Systemic Inflammatory Indices in Second-Line Soft Tissue Sarcoma Patients: Focus on Lymphocyte/Monocyte Ratio and Trabectedin
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Ethical Statement
2.3. Next-Generation Sequencing Analysis
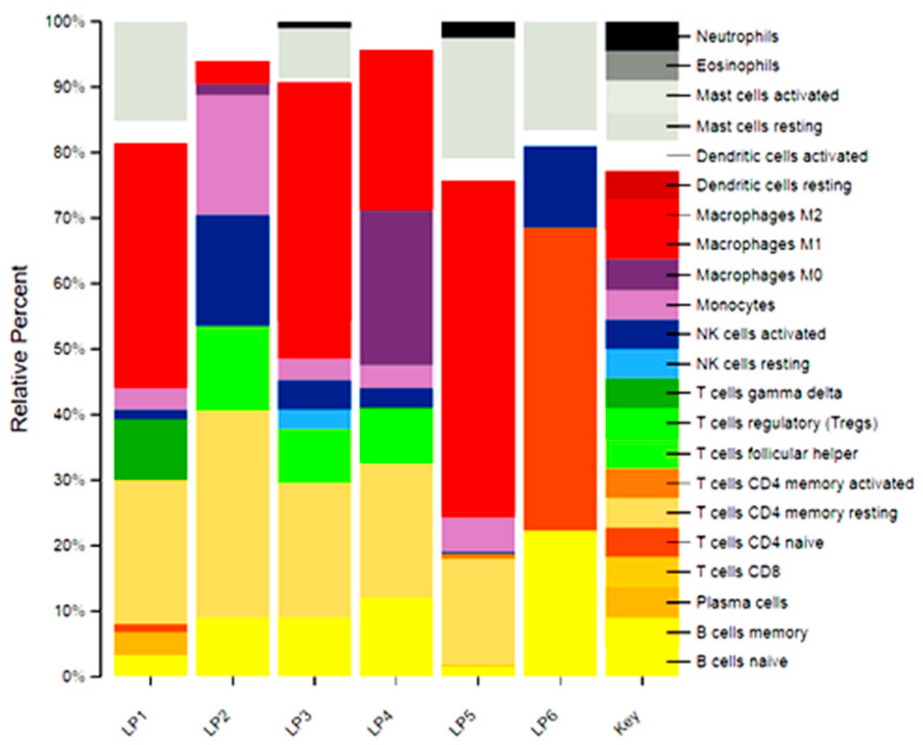
2.4. Immune Infiltrate Analysis
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
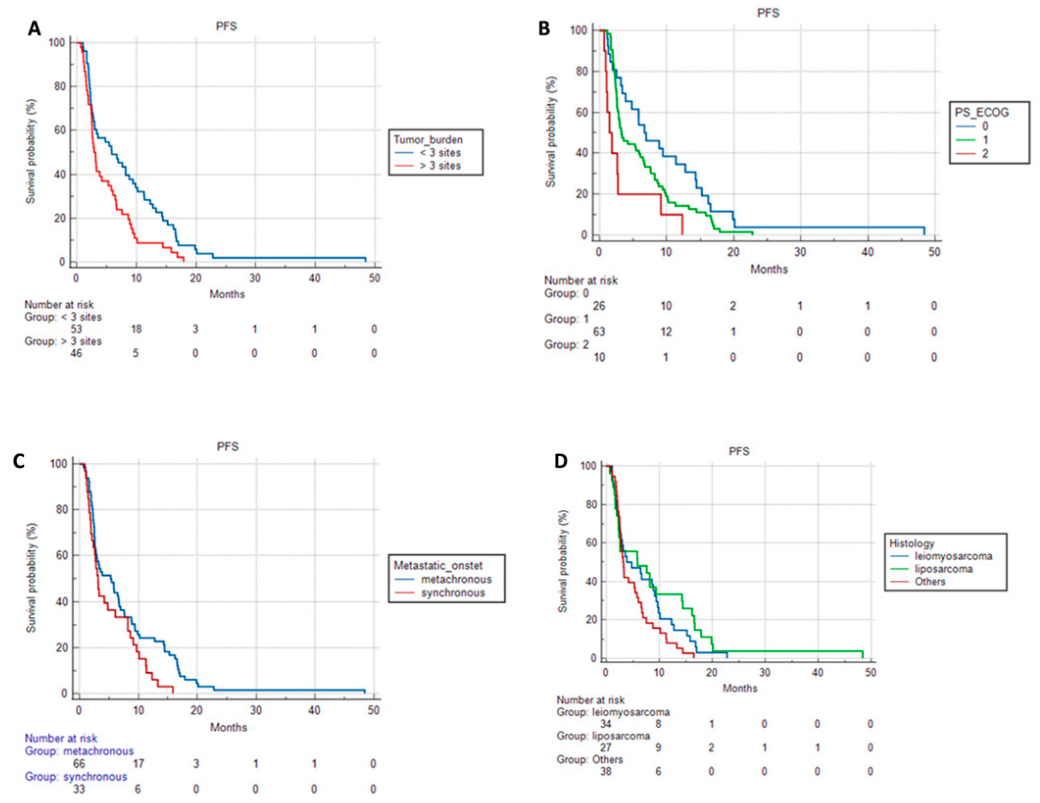
3.2. Progression-Free Survival and Overall Survival Based on Patient Characteristics and Treatments
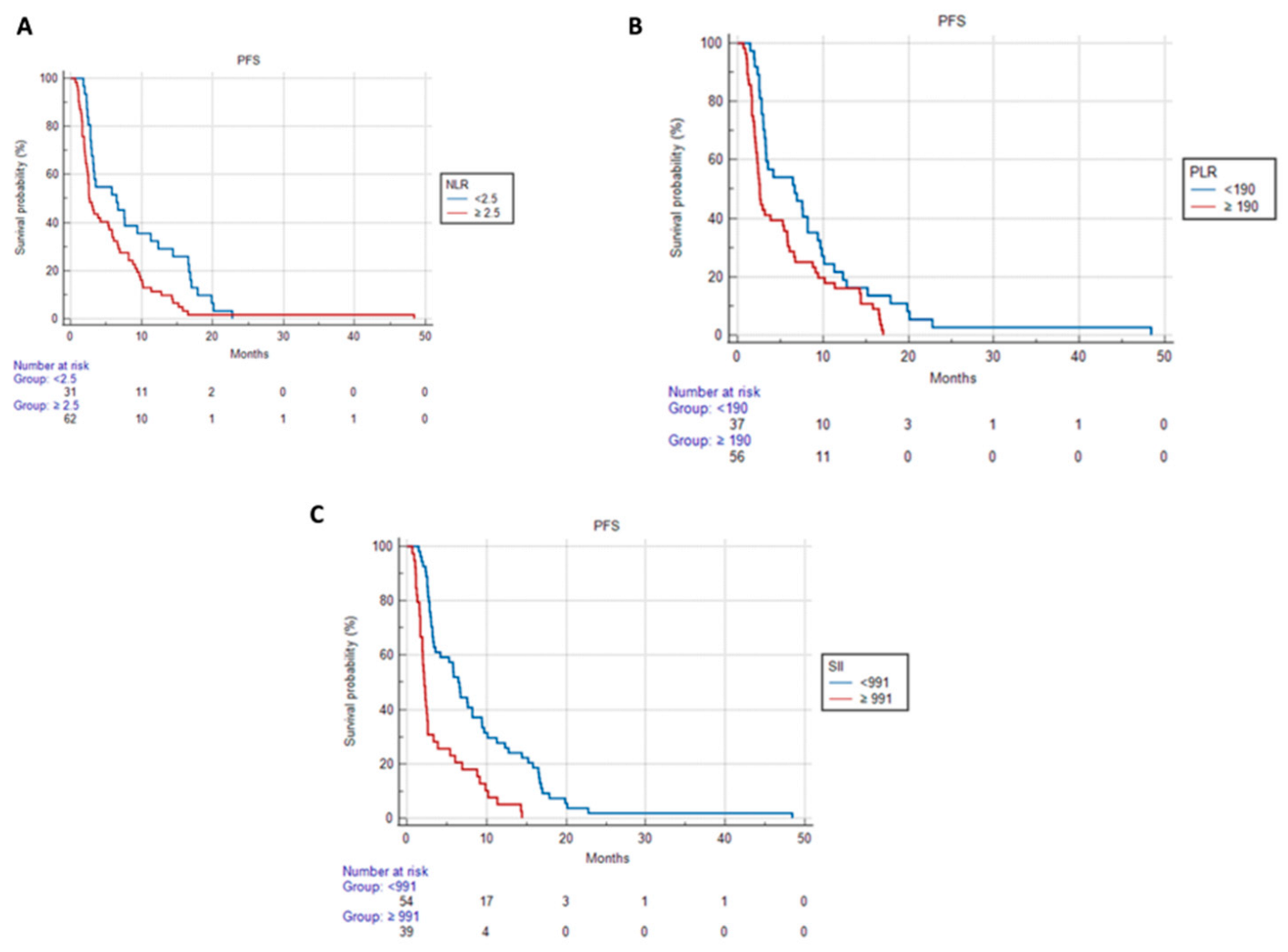
3.3. Progression-Free Survival and Overall Survival Based on Systemic Inflammatory Indices
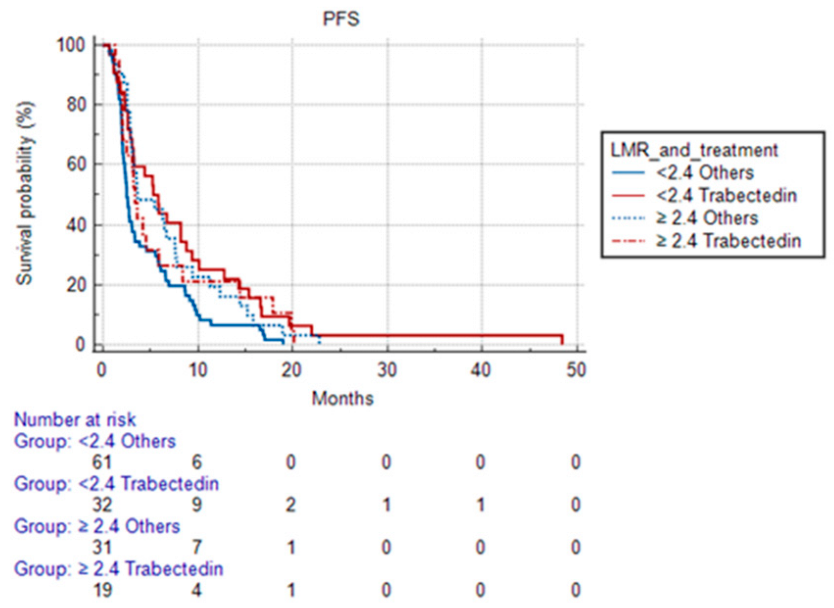
- -
- 5.83 months (95% CI 3.070 to 48.400) in patients treated with Trabectedin and with LMR < 2.4;
- -
- 3.37 months (95% CI 2.030 to 20.130) in patients treated with Trabectedin and with LMR ≥ 2.4;
- -
- 2.5 months (95% CI 2.130 to 19,000) in patients treated with other treatments and with LMR < 2.4;
- -
- 3.63 months (95% CI 2.930 to 22.800) in patients treated with other treatments and with LMR ≥ 2.4.
4. Discussion
- Has a cytotoxic effect on the monocyte-macrophage line (more than on other populations, such as the lymphocyte);
- Can inhibit the differentiation of monocytes into macrophages;
- Reduces the production of two pro-inflammatory mediators, CCL2 (responsible for recruiting monocytes to tumor sites) and IL-6 (tumor growth factor), in monocytes, macrophages, TAM, and isolated ovarian cancer cells;
- Acts through a mechanism of action involving modulation of the TME.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gatta, G.; Capocaccia, R.; Botta, L.; Mallone, S.; De Angelis, R.; Ardanaz, E.; Comber, H.; Dimitrova, N.; Leinonen, M.K.; Siesling, S.; et al. Burden and centralised treatment in Europe of rare tumours: Results of RARECAREnet-a population-based study. Lancet Oncol. 2017, 18, 1022–1039. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours: 3, 5th ed.; IARC: Lyon, France, 2020; Available online: https://publications.iarc.fr/588 (accessed on 1 May 2021).
- Blay, J.Y.; Soibinet, P.; Penel, N.; Bompas, E.; Duffaud, F.; Stoeckle, E.; Mir, O.; Adam, J.; Chevreau, C.; Bonvalot, S.; et al. Improved survival using specialized multidisciplinary board in sarcoma patients. Ann. Oncol. 2017, 28, 2852–2859. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Ford, S.; Callegaro, D.; Sangalli, C.; Colombo, C.; Radaelli, S.; Frezza, A.M.; Renne, S.L.; Casali, P.G.; Gronchi, A. Adequate Local Control in High-Risk Soft Tissue Sarcoma of the Extremity Treated with Surgery Alone at a Reference Centre: Should Radiotherapy Still be a Standard? Ann. Surg. Oncol. 2018, 25, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Callegaro, D.; Miceli, R.; Bonvalot, S.; Ferguson, P.; Strauss, D.C.; Levy, A.; Griffin, A.; Hayes, A.J.; Stacchiotti, S.; Le Pèchoux, C.; et al. Impact of perioperative chemotherapy and radiotherapy in patients with primary extremity soft tissue sarcoma: Retrospective analysis across major histological subtypes and major reference centres. Eur. J. Cancer 2018, 105, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Palassini, E.; Ferrari, S.; Verderio, P.; De Paoli, A.; Martin Broto, J.; Quagliuolo, V.; Comandone, A.; Sangalli, C.; Palmerini, E.; Lopez-Pousa, A.; et al. Feasibility of Preoperative Chemotherapy with or Without Radiation Therapy in Localized Soft Tissue Sarcomas of Limbs and Superficial Trunk in the Italian Sarcoma Group/Grupo Español de Investigación en Sarcomas Randomized Clinical Trial: Three Versus Five Cycles of Full-Dose Epirubicin Plus Ifosfamide. J. Clin. Oncol. 2015, 33, 3628–3634. [Google Scholar] [CrossRef]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Ghadjar, P.; Hohenberger, P.; Angele, M.; Salat, C.; et al. Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients with Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef]
- Frustaci, S.; Gherlinzoni, F.; De Paoli, A.; Bonetti, M.; Azzarelli, A.; Comandone, A.; Olmi, P.; Buonadonna, A.; Pignatti, G.; Barbieri, E.; et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: Results of the Italian randomized cooperative trial. J. Clin. Oncol. 2001, 19, 1238–1247. [Google Scholar] [CrossRef]
- Woll, P.J.; Reichardt, P.; Le Cesne, A.; Bonvalot, S.; Azzarelli, A.; Hoekstra, H.J.; Leahy, M.; Van Coevorden, F.; Verweij, J.; Hogendoorn, P.C.; et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): A multicentre randomised controlled trial. Lancet Oncol. 2012, 13, 1045–1054. [Google Scholar] [CrossRef]
- Gronchi, A.; Palmerini, E.; Quagliuolo, V.; Martin Broto, J.; Lopez Pousa, A.; Grignani, G.; Brunello, A.; Blay, J.Y.; Tendero, O.; Diaz Beveridge, R.; et al. Neoadjuvant Chemotherapy in High-Risk Soft Tissue Sarcomas: Final Results of a Randomized Trial From Italian (ISG), Spanish (GEIS), French (FSG), and Polish (PSG) Sarcoma Groups. J. Clin. Oncol. 2020, 38, 2178–2186. [Google Scholar] [CrossRef]
- Pasquali, S.; Palmerini, E.; Quagliuolo, V.; Martin-Broto, J.; Lopez-Pousa, A.; Grignani, G.; Brunello, A.; Blay, J.Y.; Tendero, O.; Diaz-Beveridge, R.; et al. Neoadjuvant chemotherapy in high-risk soft tissue sarcomas: A Sarculator-based risk stratification analysis of the ISG-STS 1001 randomized trial. Cancer 2022, 128, 85–93. [Google Scholar] [CrossRef]
- Pasquali, S.; Pizzamiglio, S.; Touati, N.; Litiere, S.; Marreaud, S.; Kasper, B.; Gelderblom, H.; Stacchiotti, S.; Judson, I.; Dei Tos, A.P.; et al. The impact of chemotherapy on survival of patients with extremity and trunk wall soft tissue sarcoma: Revisiting the results of the EORTC-STBSG 62931 randomised trial. Eur. J. Cancer 2019, 109, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; Klimczak, A.; Ługowska, I.; Jagielska, B.; Wągrodzki, M.; Dębiec-Rychter, M.; Pieńkowska-Grela, B.; Świtaj, T. Long-term results of treatment of advanced dermatofibrosarcoma protuberans (DFSP) with imatinib mesylate—The impact of fibrosarcomatous transformation. Eur. J. Surg. Oncol. 2017, 43, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, R.S.; Wiernik, P.H.; Bachur, N.R. Adriamycin: A new effective agent in the therapy of disseminated sarcomas. Med. Pediatr. Oncol. 1975, 1, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Borden, E.C.; Amato, D.A.; Rosenbaum, C.; Enterline, H.T.; Shiraki, M.J.; Creech, R.H.; Lerner, H.J.; Carbone, P.P. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J. Clin. Oncol. 1987, 5, 840–850. [Google Scholar] [CrossRef]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- Demetri, G.D.; von Mehren, M.; Jones, R.L.; Hensley, M.L.; Schuetze, S.M.; Staddon, A.; Milhem, M.; Elias, A.; Ganjoo, K.; Tawbi, H.; et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2016, 34, 786–793. [Google Scholar] [CrossRef]
- Kawai, A.; Araki, N.; Sugiura, H.; Ueda, T.; Yonemoto, T.; Takahashi, M.; Morioka, H.; Hiraga, H.; Hiruma, T.; Kunisada, T.; et al. Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation-related sarcoma: A randomised, open-label, phase 2 study. Lancet Oncol. 2015, 16, 406–416. [Google Scholar] [CrossRef]
- Van der Graaf, W.T.; Blay, J.Y.; Chawla, S.P.; Kim, D.W.; Bui-Nguyen, B.; Casali, P.G.; Schöffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Schöffski, P.; Chawla, S.; Maki, R.G.; Italiano, A.; Gelderblom, H.; Choy, E.; Grignani, G.; Camargo, V.; Bauer, S.; Rha, S.Y.; et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open-label, multicentre, phase 3 trial. Lancet 2016, 387, 1629–1637. [Google Scholar] [CrossRef]
- Pautier, P.; Floquet, A.; Penel, N.; Piperno-Neumann, S.; Isambert, N.; Rey, A.; Bompas, E.; Cioffi, A.; Delcambre, C.; Cupissol, D.; et al. Randomized multicenter and stratified phase II study of gemcitabine alone versus gemcitabine and docetaxel in patients with metastatic or relapsed leiomyosarcomas: A Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) French Sarcoma Group Study (TAXOGEM study). Oncologist 2012, 17, 1213–1220. [Google Scholar] [CrossRef]
- García-Del-Muro, X.; López-Pousa, A.; Maurel, J.; Martín, J.; Martínez-Trufero, J.; Casado, A.; Gómez-España, A.; Fra, J.; Cruz, J.; Poveda, A.; et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: A Spanish Group for Research on Sarcomas study. J. Clin. Oncol. 2011, 29, 2528–2533. [Google Scholar] [CrossRef] [PubMed]
- Ferraresi, V.; Ciccarese, M.; Cercato, M.C.; Nuzzo, C.; Zeuli, M.; Di Filippo, F.; Giannarelli, D.; Cognetti, F. Gemcitabine at fixed dose-rate in patients with advanced soft-tissue sarcomas: A mono-institutional phase II study. Cancer Chemother. Pharmacol. 2008, 63, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Vadhan-Raj, S.; Papadopolous, N.; Plager, C.; Burgess, M.A.; Hays, C.; Benjamin, R.S. High-dose ifosfamide in bone and soft tissue sarcomas: Results of phase II and pilot studies--dose-response and schedule dependence. J. Clin. Oncol. 1997, 15, 2378–2384. [Google Scholar] [CrossRef] [PubMed]
- van Oosterom, A.T.; Mouridsen, H.T.; Nielsen, O.S.; Dombernowsky, P.; Krzemieniecki, K.; Judson, I.; Svancarova, L.; Spooner, D.; Hermans, C.; Van Glabbeke, M.; et al. Results of randomised studies of the EORTC Soft Tissue and Bone Sarcoma Group (STBSG) with two different ifosfamide regimens in first- and second-line chemotherapy in advanced soft tissue sarcoma patients. Eur. J. Cancer 2002, 38, 2397–2406. [Google Scholar] [CrossRef]
- Chabner, B.A.; Bertino, J.; Cleary, J.; Ortiz, T.; Lane, A.; Supko, J.G.; Ryan, D. Cytotoxic Agents. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 12th ed.; Brunton, L.L., Chabner, B.A., Knollmann, B.C., Eds.; McGraw Hill: New York, NY, USA, 2011. [Google Scholar]
- Lee, S.H.; Chang, M.H.; Baek, K.K.; Han, B.; Lim, T.; Lee, J.; Park, J.O. High-dose ifosfamide as second- or third-line chemotherapy in refractory bone and soft tissue sarcoma patients. Oncology 2011, 80, 257–261. [Google Scholar] [CrossRef]
- Viñal, D.; Martinez, D.; Garcia-Cuesta, J.A.; Gutierrez-Sainz, L.; Martinez-Recio, S.; Villamayor, J.; Martinez-Marin, V.; Gallego, A.; Ortiz-Cruz, E.; Mendiola, M.; et al. Prognostic value of neutrophil-to-lymphocyte ratio and other inflammatory markers in patients with high-risk soft tissue sarcomas. Clin. Transl. Oncol. 2020, 22, 1849–1856. [Google Scholar] [CrossRef]
- Nishijima, T.F.; Muss, H.B.; Shachar, S.S.; Tamura, K.; Takamatsu, Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat. Rev. 2015, 41, 971–978. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Wang, X.; Ni, X.; Tang, G. Prognostic Role of Platelet-to-Lymphocyte Ratio in Patients with Bladder Cancer: A Meta-Analysis. Front. Oncol. 2019, 9, 757. [Google Scholar] [CrossRef]
- Idowu, O.K.; Ding, Q.; Taktak, A.F.; Chandrasekar, C.R.; Yin, Q. Clinical implication of pretreatment neutrophil to lymphocyte ratio in soft tissue sarcoma. Biomarkers 2012, 17, 539–544. [Google Scholar] [CrossRef]
- Szkandera, J.; Gerger, A.; Liegl-Atzwanger, B.; Absenger, G.; Stotz, M.; Friesenbichler, J.; Trajanoski, S.; Stojakovic, T.; Eberhard, K.; Leithner, A.; et al. The lymphocyte/monocyte ratio predicts poor clinical outcome and improves the predictive accuracy in patients with soft tissue sarcomas. Int. J. Cancer 2014, 135, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Panotopoulos, J.; Posch, F.; Alici, B.; Funovics, P.; Stihsen, C.; Amann, G.; Brodowicz, T.; Windhager, R.; Ay, C. Hemoglobin, alkalic phosphatase, and C-reactive protein predict the outcome in patients with liposarcoma. J. Orthop. Res. 2015, 33, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Jiang, S.; Situ, D.; Lin, Y.; Yang, H.; Li, Y.; Long, H.; Zhou, Z. Prognostic value of monocyte and neutrophils to lymphocytes ratio in patients with metastatic soft tissue sarcoma. Oncotarget 2015, 6, 9542–9550. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.; Zhang, Z.; Chew, W.; Tan, G.F.; Lim, C.L.; Zhou, L.; Goh, W.L.; Poon, E.; Somasundaram, N.; Selvarajan, S.; et al. Biological significance and prognostic relevance of peripheral blood neutrophil-to-lymphocyte ratio in soft tissue sarcoma. Sci. Rep. 2018, 8, 11959. [Google Scholar] [CrossRef]
- Sasaki, H.; Nagano, S.; Komiya, S.; Taniguchi, N.; Setoguchi, T. Validation of Different Nutritional Assessment Tools in Predicting Prognosis of Patients with Soft Tissue Spindle-Cell Sarcomas. Nutrients 2018, 10, 765. [Google Scholar] [CrossRef]
- Liang, Y.; Xiao, W.; Guan, Y.X.; Wang, W.; Chen, H.Y.; Fang, C.; Zhang, X.; Zhou, Z.W. Prognostic value of the C-reactive protein/Albumin Ratio (CAR) in patients with operable soft tissue sarcoma. Oncotarget 2017, 8, 98135–98147. [Google Scholar] [CrossRef]
- García-Ortega, D.Y.; Álvarez-Cano, A.; Sánchez-Llamas, L.A.; Caro-Sanchez, C.; Martínez-Said, H.; Luna-Ortiz, K.; Cuéllar-Hübbe, M. Neutrophil/lymphocyte ratio is associated with survival in synovial sarcoma. Surg. Oncol. 2018, 27, 551–555. [Google Scholar] [CrossRef]
- Chen, S.; Luo, P.; Yang, L.; Zheng, B.; Sun, Z.; Yan, W.; Wang, C. Prognostic analysis of surgically treated clear cell sarcoma: An analysis of a rare tumor from a single center. Int. J. Clin. Oncol. 2019, 24, 1605–1611. [Google Scholar] [CrossRef]
- Vasquez, L.; León, E.; Beltran, B.; Maza, I.; Oscanoa, M.; Geronimo, J. Pretreatment Neutrophil-to-Lymphocyte Ratio and Lymphocyte Recovery: Independent Prognostic Factors for Survival in Pediatric Sarcomas. J. Pediatr. Hematol. Oncol. 2017, 39, 538–546. [Google Scholar] [CrossRef]
- Cheng, Y.; Mo, F.; Pu, L.; Li, Q.; Ma, X. Pretreatment Inflammatory Indexes as Prognostic Predictors of Survival in Patients Suffering From Synovial Sarcoma. Front. Oncol. 2019, 9, 955. [Google Scholar] [CrossRef]
- Germano, G.; Frapolli, R.; Belgiovine, C.; Anselmo, A.; Pesce, S.; Liguori, M.; Erba, E.; Uboldi, S.; Zucchetti, M.; Pasqualini, F.; et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 2013, 23, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Allavena, P.; Signorelli, M.; Chieppa, M.; Erba, E.; Bianchi, G.; Marchesi, F.; Olimpio, C.O.; Bonardi, C.; Garbi, A.; Lissoni, A.; et al. Anti-inflammatory properties of the novel antitumor agent yondelis (trabectedin): Inhibition of macrophage differentiation and cytokine production. Cancer Res. 2005, 65, 2964–2971. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Khodadoust, M.S.; Liu, C.L.; Newman, A.M.; Alizadeh, A.A. Profiling Tumor Infiltrating Immune Cells with CIBERSORT. Methods Mol. Biol. 2018, 1711, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Younus, J.; Stys-Norman, D.; Haynes, A.E.; Blackstein, M.; Members of the Sarcoma Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based Care. Meta-analysis of ifosfamide-based combination chemotherapy in advanced soft tissue sarcoma. Cancer Treat. Rev. 2008, 34, 339–347. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Hefler, L.A.; Concin, N.; Hofstetter, G.; Marth, C.; Mustea, A.; Sehouli, J.; Zeillinger, R.; Leipold, H.; Lass, H.; Grimm, C.; et al. Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clin. Cancer Res. 2008, 14, 710–714. [Google Scholar] [CrossRef]
- Polterauer, S.; Grimm, C.; Tempfer, C.; Sliutz, G.; Speiser, P.; Reinthaller, A.; Hefler, L.A. C-reactive protein is a prognostic parameter in patients with cervical cancer. Gynecol. Oncol. 2007, 107, 114–117. [Google Scholar] [CrossRef]
- Zhou, L.; Cai, X.; Liu, Q.; Jian, Z.Y.; Li, H.; Wang, K.J. Prognostic Role of C-Reactive Protein in Urological Cancers: A Meta-Analysis. Sci. Rep. 2015, 5, 12733. [Google Scholar] [CrossRef]
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef]
- Tang, H.; Liu, D.; Lu, J.; He, J.; Ji, S.; Liao, S.; Wei, Q.; Lu, S.; Liu, Y. Significance of the neutrophil-to-lymphocyte ratio in predicting the response to neoadjuvant chemotherapy in extremity osteosarcoma: A multicentre retrospective study. BMC Cancer 2022, 22, 33. [Google Scholar] [CrossRef]
- García-Ortega, D.Y.; Melendez-Fernandez, A.P.; Alvarez-Cano, A.; Clara-Altamirano, M.A.; Caro-Sanchez, C.; Alamilla-Garcia, G.; Luna-Ortiz, K. Neutrophil-to-Lymphocyte ratio as a prognostic biomarker in extremities undifferentiated pleomorphic sarcoma. Surg. Oncol. 2022, 42, 101746. [Google Scholar] [CrossRef] [PubMed]
- Koseci, T.; Haksoyler, V.; Olgun, P.; Ata, S.; Nayir, E.; Duman, B.B.; Cil, T. Prognostic Importance of Inflammatory Indexes in Patients Treated by Pazopanib for Soft Tissue Sarcoma. Clin. Lab. 2022, 68, 210431. [Google Scholar] [CrossRef]
- Griffiths, T.T.; Arango, M.W.F.; Smith, I.M.; Wade, R.G. The baseline neutrophil lymphocyte ratio predicts survival in soft-tissue sarcoma: A 17-year cohort study. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Nakano, K.; Wang, X.; Fukuda, N.; Urasaki, T.; Ohmoto, A.; Hayashi, N.; Yunokawa, M.; Ono, M.; Tomomatsu, J.; et al. Pre-Treatment Neutrophil-to-Lymphocyte Ratio (NLR) as a Predictive Marker of Pazopanib Treatment for Soft-Tissue Sarcoma. Cancers 2021, 13, 6266. [Google Scholar] [CrossRef]
- Yapar, A.; Tokgöz, M.A.; Yapar, D.; Atalay, İ.B.; Ulucaköy, C.; Güngör, B.Ş. Diagnostic and prognostic role of neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and lymphocyte/monocyte ratio in patients with osteosarcoma. Jt. Dis. Relat. Surg. 2021, 32, 489–496. [Google Scholar] [CrossRef]
- Sato, Y.; Nakano, K.; Fukuda, N.; Wang, X.; Urasaki, T.; Ohmoto, A.; Yunokawa, M.; Ono, M.; Tomomatsu, J.; Hayakawa, K.; et al. Pre-treatment Neutrophil-to-Lymphocyte Ratio Predicts Efficacy of Eribulin for Soft-tissue Sarcoma. Anticancer Res. 2021, 41, 527–532. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, M.; Zhou, H.; Zhu, S.; Hu, C. Survival and prognostic analysis of preoperative indicators in patients undergoing surgical resections with rhabdomyosarcoma. Medicine 2020, 9, e22760. [Google Scholar] [CrossRef]
- Sambri, A.; Zucchini, R.; Giannini, C.; Cevolani, L.; Fiore, M.; Spinnato, P.; Bianchi, G.; Donati, D.M.; De Paolis, M. Systemic Inflammation Is Associated with Oncological Outcome in Patients with High-Grade Myxofibrosarcoma of the Extremities: A Retrospective Analysis. Oncol. Res. Treat. 2020, 43, 531–538. [Google Scholar] [CrossRef]
- Netanyahu, Y.; Gerstenhaber, F.; Shamai, S.; Sher, O.; Merimsky, O.; Klausner, J.M.; Lahat, G.; Nizri, E. Innate inflammatory markers for predicting survival in retroperitoneal sarcoma. J. Surg. Oncol. 2020, 122, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Namba, K.; Yamamoto, H.; Toji, T.; Soh, J.; Shien, K.; Suzawa, K.; Kurosaki, T.; Otani, S.; Okazaki, M.; et al. The neutrophil-to-lymphocyte ratio as a novel independent prognostic factor for multiple metastatic lung tumors from various sarcomas. Surg. Today 2021, 51, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Mirili, C.; Paydaş, S.; Guney, I.B.; Ogul, A.; Gokcay, S.; Buyuksimsek, M.; Yetisir, A.E.; Karaalioglu, B.; Tohumcuoglu, M.; Seydaoglu, G. Assessment of potential predictive value of peripheral blood inflammatory indexes in 26 cases with soft tissue sarcoma treated by pazopanib: A retrospective study. Cancer Manag. Res. 2019, 11, 3445–3453. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Okuma, T.; Oka, H.; Hirai, T.; Ohki, T.; Ikegami, M.; Sawada, R.; Shinoda, Y.; Akiyama, T.; Sato, K.; et al. Neutrophil-to-lymphocyte ratio after pazopanib treatment predicts response in patients with advanced soft-tissue sarcoma. Int. J. Clin. Oncol. 2018, 23, 368–374. [Google Scholar] [CrossRef]
- Choi, E.S.; Kim, H.S.; Han, I. Elevated preoperative systemic inflammatory markers predict poor outcome in localized soft tissue sarcoma. Ann. Surg. Oncol. 2014, 21, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; Teterycz, P.; Klimczak, A.; Bylina, E.; Szamotulska, K.; Lugowska, I. Blood neutrophil-to-lymphocyte ratio is associated with prognosis in advanced gastrointestinal stromal tumors treated with imatinib. Tumori 2018, 104, 415–422. [Google Scholar] [CrossRef]
- Sobczuk, P.; Teterycz, P.; Lugowska, I.; Klimczak, A.; Bylina, E.; Czarnecka, A.M.; Kosela-Paterczyk, H.; Osuch, C.; Streb, J.; Rutkowski, P. Prognostic value of the pretreatment neutrophil-to-lymphocyte ratio in patients with advanced gastrointestinal stromal tumors treated with sunitinib after imatinib failure. Oncol. Lett. 2019, 18, 3373–3380. [Google Scholar] [CrossRef]
- Li, Y.J.; Yang, X.; Zhang, W.B.; Yi, C.; Wang, F.; Li, P. Clinical implications of six inflammatory biomarkers as prognostic indicators in Ewing sarcoma. Cancer Manag. Res. 2017, 9, 443–451. [Google Scholar] [CrossRef]
- Luo, P.; Cai, W.; Yang, L.; Chen, S.; Wu, Z.; Chen, Y.; Zhang, R.; Shi, Y.; Yan, W.; Wang, C. Prognostic significance of pretreatment lymphocyte/monocyte ratio in retroperitoneal liposarcoma patients after radical resection. Cancer Manag. Res. 2018, 10, 4727–4734. [Google Scholar] [CrossRef]
- Hou, T.; Guo, T.; Nie, R.; Hong, D.; Zhou, Z.; Zhang, X.; Liang, Y. The prognostic role of the preoperative systemic immune-inflammation index and high-sensitivity modified Glasgow prognostic score in patients after radical operation for soft tissue sarcoma. Eur. J. Surg. Oncol. 2020, 46, 1496–1502. [Google Scholar] [CrossRef]
- Ma, C.; Yu, R.; Li, J.; Guo, J.; Xu, J.; Wang, X.; Liu, P. Preoperative prognostic nutritional index and systemic immune-inflammation index predict survival outcomes in osteosarcoma: A comparison between young and elderly patients. J. Surg. Oncol. 2022, 125, 754–765. [Google Scholar] [CrossRef]
- Ouyang, H.; Wang, Z. Predictive value of the systemic immune-inflammation index for cancer-specific survival of osteosarcoma in children. Front. Public Health 2022, 10, 879523. [Google Scholar] [CrossRef] [PubMed]
- Goubran, H.A.; Burnouf, T.; Radosevic, M.; El-Ekiaby, M. The platelet-cancer loop. Eur. J. Intern. Med. 2013, 24, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Gay, L.J.; Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 2011, 11, 123–134. [Google Scholar] [CrossRef]
- De Larco, J.E.; Wuertz, B.R.; Furcht, L.T. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin. Cancer Res. 2004, 10, 4895–4900. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Qian, B.Z.; Pollard, J.W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 2015, 15, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Shimada, E.; Endo, M.; Matsumoto, Y.; Tsuchihashi, K.; Ito, M.; Kusaba, H.; Nabeshima, A.; Nawata, T.; Maekawa, A.; Matsunobu, T.; et al. Does the Use of Peripheral Immune-Related Markers Indicate Whether to Administer Pazopanib, Trabectedin, or Eribulin to Advanced Soft Tissue Sarcoma Patients? J. Clin. Med. 2021, 26, 4972. [Google Scholar] [CrossRef]
- Bingle, L.; Brown, N.J.; Lewis, C.E. The role of tumour-associated macrophages in tumour progression: Implications for new anticancer therapies. J. Pathol. 2002, 196, 254–265. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Liu, L.; Gong, C.Y.; Shi, H.S.; Zeng, Y.H.; Wang, X.Z.; Zhao, Y.W.; Wei, Y.Q. Prognostic significance of tumor-associated macrophages in solid tumor: A meta-analysis of the literature. PLoS ONE 2012, 7, e50946. [Google Scholar] [CrossRef]
- Tazzari, M.; Bergamaschi, L.; De Vita, A.; Collini, P.; Barisella, M.; Bertolotti, A.; Ibrahim, T.; Pasquali, S.; Castelli, C.; Vallacchi, V. Molecular Determinants of Soft Tissue Sarcoma Immunity: Targets for Immune Intervention. Int. J. Mol. Sci. 2021, 22, 7518. [Google Scholar] [CrossRef]
- Mantovani, A.; Bottazzi, B.; Colotta, F.; Sozzani, S.; Ruco, L. The origin and function of tumor-associated macrophages. Immunol. Today 1992, 13, 265–270. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive and Integrated Genomic Characterization of Adult Soft Tissue Sarcomas. Cell 2017, 171, 950–965.e28. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Espinosa, I.; Vrijaldenhoven, S.; Subramanian, S.; Montgomery, K.D.; Zhu, S.; Marinelli, R.J.; Peterse, J.L.; Poulin, N.; Nielsen, T.O.; et al. Prognostic significance of macrophage infiltration in leiomyosarcomas. Clin. Cancer Res. 2008, 14, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Ganjoo, K.N.; Witten, D.; Patel, M.; Espinosa, I.; La, T.; Tibshirani, R.; van de Rijn, M.; Jacobs, C.; West, R.B. The prognostic value of tumor-associated macrophages in leiomyosarcoma: A single institution study. Am. J. Clin. Oncol. 2011, 34, 82–86. [Google Scholar] [CrossRef]
- De Vita, A.; Recine, F.; Miserocchi, G.; Pieri, F.; Spadazzi, C.; Cocchi, C.; Vanni, S.; Liverani, C.; Farnedi, A.; Fabbri, F.; et al. The potential role of the extracellular matrix in the activity of trabectedin in UPS and L-sarcoma: Evidence from a patient-derived primary culture case series in tridimensional and zebrafish models. J. Exp. Clin. Cancer Res. 2021, 40, 165. [Google Scholar] [CrossRef]
- de Nonneville, A.; Barbolosi, D.; Andriantsoa, M.; El-Cheikh, R.; Duffaud, F.; Bertucci, F.; Salas, S. Validation of Neutrophil Count as An Algorithm-Based Predictive Factor of Progression-Free Survival in Patients with Metastatic Soft Tissue Sarcomas Treated with Trabectedin. Cancers 2019, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Nakano, K.; Kawaguchi, K.; Fukuda, N.; Wang, X.; Urasaki, T.; Ohmoto, A.; Hayashi, N.; Yunokawa, M.; Ono, M.; et al. Changes in Neutrophil-to-lymphocyte Ratio Predict Efficacy of Trabectedin for Soft-tissue Sarcoma. Cancer Diagn. Progn. 2021, 1, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yu, X.; Wang, Z.; Wu, P.; Huang, J. Anthracyclines potentiate anti-tumor immunity: A new opportunity for chemoimmunotherapy. Cancer Lett. 2015, 369, 331–335. [Google Scholar] [CrossRef]

| Sex | |
|---|---|
| Male | 43 (43.4) |
| Female | 56 (56.6) |
| Age at treatment | |
| Median (range) | 64 (26–83) |
| Metastatic onset | |
| Metachronous | 66 (66.6) |
| Synchronous | |
| Primary site | |
| Retroperitoneum | 8 (8) |
| Limbs | 65(65.6) |
| Head and Neck | 1(1.1) |
| Trunk | 4(4.1) |
| Uterus | 21 (21.2) |
| Histotype | |
| Leiomyosarcoma | 34 (34.4) |
| Leiomyosarcoma nas | 17 |
| Uterine leiomyosarcoma | 17 |
| Liposarcoma | 27 (27.3) |
| Well differentiated liposarcoma | 7 |
| De differentiated liposarcoma | 14 |
| Myxoid liposarcoma | 2 |
| Pleomorphic liposarcoma | 4 |
| Others | 38 (38.3) |
| Angiosarcoma | 7 |
| DSRC | 2 |
| Fibrosarcoma | 2 |
| Myxofibrosarcoma | 4 |
| MPNST | 1 |
| Rhabdomyosarcoma | 1 |
| Alveolar sarcoma | 1 |
| Epithelioid sarcoma | 1 |
| Synovial sarcoma | 4 |
| High grade SES | 1 |
| SFT | 4 |
| UPS | 10 |
| Tumor burden | |
| Single metastasis | 1 (1.0) |
| Oligometastatic | 52 (53.1) |
| Disseminated | 45 (45.9) |
| Grading (FNCLCC) | |
| G1 | 6 (6.2) |
| G2 | 22 (22.7) |
| G3 | 69 (71.1) |
| PS ECOG | |
| 0 | 6 (6.2) |
| 1 | 63 (63.7) |
| 2 | 30 (30.1) |
| First-line treatment | |
| Adriamycin | 39 (39.4) |
| Adriamycin + Dacarbazine | 11 (11.1) |
| EI | 47 (47.5) |
| VAI-IE | 2 (2.1) |
| II line | PD | SD | PR | TOT | mPFS (mo) | mOS (mo) |
|---|---|---|---|---|---|---|
| Trabectedin | 11 (34.38%) | 16 (50%) | 5 (15.63%) | 32 (100%) | 6.73 | 13.9 |
| Dacarbazine | 7 (77.78%) | 2 (22.22%) | 0 | 9 (100%) | 2.54 | 4.37 |
| Gemcitabine-based | 19 (52.78%) | 12 (33.33%) | 5 (13.89%) | 36 (100%) | 3.37 | 11.57 |
| Ifo-HD | 8 (57.14%) | 2 (14.29%) | 4 (28.57%) | 14 (100%) | 2.6 | 14.57 |
| Pazopanib | 3 (60%) | 2 (40%) | 0 | 5 (100%) | 2.73 | 8.93 |
| Others | 2 (66.67%) | 0 | 1 (33.33%) | 3 (100%) | 2 | 15.13 |
| TOT | 50 (50.51%) | 34 (34.34%) | 15 (15.15%) | 99 (100%) |
| Mixture | LP1 | LP2 | LP3 | LP4 | LP5 | LP6 |
|---|---|---|---|---|---|---|
| NLR | 2.89 | 2.45 | 3.06 | 2.22 | 6.32 | 3.22 |
| PLR | 142.28 | 125.5 | 147 | 104.22 | 278.76 | 104.22 |
| LMR | 2.94 | 4.87 | 2.96 | 3.23 | 0.85 | 4.94 |
| B cells naive | 0.92 | 9.03 | 8.99 | 11.95 | 1.38 | 22.27 |
| B cells memory | 2.35 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Plasma cells | 3.41 | 0.00 | 0.00 | 0.00 | 0.27 | 0.00 |
| T cells CD8 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| T cells CD4 naive | 1.36 | 0.00 | 0.00 | 0.00 | 0.00 | 46.14 |
| T cells CD4 memory resting | 21.96 | 31.58 | 20.61 | 20.52 | 16.43 | 0.00 |
| T cells CD4 memory activated | 0.00 | 0.00 | 0.00 | 0.00 | 0.56 | 0.00 |
| T cells follicular helper | 0.00 | 13.02 | 8.26 | 0.00 | 0.00 | 0.00 |
| T cells regulatory (Tregs) | 0.00 | 0.00 | 0.00 | 8.50 | 0.00 | 0.00 |
| T cells gamma delta | 9.33 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| NK cells resting | 0.00 | 0.00 | 2.98 | 0.00 | 0.07 | 0.00 |
| NK cells activated | 1.33 | 17.00 | 4.32 | 3.11 | 0.17 | 12.67 |
| Monocytes | 3.33 | 18.01 | 3.38 | 3.49 | 5.36 | 0.00 |
| Macrophages M0 | 0.00 | 1.83 | 0.00 | 23.58 | 0.00 | 0.00 |
| Macrophages M1 | 0.69 | 0.00 | 5.78 | 0.00 | 1.13 | 0.00 |
| Macrophages M2 | 36.75 | 3.61 | 36.40 | 24.55 | 50.33 | 0.00 |
| Dendritic cells resting | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Dendritic cells activated | 3.27 | 5.92 | 0.59 | 4.29 | 3.41 | 2.25 |
| Mast cells resting | 15.29 | 0.00 | 7.62 | 0.00 | 18.31 | 16.68 |
| Mast cells activated | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Eosinophils | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Neutrophils | 0.00 | 0.00 | 1.07 | 0.00 | 2.59 | 0.00 |
| Ref | Author | N° of patients | Setting | Group | Index | Cutoff (≥) | Outcome | P |
|---|---|---|---|---|---|---|---|---|
| [54] | Garcìa-Ortega | 112 | Mixed | UPS | NLR | 3.09 | worse OS | 0.04 |
| [55] | Koseci | 30 | Recurrent or metastatic treated with Pazopanib | STS | NLR | 3 | worse PFS and OS | 0.04; 0.015 |
| [56] | Griffiths | 401 | Mixed | extremity STS | NLR | 3 | worse OS | / |
| [57] | Sato | 141 | Recurrent or metastatic | STS | NLR | 3 | worse OS | 0.01 |
| [58] | Yapar | 172 | Mixed | osteosarcoma | NLR | 3.28 | worse OS | <0.001 |
| [59] | Sato | 53 | recurrent or metastatic treated with Eribulin | STS | NLR | 3 | worse | 0.01 |
| [60] | Jin | 55 | Localized | RMS | NLR | 2.843 | worse PFS and OS | 0.029; 0.005 |
| [61] | Sambri | 126 | Localized | MFS | NLR | 3.5 | worse DSS | <0.001 |
| [62] | Netanyahu | 78 | Localized | RPS | NLR | 2.1 | worse PFS and OS | 0.06, 0.3 |
| [63] | Yamamoto | 158 | Advanced | STS | NLR | 2.26 | worse OS | / |
| [28] | Vinal | 79 | Mixed | STS | NLR | 2.83 | worse PFS and OS | <0.001; 0.01 |
| [42] | Cheng | 103 | Mixed | SS | NLR | 2.7 | worse OS | 0.03 |
| [40] | Chen | 42 | After radical surgery | Clear Cell Sarcoma | NLR | 2.73 | worse OS | 0.01 |
| [64] | Mirili | 26 | Recurrent or metastatic treated with Pazopanib | STS | NLR | 4.8 | worse OS | 0.02 |
| [39] | Garcìa-Ortega | 169 | Presurgery | SS | NLR | 3.5 | worse OS | 0.00 |
| [36] | Chan | 712 | Localized | STS | NLR | 2.5 | worse PFS and OS | 0.0125; 0.0112 |
| [36] | Chan | metastatic/unresectable | STS | NLR | 2.5 | worse OS | 0.01 | |
| [65] | Kobayashi | 25 | Advanced | STS | NLR | 3.8 | worse PFS and OS | 0.001; 0.0006 |
| [41] | Vasquez | 100 | Mixed | pediatric sarcomas (OS, RMS, ES) | NLR | 2 | worse OS | 0.0237 RMS; 0.046 OS |
| [66] | Choi | 162 | Localized | STS | NLR | 2.5 | worse DFS | 0.03 |
| [67] | Rutkowski | 385 | Advanced | GIST | NLR | 2.7 | worse PFS and OS | <0.001; 0.001 |
| [68] | Sobczuk | 146 | unresectable/metastatic GIST treated with sunitinib after failure of imatinib | GIST | NLR | 2.4 | worse PFS and OS | 0.075; 0.002 |
| [69] | Li | 122 | Mixed | ES | NLR | 2.38 | worse OS | 0.01 |
| [58] | Yapar | 172 | Mixed | OS | PLR | 128 | worse OS | 0.01 |
| [60] | Jin | 55 | Localized | RMS | PLR | 162.96 | worse PFS and OS | 0.08; 0.05 |
| [40] | Chen | 42 | After radical surgery | Clear Cell Sarcoma | PLR | 103.89 | worse OS | 0.0147 |
| [64] | Mirili | 26 | Recurrent or metastatic treated with Pazopanib | STS | PLR | 195 | worse OS | / |
| [69] | Li | 122 | Mixed | ES | PLR | 131 | worse OS | 0.032 |
| [58] | Yapar | 172 | Mixed | OS | LMR | 4.22 | Better OS | 0.004 |
| [42] | Cheng | 103 | Mixed | SS | LMR | 4.16 | Better PFS | 0.025 |
| [40] | Chen | 42 | After radical surgery | Clear Cell Sarcoma | LMR | 4.7 | Better OS | 0.0445 |
| [70] | Luo | 100 | Presurgery | RPLS | LMR | 3 | Better OS | 0.002 |
| [71] | Hou | 454 | After radical surgery | STS | SII | unk | worse OS | / |
| [72] | Ma | 125 | Localized | OS | SII | 607.3 | worse OS | / |
| [73] | Ouyang | 86 | Mixed | Pediatric OS | SII | unk | worse OS | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fausti, V.; De Vita, A.; Vanni, S.; Ghini, V.; Gurrieri, L.; Riva, N.; Casadei, R.; Maraldi, M.; Ercolani, G.; Cavaliere, D.; et al. Systemic Inflammatory Indices in Second-Line Soft Tissue Sarcoma Patients: Focus on Lymphocyte/Monocyte Ratio and Trabectedin. Cancers 2023, 15, 1080. https://doi.org/10.3390/cancers15041080
Fausti V, De Vita A, Vanni S, Ghini V, Gurrieri L, Riva N, Casadei R, Maraldi M, Ercolani G, Cavaliere D, et al. Systemic Inflammatory Indices in Second-Line Soft Tissue Sarcoma Patients: Focus on Lymphocyte/Monocyte Ratio and Trabectedin. Cancers. 2023; 15(4):1080. https://doi.org/10.3390/cancers15041080
Chicago/Turabian StyleFausti, Valentina, Alessandro De Vita, Silvia Vanni, Virginia Ghini, Lorena Gurrieri, Nada Riva, Roberto Casadei, Marco Maraldi, Giorgio Ercolani, Davide Cavaliere, and et al. 2023. "Systemic Inflammatory Indices in Second-Line Soft Tissue Sarcoma Patients: Focus on Lymphocyte/Monocyte Ratio and Trabectedin" Cancers 15, no. 4: 1080. https://doi.org/10.3390/cancers15041080
APA StyleFausti, V., De Vita, A., Vanni, S., Ghini, V., Gurrieri, L., Riva, N., Casadei, R., Maraldi, M., Ercolani, G., Cavaliere, D., Pacilio, C. A., Pieri, F., Foca, F., Bongiovanni, A., Ranallo, N., Calpona, S., Frassineti, G. L., Ibrahim, T., & Mercatali, L. (2023). Systemic Inflammatory Indices in Second-Line Soft Tissue Sarcoma Patients: Focus on Lymphocyte/Monocyte Ratio and Trabectedin. Cancers, 15(4), 1080. https://doi.org/10.3390/cancers15041080










