Whole-Body Composition Features by Computed Tomography in Ovarian Cancer: Pilot Data on Survival Correlations
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Clinical Data Recorded
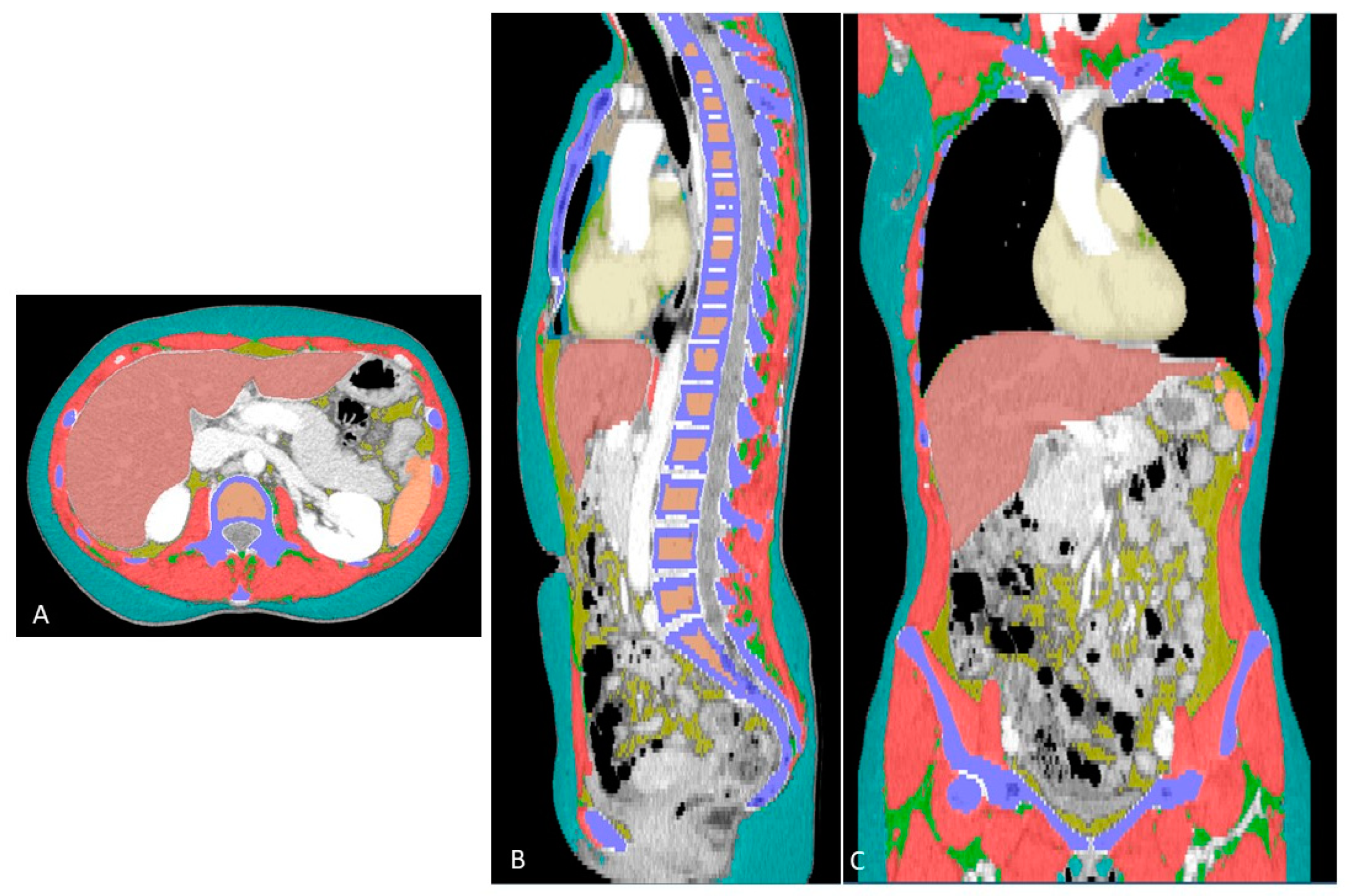
2.3. Extraction of Whole-Body Composition Features
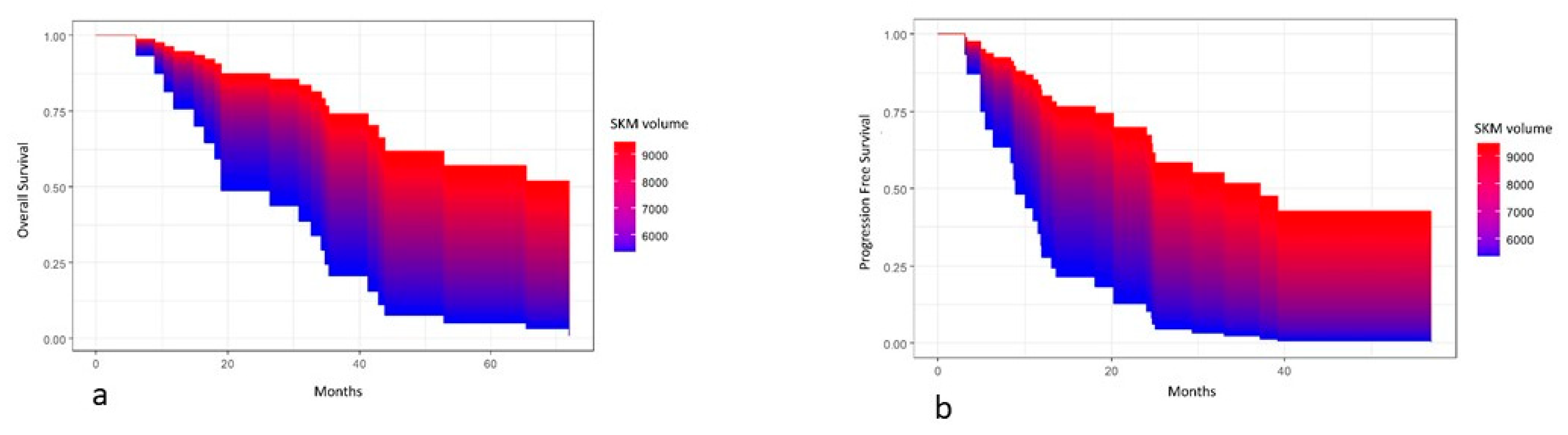
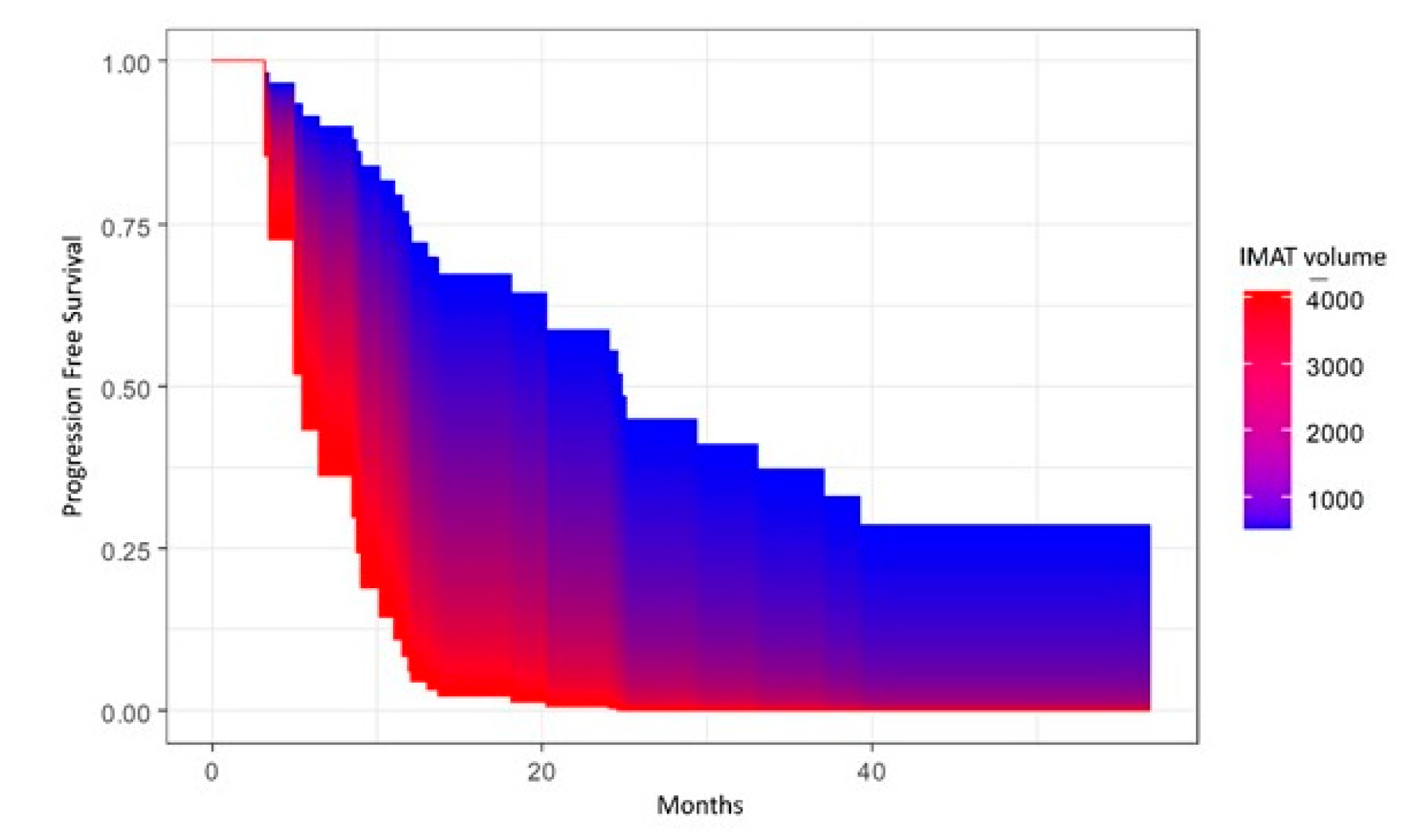
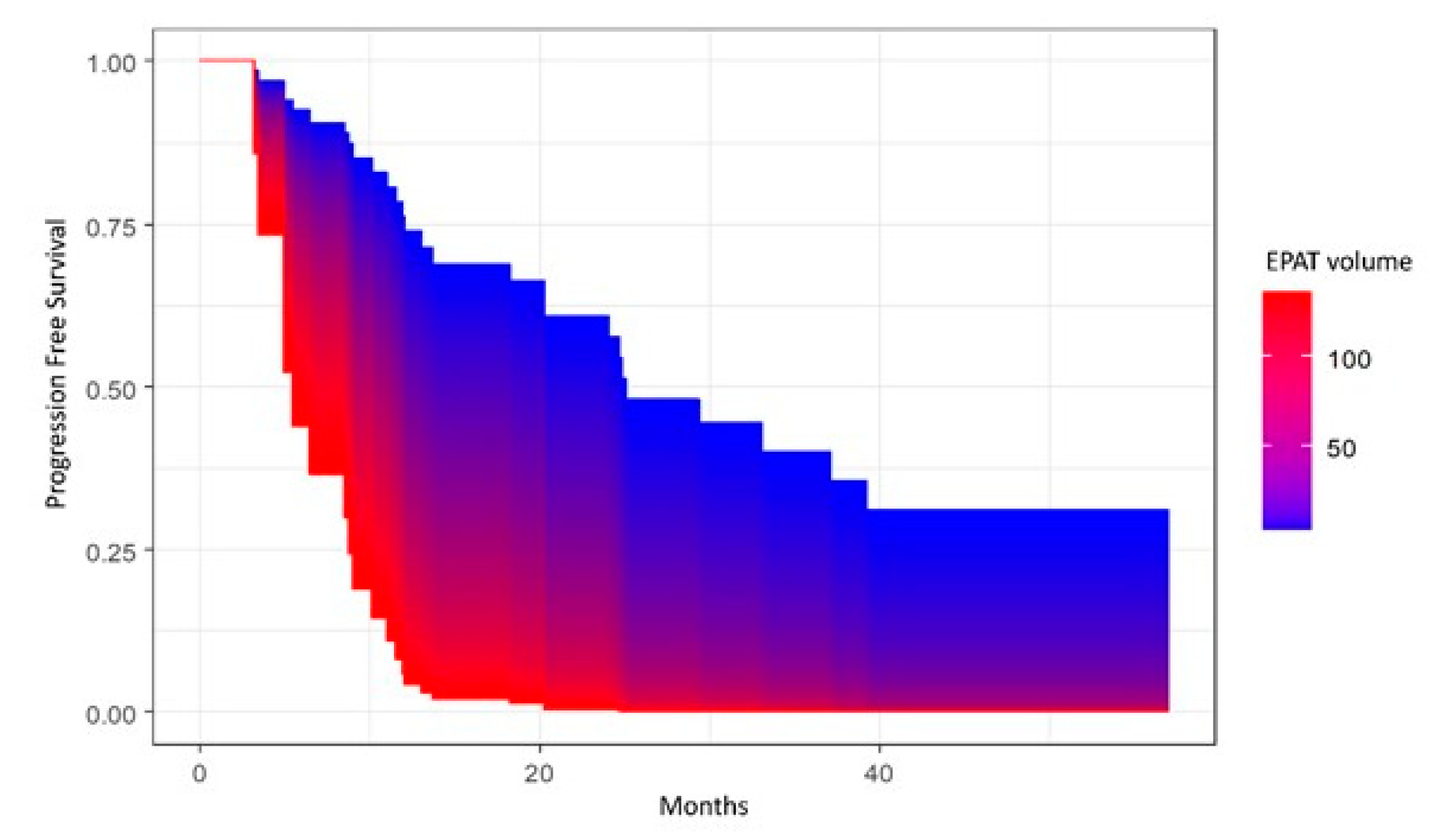
2.4. Statistical Analysis
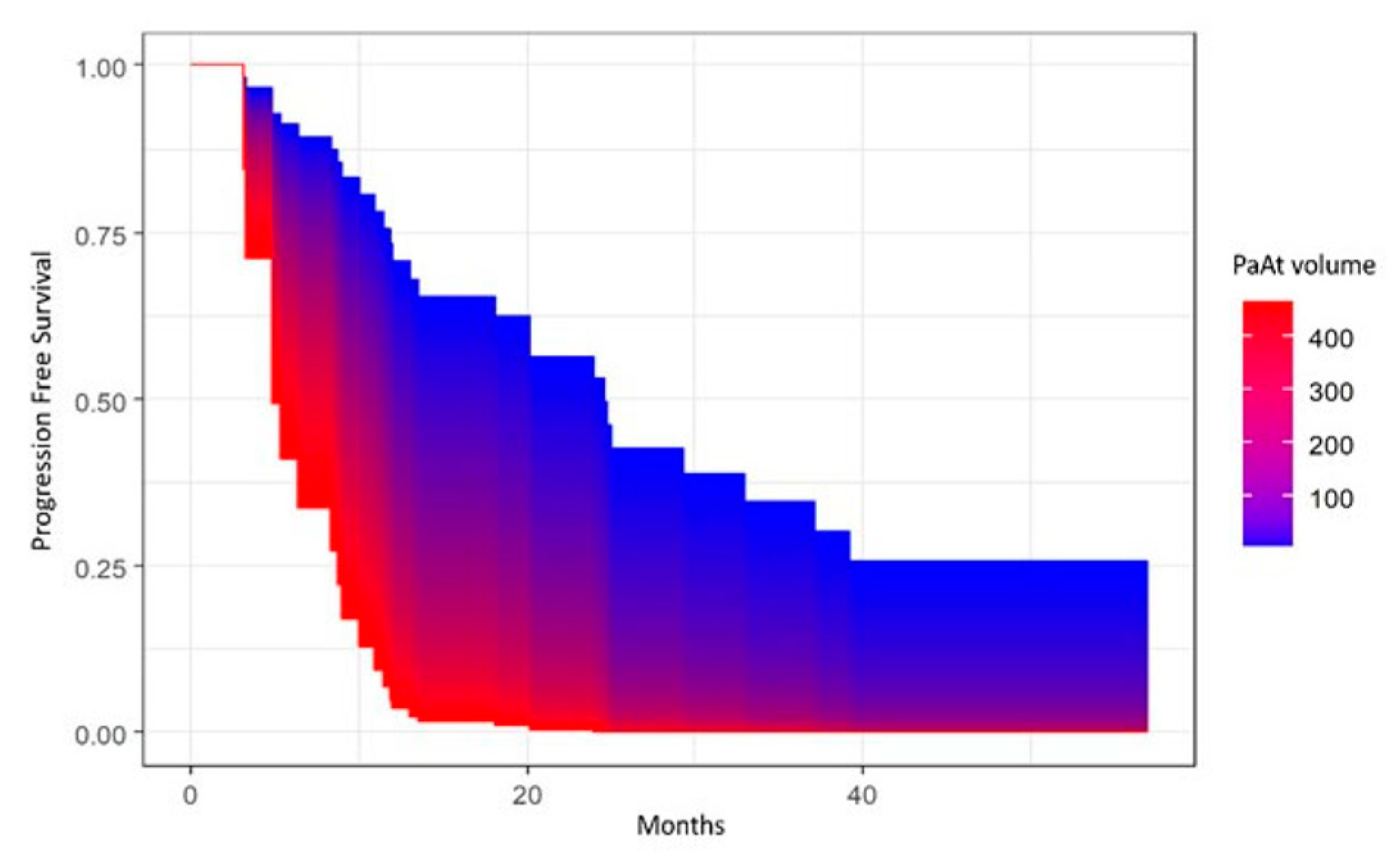
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Sessa, C.; Bois, A.D.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- Vergote, I.; Leunen, K.; Amant, F. Primary surgery or neoadjuvant chemotherapy in ovarian cancer: What is the value of comparing apples with oranges? Gynecol. Oncol. 2012, 124, 1–2. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, P.; Banerjee, S.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. SOLO1 Investigators. Overall Survival with Maintenance Olaparib at a 7-Year Follow-Up in Patients with Newly Diagnosed Advanced Ovarian Cancer and a BRCA Mutation: The SOLO1/GOG 3004 Trial. J. Clin. Oncol. 2023, 41, 609–617. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Brown, J.; Barnicle, A.; Wessen, J.; Lao-Sirieix, P.; Criscione, S.W.; du Bois, A.; Lorusso, D.; Romero, I.; Petru, E.; et al. Homologous Recombination Repair Gene Mutations to Predict Olaparib Plus Bevacizumab Efficacy in the First-Line Ovarian Cancer PAOLA-1/ENGOT-ov25 Trial. JCO Precis. Oncol. 2023, 7, e2200258. [Google Scholar] [CrossRef] [PubMed]
- O’Cearbhaill, R.E.; Pérez-Fidalgo, J.A.; Monk, B.J.; Tusquets, I.; McCormick, C.; Fuentes, J.; Moore, R.G.; Vulsteke, C.; Shahin, M.S.; Forget, F.; et al. Efficacy of niraparib by time of surgery and postoperative residual disease status: A post hoc analysis of patients in the PRIMA/ENGOT-OV26/GOG-3012 study. Gynecol. Oncol. 2022, 166, 36–43. [Google Scholar] [CrossRef] [PubMed]
- de Jong, E.E.C.; van Elmpt, W.; Rizzo, S.; Colarieti, A.; Spitaleri, G.; Leijenaar, R.T.H.; Jochems, A.; Hendriks, L.E.L.; Troost, E.G.C.; Reymen, B.; et al. Applicability of a prognostic CT-based radiomic signature model trained on stage I-III non-small cell lung cancer in stage IV non-small cell lung cancer. Lung Cancer 2018, 124, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.M.; Kalra, M.K.; Schmidt, B.; Raupach, R.; Maher, M.M.; Blake, M.A.; Saini, S. CT images of abdomen and pelvis: Effect of nonlinear three-dimensional optimized reconstruction algorithm on image quality and lesion characteristics. Radiology 2005, 237, 309–315. [Google Scholar] [CrossRef]
- Petrella, F.; Rizzo, S.; Radice, D.; Borri, A.; Galetta, D.; Gasparri, R.; Solli, P.; Veronesi, G.; Bellomi, M.; Spaggiari, L. Predicting prolonged air leak after standard pulmonary lobectomy: Computed tomography assessment and risk factors stratification. Surgeon 2011, 9, 72–77. [Google Scholar] [CrossRef]
- Rietjens, M.; Villa, G.; Toesca, A.; Rizzo, S.; Raimondi, S.; Rossetto, F.; Sangalli, C.; De Lorenzi, F.; Manconi, A.; Matthes, A.G.Z.; et al. Appropriate use of magnetic resonance imaging and ultrasound to detect early silicone gel breast implant rupture in postmastectomy reconstruction. Plast. Reconstr. Surg. 2014, 134, 13e–20e. [Google Scholar] [CrossRef]
- Cassano, E.; Rizzo, S.; Bozzini, A.; Menna, S.; Bellomi, M. Contrast enhanced ultrasound of breast cancer. Cancer Imaging 2006, 6, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Zaffina, C.; Wyttenbach, R.; Pagnamenta, A.; Grasso, R.F.; Biroli, M.; Del Grande, F.; Rizzo, S. Body composition assessment: Comparison of quantitative values between magnetic resonance imaging and computed tomography. Quant. Imaging Med. Surg. 2022, 12, 1450–1466. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.; Mallio, C.A. Artificial intelligence and abdominal adipose tissue analysis: A literature review. Quant. Imaging Med. Surg. 2021, 11, 4461–4474. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.; Dinkel, C.; Mahajan, A.; Siddique, M.; Cook, G.J.; Goh, V. Imaging body composition in cancer patients: Visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging 2015, 6, 489–497. [Google Scholar] [CrossRef]
- Medici, F.; Rizzo, S.; Buwenge, M.; Arcelli, A.; Ferioli, M.; Macchia, G.; Deodato, F.; Cilla, S.; De Iaco, P.; Perrone, A.M.; et al. Everything You Always Wanted to Know about Sarcopenia but Were Afraid to Ask: A Quick Guide for Radiation Oncologists (Impact of Sarcopenia in Radiotherapy: The AFRAID Project). Curr. Oncol. 2022, 29, 8513–8528. [Google Scholar] [CrossRef]
- Rizzo, S.; Scala, I.; Robayo, A.R.; Cefalì, M.; De Dosso, S.; Cappio, S.; Xhepa, G.; Del Grande, F. Body composition as a predictor of chemotherapy-related toxicity in pancreatic cancer patients: A systematic review. Front. Oncol. 2022, 12, 974116. [Google Scholar] [CrossRef]
- Rizzo, S.; Petrella, F.; Bardoni, C.; Bramati, L.; Cara, A.; Mohamed, S.; Radice, D.; Raia, G.; Del Grande, F.; Spaggiari, L. CT-Derived Body Composition Values and Complications After Pneumonectomy in Lung Cancer Patients: Time for a Sex-Related Analysis? Front. Oncol. 2022, 12, 826058. [Google Scholar] [CrossRef]
- Huber, F.A.; Del Grande, F.; Rizzo, S.; Guglielmi, G.; Guggenberger, R. MRI in the assessment of adipose tissues and muscle composition: How to use it. Quant. Imaging Med. Surg. 2020, 10, 1636–1649. [Google Scholar] [CrossRef]
- Cuello, M.A.; Gómez, F.; Wichmann, I.; Suárez, F.; Kato, S.; Orlandini, E.; Brañes, J.; Ibañez, C. Body Composition and Metabolic Dysfunction Really Matter for the Achievement of Better Outcomes in High-Grade Serous Ovarian Cancer. Cancers 2023, 15, 1156. [Google Scholar] [CrossRef]
- Ham, S.; Choi, J.H.; Shin, S.G.; Lee, E.J. High visceral fat-to-muscle ratio is an independent factor that predicts worse overall survival in patients with primary epithelial ovarian, fallopian tube, and peritoneal cancer. J. Ovarian. Res. 2023, 16, 19. [Google Scholar] [CrossRef]
- Wood, N.; Morton, M.; Shah, S.N.; Yao, M.; Barnard, H.; Tewari, S.; Suresh, A.; Kollikonda, S.; AlHilli, M.M. Association between CT-based body composition assessment and patient outcomes during neoadjuvant chemotherapy for epithelial ovarian cancer. Gynecol. Oncol. 2023, 169, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Stelten, S.; Schofield, C.; Hartman, Y.A.W.; Lopez, P.; Kenter, G.G.; Newton, R.U.; Galvão, D.A.; Hoedjes, M.; Taaffe, D.R.; van Lonkhuijzen, L.R.C.W.; et al. Association between Energy Balance-Related Factors and Clinical Outcomes in Patients with Ovarian Cancer: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 4567. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Cartmel, B.; Li, F.Y.; Gottlieb, L.T.; Harrigan, M.; Ligibel, J.A.; Gogoi, R.; Schwartz, P.E.; Irwin, M.L.; Ferrucci, L.M. Effect of exercise on body composition among women with ovarian cancer. J. Cancer Surviv. 2022; Epub ahead of print. [Google Scholar] [CrossRef]
- Ubachs, J.; van de Worp, W.R.P.H.; Vaes, R.D.W.; Pasmans, K.; Langen, R.C.; Meex, R.C.R.; van Bijnen, A.A.J.H.M.; Lambrechts, S.; Van Gorp, T.; Kruitwagen, R.F.P.M.; et al. Ovarian cancer ascites induces skeletal muscle wasting in vitro and reflects sarcopenia in patients. J. Cachexia Sarcopenia Muscle 2022, 13, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Del Grande, M.; Rizzo, S.; Nicolino, G.M.; Colombo, I.; Rossi, L.; Manganaro, L.; Del Grande, F. Computed Tomography-Based Body Composition in Patients with Ovarian Cancer: Association with Chemotoxicity and Prognosis. Front. Oncol. 2021, 1, 718815. [Google Scholar] [CrossRef]
- Rizzo, S.; Raia, G.; Del Grande, M.; Gasparri, M.L.; Colombo, I.; Manganaro, L.; Papadia, A.; Del Grande, F. Body composition as a predictor of chemotherapy-related toxicity in ovarian cancer patients: A systematic review. Front. Oncol. 2022, 12, 1057631. [Google Scholar] [CrossRef]
- Jin, Y.; Ma, X.; Yang, Z.; Zhang, N. Low L3 skeletal muscle index associated with the clinicopathological characteristics and prognosis of ovarian cancer: A meta-analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 697–705. [Google Scholar] [CrossRef]
- Dalal, T.; Kalra, M.K.; Rizzo, S.M.; Schmidt, B.; Suess, C.; Flohr, T.; Blake, M.A.; Saini, S. Metallic prosthesis: Technique to avoid increase in CT radiation dose with automatic tube current modulation in a phantom and patients. Radiology 2005, 236, 671–675. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Denz, R.; Nina, T. Visualizing the Causal Effect of a Continuous Variable on a Time-To-Event Outcome. arXiv 2022, arXiv:2208.04644. [Google Scholar]
- Shiri, P.; Ramezanpour, S.; Amani, A.M.; Dehaen, W. A patent review on efficient strategies for the total synthesis of pazopanib, regorafenib and lenvatinib as novel anti-angiogenesis receptor tyrosine kinase inhibitors for cancer therapy. Mol. Divers 2022, 26, 2981–3002. [Google Scholar] [CrossRef]
- Kumar, A.; Moynagh, M.R.; Multinu, F.; Cliby, W.A.; McGree, M.E.; Weaver, A.L.; Young, P.M.; Bakkum-Gamez, J.N.; Langstraat, C.L.; Dowdy, S.C.; et al. Muscle composition measured by CT scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecol. Oncol. 2016, 142, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Kim, T.M.; Lee, M.; Kim, H.S.; Chung, H.H.; Cho, J.Y.; Song, Y.S. Impact of CT-Determined Sarcopenia and Body Composition on Survival Outcome in Patients with Advanced-Stage High-Grade Serous Ovarian Carcinoma. Cancers 2020, 12, 559. [Google Scholar] [CrossRef] [PubMed]
- Ataseven, B.; Luengo, T.G.; du Bois, A.; Waltering, K.U.; Traut, A.; Heitz, F.; Alesina, P.F.; Prader, S.; Meier, B.; Schneider, S.; et al. Skeletal Muscle Attenuation (Sarcopenia) Predicts Reduced Overall Survival in Patients with Advanced Epithelial Ovarian Cancer Undergoing Primary Debulking Surgery. Ann. Surg. Oncol. 2018, 25, 3372–3379. [Google Scholar] [CrossRef]
- Bronger, H.; Hederich, P.; Hapfelmeier, A.; Metz, S.; Noël, P.B.; Kiechle, M.; Schmalfeldt, B. Sarcopenia in Advanced Serous Ovarian Cancer. Int. J. Gynecol. Cancer 2017, 27, 223–232. [Google Scholar] [CrossRef]
- Rutten, I.J.; Ubachs, J.; Kruitwagen, R.F.; van Dijk, D.P.; Beets-Tan, R.G.; Massuger, L.F.; Damink, S.O.; Van Gorp, T. The influence of sarcopenia on survival and surgical complications in ovarian cancer patients undergoing primary debulking surgery. Eur. J. Surg. Oncol. 2017, 43, 717–724. [Google Scholar] [CrossRef]
- Liu, J.; Fox, C.S.; Hickson, D.A.; May, W.D.; Hairston, K.G.; Carr, J.J.; Taylor, H.A. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: The Jackson Heart Study. J. Clin. Endocrinol. Metab. 2010, 95, 5419–5426. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.; Schreiner, P.J.; Terry, J.G. The relation between visceral fat measurement and torso level—Is one level better than another? The Atherosclerosis Risk in Communi-ties Study, 1990–1992. Am. J. Epidemiol. 2006, 163, 352–358. [Google Scholar] [CrossRef]
- Bertaso, A.G.; Bertol, D.; Duncan, B.B.; Foppa, M. Epicardial fat: Definition, measurements and systematic review of main outcomes. Arq. Bras. Cardiol. 2013, 101, e18–e28. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Basta, G. Ectopic fat and cardiovascular disease: What is the link? Nutr. Metab. Cardiovasc. Dis. 2010, 20, 481–490. [Google Scholar] [CrossRef]
- Ding, J.; Hsu, F.C.; Harris, T.B.; Liu, Y.; Kritchevsky, S.B.; Szklo, M.; Ouyang, P.; Espeland, M.A.; Lohman, K.K.; Criqui, M.H.; et al. The association of pericardial fat with incident coronary heart disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Clin. Nutr. 2009, 90, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Barbaro, G.; Gerstein, H.C. Relationship of epicardial fat thickness and fasting glucose. Int. J. Cardiol. 2008, 128, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt, N.; Toschke, A.M.; Leite, D.; Rocha, J.; Carvalho, M.; Sampaio, F.; Xará, S.; Leite-Moreira, A.; Nagel, E.; Gama, V. Epicardial adipose tissue is an independent predictor of coronary atherosclerotic burden. Int. J. Cardiol. 2012, 158, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Momesso, D.P.; Bussade, I.; Epifanio, M.A.; Schettino, C.D.; Russo, L.A.; Kupfer, R. Increased epicardial adipose tissue in type 1 diabetes is associated with central obesity and metabolic syndrome. Diabetes Res. Clin. Pract. 2011, 91, 47–53. [Google Scholar] [CrossRef]
- Gaborit, B.; Jacquier, A.; Kober, F.; Abdesselam, I.; Cuisset, T.; Boullu-Ciocca, S.; Emungania, O.; Alessi, M.C.; Clément, K.; Bernard, M.; et al. Effects of bariatric surgery on cardiac ectopic fat: Lesser decrease in epicardial fat compared to visceral fat loss and no change in myo-cardial triglyceride content. J. Am. Coll. Cardiol. 2012, 60, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, J.M.; Longenecker, J.Z.; Ramkumar, S.; Xu, X.; Dorn, L.E.; Bratasz, A.; Yu, L.; Maurya, S.; Tolstikov, V.; Bussberg, V.; et al. Paracardial fat remodeling affects systemic metabolism through alcohol dehydrogenase 1. J. Clin. Investig. 2021, 131, e141799. [Google Scholar] [CrossRef] [PubMed]

| N (%) | |
|---|---|
| Age at diagnosis, median (IQR) | 64.9 (55.4;75.4) |
| FIGO Stage IIB IIIA IIIC IV | 1 (2.9) 2 (5.9) 18 (52.9) 13 (38.3) |
| NACT 0 1 | 21 (61.8) 13 (38.2) |
| Outcome R0 (no residual disease) R1 (residual disease < 1 cm) R2 (residual disease > 1 cm) NA | 20 (58.8) 7 (20.6) 4 (11.8) 3 (8.8) |
| BMI, median (IQR) | 22.9 (21.7; 26.2) |
| Values | Median (IQR) |
|---|---|
| SKM Volume (mm3) HU mean HU standard deviation | 7793.3 (6829.1; 8286.4) 46.0 (42.8; 51.9) 30.2 (29.0; 31.4) |
| IMAT Volume (mm3) HU mean HU standard deviation | 1143.5 (935.9; 1392.0) −50.77 (−55.3; −47.7) 29.4 (28.3; 31.2) |
| VAT Volume HU mean HU s standard deviation | 1624.6 (942.6; 2028.2) −74.3 (−80.3; −65.0) 24.0 (22.2; 26.2) |
| SAT Volume (mm3) HU mean HU standard deviation | 9908.5 (7749.1; 14,534.1) −94.8 (−102.1; −90.5) 21.4 (19.8; 23.5) |
| VAT U SAT Volume (mm3) HU mean HU standard deviation | 11,503.6 (8957.5; 17,146.9) −92.1 (−99.6; −87.4) 23.0 (21.9; 25.06) |
| EpAT Volume (mm3) HU mean HU standard deviation | 31.4 (15.8; 45.0) −58.0 (−66.5; −49.8) 27.7 (26.5; 30.3) |
| PaAT Volume (mm3) HU mean HU standard deviation | 79.1 (46.3; 105.3) −71.9 (−79.5; −61.5) 27.1 (25.9; 28.7) |
| ThAT Volume (mm3) HU mean HU standard deviation | 19.7 (12.4; 39.1) −68.7 (−79.6; −61.5) 27.1 (25.2; 28.5) |
| Bone HU mean HU standard deviation | 378.1 (316.5; 429.9) 248.3 (233.8; 303.3) |
| TRBCLR HU mean HU standard deviation | 137.2 (125.1; 166.8) 89.2 (80.3; 94.3) |
| LIV Volume (mm3) HU mean HU standard deviation | 1398.0 (1239.8; 1655.2) 118.7 (108.4; 126.8) 22.3 (19.6; 25.2) |
| SPL Volume (mm3) HU mean HU standard deviation | 162.7 (123.2; 195.4) 116.5 (105.2; 126.7) 23.9 (19.9; 29.1) |
| AOC Volume (mm3) | 0.23 (0.008; 0.82) |
| HRT Volume (mm3) | 589.3 (537.3; 651.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raia, G.; Del Grande, M.; Colombo, I.; Nerone, M.; Manganaro, L.; Gasparri, M.L.; Papadia, A.; Del Grande, F.; Rizzo, S. Whole-Body Composition Features by Computed Tomography in Ovarian Cancer: Pilot Data on Survival Correlations. Cancers 2023, 15, 2602. https://doi.org/10.3390/cancers15092602
Raia G, Del Grande M, Colombo I, Nerone M, Manganaro L, Gasparri ML, Papadia A, Del Grande F, Rizzo S. Whole-Body Composition Features by Computed Tomography in Ovarian Cancer: Pilot Data on Survival Correlations. Cancers. 2023; 15(9):2602. https://doi.org/10.3390/cancers15092602
Chicago/Turabian StyleRaia, Giorgio, Maria Del Grande, Ilaria Colombo, Marta Nerone, Lucia Manganaro, Maria Luisa Gasparri, Andrea Papadia, Filippo Del Grande, and Stefania Rizzo. 2023. "Whole-Body Composition Features by Computed Tomography in Ovarian Cancer: Pilot Data on Survival Correlations" Cancers 15, no. 9: 2602. https://doi.org/10.3390/cancers15092602
APA StyleRaia, G., Del Grande, M., Colombo, I., Nerone, M., Manganaro, L., Gasparri, M. L., Papadia, A., Del Grande, F., & Rizzo, S. (2023). Whole-Body Composition Features by Computed Tomography in Ovarian Cancer: Pilot Data on Survival Correlations. Cancers, 15(9), 2602. https://doi.org/10.3390/cancers15092602








