oral lesions in subjects with and without HPV vaccination
- ➢
- Evaluating the oral lesions in relation to the HPV vaccine type.
2.6. Quality Assessment
The risk of bias in the studies included in this systematic review was assessed by three independent reviewers (F.D.S, M.P.D.P., G.P.) using the Risk of Bias Instrument for Non-randomized Studies of Exposures [16], which is a modified version of the ROBINS -I (Risk Of Bias In Non-randomized Studies of Interventions) instrument [17], which takes into account the bias due to confounding, participant selection, intervention classification, deviations from planned interventions, missing data, selection of reported outcomes, and bias in outcome measurement.
The risk was classified as “low” or “moderate” if the risk of bias was low or low or moderate in all domains, respectively. A “serious” risk of bias was defined if a serious risk of bias was identified in at least one domain, but a critical risk of bias was not identified in any domain. The study was considered “critical” if the risk of bias was found to be critical in at least one area.
3. Results
3.1. Study Selection
Electronic searches retrieved a total of 83 records, 47 from MEDLINE/PubMed, 2 from Scopus, and 34 from BioMed Central databases; 3 duplicates were subsequently removed. The remaining 80 titles/abstracts were screened, and 37 were excluded because: 21 did not focus on HPV (6 focused on HIV); 5 reported oral HPV-related infection or lesions in adult women; 5 were in vitro studies; 2 did not report oral lesions (extra-oral lesions only); 2 were questionnaires assessing HPV knowledge; 1 was an animal study; 1 assessed oral HPV infection without associated oral lesions.
Of the 43 abstracts that were relevant to the present systematic review and met the eligibility criteria, the full texts were screened, and an additional 35 articles were excluded, specifically because: 5 studies did not report oral lesions; 15 did not involve pediatric patients, or it was not possible to extract data from them; 10 involved >18-year-old subjects; 2 described oral lesions that were not HPV-related; 3 were reviews.
A total of eight studies [18,19,20,21,22,23,24,25] were found in the electronic search and included in this systematic review.
Fifty-two additional records [1,2,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75] that were manually retrieved through the reference lists of the eight articles already included in the present systematic review [16,17,18,19,20,21,22,23] and met the currently applied eligibility criteria were presently included.
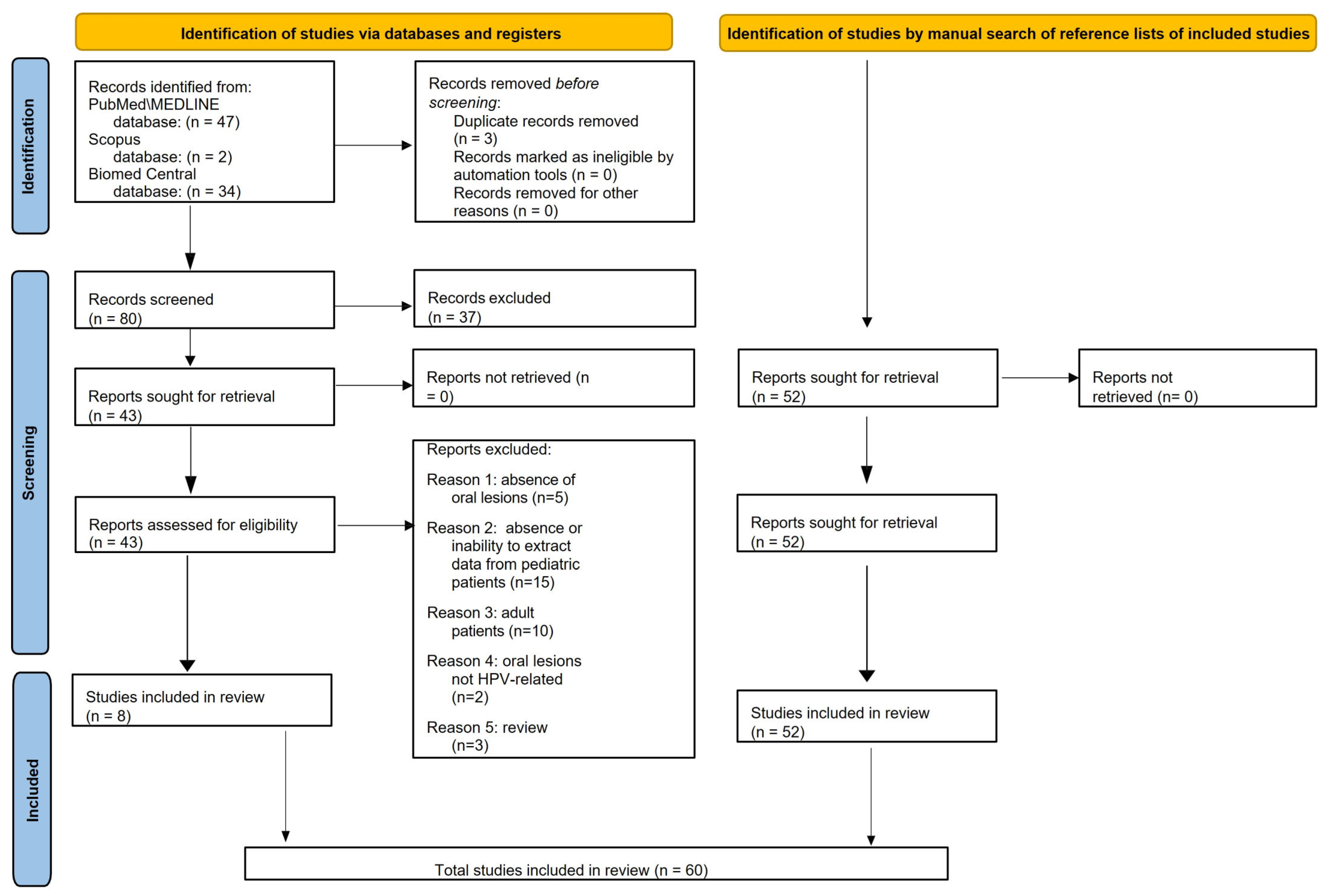
Finally, the present systematic review included 60 articles on oral HPV-related lesions in pediatric subjects (≤18 years of age) (Figure 1).
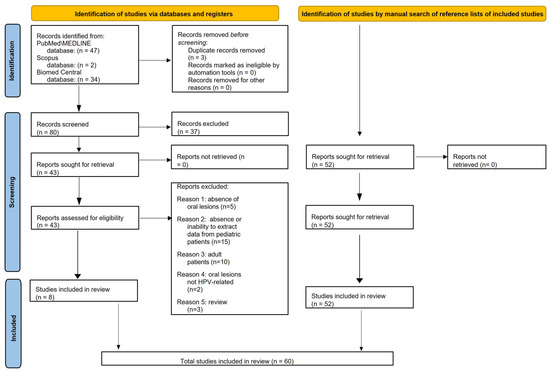
Figure 1.
PRISMA 2020 flow diagram for electronically and manually retrieved records.
Data from 60 studies describing oral HPV-related lesions diagnosed through clinical examination and confirmed based on histopathologic analysis in pediatric subjects were extracted and synthesized.
Of the 60 included studies, 36 were case reports, 19 were case series, 3 were retrospective, and 2 were prospective studies involving a total of 153 cases diagnosed with HPV-related oral lesions in pediatric (≤18 years of age) subjects.
The sources, methods, and qualitative synthesis of oral outcomes from the included studies were pooled. Only data that met eligibility criteria were extracted and analyzed; therefore, data from individuals who were >18-years-old were not detailed.
3.2. Studies Reporting Pediatric Cases Diagnosed with Oral Papilloma, Verruca Vulgaris, or Condyloma Acuminata
Table 1 summarizes data from included studies describing pediatric (≤18 years of age) cases diagnosed with oral papilloma, verruca vulgaris, or condyloma acuminata.

Table 1.
Data extracted and collected from studies reporting pediatric cases diagnosed with oral papilloma, verruca vulgaris, or condyloma acuminata. Study characteristics: first author, year, and journal of publication; study design; the reference number; funding. Study methods: sample size (n.), mean age (y.o.), gender ratio (M/F), country, comorbidities and associated ongoing treatments, relatives with similar lesions, HPV exposure (if any), time to onset of oral lesions, type of HPV vaccine administered (if any). Oral HPV-related lesions: macroscopic and microscopic features, number (single/multiple), distribution (unilateral/bilateral, asymmetric or symmetric), location, extra-oral involvement, HPV genotype. Diagnosis: definitive diagnosis, diagnostic procedure(s) performed, therapy, progression.
3.2.1. Oral Papilloma, Verruca Vulgaris, or Condyloma Acuminata: Case Characteristics
A total of 76 pediatric subjects were diagnosed with oral HPV-related lesions, which were divided into 37 papillomas [17,20,21,23,25,28,29,30,31,33,35,40,41,42,45,46], 19 verrucae vulgaris cases [2,22,24,36], and 13 condyloma acuminata cases [2,26,27,32,34,37,38,43,44].
The study population included 35 males and 33 females between the ages of 1.5 and 18 years, with a mean age of 8.9. However, the gender and age of six subjects were not reported [40].
Country of origin or ethnicity was reported for 28 patients, as follows: 7 Black [21,23]; 2 Latino [23]; 16 Caucasian [23,27,38,43,44,46], including 1 from Denmark [27]; 1 from Somalia [37]; 1 from North Kerala [41].
Patients from 11 studies had no comorbidities [17,20,25,26,28,29,31,41,43,44,45], while in one case the patient had impetigo and gonorrhea [34] and in another case, acute respiratory illness [43].
Associated treatments for comorbidities were antibiotics [34], aciclovir [43], and podophyllin for skin warts [44].
HPV exposure was associated with the following: suspected abuse [26,34,46]; sexual abuse in three cases [38,43]; vaccination in two cases [39]; vertical transmission in eight cases [40]. Relatives with similar HPV-related lesions on any body site were as follows: the father [26], the mother [43], and a foster child [37]; no information was provided for four reported cases [17,34,41,43].
The mean time to the onset of oral lesions was 11.4 months and ranged from 14 days to 7 years. Only one study [28] reported that the patient had not received an HPV vaccine.
3.2.2. Oral Papilloma, Verruca Vulgaris, or Condyloma Acuminata: Macroscopic and Microscopic Features, Extra-Oral Involvement, HPV Genotype
Macroscopic features were reported in 22 [2,17,20,25,26,28,29,30,31,32,33,34,35,37,38,39,40,41,42,43,44,45], while microscopic ones were reported in 20 studies [2,17,20,21,24,25,28,29,30,31,32,33,34,35,37,38,39,41,42,44], respectively.
HPV-related oral manifestations occurred as a single lesion in 22 cases [2,20,25,26,29,30,31,32,33,35,40,43,45,46] and as multiple lesions in 10 cases [17,28,34,37,38,41,42,43,44], with a unilateral distribution in 18 cases [25,26,29,30,31,32,33,34,35,38,41,42,45,46], bilateral distribution in 11 cases [2,17,28,37,40,43], and asymmetric pattern in 2 cases [28,37]; the lesions tended to coalesce in 1 reported case [44].
The affected sites were as follows: lower lip (n = 8) [24,28,30,34,37,41] and upper lip (n = 9) [17,24,26,37,39,41,44]; palate (n = 13) [20,31,35,38,39,40,42,43]; tongue (n = 9) [17,30,36,37,38,42,43,44]; commissures (n = 6) [24,28,30,39,43]; cheeks (n = 5) [17,28,37,43]; gingiva (n = 6) [2,17,21,27,29,36]; uvula (n = 3) [25,33,45]; tonsils (n = 2) [46]; vermilion (n = 2) [2]; anterior faucial columns (n = 2) [27,40]; retromolar area (n = 1) [17]; vestibule (n = 1) [42]; N\D oral mucosa (n = 1) [36].
Thirteen patients had no extra-oral involvement on examination [17,27,31,35,36,37,42,43], while eleven others also had extra-oral involvement, namely: warts on the hand or fingers in six cases [27,28,36,39]; genital warts in two cases [26,44]; skin warts in one case [34]; warts on the forehead in one case [21]; laryngeal papillomas in one case [46]
HPV genotypes detected in oral lesions were as follows: HPV-2 in six cases [24,36]; HPV-6 in nine cases [2,21,26,27,28,37,46]; HPV-11 in seven cases [2,26,28,37,46]; HPV-57 in three cases [36]; HPV-16 in two cases [17,40]; HPV-32 in one case [41]. In 16 cases, the viral genotypes studied were not further described [2,21,24,27,36,38,39].
3.2.3. Oral Papilloma, Verruca Vulgaris, or Condyloma Acuminata: Definitive Diagnosis, Diagnostic Procedure(s), Therapy, Progression
The diagnostic procedures performed included the following: biopsy (n = 48) [17,20,21,23,26,27,28,29,30,31,32,33,35,36,37,38,39,40,41,42,44]; in situ hybridization (n = 28) [2,21,22,24,26,27,28,36,37,40,43,46]; immunoperoxidase assays for viral antigens (n = 2) [21]; PCR analysis (n = 3) [17,40,41]; immunohistochemistry for the p53 protein (n = 1) [30]; cytologic scrapes (n = 8) [40]; vaginal culture (n = 1) [34] testing positive for Neisseria (N.) lactamina, N. meningitides, N. gonorrhoeae; swabs (n = 3) [43]; radiographs (n = 1) [45]; direct laryngoscopy (n = 1) [45]; serological tests (n = 5) [17,37,43] yielding negative results for HIV in four cases [17,43], Treponema pallidum and HBV in three cases [43], antisyphilis antibodies [17]. The following therapies were performed: Electrocautery in 2 cases [25,41]; CO2 laser surgery in 2 [28]; laser surgery in 7 [20,29,43]; excisional biopsy in 10 [31,32,33,34,40,42,44,45]; oral surgery in 1 [17]; cimetidine in 1 [43]; interferon alpha-2a in 1 [37]; podophyllin in 2 [37,44].
3.3. Studies Reporting Pediatric Cases Diagnosed with Focal Epithelial Hyperplasia
Table 2 shows data from retrieved studies describing pediatric (≤18 years of age) cases diagnosed with oral focal epithelial hyperplasia.

Table 2.
Extracted and collected data from studies reporting pediatric cases diagnosed with oral focal epithelial hyperplasia. Study characteristics: first author, year, and journal of publication; study design; the reference number; funding. Study methods: sample size (n.), mean age (y.o.), gender ratio (M/F), country, comorbidities and associated ongoing treatments, relatives with similar lesions, HPV exposure (if any), time to onset of oral lesions, type of HPV vaccine administered (if any). Oral HPV-related lesions: macroscopic and microscopic features, number (single/multiple), distribution (unilateral/bilateral, asymmetric or symmetric), location, extra-oral involvement, HPV genotype. Diagnosis: definitive diagnosis, diagnostic procedure(s) performed, therapy, progression.
3.3.1. Focal Epithelial Hyperplasia: Case Characteristics
Seventy-five pediatric subjects were diagnosed with focal epithelial hyperplasia [2,18,21,29,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75]. Two studies [56,62] included in this systematic review described cases of FEH in adult patients who reported having the condition since childhood; data from these two studies are shown in Table 2 but were not included in the data analysis. The study population included 18 males and 45 females aged 0–18 years, with a mean age of 9.97 years. However, gender was not specified for 10 subjects [21].
Country of origin or ethnicity was reported for 52 patients, specifically as follows: 2 Hispanic [50,69]; 1 Caucasian [67]; 1 Black [74]; 1 African [51]; 1 Greenlandic Eskimo [29]; 9 Venezuelan [24]; 2 Dutch [72]; 1 Ecuadorian [52]; 6 Brazilian [53,63]; 2 Mexican [54]; 8 Iranian [57,64]; 1 Moroccan [61]; 1 Algerian [61]; 1 Turkish [68]; 1 Libyan [58]; 3 Australian [59]; 1 Chinese [60]; 6 Ghanaian [66]; 2 Ikalouit [73]; 2 Guyanese [71].
HPV exposure was associated with the following: 1 suspected abuse [55]; 1 horizontal transmission [71]. Relatives with similar HPV-related lesions anywhere on the body were as follows: siblings in 13 cases [24,53,55,58,59,61,71,72,73]; mother in 2 cases [59,71]; father [73] and cousin [58] in 1 case, each; none in 7 reported cases [52,60,63,66,74,75].
The median time to onset of oral lesions was 19.9 months and ranged from 1 month to 8 years. No study reported whether or not patients were vaccinated against HPV.
3.3.2. Focal Epithelial Hyperplasia: Macroscopic and Microscopic Features, Extra-Oral Involvement, HPV Genotype
Macroscopic and microscopic features were reported in 27 [18,24,49,50,52,53,54,55,57,58,59,60,61,63,75] and 24 studies, respectively [18,24,50,51,52,54,55,57,58,59,60,61,63,64,65,66,67,68,69,70,72,74,75].
Oral FEH occurred in 54 pediatric cases [2,18,24,49,50,51,52,53,54,55,57,58,59,61,63,64,65,66,67,68,69,70,71,72,73,74,75], with a unilateral distribution in 4 cases [64,69,71], bilateral distribution in 10 cases [18,54,60,61,64,70,71,73], asymmetric pattern in 13 cases [51,52,55,57,58,59,65,75]; lesions tended to coalesce in 5 cases [49,57,61,71,75].
The affected sites were as follows: lips (n = 51) [2,18,24,29,49,50,51,52,53,54,55,57,58,59,60,61,64,65,66,67,68,69,70,72,74,75], specifically lower lip (n = 21) [18,29,49,51,53,54,55,58,59,64,65,68,72,75] and upper lip (n = 15) [24,51,53,54,58,64,67,68,72,75]; cheeks (n = 30) [18,49,50,52,53,54,58,60,61,64,66,67,68,69,70,71,72,73,74,75]; tongue (n = 19) [24,49,51,52,53,54,55,59,61,65,66,71,72,73]; commissures (n = 9) [58,66,68,72]; gingiva (n = 8) [49,50,54,61,63,66,72]; palate (n = 5) [61,72,75]; the floor of mouth (n = 3) [54,66,72]; vermillion (n = 3) [58]; vestibule (n = 1) [70]; anterior faucial columns (n = 1) [72]; retromolar area (n = 1) [74].
Nine patients did not show extra-oral involvement on examination [51,52,54,60,65,67,70,73], while nine also showed extra-oral involvement; notably, skin warts were described in 12 cases [29,58,61,68], and 3 of them were located on the hand [29,58,61].
The HPV genotypes detected were as follows: HPV-13 in 15 cases [24,29,50,52,53,54,57,58,60,63,68,71]; HPV-32 in 4 cases [51,57,65,67]; HPV-6 and 11 in 1 case [2].
3.3.3. Focal Epithelial Hyperplasia: Definitive Diagnosis, Diagnostic Procedure(s), Therapy, Progression
In five cases, no therapies were performed [50,57,60,66,67]. The treatments that were performed in the remaining cases were as follows: TCA in 12 cases [21,75]; electrocautery [61,75], laser surgery [52,54,65], and interferon alpha-2b [49] in 3 cases each; CO2 laser surgery [18,49]; shaving [61], excisional biopsy [63,69], cryotherapy [55,75], and levamisole [49,75] in 2 cases each; Quantum Molecular Resonance Scalpel [70], oral surgery [54], acitretin [49], podophyllin [55], vitamin A, and imiquimod [75] in 1 case each.
Regarding the progression of oral FEH lesions: in 14 cases, lesions healed after an average of 15.95 months, ranging from 14 days to 3 years; in 17 cases, no recurrence occurred after an average of 11 months, with a follow-up ranging from 1.5 months to 1 year. Improvement was noted in seven cases after an average of 18.4 months, ranging from 1 month to 5 years. Recurrence occurred in two cases, after six months in 1 case, while deterioration was declared in 1 case and no improvement occurred in 9 cases with an average follow-up time of 27 months.
3.4. Studies Reporting Pediatric Cases Diagnosed with Oral Squamous Cell Carcinoma
Table 3 summarizes data from identified studies characterizing pediatric (≤18 years of age) cases diagnosed with oral squamous cell carcinoma.

Table 3.
Extracted and collected data from studies reporting pediatric cases diagnosed with oral squamous cell carcinoma. Study characteristics: first author, year, and journal of publication; study design; the reference number; funding. Study methods: sample size (n.), mean age (y.o.), gender ratio (M/F), country, comorbidities and associated ongoing treatments, relatives with similar lesions, HPV exposure (if any), time to onset of oral lesions, type of HPV vaccine administered (if any). Oral HPV-related lesions: macroscopic and microscopic features, number (single/multiple), distribution (unilateral/bilateral, asymmetric or symmetric), location, extra-oral involvement, HPV genotype. Diagnosis: definitive diagnosis, diagnostic procedure(s) performed, therapy, progression.
3.4.1. Oral Squamous Cell Carcinoma: Case Characteristics
A total of two pediatric subjects were diagnosed with oral malignant HPV-related lesions categorized as oral squamous cell carcinoma (OSCC) [1,20].
The study population included two males aged 5 to 8 years, with a mean age of 6.5 years, one of whom was of Caucasian origin [20], and both were without comorbidities.
In one case, HPV exposure through self-vaccination was suspected [18], which was supported by evidence that his two brothers also had HPV-related lesions [18].
3.4.2. Oral Squamous Cell Carcinoma: Macroscopic and Microscopic Features, Extra-Oral Involvement, HPV Genotype
Macroscopic and microscopic features were reported in both studies [1,18], and in two cases, the lesions appeared to be solitary [1,18] and unilateral [1,18] and involved the maxillary ridge [1,18].
Extra-oral involvement was described in one case [18] as warts on the hands, chin, philtrum, and commissures.
The HPV genotype involved was not reported, but p16 positivity was noted in both cases; HPV association with pediatric OSCC was therefore reported based on the p16 status of the lesions.
3.4.3. Oral Squamous Cell Carcinoma: Definitive Diagnosis, Diagnostic Procedure(s), Therapy, Progression
The diagnostic procedures performed were as follows: two biopsies [1,18]; one periapical radiograph [18]; two CBCT [1,18]; one PCR analysis [18]; one CT [1], and one RMI [1].
Therapies performed were as follows: one pharmacologic therapy with antibiotics [18]; one tooth extraction [18]; biopsies [1,18]; and two partial maxillectomies [1,18].
In one case, the patient healed after 24 months [1] since the partial maxillectomy, while in one case, the patient had two recurrences [18] and one exacerbation [18] and healed only 4 weeks after the partial maxillectomy [18].
3.5. Prevalence of Reported Oral HPV-Related Lesions in Pediatric Subjects
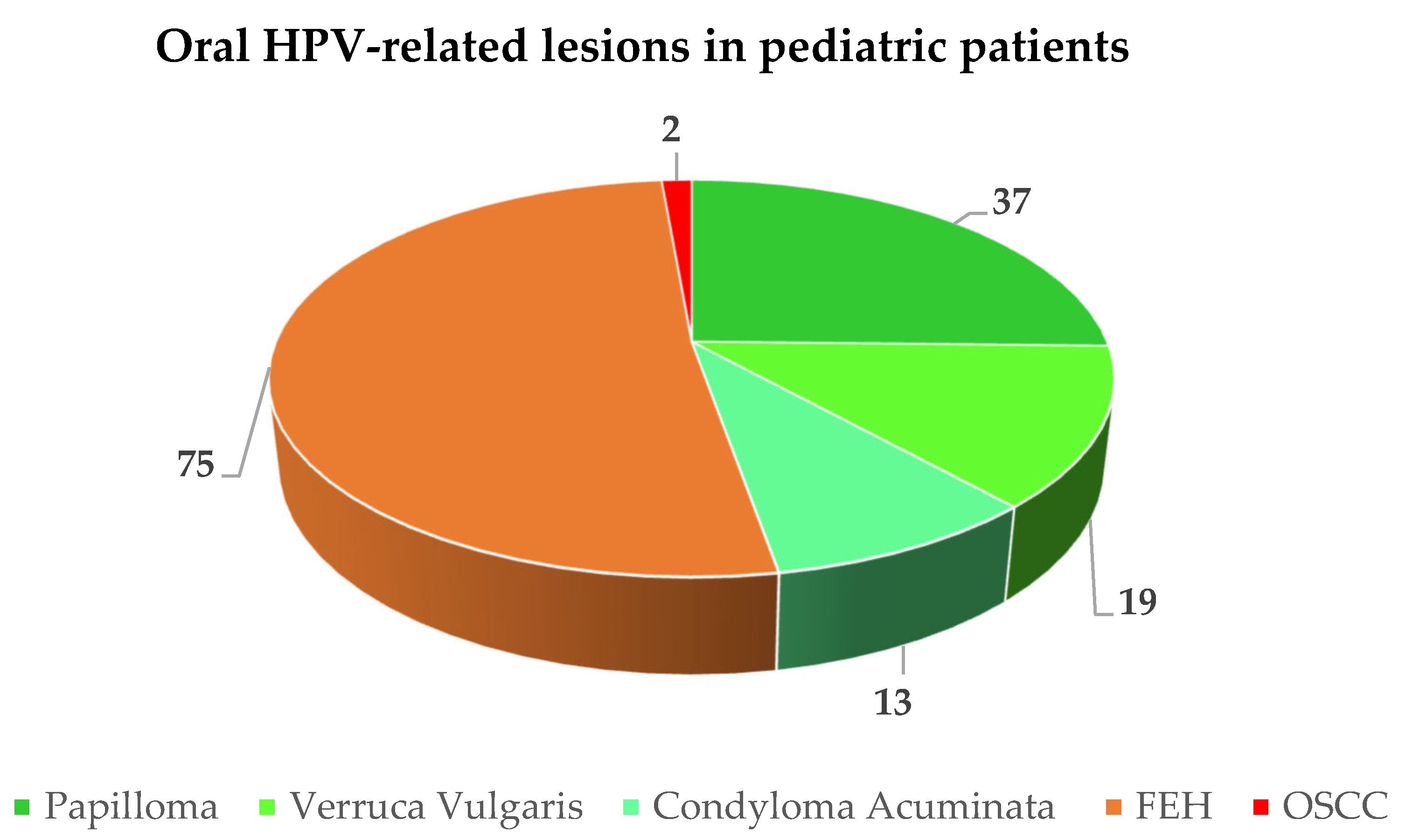
A total of 146 HPV-related oral lesions were diagnosed in 153 pediatric subjects (M:F ratio = 1:1.4) with a mean age of 8.46 years, including 47.26% (n = 69) verrucae vulgaris, squamous cell papillomas, and condyloma acuminata, 51.37% (n = 75) FEH, and 1.37% (n = 2) OSSC (Figure 2). In addition, seven poorly described benign HPV-related oral lesions were reported but could not be categorized.
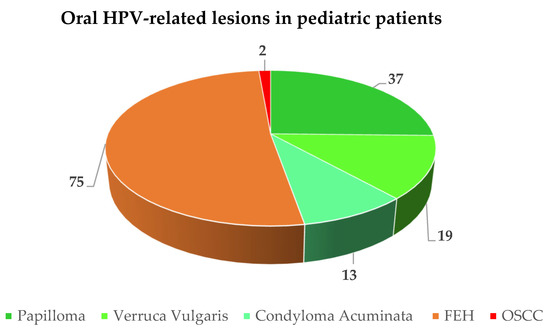
Figure 2.
Frequency of reported oral HPV-related lesions in pediatric patients.
On average, patients visited a physician 15.65 months after the appearance of oral lesions, and in no case was HPV vaccination indicated.
The most frequently affected sites were as follows, in descending order: lips (36.6%) in 74 cases (upper lip was reported in 29 and lower lip in 24 cases), followed by cheeks (17.3%) in 35 cases, tongue (13.9%) in 28 cases, palate (8.9%) in 18 cases, labial commissures (7.4%) in 15 cases, gingiva in 14 cases, vermilion (2.5%) in 5 cases, anterior faucial columns (1.5%), uvula (1.5%), and floor of mouth (1.5%) in 3 cases, tonsils (0.99%), the vestibule (0.99%) and alveolar ridge (0.99%) in 2 cases; N\D oral mucosa in 1 (0.5%).
3.6. HPV Genotypes Detected in Oral HPV-Related Lesions in Pediatric Subjects
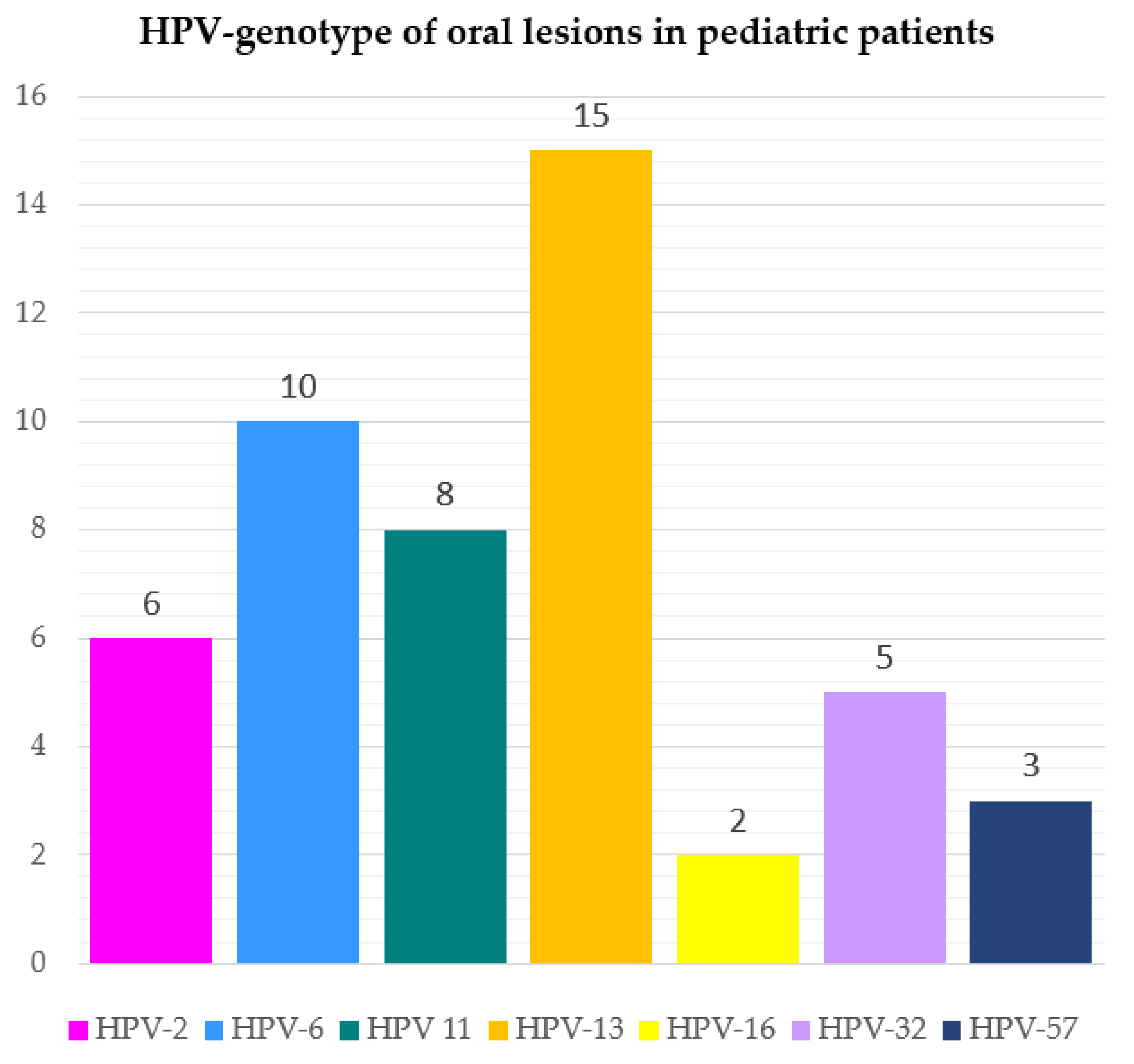
The HPV genotypes detected were as follows: HPV-13 in 15 cases (30.61%); HPV-6 in 10 cases (20.41%); HPV-11 in 8 cases (16.33%); HPV-2 in 6 cases (12.24%); HPV-32 in 5 cases (10.20%); HPV-57 in 3 cases (6.12%); HPV-16 in 2 cases (4.08%), as shown in Figure 3 and mapped in Figure 4 in relation to the type of oral lesion.
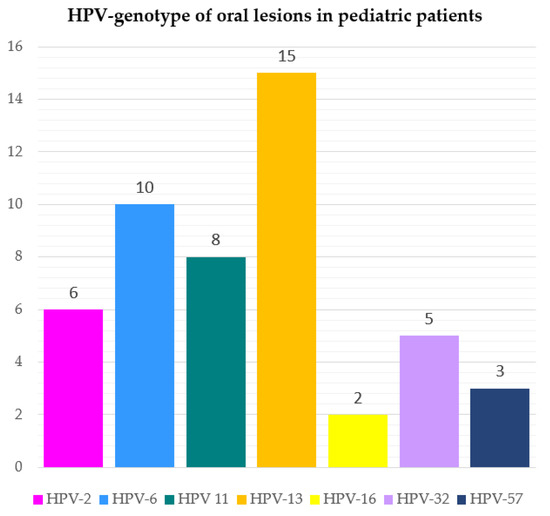
Figure 3.
Frequency of viral genotypes detected in HPV-related oral lesions in pediatric patients.
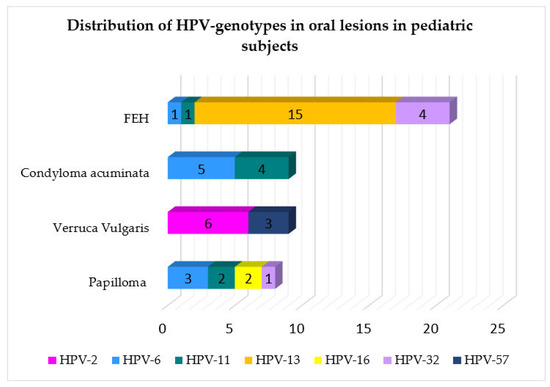
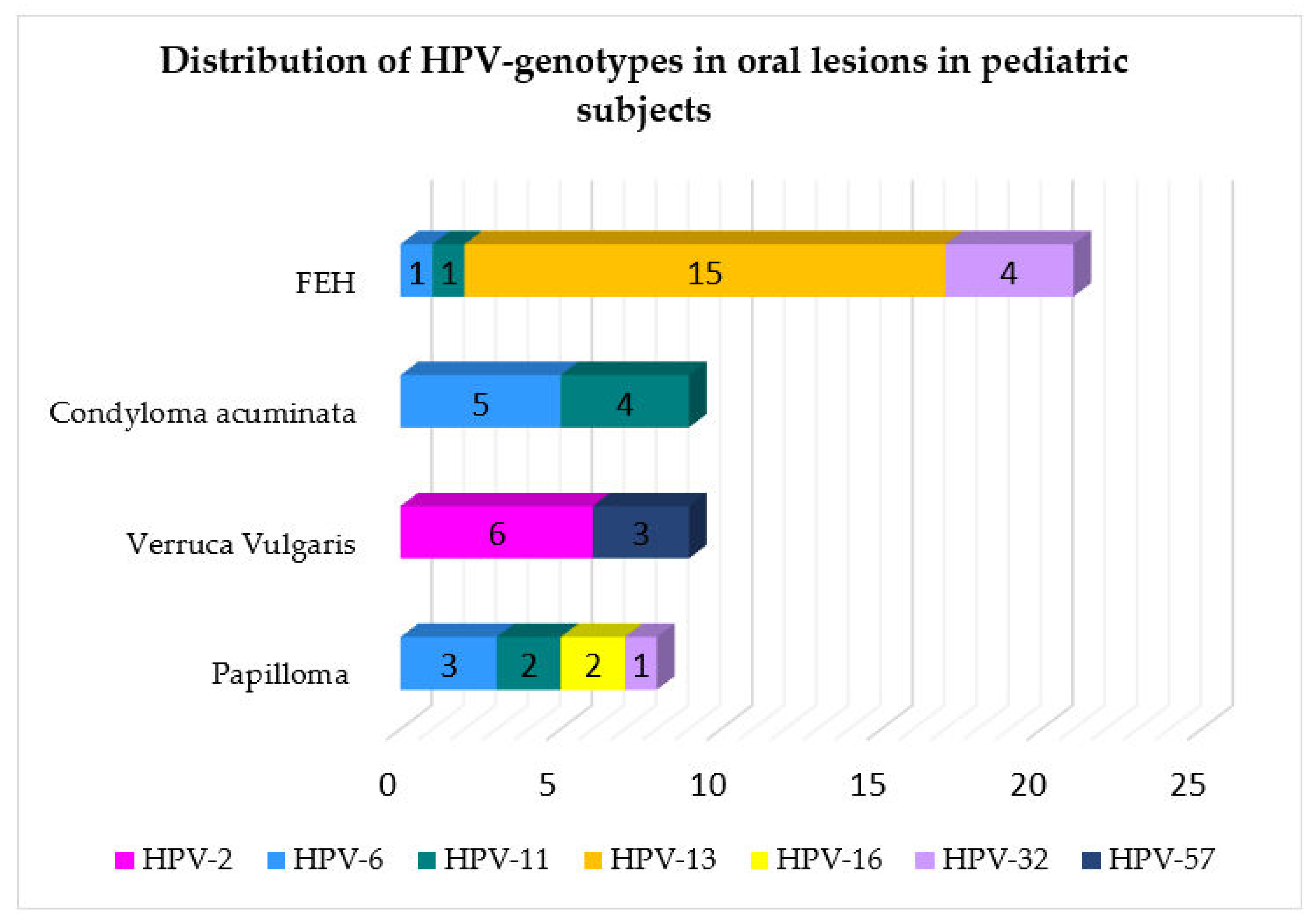
Figure 4.
Distribution of HPV genotype in relation to the type of oral lesions reported in pediatric patients.
3.7. Quality Assessment
The risk of bias of the non-randomized studies included in the present systematic review is reported in Table 4.

Table 4.
Risk of bias of all studies included in the systematic review and listed in alphabetical order. For each of the seven domains assessed: Y = low risk of bias, PY = moderate risk of bias, PN = serious risk, N = critical risk of bias, and NI = no information.
4. Discussion
The present systematic review aimed to assess the prevalence of oral HPV-related lesions in pediatric subjects (≤18 years of age), categorize them as benign and malignant, and rank them in descending order of occurrence. The secondary objectives were to evaluate the frequency and types of oral lesions described in relation to HPV genotypes and the HPV vaccine type (if any).
Sixty studies were considered, and along with seven poorly described benign HPV-related oral lesions that could not be categorized, a total of 146 HPV-related oral lesions, namely 47.26% (n = 69) VV, SP, and CA, 51.37% (n = 75) FEH, and 1.37% (n = 2) OSSC, were diagnosed in 153 pediatric subjects (M:F ratio = 1:1.4) with a mean age of lesion onset of 8.46 years. The viral genotypes detected were HPV-13 (30.61%), -6 (20.41%), -11 (16.33%), HPV-2 (12.24%), -32 (10.20%), -57 (6.12%), and -16 (4.08%). No HPV vaccination was reported in any case.
4.1. Oral Benign HPV-Related Lesions in Pediatric Subjects: Verruca Vulgaris, Squamous Cell Papilloma, and Condyloma Acuminatum
The investigated population with oral benign HPV-related proliferation was almost evenly distributed between the female and male genders, with an M:F ratio = 1.06:1. A similar distribution has been reported for other viral infectious diseases associated with oral manifestations in the pediatric population, such as HIV (M:F = 1:1) [76] and SARS-CoV-2 (M:F = 1.3:1) [77].
The pediatric cases diagnosed with benign oral HPV lesions had a mean age of 8.9 years, slightly lower than the mean age found for FEH (9.97 years), as described later, and lower than the range reported for the highest incidence of verruca vulgaris, between 12 and 16 years of age [74].
In general, HPV-related oral benign lesions are more common in adults [78], similar to the rate of detectable oral HPV infection, which also increases in adulthood [79]. More specifically, detectable oral HPV infection has two peaks in prevalence at ages 30–34 and 60–64 years [79]. It has been hypothesized that the peak at older ages may be related to the normal process of immunosenescence [80], leading to the possible reactivation of latent HPV infections [81]. Nevertheless, benign HPV-related oral lesions have also shown a similar bimodal peak, with condylomata acuminata showing a higher incidence between the third and fourth decades of life and squamous cell papillomas between the third and seventh decades of life [78].
The main transmission route for HPV is skin-to-skin or skin-to-mucosa contact [82]. Sexual transmission has also been adequately documented [80]. Accordingly, in the present systematic review, three cases were sexually abused [40,45] and three cases were investigated for suspected sexual abuse [28,36,48]. Horizontal transmission can occur via fomites, fingers, mouth, and skin contact [82]. Most skin warts in children result from horizontal transmission [15]. For oral mucosal lesions, few studies in the present review also investigated the presence of potentially HPV-related lesions on the body in the patient’s family members, and only three cases [28,39,45] were found in at least one family member, suggesting presumed horizontal transmission. In two patients in the present study, self-inoculation was suspected in virgin women and children with genital warts without suspected sexual abuse [82]. Self-inoculation is typical of vulvar warts in children who suck their thumbs or carry their fingers in their mouths [78]. Simultaneous evidence of lesions in the genital area and oral cavity may indicate sexual transmission [78]. Another route of transmission is vertical mother-to-child transmission [82], which is the main route of HPV infection in newborns [15]. Finally, several studies have suggested the possibility of viral transmission through the amniotic fluid or placenta or through contact with the genital mucosa of the mother during delivery [82]. The results of the present study do not suggest a higher prevalence of one route of transmission than the other. However, most of the included studies did not investigate this aspect of infection.
Squamous papillomas are estimated to be the most common benign oral epithelial le-sions in both pediatric and adult populations [78], which is consistent with the present results (n = 37).
Second, in overall frequency, verrucae vulgaris is the predominant cutaneous manifestation of HPV infection, with a total incidence of 10% in children and young adults [74]. Although considered uncommon in the oral cavity [74], VV cases have currently been observed in 19 cases, accounting for 12.42% of all diagnoses.
Condyloma acuminata is more common anogenitally and is the most common manifestation of sexually transmitted infections in the United States and the United Kingdom [74]. It is also estimated that CA cases are not common in the oral cavity [74], which is consistent with the present results showing that CA is the least frequently reported oral manifestation in pediatric subjects (n = 13).
Rarely, multiple verrucae vulgaris occurs together in the oral cavity or clusters [74]; the same is true for SPs. Indeed, in the study by Frigerio et al. [83], which examined 205 oral SPs, only four patients had more than two lesions simultaneously.
Despite the fact that CAs are the benign HPV-related oral lesions that most commonly occur as multiple lesions and tend to coalesce and although Panici et al. [84], examining 101 oral Cas, found 61% of patients with more than five lesions, the retrieved data revealed a higher prevalence of single (22 cases) compared with multiple lesions (10 cases), most of which were represented by CA [2,36,39,40,45,46].
The palate and tongue are considered the sites most commonly affected by SPs [78]. VV, on the other hand, occurs most frequently on the labial mucosa and palate [74], whereas CA occurs on the tongue and upper lip [74]. In line with these findings, the results of the present systematic review showed that benign HPV-related oral lesions most frequently affected the lips (n = 23; 31.08%), with apparently no preference for the upper or lower lip, palate (n = 13; 17.57%), and tongue (n = 9; 12.16%).
In the normal oral mucosa, latent infection is most commonly maintained by HPV-6 and -11 [3], and oral squamous cell papillomas are generally associated with specific viral genotypes. Consistent with reported evidence [2,3], findings of the present systematic review pointed out that HPV-6 was the most frequently detected genotype: in SPs, HPV-6 (n = 3), followed by HPV-11 (n = 2), 16 (n = 2), and 32 (n = 1); in CA, HPV-6 (n = 5) and HPV-11 (n = 4); in VV, HPV-2 (n = 6) and HPV-57 (n = 3).
Only one study [30] reported that the child had not received HPV vaccination, whereas such information was not detailed elsewhere. Especially, in this pediatric population, dentists should make patients and their parents and caregivers aware of the importance of HPV vaccination, even in children who already have infections, regardless of their HPV status. The American Cancer Society (ACS) has developed new recommendations for HPV vaccination that should be routinely administered between the ages of 9 and 12 to achieve the greatest vaccine efficacy and increase the number of cancers prevented [11]. Healthcare providers should encourage vaccination as early as 9 to 10 years of age and if appropriate, advise those who have not been vaccinated or have not completed vaccination that vaccine administration is less effective in reducing cancer risk at an older age [9]. It is therefore crucial that the pediatric population is encouraged to receive HPV vaccination, as vaccines are critical prevention measures, as was also demonstrated during the COVID-19 pandemic, in which vaccines significantly reduced the risk of virus transmission and infection and prevented severe forms of the disease [85].
4.2. Oral Benign HPV-Related Lesions in Pediatric Subjects: Focal Epithelial Hyperplasia
Focal Epithelial Hyperplasia (FEH), also known as Heck’s disease, is an asymptomatic and benign condition caused by HPV-13 and/or -32 genotypes [56,86] that presents in the oral cavity as multiple, well-circumscribed, raised, soft papules, and nodules with the same color as the surrounding oral mucosa [56,87].
FEH can affect children and adults [15] but generally shows a higher prevalence in younger individuals, with the first and second decades of life being most commonly affected [87]. Accordingly, the mean age currently calculated was 9.97 years. These data are consistent with those of Sethi et al. [88], who studied a broader age range and included subjects aged 3 to 92 years with a mean age of 23.1.
The same study [88] recorded a male-to-female ratio of 3:4. The higher prevalence of FEH in females has also been described in other studies [86,88,89] with a ratio of 3:4 to 1:5, which is consistent with the findings of the present systematic review that recorded a male-to-female ratio of 2:5. The reason for the higher incidence of FEH in females is still unclear and may be due to the poor hygienic conditions in which women live in certain ethnic groups [90], especially in some regions with a higher FEH prevalence.
In fact, there are no accurate data on the prevalence of FEH in the general population, but it is considered a rare condition [15]. According to initial studies, it occurs most frequently in Native Americans, Mexican Indians, South Americans, and Eskimos [15,89]. Accordingly, the data extracted in this systematic review showed that the country of origin from which most cases of FEH were reported in pediatric patients was Venezuela (n = 9) [24], followed by Iran (n = 8) [57,64], Brazil, and Ghana (n = 6) [53,63,66]. Overall, 20 (26.7%) of the 75 FEH cases were registered in South America, an epidemiological finding consistent with the initial estimate of the geographic distribution of FEH [13]. The involvement of the African continent also seems consistent, with 10 (13.3%) of the cases included in this systematic review (Morocco (n = 1) [61], Libya (n = 1) [58], Algeria (n = 1) [59], Africa (n = 1) [51]; Ghana (n = 6) [66]). In addition, data from the present study also show a high incidence of FEH in Iran, where 8 (10.7%) of the 75 cases occurred [57,64]. This suggests that Heck’s disease is generally rare and likely more common in certain ethnic and racial groups [89] living in more densely populated and developing countries. These contexts are even referred to as ‘prisons’, underscoring the restricted living conditions that place them at high risk for infection [NO_PRINTED_FORM]. Coherently, fewer cases have been diagnosed with oral FEH in Europe and North America, although they have slightly increased between 2001 and 2019 (20 published cases) compared to numbers in 1966–2005 (9 published cases) [86].
The mean time to the appearance of oral lesions was 19.9 months, ranging from 1 to 8 years, as on average, more than 1.5 years elapsed before patients saw a specialist for control. In two cases [56,62], a specialist was even not seen until 10 to 25 years after the lesions appeared. These data suggest that the surveyed population lacks knowledge about Heck’s disease and awareness and concern about oral mucosal changes. In addition, it can be assumed that the cases of FEH probably waited an average of one and a half years after the appearance of the lesions before seeing a specialist because the lesions were asymptomatic and did not affect oral functions and esthetics [60]. Consequently, it seems necessary to make the population aware of the importance of regular oral and dental checkups, even more so in the case of oral mucosal changes. These findings can be a more comprehensive alarm signal, even if they are associated with a benign pathology, such as FEH. Indeed, oral lesions, even if asymptomatic, should never be underestimated, as they can be an early sign of other systemic diseases or malignancies. Thus, raising awareness of this issue goes hand in hand with encouraging patients to be vaccinated against HPV.
In this regard, no study reported whether patients with manifestations of Heck’s disease received the vaccine. This may be in part because FEH is associated with low-risk HPV-13 and -32 genotypes, whereas HPV vaccines target high-risk HPV genotypes [91].
However, although FEH is a benign condition caused by low-risk HPV genotypes, diagnosed cases may be at higher risk for infection with other viral genotypes. Of note, coinfection with multiple HPV genotypes is recognized as a risk factor for invasive cervical cancer (ICC) and high-grade squamous intraepithelial lesions (HSILs) [92], as is HPV-HIV coinfection for various squamous cell carcinomas [93]. Therefore, awareness, particularly of pediatric patients, who are preferentially targeted by HPV vaccination, remains critical to curbing coinfection, which can further increase the risk of malignancies.
Moreover, HPV vaccination also plays an essential role because Highly Active Antiretroviral Therapy (HAART) has been shown to be less effective for HIV–HPV coinfection-related malignancies, and to date, no vaccine against HIV is available [92].
Furthermore, the rate of migration from areas at a higher risk of infection to high-income countries has generally increased, proportionally increasing the number of potentially underdiagnosed cases.
Because oral FEH lesions often regress spontaneously [89] and have no cosmetic or functional consequences [84], treatment is often unnecessary [87]. However, it may be considered if the lesions become painful, interfere with occlusion, or present esthetic complications or social stigma [89,94]. Possible therapies include traditional surgery, laser surgery, electrocautery, cryotherapy, and trichloroacetic acid, excision of the lesion(s), or drug therapy with interferon, podophyllin, vitamin A, levamisole, or imiquimod [49,52,54,55,61,63,65,69,75,95]. These techniques, heterogeneously applied and associated with different success and recurrence rates, were also recorded in this systematic review [94], probably due to the lack of guidelines.
The surgical approach of excising lesions should be limited to single and limited lesions. However, in most cases, FEH occurs as multiple nodulo-papular lesions involving large and diverse mucosa and perilabial tissue areas.
Coherently, in this systematic review, single lesions accounted for 5.3% of cases, while the remaining 94.7% had multiple lesions, predominantly with bilateral and asymmetric patterns. In cases with greater mucosal involvement and considering the often young age of patients (first to second decade of life), conservative and atraumatic approaches may be preferred to preserve esthetics and functions.
From this perspective, a new therapeutic approach using nano-pulse stimulation (NPS) has shown promising results [94]. This technique uses ultrashort electrical pulses to induce localized cell death [94], in contrast to cryotherapy, electrodesiccation, or laser techniques, which produce less precise and effective thermal necrosis [94]. In addition, postoperative complications were minimal [94]. However, further studies on FEH cases treated with NPS are needed to confirm the true efficacy for lesions in the oral cavity, but this technique has already shown optimal safety and efficacy in the treatment of other non-genital warts.
4.3. Oral Malignant HPV-Related Lesions in Pediatric Subjects: Oral Squamous Cell Carcinoma
Oral squamous cell carcinoma is a malignant epithelial neoplasm of the head and neck and ranks seventh in worldwide cancer prevalence [96]. However, it remains rare in the pediatric population [20], often associated with congenital syndromes correlated with an increased cancer risk, such as Fanconi anemia, Li-Fraumeni syndrome, Bloom syndrome, ataxia telangiectasia, and xeroderma pigmentosum [97,98], but not others associated with various oral mucosal changes. According to a recent literature review by Lee et al. [18], there were 25 reported cases of OSCC in non-syndromic patients under 16 years of age, while the review by Magalhaes et al. [1] listed a total of 42 cases of OSCC in individuals under 20 years of age in the literature since 1976.
Although its oncogenic role is still not well understood [5], HPV appears to be responsible for 3% of oral carcinomas in adults [6]. To our knowledge, two pediatric cases of OSCC [1,18] have been identified in which HPV, although the viral genotype was not reported, or HPV-positive predictive proteins have been detected. In detail, p16 positivity was detected in both cases [1,18]. The overexpression of p16 is induced by the E7 viral protein of high-risk HPV [99], such as HPV-16 and HPV-18, which are most commonly associated with OSCC [6]. Consequently, detecting high levels of p16 predicts high-risk HPV infection [5]. However, on the one hand, elevated p16 levels were also found in HPV-negative tumors, and on the other hand, many HPV-positive OSCCs were negative for p16 [4], revealing a discrepancy between HPV DNA positivity and oncogenic activity [4]. Given these considerations, although the HPV association with pediatric oral cancer has been reported based on the p16 status of the tumors, the role of HPV in oral carcinogenesis in these cases is unknown, and HPV-associated OSCC would be extremely rare in a pediatric population.
The two young subjects with HPV-related OSCC were both males, which is consistent with other studies reporting a higher prevalence of OSCC in pediatric male patients [1,18] and of HPV-OSCC in adult males (70% of cases) [100], likely related to the higher rate of oral HPV infection in males, who usually experience oral rather than anogenital infections, compared with those in females [101].
In those two cases [1,18], lesions occurred in the maxillary alveolar ridge region, similar to 12 of 42 OSCC pediatric cases with the involvement of the gingival alveolar ridge described by Magalhaes et al. [1], probably due to embryonic tissue and increased stem cell activity implicated in odontogenesis until tooth eruption [1,98].
In both studies reporting HPV-related OSCC in pediatric subjects [1,18], information about possible HPV vaccination was not reported. Nevertheless, raising awareness of HPV vaccination among children and their parents and caregivers may be of even greater importance, especially in patients exposed to high-risk HPV [102]. In fact, persistent high-risk-HPV infections are associated with more than 90% of cervical cancers and several vaginal, anal, vulvar, penile, and oropharyngeal cancers [103].
5. Conclusions
The present systematic review showed that healthy pediatric patients (≤18 years of age) with HPV-related oral lesions had a mean age of 8.46 years and were most frequently diagnosed with FEH (51%), followed by squamous cell papillomas (25%), verrucae vulgaris (13%), and condyloma acuminata (9%). The HPV association with oral squamous cell carcinomas was described in two cases based on the p16 status of the tumors, and no viral genotype was reported; thus, the role of HPV in oral carcinogenesis in these cases remains unknown, and HPV-related OSCC would be extremely rare in a pediatric population [9]. The viral genotypes detected were HPV-13 (30.61%), -6 (20.41%), -11 (16.33%), HPV-2 (12.24%), -32 (10.20%), -57 (6.12%), and -16 (4.08%). No HPV vaccination was reported in any case.
Overall, these findings and the possible independent role of HPV in oral carcinogenesis underscore the importance of HPV vaccination. The currently available vaccines (bivalent, quadrivalent, and nonavalent) are the primary means of protection against HPV infection. Since the COVID-19 pandemic abruptly halted the HPV vaccination campaign, it is advisable to revive and exceed the vaccination schedule in time for the first pandemic peak.
Oral healthcare providers, who frequently encounter benign HPV-related lesions, should also take a leading role in this scenario, both in the early diagnosis and treatment of oral HPV-related lesions and in raising awareness of HPV vaccination among pediatric patients and their parents and caregivers.
Author Contributions
Conceptualization, F.D.S., M.P.D.P. and M.A.; Methodology, A.A. and G.P.; Validation, A.R. and M.A.; Formal Analysis, F.D.S., A.A. and A.R.; Data Curation, F.D.S., M.P.D.P., G.P. and A.A.; Writing—Original Draft Preparation, F.D.S., M.P.D.P. and G.P.; Writing—Review & Editing, A.A., A.R. and M.A.; Supervision. M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available in MEDLINE/PubMed, Scopus, and BioMed Central databases.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Magalhaes, M.A.O.; Somers, G.R.; Sikorski, P.; Forte, V.; Abouzgia, M.; Barrett, E.; Bradley, G. Unusual Presentation of Squamous Cell Carcinoma of the Maxilla in an 8-Year-Old Child. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, e179–e185. [Google Scholar] [CrossRef] [PubMed]
- Pina, A.; Fonseca, F.; Pontes, F.; Pontes, H.; Pires, F.; Taylor, A.; Aguirre-Urizar, J.; de Almeida, O. Benign Epithelial Oral Lesions—Association with Human Papillomavirus. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e290–e295. [Google Scholar] [CrossRef] [PubMed]
- Castro, T.P.P.G.; Filho, I.B. Prevalence of Human Papillomavirus (HPV) in Oral Cavity and Oropharynx. Braz. J. Otorhinolaryngol. 2006, 72, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus–Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Hübbers, C.U.; Akgül, B. HPV and Cancer of the Oral Cavity. Virulence 2015, 6, 244–248. [Google Scholar] [CrossRef]
- Sri, S.; Ramani, P.; Premkumar, P.; Ramshankar, V.; Ramasubramanian, A.; Krishnan, R. Prevalence of Human Papillomavirus (HPV) 16 and 18 in Oral Malignant and Potentially Malignant Disorders: A Polymerase Chain Reaction Analysis—A Comparative Study. Ann. Maxillofac. Surg. 2021, 11, 6. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Freedman, N.D.; Abnet, C.C. The Evolving Epidemiology of Oral Cavity and Oropharyngeal Cancers. Cancer Res. 2022, 82, 2821–2823. [Google Scholar] [CrossRef]
- Yete, S.; D’Souza, W.; Saranath, D. High-Risk Human Papillomavirus in Oral Cancer: Clinical Implications. Oncology 2018, 94, 133–141. [Google Scholar] [CrossRef]
- Muzio, L.L.; Ballini, A.; Cantore, S.; Bottalico, L.; Charitos, I.A.; Ambrosino, M.; Nocini, R.; Malcangi, A.; Dioguardi, M.; Cazzolla, A.P.; et al. Overview of Candida Albicans and Human Papillomavirus (HPV) Infection Agents and Their Biomolecular Mechanisms in Promoting Oral Cancer in Pediatric Patients. BioMed Res. Int. 2021, 2021, 7312611. [Google Scholar] [CrossRef]
- Petrosky, E.; Bocchini, J.A.; Hariri, S.; Chesson, H.; Curtis, C.R.; Saraiya, M.; Unger, E.R.; Markowitz, L.E.; Centers for Disease Control and Prevention (CDC). Use of 9-Valent Human Papillomavirus (HPV) Vaccine: Updated HPV Vaccination Recommendations of the Advisory Committee on Immunization Practices. Morb. Mortal. Wkly. Rep. 2015, 64, 300–304. [Google Scholar]
- Saslow, D.; Andrews, K.S.; Manassaram-Baptiste, D.; Smith, R.A.; Fontham, E.T.H. Human Papillomavirus Vaccination 2020 Guideline Update: American Cancer Society Guideline Adaptation. CA Cancer J. Clin. 2020, 70, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2008; ISBN 9780470699515. [Google Scholar]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A Framework for Formulating Good Questions to Explore the Association of Environmental and Other Exposures with Health Outcomes. Environ. Int. 2018, 121, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, S. Oral Manifestations of Human Papillomavirus Infections. Eur. J. Oral Sci. 2018, 126, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.L.; Thayer, K.A.; Santesso, N.; Holloway, A.C.; Blain, R.; Eftim, S.E.; Goldstone, A.E.; Ross, P.; Guyatt, G.; Schünemann, H.J. Evaluation of the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) and the ‘Target Experiment’ Concept in Studies of Exposures: Rationale and Preliminary Instrument Development. Environ. Int. 2018, 120, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Hashemipour, M.A.; Shoryabi, A.; Adhami, S.; Mehrabizadeh Honarmand, H. Extensive Focal Epithelial Hyperplasia. Arch. Iran. Med. 2010, 13, 48–52. [Google Scholar]
- Liu, N.; Wang, J.; Lei, L.; Li, Y.; Zhou, M.; Dan, H.; Zeng, X.; Chen, Q. Human Papillomavirus-32-Associated Focal Epithelial Hyperplasia Accompanying HPV-16-Positive Papilloma-Like Lesions in Oral Mucosa. J. Craniofacial Surg. 2013, 24, 905–908. [Google Scholar] [CrossRef]
- Lee, N.V.; Kang, E.T.B.; Senger, C.; Poh, C.F. Oral Cancer in a 5-Year-Old Boy: A Rare Case Report and Review of Literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, e10–e19. [Google Scholar] [CrossRef]
- Carmona Lorduy, M.; Harris Ricardo, J.; Hernández Arenas, Y.; Medina Carmona, W. Use of Trichloroacetic Acid for Management of Oral Lesions Caused by Human Papillomavirus. Gen Dent 2018, 66, 47–49. [Google Scholar]
- Misir, A.F.; Demiriz, L.; Barut, F. Laser Treatment of an Oral Squamous Papilloma in a Pediatric Patient: A Case Report. J. Indian Soc. Pedod. Prev. Dent. 2013, 31, 279. [Google Scholar] [CrossRef] [PubMed]
- Naghashfar, Z.; Sawada, E.; Kutcher, M.J.; Swancar, J.; Gupta, J.; Daniel, R.; Kashima, H.; Woodruff, J.D.; Shah, K. Identification of Genital Tract Papillomaviruses HPV-6 and HPV-16 in Warts of the Oral Cavity. J. Med. Virol. 1985, 17, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Premoli-de-Percoco, G.; Galindo, I.; Ramirez, J.L. In Situ Hybridization with Digoxigenin-Labelled DNA Probes for the Detection of Human Papillomavirus-Induced Focal Epithelial Hyperplasia among Venezuelans. Virchows Arch. A Pathol. Anat. Histopathol. 1992, 420, 295–300. [Google Scholar] [CrossRef]
- Sinclair, K.A.; Woods, C.R.; Kirse, D.J.; Sinal, S.H. Anogenital and Respiratory Tract Human Papillomavirus Infections among Children: Age, Gender, and Potential Transmission through Sexual Abuse. Pediatrics 2005, 116, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Adler-Storthz, K.; Newland, J.R.; Tessin, B.A.; Yeudall, W.A.; Shillitoe, E.J. Identification of Human Papillomavirus Types in Oral Verruca Vulgaris. J. Oral Pathol. Med. 1986, 15, 230–233. [Google Scholar] [CrossRef]
- Aldhafeeri, K.; Alshaikh, M.; Kilany, F.; AlKhaldi, S.; Alamri, A. Unusual Manifestation of Benign Squamous Papilloma of the Uvula: A Case Report and Review of Literature. Cureus 2020, 12, e6716. [Google Scholar] [CrossRef]
- Babich, S.B.; Haber, S.D.; Caviedes, E.Y.; Teplitsky, P. Condylomata Acuminata in a Boy. J. Am. Dent. Assoc. 2003, 134, 331–334. [Google Scholar] [CrossRef][Green Version]
- Beaudenon, S.; Praetorius, F.; Kremsdorf, D.; Lutzner, M.; Worsaae, N.; Pehau-Arnaudet, G.; Orth, G. A New Type of Human Papillomavirus Associated with Oral Focal Epithelial Hyperplasia. J. Investig. Dermatol. 1987, 88, 130–135. [Google Scholar] [CrossRef]
- Benyo, S.; Keane, A.; Warrick, J.; Choi, K.Y. HPV-positive Oral Papillomas in an Adolescent—A Diagnostic Dilemma. Clin. Case Rep. 2021, 9, e04546. [Google Scholar] [CrossRef]
- Boj, J.R.; Hernandez, M.; Espasa, E.; Poirier, C. Laser Treatment of an Oral Papilloma in the Pediatric Dental Office: A Case Report. Quintessence Int. 2007, 38, 307–312. [Google Scholar]
- Carneiro, T.E.; Marinho, S.A.; Verli, F.D.; Mesquita, A.T.M.; Lima, N.L.; Miranda, J.L. Oral Squamous Papilloma: Clinical, Histologic and Immunohistochemical Analyses. J. Oral Sci. 2009, 51, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, P.; Martha, S.; Punithvathy, R.; Reddy, M. Squamous Papilloma on Hard Palate: Case Report and Literature Review. Int. J. Clin. Pediatr. Dent. 2018, 11, 244–246. [Google Scholar] [CrossRef] [PubMed]
- de Meneses, R.K.L.; Sousa, C.G.; Gomes, D.Q.D.C.; Pereira, J.V.; Filho, T.J.D.S.; Nonaka, C.F.W.; Alves, P.M. Oral condyloma acuminatum in children: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, e172. [Google Scholar] [CrossRef]
- Devi, R.S.; Rajsekhar, B.; Srinivas, G.V.; Moon, N.J. Unusual Length of Pedicle: Pedunculated Squamous Papilloma of Uvula Causing Unusual Dysphagia of Long Duration in a Child of 10 Years. Case Rep. Dent. 2014, 2014, 313506. [Google Scholar] [CrossRef]
- Emmanouil, D.E.; Post, A.C. Oral Condyloma Acuminatum in a Child: Case Report. Pediatr. Dent. 1987, 9, 232–235. [Google Scholar] [PubMed]
- Orenuga, O.O.; Oluwo, A.; Oluwakuyide, R.T.; Olawuyi, A.B. Recurrent Oral Squamous Papilloma in a Pediatric Patient: Case Report and Review of the Literature. Niger. J. Clin. Pract. 2018, 21, 1674–1677. [Google Scholar] [CrossRef]
- Padayachee, A. Human Papillomavirus (HPV) Types 2 and 57 in Oral Verrucae Demonstrated by in Situ Hybridization. J. Oral Pathol. Med. 1994, 23, 413–417. [Google Scholar] [CrossRef]
- Paradisi, M.; Mostaccioli, S.; Celano, G.; Angelo, C.; Ruatti, P.; Muda, A.O.; Faraggiana, T. Infantile Condylomata of the Oral Cavity. Pediatr. Dermatol. 1992, 9, 107–111. [Google Scholar] [CrossRef]
- Percinoto, A.C.C.; Danelon, M.; Crivelini, M.M.; Cunha, R.F.; Percinoto, C. Condyloma Acuminata in the Tongue and Palate of a Sexually Abused Child: A Case Report. BMC Res. Notes 2014, 7, 467. [Google Scholar] [CrossRef]
- Premoli-de-Percoco, G.; Galindo, I.; Ramirez, J.L.; Perrone, M.; Rivera, H. Detection of Human Papillomavirus-Related Oral Verruca Vulgaris among Venezuelans. J. Oral Pathol. Med. 1993, 22, 113–116. [Google Scholar] [CrossRef]
- Puranen, M.; Yliskoski, M.; Saarikoski, S.; Syrjänen, K.; Syrjänen, S. Vertical Transmission of Human Papillomavirus from Infected Mothers to Their Newborn Babies and Persistence of the Virus in Childhood. Am. J. Obstet. Gynecol. 1996, 174, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Sabeena, S.; Pallade, S.R.; Kamath, N.; Mathew, M.; Arunkumar, G. Papilloma of Lip Associated with Human Papilloma Viruses-32 Infection in a Child. Indian J. Med. Microbiol. 2016, 34, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Sadaksharam, J.; Joshi, B. Multiple Papillomas in Oral Cavity of a Six Year Old Child. Indian J. Med. Res. 2019, 149, 680. [Google Scholar] [CrossRef]
- Squires, J.; Persaud, D.I.; Simon, P.; Sinn, D.P. Oral Condylomata in Children. Arch. Pediatr. Adolesc. Med. 1999, 153, 651. [Google Scholar] [CrossRef] [PubMed]
- Swan, R.H.; McDaniel, R.K.; Dreiman, B.B.; Rome, W.C. Condyloma Acuminatum Involving the Oral Mucosa. Oral Surg. Oral Med. Oral Pathol. 1981, 51, 503–508. [Google Scholar] [CrossRef]
- Wadhera, R.; Kalra, V.; Gulati, S.P.; Ghai, A. A Big Solitary Oropharyngeal Papilloma in a Child. Egypt. J. Ear Nose Throat Allied Sci. 2012, 13, 131–132. [Google Scholar] [CrossRef][Green Version]
- Yoshpe, N.S. Oral and Laryngeal Papilloma: A Pediatric Manifestation of Sexually Transmitted Disease? Int. J. Pediatr. Otorhinolaryngol. 1995, 31, 77–83. [Google Scholar] [CrossRef]
- Akyol, A.; Anadolu, R.; Anadolu, Y.; Ekmekci, P.; Gurgey, E.; Akay, N. Multifocal Papillomavirus Epithelial Hyperplasia: Successful Treatment with CO2 Laser Therapy Combined with Interferon Alpha-2b. Int. J. Dermatol. 2003, 42, 733–735. [Google Scholar] [CrossRef]
- Bennett, L.K.; Hinshaw, M. Heck’s Disease: Diagnosis and Susceptibility. Pediatr. Dermatol. 2009, 26, 87–89. [Google Scholar] [CrossRef]
- Binder, B.; Wieland, U.; Smolle, J. Focal Epithelial Hyperplasia (Heck Disease) in a Black Child. Pediatr. Dermatol. 2007, 24, E31–E32. [Google Scholar] [CrossRef]
- Bombeccari, G.P.; Pallotti, F.; Guzzi, G.; Spadari, F. Diode Laser Therapy for Heck’s Disease Associated with HPV13 Infection. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Borborema-Santos, C.M.; de Castro, M.M.; dos Santos, P.J.B.; Talhari, S.; Astolfi-Filho, S. Oral Focal Epithelial Hyperplasia: Report of Five Cases. Braz. Dent. J. 2006, 17, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Brehm, M.A.; Gordon, K.; Firan, M.; Rady, P.; Agim, N. Case Report of Focal Epithelial Hyperplasia (Heck’s Disease) with Polymerase Chain Reaction Detection of Human Papillomavirus 13. Pediatr. Dermatol. 2016, 33, e224–e225. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.R.; Hebert, A.A.; Adler-Storthz, K. Focal Epithelial Hyperplasia: Heck Disease. Pediatr. Dermatol. 1993, 10, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Durso, B.C.; Pinto, J.M.V.; Jorge, J.; de Almeida, O.P. Extensive Focal Epithelial Hyperplasia: Case Report. J. Can. Dent. Assoc. 2005, 71, 769–771. [Google Scholar] [PubMed]
- Falaki, F.; Amir Chaghmaghi, M.; Pakfetrat, A.; Delavarian, Z.; Mozaffari, P.M.; Pazooki, N. Detection of Human Papilloma Virus DNA in Seven Cases of Focal Epithelial Hyperplasia in Iran. J. Oral Pathol. Med. 2009, 38, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Garlick, J.A.; Calderon, S.; Buchner, A.; Mitrani-Rosenbaum, S. Detection of Human Papillomavirus (HPV) DNA in Focal Epithelial Hyperplasia. J. Oral Pathol. Med. 1989, 18, 172–177. [Google Scholar] [CrossRef]
- Hall, C.; McCullough, M.; Angel, C.; Manton, D. Multifocal Epithelial Hyperplasia: A Case Report of a Family of Somalian Descent Living in Australia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, e20–e24. [Google Scholar] [CrossRef]
- Liu, N.; Li, Y.; Zhou, Y.; Zeng, X. Focal Epithelial Hyperplasia (Heck’s Disease) in Two Chinese Females. Int. J. Oral Maxillofac. Surg. 2012, 41, 1001–1004. [Google Scholar] [CrossRef]
- Lutzner, M.; Kuffer, R.; Blanchet-Bardon, C.; Croissant, O. Different Papillomaviruses as the Causes of Oral Warts. Arch. Dermatol. 1982, 118, 393–399. [Google Scholar] [CrossRef]
- Mansouri, Z.; Bakhtiari, S.; Noormohamadi, R. Extensive Focal Epithelial Hyperplasia: A Case Report. Iran. J. Pathol. 2015, 10, 300–305. [Google Scholar] [PubMed]
- Martins, W.D.; de Lima, A.A.S.; Vieira, S. Focal Epithelial Hyperplasia (Heck’s Disease): Report of a Case in a Girl of Brazilian Indian Descent. Int. J. Paediatr. Dent. 2006, 16, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, S. Focal Epithelial Hyperplasia: Report of Two Cases and Review of Literature. J. Am. Dent. Assoc. 1986, 113, 900–902. [Google Scholar] [CrossRef] [PubMed]
- Nallanchakrava, S.; Sreebala, N.; Basavaraj; Sindgi, F. Laser Excision of Focal Epithelial Hyperplasia (Heck’s Disease): A Rare Case Report. Int. J. Clin. Pediatr. Dent. 2018, 11, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Nartey, N.; Newman, M.; Nyako, E. Focal Epithlieltal Hyperplasia: Report of Six Cases from Ghana, West Africa. J. Clin. Pediatr. Dent. 2003, 27, 63–66. [Google Scholar] [CrossRef]
- Ozden, B.; Gunduz, K.; Gunhan, O.; Ozden, F.O. A Case Report of Focal Epithelial Hyperplasia (Heck’s Disease) with PCR Detection of Human Papillomavirus. J. Maxillofac. Oral Surg. 2011, 10, 357–360. [Google Scholar] [CrossRef]
- Pfister, H.; Hettich, I.; Runne, U.; Gissmann, L.; Chilf, G.N. Characterization of Human Papillomavirus Type 13 from Focal Epithelial Hyperplasia Heck Lesions. J. Virol. 1983, 47, 363–366. [Google Scholar] [CrossRef]
- Puriene, A.; Rimkevicius, A.; Gaigalas, M. Focal Epithelial Hyperplasia: Case Report. Stomatologija 2011, 13, 102–104. [Google Scholar]
- Sarraj, A.; Mergoni, G.; Manfredi, M.; Meleti, M.; Vescovi, P. A Case of Heck’s Disease Treated with Quantum Molecular Resonance Scalpel. Ann. Stomatol. 2013, 4, 38–39. [Google Scholar]
- Saunders, N.R.; Scolnik, D.; Rebbapragada, A.; Koelink, E.; Craw, L.; Roth, S.; Aronson, L.; Perusini, S.; Silverman, M.S. Focal Epithelial Hyperplasia Caused by Human Papillomavirus 13. Pediatr. Infect. Dis. J. 2010, 29, 550–552. [Google Scholar] [CrossRef]
- Starink, T.M.; Woerdeman, M.J. Focal Epithelial Hyperplasia of the Oral Mucosa. Br. J. Dermatol. 1977, 96, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Tewfik, T.; Moroz, B.; Thibault, M.; Watters, K. Focal Epithelial Hyperplasia. Otolaryngol.—Head Neck Surg. 1995, 112, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.R. Focal Epithelial Hyperplasia (Heck’s Disease): Report of Case. J. Am. Dent. Assoc. 1976, 93, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Yasar, S.; Mansur, A.T.; Serdar, Z.A.; Goktay, F.; Aslan, C. Treatment of Focal Epithelial Hyperplasia with Topical Imiquimod: Report of Three Cases. Pediatr. Dermatol. 2009, 26, 465–468. [Google Scholar] [CrossRef]
- Gallottini Magalhães, M.; Franco Bueno, D.; Serra, E.; Gonçalves, R. Oral Manifestations of HIV Positive Children. J. Clin. Pediatr. Dent. 2001, 25, 103–106. [Google Scholar] [CrossRef]
- Di Spirito, F.; Caggiano, M.; di Palo, M.P.; Contaldo, M.; D’Ambrosio, F.; Martina, S.; Amato, A. Oral Lesions in Pediatric Subjects: SARS-CoV-2 Infection and COVID-19 Vaccination. Appl. Sci. 2022, 12, 8995. [Google Scholar]
- Betz, S.J. HPV-Related Papillary Lesions of the Oral Mucosa: A Review. Head Neck Pathol. 2019, 13, 80–90. [Google Scholar] [CrossRef]
- Wierzbicka, M.; Klussmann, J.P.; San Giorgi, M.R.; Wuerdemann, N.; Dikkers, F.G. Oral and Laryngeal HPV Infection: Incidence, Prevalence and Risk Factors, with Special Regard to Concurrent Infection in Head, Neck and Genitals. Vaccine 2021, 39, 2344–2350. [Google Scholar] [CrossRef]
- Di Spirito, F.; Iandolo, A.; Amato, A.; Caggiano, M.; Raimondo, A.; Lembo, S.; Martina, S. Prevalence, features and degree of association of oral lesions in COVID-19: A systematic review of systematic reviews. Int. J. Environ. Res. Public Health 2022, 19, 7486. [Google Scholar] [CrossRef]
- Gillison, M.L.; Broutian, T.; Pickard, R.K.L.; Tong, Z.; Xiao, W.; Kahle, L.; Graubard, B.I.; Chaturvedi, A.K. Prevalence of Oral HPV Infection in the United States, 2009–2010. JAMA 2012, 307, 693. [Google Scholar] [CrossRef]
- Petca, A.; Borislavschi, A.; Zvanca, M.; Petca, R.-C.; Sandru, F.; Dumitrascu, M. Non-Sexual HPV Transmission and Role of Vaccination for a Better Future (Review). Exp. Ther. Med. 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, M.; Martinelli-Kläy, C.P.; Lombardi, T. Clinical, Histopathological and Immunohistochemical Study of Oral Squamous Papillomas. Acta Odontol. Scand. 2015, 73, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Panici, P.B.; Scambia, G.; Perrone, L.; Battaglia, F.; Cattani, P.; Rabitti, C.; Dettori, G.; Capelli, A.; Sedlis, A.; Mancuso, S. Oral Condyloma Lesions in Patients with Extensive Genital Human Papillomavirus Infection. Am. J. Obstet. Gynecol. 1992, 167, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Di Spirito, F.; Amato, A.; di Palo, M.P.; Contaldo, M.; D’Ambrosio, F.; lo Giudice, R.; Amato, M. Oral Lesions Following Anti-SARS-CoV-2 Vaccination: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 10228. [Google Scholar]
- Bendtsen, S.K.; Jakobsen, K.K.; Carlander, A.-L.F.; Grønhøj, C.; von Buchwald, C. Focal Epithelial Hyperplasia. Viruses 2021, 13, 1529. [Google Scholar] [CrossRef] [PubMed]
- Prabhat, M.P.V.; Raja Lakshmi, C.; Sai Madhavi, N.; Bhavana, S.M.; Sarat, G.; Ramamohan, K. Multifocal Epithelial Hyperplasia of Oral Cavity Expressing HPV 16 Gene: A Rare Entity. Case Rep. Dent. 2013, 2013, 871306. [Google Scholar] [CrossRef]
- Sethi, S.; Ali, A.; Ju, X.; Antonsson, A.; Logan, R.; Jamieson, L. An Update on Heck’s Disease—a Systematic Review. J. Public Health 2022, 44, 269–285. [Google Scholar] [CrossRef]
- Said, A.K.; Leao, J.C.; Fedele, S.; Porter, S.R. Focal Epithelial Hyperplasia—An Update. J. Oral Pathol. Med. 2013, 42, 435–442. [Google Scholar] [CrossRef]
- Segura-Saint-Gerons, R.; Toro-Rojas, M.; Ceballos-Salobreña, A.; Aparicio-Soria, J.L.; Fuentes-Vaamonde, H. Focal Epithelial Hyperplasia. A Rare Disease in Our Area. Med. Oral Patol. Oral Cir. Bucal 2005, 10, 128–131. [Google Scholar]
- de Oliveira, C.M.; Fregnani, J.H.T.G.; Villa, L.L. HPV Vaccine: Updates and Highlights. Acta Cytol. 2019, 63, 159–168. [Google Scholar] [CrossRef]
- Carrillo-García, A.; Ponce-de-León-Rosales, S.; Cantú-de-León, D.; Fragoso-Ontiveros, V.; Martínez-Ramírez, I.; Orozco-Colín, A.; Mohar, A.; Lizano, M. Impact of Human Papillomavirus Coinfections on the Risk of High-Grade Squamous Intraepithelial Lesion and Cervical Cancer. Gynecol. Oncol. 2014, 134, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Vangipuram, R.; Tyring, S.K. AIDS-Associated Malignancies. Cancer Treat. Res. 2019, 177, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Elgash, M.; Bar, A.; Dhossche, J. Refractory Focal Epithelial Hyperplasia Successfully Treated with Novel Use of Nano-pulse Stimulation Technology. Pediatr. Dermatol. 2022, 39, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Santella, B.; Schettino, M.T.; Franci, G.; De Franciscis, P.; Colacurci, N.; Schiattarella, A.; Galdiero, M. Microbiota and HPV: The role of viral infection on vaginal microbiota. J. Med. Virol. 2022, 94, 4478–4484. [Google Scholar] [CrossRef]
- Di Spirito, F.; Amato, A.; Romano, A.; Dipalma, G.; Xhajanka, E.; Baroni, A.; Serpico, R.; Inchingolo, F.; Contaldo, M. Analysis of Risk Factors of Oral Cancer and Periodontitis from a Sex- and Gender-Related Perspective: Gender Dentistry. Appl. Sci. 2022, 12, 9135. [Google Scholar] [CrossRef]
- Sarode, G.S.; Batra, A.; Sarode, S.C.; Yerawadekar, S.; Patil, S. Oral Cancer-Related Inherited Cancer Syndromes: A Comprehensive Review. J. Contemp. Dent. Pract. 2016, 17, 504–510. [Google Scholar] [CrossRef]
- Bodner, L.; Manor, E.; Friger, M.D.; van der Waal, I. Oral Squamous Cell Carcinoma in Patients Twenty Years of Age or Younger—Review and Analysis of 186 Reported Cases. Oral Oncol. 2014, 50, 84–89. [Google Scholar] [CrossRef]
- Geißler, C.; Tahtali, A.; Diensthuber, M.; Gassner, D.; Stöver, T.; Wagenblast, J. The Role of P16 Expression as a Predictive Marker in HPV-Positive Oral SCCHN--a Retrospective Single-Center Study. Anticancer Res. 2013, 33, 913–916. [Google Scholar]
- Boguñá, N.; Capdevila, L.; Jané-Salas, E. El Virus Del Papiloma Humano y Su Relación Con La Patología de La Cavidad Oral. Med. Clin. 2019, 153, 157–164. [Google Scholar] [CrossRef]
- Khariwala, S.S.; Moore, M.G.; Malloy, K.M.; Gosselin, B.; Smith, R.V. The “HPV Discussion”. Otolaryngol.–Head Neck Surg. 2015, 153, 518–525. [Google Scholar] [CrossRef]
- Glenn, B.A.; Nonzee, N.J.; Tieu, L.; Pedone, B.; Cowgill, B.O.; Bastani, R. Human Papillomavirus (HPV) Vaccination in the Transition between Adolescence and Adulthood. Vaccine 2021, 39, 3435–3444. [Google Scholar] [CrossRef] [PubMed]
- Gay, J.; Johnson, N.; Kavuru, V.; Phillips, M. Utility of the Human Papillomavirus Vaccination in Management of HPV-Associated Cutaneous Lesions. Skin Ther. Lett. 2021, 26, 6–8. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).