Associations of Total Body Fat Mass and Skeletal Muscle Index with All-Cause and Cancer-Specific Mortality in Cancer Survivors
Abstract
Simple Summary
Abstract
1. Introduction
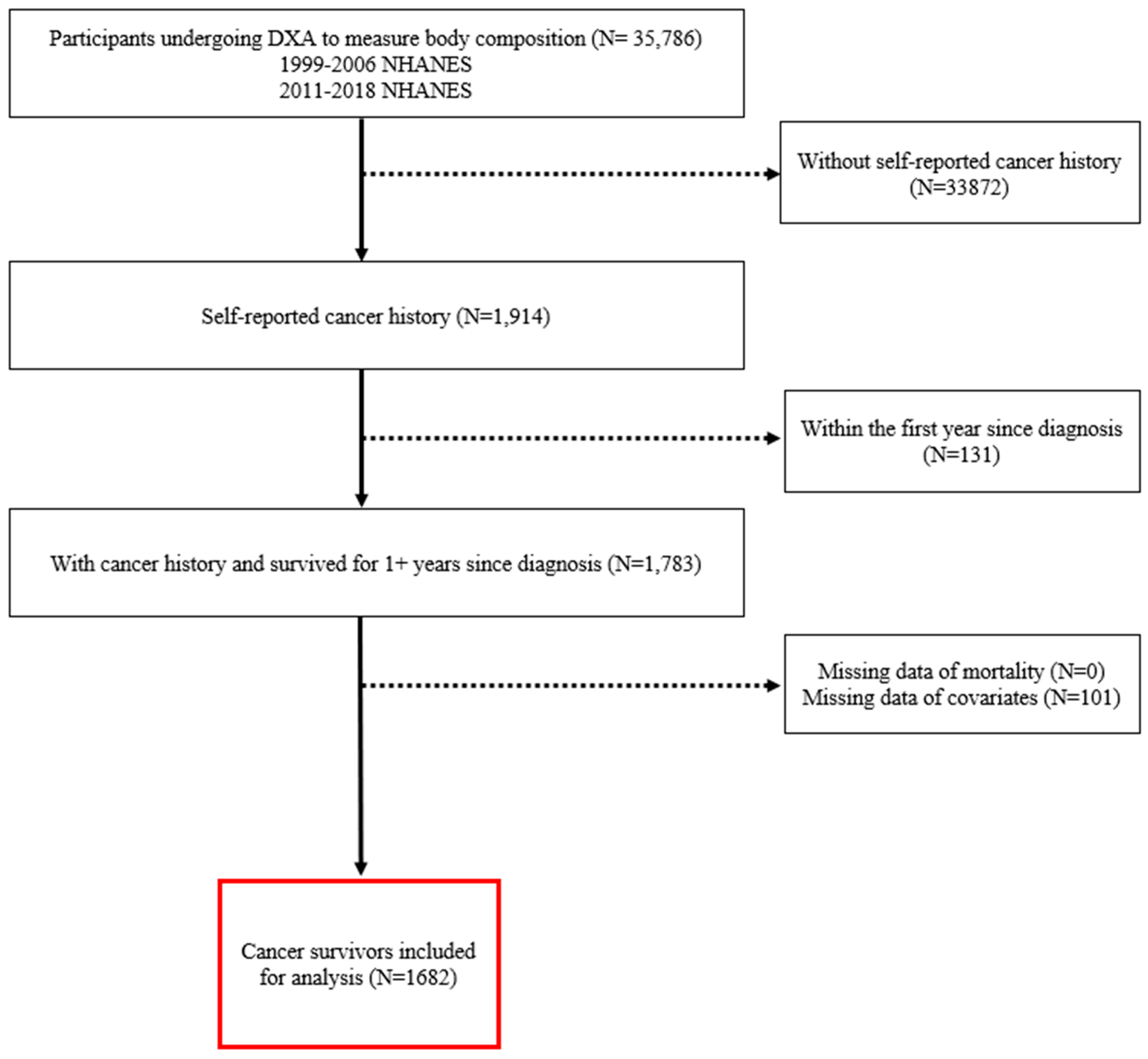
2. Materials and Methods
2.1. Exposures of Interest
2.2. Outcomes
2.3. Other Covariates
2.4. Statistical Analysis
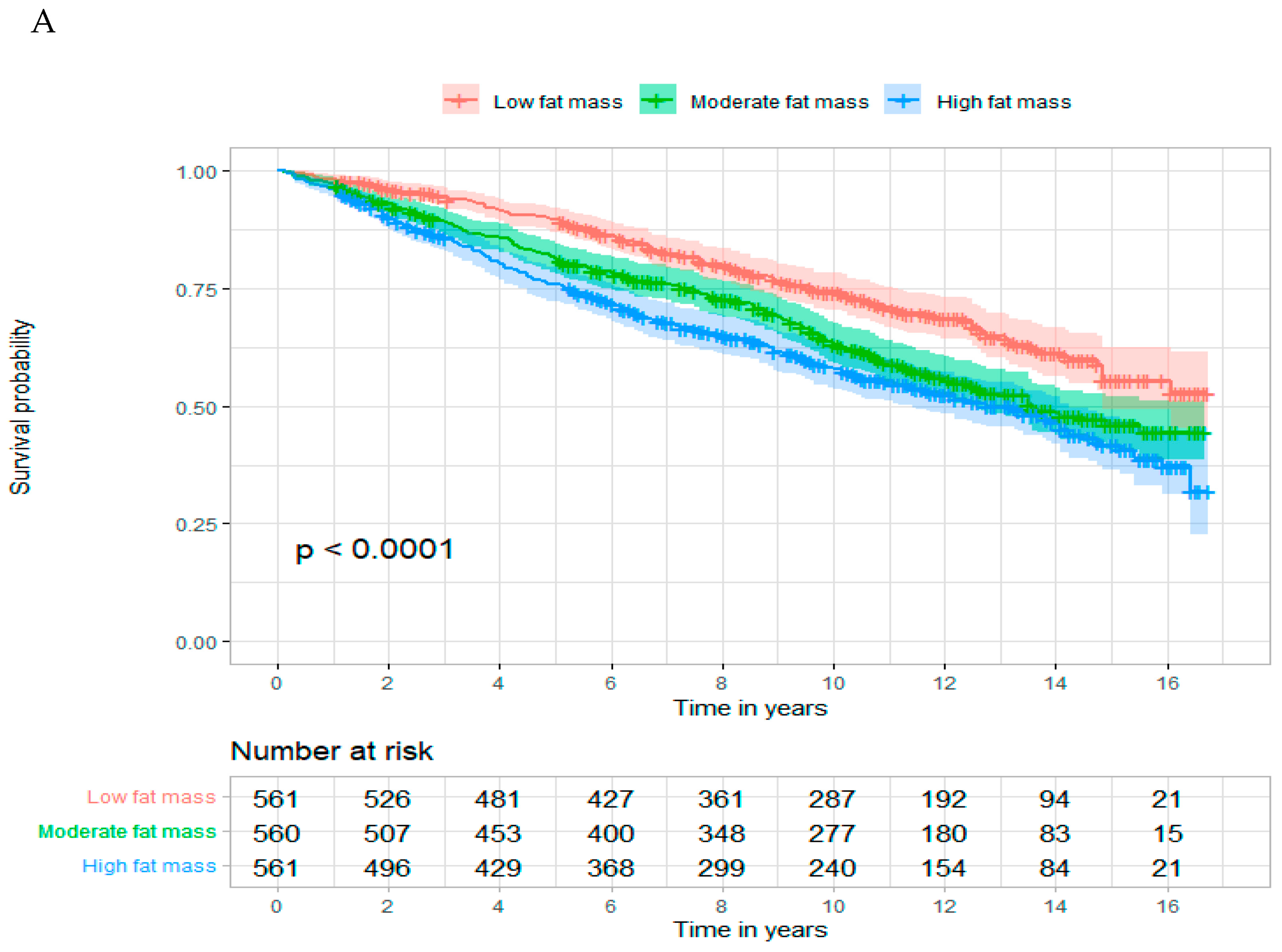
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Obesity and Cancer Fact Sheet—NCI. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/obesity-fact-sheet (accessed on 16 May 2022).
- Caan, B.J.; Cespedes Feliciano, E.M.; Kroenke, C.H. The Importance of Body Composition in Explaining the Overweight Paradox in Cancer-Counterpoint. Cancer Res. 2018, 78, 1906–1912. [Google Scholar] [CrossRef]
- Caan, B.J.; Cespedes Feliciano, E.M.; Prado, C.M.; Alexeeff, S.; Kroenke, C.H.; Bradshaw, P.; Quesenberry, C.P.; Weltzien, E.K.; Castillo, A.L.; Olobatuyi, T.A.; et al. Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol. 2018, 4, 798–804. [Google Scholar] [CrossRef]
- Yip, C.; Dinkel, C.; Mahajan, A.; Siddique, M.; Cook, G.J.R.; Goh, V. Imaging Body Composition in Cancer Patients: Visceral Obesity, Sarcopenia and Sarcopenic Obesity May Impact on Clinical Outcome. Insights Imaging 2015, 6, 489–497. [Google Scholar] [CrossRef]
- Alavi, D.H.; Henriksen, H.B.; Lauritzen, P.M.; Kværner, A.S.; Sakinis, T.; Langleite, T.M.; Henriksen, C.; Bøhn, S.K.; Paur, I.; Wiedswang, G.; et al. Quantification of Adipose Tissues by Dual-Energy X-Ray Absorptiometry and Computed Tomography in Colorectal Cancer Patients. Clin. Nutr. ESPEN 2021, 43, 360–368. [Google Scholar] [CrossRef]
- Arthur, R.S.; Xue, X.; Kamensky, V.; Chlebowski, R.T.; Simon, M.; Luo, J.; Shadyab, A.H.; Neuhouser, M.L.; Banack, H.; Ho, G.Y.F.; et al. The Association between DXA-derived Body Fat Measures and Breast Cancer Risk among Postmenopausal Women in the Women’s Health Initiative. Cancer Med. 2019, 9, 1581–1599. [Google Scholar] [CrossRef]
- Fukushima, H.; Yokoyama, M.; Nakanishi, Y.; Tobisu, K.; Koga, F. Sarcopenia as a Prognostic Biomarker of Advanced Urothelial Carcinoma. PLoS ONE 2015, 10, e0115895. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, Y.S.; Park, I.; Ahn, H.K.; Cho, E.K.; Jeong, Y.M. Prognostic Significance of CT-Determined Sarcopenia in Patients with Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 1795–1799. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, Y.S.; Kim, E.Y.; Jin, W. Prognostic Significance of CT-Determined Sarcopenia in Patients with Advanced Gastric Cancer. PLoS ONE 2018, 13, e0202700. [Google Scholar] [CrossRef]
- Prado, C.; Baracos, V.; McCargar, L.; Reiman, T.; Tonkin, K.; Mackey, J.; Koski, S.; Pituskin, E.; Sawyer, M. Sarcopenia as a Determinant of Chemotherapy Toxicity and Time to Tumor Progression in Metastatic Breast Cancer Patients Receiving Capecitabine Treatment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 2920–2926. [Google Scholar] [CrossRef]
- Dalal, S.; Hui, D.; Bidaut, L.; Lem, K.; Del Fabbro, E.; Crane, C.; Reyes-Gibby, C.C.; Bedi, D.; Bruera, E. Relationships among Body Mass Index, Longitudinal Body Composition Alterations, and Survival in Patients with Locally Advanced Pancreatic Cancer Receiving Chemoradiation: A Pilot Study. J. Pain Symptom Manag. 2012, 44, 181–191. [Google Scholar] [CrossRef]
- Parsons, H.A.; Baracos, V.E.; Dhillon, N.; Hong, D.S.; Kurzrock, R. Body Composition, Symptoms, and Survival in Advanced Cancer Patients Referred to a Phase I Service. PLoS ONE 2012, 7, e29330. [Google Scholar] [CrossRef]
- Bigaard, J.; Frederiksen, K.; Tjønneland, A.; Thomsen, B.L.; Overvad, K.; Heitmann, B.L.; Sørensen, T.I.A. Body Fat and Fat-Free Mass and All-Cause Mortality. Obes. Res. 2004, 12, 1042–1049. [Google Scholar] [CrossRef]
- Toss, F.; Wiklund, P.; Nordström, P.; Nordström, A. Body Composition and Mortality Risk in Later Life. Age Ageing 2012, 41, 677–681. [Google Scholar] [CrossRef]
- Kelly, T.L.; Wilson, K.E.; Heymsfield, S.B. Dual Energy X-Ray Absorptiometry Body Composition Reference Values from NHANES. PLoS ONE 2009, 4, e7038. [Google Scholar] [CrossRef]
- Heo, M.; Kabat, G.C.; Gallagher, D.; Heymsfield, S.B.; Rohan, T.E. Optimal Scaling of Weight and Waist Circumference to Height for Maximal Association with DXA-Measured Total Body Fat Mass by Sex, Age and Race/Ethnicity. Int. J. Obes. 2013, 37, 1154–1160. [Google Scholar] [CrossRef]
- Park, S.-Y.; Wilkens, L.R.; Murphy, S.P.; Monroe, K.R.; Henderson, B.E.; Kolonel, L.N. Body Mass Index and Mortality in an Ethnically Diverse Population: The Multiethnic Cohort Study. Eur. J. Epidemiol. 2012, 27, 489–497. [Google Scholar] [CrossRef]
- Patel, A.V.; Hildebrand, J.S.; Gapstur, S.M. Body Mass Index and All-Cause Mortality in a Large Prospective Cohort of White and Black U.S. Adults. PLoS ONE 2014, 9, e109153. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.-K.; Fielding, R.A.; Martin, F.C.; Michel, J.-P.; et al. Prevalence of and Interventions for Sarcopenia in Ageing Adults: A Systematic Review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef]
- Goodman, M.J.; Ghate, S.R.; Mavros, P.; Sen, S.; Marcus, R.L.; Joy, E.; Brixner, D.I. Development of a Practical Screening Tool to Predict Low Muscle Mass Using NHANES 1999–2004. J. Cachexia Sarcopenia Muscle 2013, 4, 187–197. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of Sarcopenia among the Elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Padwal, R.; Leslie, W.D.; Lix, L.M.; Majumdar, S.R. Relationship Among Body Fat Percentage, Body Mass Index, and All-Cause Mortality: A Cohort Study. Ann. Intern. Med. 2016, 164, 532–541. [Google Scholar] [CrossRef]
- Zong, G.; Zhang, Z.; Yang, Q.; Wu, H.; Hu, F.B.; Sun, Q. Total and Regional Adiposity Measured by Dual-Energy X-Ray Absorptiometry and Mortality in NHANES 1999–2006. Obesity 2016, 24, 2414–2421. [Google Scholar] [CrossRef]
- Allison, D.B.; Zhu, S.K.; Plankey, M.; Faith, M.S.; Heo, M. Differential Associations of Body Mass Index and Adiposity with All-Cause Mortality among Men in the First and Second National Health and Nutrition Examination Surveys (NHANES I and NHANES II) Follow-up Studies. Int. J. Obes. 2002, 26, 410–416. [Google Scholar] [CrossRef]
- Lee, D.H.; Keum, N.; Hu, F.B.; Orav, E.J.; Rimm, E.B.; Willett, W.C.; Giovannucci, E.L. Predicted Lean Body Mass, Fat Mass, and All Cause and Cause Specific Mortality in Men: Prospective US Cohort Study. BMJ 2018, 362, k2575. [Google Scholar] [CrossRef]
- Heitmann, B.L.; Erikson, H.; Ellsinger, B.M.; Mikkelsen, K.L.; Larsson, B. Mortality Associated with Body Fat, Fat-Free Mass and Body Mass Index among 60-Year-Old Swedish Men-a 22-Year Follow-up. The Study of Men Born in 1913. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2000, 24, 33–37. [Google Scholar] [CrossRef]
- Rolland, Y.; Gallini, A.; Cristini, C.; Schott, A.-M.; Blain, H.; Beauchet, O.; Cesari, M.; Lauwers-Cances, V. Body-Composition Predictors of Mortality in Women Aged ≥75 y: Data from a Large Population-Based Cohort Study with a 17-y Follow-Up. Am. J. Clin. Nutr. 2014, 100, 1352–1360. [Google Scholar] [CrossRef]
- Auyeung, T.W.; Lee, J.S.W.; Leung, J.; Kwok, T.; Leung, P.C.; Woo, J. Survival in Older Men May Benefit From Being Slightly Overweight and Centrally Obese—A 5-Year Follow-up Study in 4000 Older Adults Using DXA. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65A, 99–104. [Google Scholar] [CrossRef]
- Cesari, M.; Pahor, M.; Lauretani, F.; Zamboni, V.; Bandinelli, S.; Bernabei, R.; Guralnik, J.M.; Ferrucci, L. Skeletal Muscle and Mortality Results from the InCHIANTI Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64A, 377–384. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Shaper, A.G.; Lennon, L.; Whincup, P.H. Decreased Muscle Mass and Increased Central Adiposity Are Independently Related to Mortality in Older Men. Am. J. Clin. Nutr. 2007, 86, 1339–1346. [Google Scholar] [CrossRef]
- Genton, L.; Graf, C.E.; Karsegard, V.L.; Kyle, U.G.; Pichard, C. Low Fat-Free Mass as a Marker of Mortality in Community-Dwelling Healthy Elderly Subjects†. Age Ageing 2013, 42, 33–39. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Brown, J.C.; Yang, S.; Mire, E.F.; Wu, X.-C.; Miele, L.; Ochoa, A.C.; Zabaleta, J. Association of Abdominal Visceral Adiposity and Total Fat Mass with Cancer Incidence and Mortality in White and Black Adults. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1532–1538. [Google Scholar] [CrossRef]
- Velho, S.; Costa Santos, M.P.; Cunha, C.; Agostinho, L.; Cruz, R.; Costa, F.; Garcia, M.; Oliveira, P.; Maio, R.; Baracos, V.E.; et al. Body Composition Influences Post-Operative Complications and 90-Day and Overall Survival in Pancreatic Surgery Patients. GE Port. J. Gastroenterol. 2021, 28, 13–25. [Google Scholar] [CrossRef]
- Lopez, P.; Newton, R.U.; Taaffe, D.R.; Singh, F.; Buffart, L.M.; Spry, N.; Tang, C.; Saad, F.; Galvão, D.A. Associations of Fat and Muscle Mass with Overall Survival in Men with Prostate Cancer: A Systematic Review with Meta-Analysis. Prostate Cancer Prostatic Dis. 2021, 25, 615–626. [Google Scholar] [CrossRef]
- Cheng, E.; Kirley, J.; Cespedes Feliciano, E.M.; Caan, B.J. Adiposity and Cancer Survival: A Systematic Review and Meta-Analysis. Cancer Causes Control CCC 2022, 33, 1219–1246. [Google Scholar] [CrossRef]
- Kuk, J.L.; Ardern, C.I. Influence of Age on the Association between Various Measures of Obesity and All-Cause Mortality. J. Am. Geriatr. Soc. 2009, 57, 2077–2084. [Google Scholar] [CrossRef]
- Metter, E.J.; Talbot, L.A.; Schrager, M.; Conwit, R. Skeletal Muscle Strength as a Predictor of All-Cause Mortality in Healthy Men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, B359–B365. [Google Scholar] [CrossRef]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, but Not Muscle Mass, Is Associated with Mortality in the Health, Aging and Body Composition Study Cohort. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 72–77. [Google Scholar] [CrossRef]
- Simpson, J.A.; MacInnis, R.J.; Peeters, A.; Hopper, J.L.; Giles, G.G.; English, D.R. A Comparison of Adiposity Measures as Predictors of All-Cause Mortality: The Melbourne Collaborative Cohort Study. Obesity 2007, 15, 994–1003. [Google Scholar] [CrossRef]
- Adams, K.F.; Schatzkin, A.; Harris, T.B.; Kipnis, V.; Mouw, T.; Ballard-Barbash, R.; Hollenbeck, A.; Leitzmann, M.F. Overweight, Obesity, and Mortality in a Large Prospective Cohort of Persons 50 to 71 Years Old. N. Engl. J. Med. 2006, 355, 763–778. [Google Scholar] [CrossRef]
- McTigue, K.; Larson, J.C.; Valoski, A.; Burke, G.; Kotchen, J.; Lewis, C.E.; Stefanick, M.L.; Van Horn, L.; Kuller, L. Mortality and Cardiac and Vascular Outcomes in Extremely Obese Women. JAMA 2006, 296, 79–86. [Google Scholar] [CrossRef]
- Pischon, T.; Boeing, H.; Hoffmann, K.; Bergmann, M.; Schulze, M.B.; Overvad, K.; van der Schouw, Y.T.; Spencer, E.; Moons, K.G.M.; Tjønneland, A.; et al. General and Abdominal Adiposity and Risk of Death in Europe. N. Engl. J. Med. 2008, 359, 2105–2120. [Google Scholar] [CrossRef]
- Freedman, D.M.; Ron, E.; Ballard-Barbash, R.; Doody, M.M.; Linet, M.S. Body Mass Index and All-Cause Mortality in a Nationwide US Cohort. Int. J. Obes. 2006, 30, 822–829. [Google Scholar] [CrossRef]
- Gelber, R.P.; Kurth, T.; Manson, J.E.; Buring, J.E.; Gaziano, J.M. Body Mass Index and Mortality in Men: Evaluating the Shape of the Association. Int. J. Obes. 2007, 31, 1240–1247. [Google Scholar] [CrossRef]
- Koster, A.; Leitzmann, M.F.; Schatzkin, A.; Adams, K.F.; van Eijk, J.T.M.; Hollenbeck, A.R.; Harris, T.B. The Combined Relations of Adiposity and Smoking on Mortality. Am. J. Clin. Nutr. 2008, 88, 1206–1212. [Google Scholar] [CrossRef]
- Pednekar, M.S.; Hakama, M.; Hebert, J.R.; Gupta, P.C. Association of Body Mass Index with All-Cause and Cause-Specific Mortality: Findings from a Prospective Cohort Study in Mumbai (Bombay), India. Int. J. Epidemiol. 2008, 37, 524–535. [Google Scholar] [CrossRef]
- Ringbäck Weitoft, G.; Eliasson, M.; Rosén, M. Underweight, Overweight and Obesity as Risk Factors for Mortality and Hospitalization. Scand. J. Public Health 2008, 36, 169–176. [Google Scholar] [CrossRef]
- Boggs, D.A.; Rosenberg, L.; Cozier, Y.C.; Wise, L.A.; Coogan, P.F.; Ruiz-Narvaez, E.A.; Palmer, J.R. General and Abdominal Obesity and Risk of Death among Black Women. N. Engl. J. Med. 2011, 365, 901–908. [Google Scholar] [CrossRef]
- Stevens, J. Obesity and Mortality in African Americans. Nutr. Rev. 2000, 58, 346–353. [Google Scholar] [CrossRef]
- Reis, J.P.; Araneta, M.R.; Wingard, D.L.; Macera, C.A.; Lindsay, S.P.; Marshall, S.J. Overall Obesity and Abdominal Adiposity as Predictors of Mortality in u.s. White and Black Adults. Ann. Epidemiol. 2009, 19, 134–142. [Google Scholar] [CrossRef]
- Calle, E.E.; Thun, M.J.; Petrelli, J.M.; Rodriguez, C.; Heath, C.W. Body-Mass Index and Mortality in a Prospective Cohort of U.S. Adults. N. Engl. J. Med. 1999, 341, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Ziolkowski, S.L.; Long, J.; Baker, J.F.; Chertow, G.M.; Leonard, M.B. Relative Sarcopenia and Mortality and the Modifying Effects of Chronic Kidney Disease and Adiposity. J. Cachexia Sarcopenia Muscle 2019, 10, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Hurst, L.; Stafford, M.; Cooper, R.; Hardy, R.; Richards, M.; Kuh, D. Lifetime Socioeconomic Inequalities in Physical and Cognitive Aging. Am. J. Public Health 2013, 103, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Fanelli Kuczmarski, M.; Mason, M.A.; Beydoun, M.A.; Allegro, D.; Zonderman, A.B.; Evans, M.K. Dietary Patterns and Sarcopenia in an Urban African American and White Population in the United States. J. Nutr. Gerontol. Geriatr. 2013, 32, 291–316. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Weinberg, R.A. A Perspective on Cancer Cell Metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Pak, S.; Kim, M.S.; Park, E.Y.; Kim, S.H.; Lee, K.H.; Joung, J.Y. Association of Body Composition With Survival and Treatment Efficacy in Castration-Resistant Prostate Cancer. Front. Oncol. 2020, 10, 558. [Google Scholar] [CrossRef]
- Ying, P.; Jin, W.; Wu, X.; Cai, W. Association between CT-Quantified Body Composition and Recurrence, Survival in Nonmetastasis Colorectal Cancer Patients Underwent Regular Chemotherapy after Surgery. BioMed Res. Int. 2021, 2021, 6657566. [Google Scholar] [CrossRef]
- Han, J.S.; Ryu, H.; Park, I.J.; Kim, K.W.; Shin, Y.; Kim, S.O.; Lim, S.-B.; Kim, C.W.; Yoon, Y.S.; Lee, J.L.; et al. Association of Body Composition with Long-Term Survival in Non-Metastatic Rectal Cancer Patients. Cancer Res. Treat. 2020, 52, 563–572. [Google Scholar] [CrossRef]
- Cespedes Feliciano, E.M.; Kroenke, C.H.; Meyerhardt, J.A.; Prado, C.M.; Bradshaw, P.T.; Kwan, M.L.; Xiao, J.; Alexeeff, S.; Corley, D.; Weltzien, E.; et al. Association of Systemic Inflammation and Sarcopenia With Survival in Nonmetastatic Colorectal Cancer: Results From the C SCANS Study. JAMA Oncol. 2017, 3, e172319. [Google Scholar] [CrossRef]
- Deluche, E.; Leobon, S.; Desport, J.C.; Venat-Bouvet, L.; Usseglio, J.; Tubiana-Mathieu, N. Impact of Body Composition on Outcome in Patients with Early Breast Cancer. Support. Care Cancer 2018, 26, 861–868. [Google Scholar] [CrossRef]
- Pak, S.; Park, S.Y.; Shin, T.J.; You, D.; Jeong, I.G.; Hong, J.H.; Kim, C.-S.; Ahn, H. Association of Muscle Mass with Survival after Radical Prostatectomy in Patients with Prostate Cancer. J. Urol. 2019, 202, 525–532. [Google Scholar] [CrossRef]
- Narici, M.V.; Maffulli, N. Sarcopenia: Characteristics, Mechanisms and Functional Significance. Br. Med. Bull. 2010, 95, 139–159. [Google Scholar] [CrossRef]
- Avgerinou, C. Sarcopenia: Why It Matters in General Practice. Br. J. Gen. Pract. 2020, 70, 200–201. [Google Scholar] [CrossRef]



| Cancer Survivors (N = 1682) No. (%) | ||||
|---|---|---|---|---|
| Overall | Low Total Fat Mass | Moderate Total Fat Mass | High Total Fat Mass | |
| Participant Characteristics | 1682 (100) | 561 (33.3) | 560 (33.3) | 561 (33.3) |
| Age (year) | ||||
| <50 | 402 (23.9) | 161 (28.7) | 101 (18.0) | 140 (25.0) |
| 50–64 | 421 (25.0) | 169 (30.1) | 127 (22.7) | 125 (22.3) |
| 65–74 | 426 (25.3) | 150 (26.7) | 154 (27.5) | 122 (21.7) |
| ≥75 | 433 (25.7) | 81 (14.4) | 178 (31.8) | 174 (31.0) |
| Sex | ||||
| Female | 737 (43.8) | 164 (29.2) | 255 (45.5) | 318 (56.7) |
| Male | 945 (56.2) | 397 (70.8) | 305 (54.5) | 243 (43.3) |
| Race | ||||
| White | 1213 (72.1) | 380 (67.7) | 410 (73.2) | 423 (75.4) |
| Black | 216 (12.8) | 94 (16.8) | 58 (10.4) | 64 (11.4) |
| Other | 253 (15.0) | 87 (15.5) | 92 (16.4) | 74 (13.2) |
| Education | ||||
| High school or less | 810 (48.2) | 272 (48.5) | 265 (47.3) | 273 (48.8) |
| Attended college | 473 (28.2) | 179 (31.9) | 148 (26.4) | 146 (26.1) |
| Graduated from college | 397 (23.6) | 110 (1.6) | 147 (26.3) | 140 (25.0) |
| Marital status | ||||
| Not married | 672 (40.6) | 235 (42.4) | 215 (39.2) | 222 (40.1) |
| Married or living with partner | 983 (59.4) | 319 (57.6) | 333 (60.8) | 331 (59.9) |
| Smoking status | ||||
| Never | 664 (68.2) | 223 (70.3) | 229 (74.4) | 212 (60.7) |
| Current | 275 (28.2) | 82 (25.9) | 70 (22.7) | 123 (35.2) |
| Former | 35 (3.6) | 12 (3.8) | 9 (2.9) | 14 (4.0) |
| BMI | ||||
| Underweight | 28 (2.1) | 28 (6.2) | 0 (0.0) | 0 (0.0) |
| Normal | 411 (30.7) | 307 (67.9) | 103 (22.4) | 1 (0.2) |
| Overweight | 478 (35.8) | 116 (25.7) | 284 (61.7) | 78 (18.4) |
| Obese | 420 (31.4) | 1 (0.2) | 73 (15.9) | 346 (81.4) |
| Energy intake (kcal/day) | ||||
| <1359.0 | 345 (26.4) | 117 (28.0) | 114 (25.5) | 114 (25.9) |
| 1360.0–1784 | 323 (24.7) | 107 (25.6) | 112 (25.1) | 104 (23.6) |
| 1785–2348 | 354 (27.1) | 112 (26.8) | 120 (26.8) | 122 (27.7) |
| ≥2349.0 | 284 (21.7) | 82 (19.6) | 101 (22.6) | 101 (22.9) |
| ASMI | ||||
| Without sarcopenia | 913 (75.0) | 326 (88.8) | 328 (78.3) | 259 (60.1) |
| With sarcopenia | 304 (25.0) | 41 (11.2) | 91 (21.7) | 172 (39.9) |
| No. comorbidities | ||||
| 0 | 519 (30.9) | 135 (24.1) | 178 (31.8) | 206 (36.7) |
| 1 | 450 (26.8) | 164 (29.2) | 156 (27.9) | 130 (23.2) |
| ≥2 | 713 (42.4) | 262 (46.7) | 226 (40.4) | 225 (40.1) |
| Stroke | ||||
| No | 1566 (93.3) | 525 (93.6) | 522 (93.7) | 519 (92.5) |
| Yes | 113 (6.7) | 36 (6.4) | 35 (6.3) | 42 (7.5) |
| Coronary heart disease | ||||
| No | 1215 (89.8) | 395 (91.4) | 418 (89.7) | 402 (88.4) |
| Yes | 138 (10.2) | 37 (8.6) | 48 (10.3) | 53 (11.6) |
| Congestive heart failure | ||||
| No | 1252 (91.5) | 401 (90.7) | 431 (91.7) | 420 (91.9) |
| Yes | 117 (8.5) | 41 (9.3) | 39 (8.3) | 37 (8.1) |
| Heart attack | ||||
| No | 1521 (90.9) | 520 (93.4) | 499 (89.4) | 502 (89.8) |
| Yes | 135 (9.1) | 37 (6.6) | 59 (10.6) | 57 (10.2) |
| Continuous variables, mean (SD) | ||||
| Waist circumference | 99.4 (15.0) | 113 (12.9) | 98.4 (9.3) | 87.1 (9.4) |
| Height | 167 (9.8) | 167 (9.6) | 167 (10.2) | 167 (9.7) |
| Weight | 79.5 (19.2) | 97.0 (17.3) | 76.8 (12.0) | 64.8 (11.4) |
| No. Death/Person-Years | Age-Adjusted HR (95% CI) | aHR (95% CI) | aHR (95% CI) | |
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| All-cause mortality | ||||
| Total fat mass | ||||
| Low | 169/1602.3 | Ref | Ref | Ref |
| Medium | 237/2144.8 | 1.48 (1.21–1.80) | 1.09 (0.89–1.34) | 1.09 (0.89–1.34) |
| High | 262/2207.4 | 1.76 (1.45–2.13) | 1.30 (1.06–1.61) | 1.31 (1.06–1.61) |
| Appendicular skeletal muscle mass | ||||
| Without sarcopenia | 253/8624.7 | Ref | Ref | Ref |
| With sarcopenia | 155/2422.8 | 1.58 (1.28–1.94) | 1.51 (1.22–1.88) | 1.60 (1.28–1.99) |
| Waist circumference | ||||
| Normal | 211/6320.0 | Ref | Ref | Ref |
| High | 457/8801.2 | 0.85 (1.17–0.73) | 1.23 (0.98–1.54) | 1.01 (0.82–1.47) |
| Body Mass Index | ||||
| Normal | 219/4450.1 | Ref | Ref | Ref |
| obese | 171/5007.4 | 0.68 (0.58–0.80) | 0.71 (0.50–1.10) | 0.66 (0.41–1.14) |
| Cancer-specific mortality | ||||
| Total fat mass | ||||
| Low | 58/4768.8 | Ref | Ref | Ref |
| Medium | 72/4416.3 | 1.04 (0.73–1.47) | 1.01 (0.71–1.44) | 0.99 (0.70–1.42) |
| High | 83/4195.8 | 1.42 (1.01–2.00) | 1.24 (0.87–1.77) | 1.23 (0.86–1.75) |
| Appendicular skeletal muscle mass | ||||
| Without sarcopenia | 83/7840.7 | Ref | Ref | Ref |
| With sarcopenia | 56/1976.5 | 1.88 (1.33–2.65) | 1.74 (1.23–2.29) | 1.84 (1.28–2.65) |
| Waist circumference | ||||
| Normal | 66/6320.0 | Ref | Ref | Ref |
| High | 147/8801.2 | 0.72 (0.54–0.96) | 0.67 (0.50–0.90) | 0.62 (0.43–0.89) |
| Body Mass Index | ||||
| Normal | 82/4737.7 | Ref | Ref | Ref |
| Overweight or obese | 126/10,104.8 | 0.63 (0.48–0.83) | 0.58 (0.43–0.78) | 0.51 (0.35–0.73) |
| Measures of Body Composition | Obesity-Related Cancers (n = 699) | Non-Obesity-Related Cancers (n= 983) | ||
|---|---|---|---|---|
| Model 1 Age Adjusted HR (95 CI) | Model 2 Multivariable HR (95 CI) | Model 1 Age Adjusted HR (95 CI) | Model 2 Multivariable HR (95 CI) | |
| All-cause mortality | ||||
| Total fat mass | ||||
| Low | Ref | Ref | Ref | Ref |
| Medium | 1.14 (0.84–1.54) | 1.26 (0.93–1.71) | 1.02 (0.77–1.35) | 0.99 (0.74–1.32) |
| High | 1.81 (1.34–2.43) | 1.69 (1.23–2.32) | 1.27 (0.97–1.67) | 1.14 (0.86–1.52) |
| Appendicular skeletal muscle mass | ||||
| Without sarcopenia | Ref | Ref | Ref | Ref |
| With sarcopenia | 1.49 (1.09–2.03) | 1.53 (1.10–2.13) | 1.79 (1.35–2.36) | 1.61 (1.18–2.19) |
| Cancer-specific mortality | ||||
| Whole body fat mass | ||||
| Low | Ref | Ref | Ref | Ref |
| Medium | 1.09 (0.65–1.81) | 1.08 (0.65–1.81) | 1.00 (0.60–1.67) | 0.99 (0.59–1.67) |
| High | 1.51 (0.91–2.52) | 1.42 (0.84–2.41) | 1.54 (0.95–2.48) | 1.35 (0.81–2.23) |
| Appendicular skeletal muscle mass | ||||
| Without sarcopenia | ||||
| With sarcopenia | Ref | Ref | Ref | Ref |
| 1.65 (0.98–2.77) | 1.76 (1.03–3.02) | 2.23 (1.38–3.62) | 1.80 (1.04–3.12) | |
| Measures of Body Composition | Whites | Blacks | ||
|---|---|---|---|---|
| Model 1 Age Adjusted HR (95 CI) | Model 2 Multivariable HR (95 CI) | Model 1 Age Adjusted HR (95 CI) | Model 2 Multivariable HR (95 CI) | |
| All-cause mortality | ||||
| Total fat mass | ||||
| Low | Ref | Ref | Ref | Ref |
| Medium | 1.27 (0.97–1.56) | 1.24 (0.97–1.57) | 1.02 (0.98–0.59) | 0.78 (0.44–1.38) |
| High | 1.50 (1.18–1.50) | 1.37 (1.07–1.75) | 1.80 (1.12–1.78) | 1.27 (0.73–2.22) |
| Appendicular skeletal muscle mass index | ||||
| Without sarcopenia | Ref | Ref | Ref | Ref |
| With sarcopenia | 1.57 (1.23–1.98) | 1.53 (1.19–1.95) | 2.76 (1.44–5.32) | 2.99 (1.39–6.06) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aduse-Poku, L.; Karanth, S.D.; Wheeler, M.; Yang, D.; Washington, C.; Hong, Y.-R.; Manini, T.M.; Fabregas, J.C.; Cheng, T.-Y.D.; Braithwaite, D. Associations of Total Body Fat Mass and Skeletal Muscle Index with All-Cause and Cancer-Specific Mortality in Cancer Survivors. Cancers 2023, 15, 1081. https://doi.org/10.3390/cancers15041081
Aduse-Poku L, Karanth SD, Wheeler M, Yang D, Washington C, Hong Y-R, Manini TM, Fabregas JC, Cheng T-YD, Braithwaite D. Associations of Total Body Fat Mass and Skeletal Muscle Index with All-Cause and Cancer-Specific Mortality in Cancer Survivors. Cancers. 2023; 15(4):1081. https://doi.org/10.3390/cancers15041081
Chicago/Turabian StyleAduse-Poku, Livingstone, Shama D. Karanth, Meghann Wheeler, Danting Yang, Caretia Washington, Young-Rock Hong, Todd M. Manini, Jesus C. Fabregas, Ting-Yuan David Cheng, and Dejana Braithwaite. 2023. "Associations of Total Body Fat Mass and Skeletal Muscle Index with All-Cause and Cancer-Specific Mortality in Cancer Survivors" Cancers 15, no. 4: 1081. https://doi.org/10.3390/cancers15041081
APA StyleAduse-Poku, L., Karanth, S. D., Wheeler, M., Yang, D., Washington, C., Hong, Y.-R., Manini, T. M., Fabregas, J. C., Cheng, T.-Y. D., & Braithwaite, D. (2023). Associations of Total Body Fat Mass and Skeletal Muscle Index with All-Cause and Cancer-Specific Mortality in Cancer Survivors. Cancers, 15(4), 1081. https://doi.org/10.3390/cancers15041081