Malignant Pleural Mesothelioma: Preliminary Toxicity Results of Adjuvant Radiotherapy Hypofractionation in a Prospective Trial (MESO-RT)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
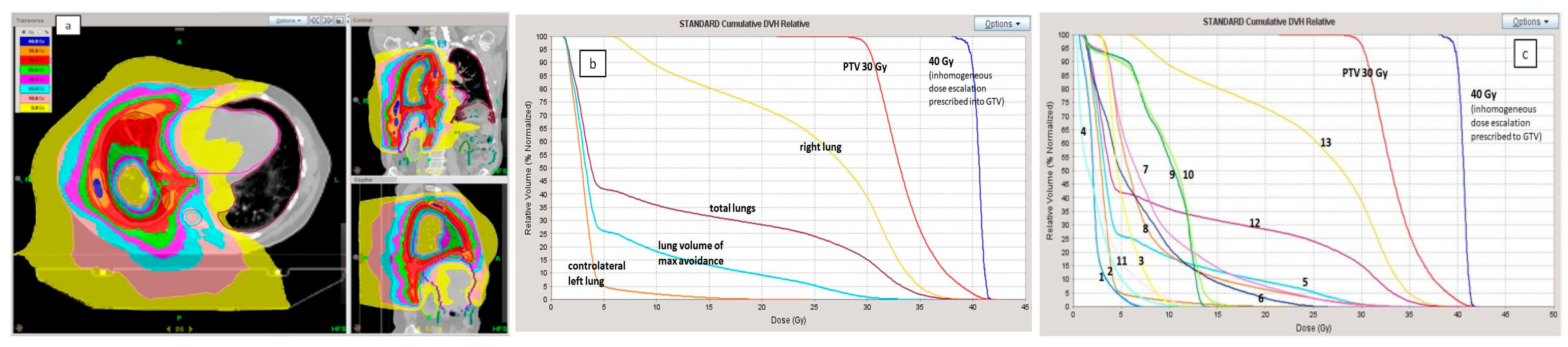
2.1. Planning Procedure and Treatment Delivery
2.2. Assessing Treatment Outcome
2.3. Statistical Analysis
3. Results
3.1. Radiation Treatments Details
3.2. Acute Toxicity
3.3. Late Toxicity
3.4. Pulmonary Function Tests
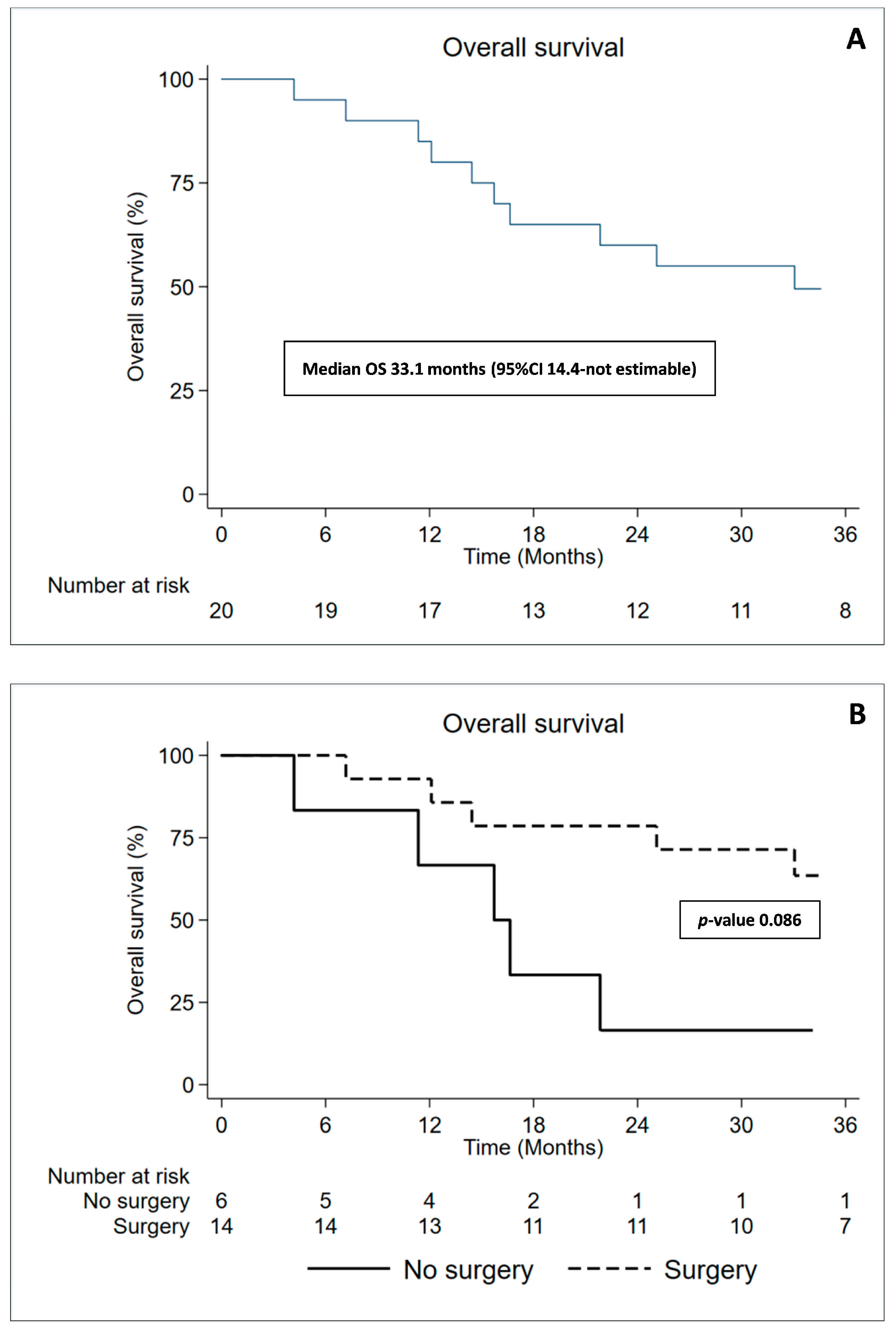
3.5. Overall Survival and Time to Progression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, A.J.; Parker, R.J.; Wiggins, J. Malignant mesothelioma. Orphanet J. Rare Dis. 2008, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Muers, M.F.; Stephens, R.J.; Fisher, P.; Darlison, L.; Higgs, C.M.; Lowry, E.; Nicholson, A.G.; O’Brien, M.; Peake, M.; Rudd, R.; et al. Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): A multicentre randomised trial. Lancet 2008, 371, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Scherpereel, A.; Opitz, I.; Berghmans, T.; Psallidas, I.; Glatzer, M.; Rigau, D.; Astoul, P.; Bölükbas, S.; Boyd, J.; Coolen, J.; et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesotelioma. Eur. Respir. J. 2020, 55, 1900953. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, D.J.; Flores, R.M.; Jaklitsch, M.T.; Richards, W.G.; Strauss, G.M.; Corson, J.M.; DeCamp, M.M., Jr.; Swanson, S.J.; Bueno, R.; Lukanich, J.M.; et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: Results in 183 patients. J. Thorac. Cardiovasc. Surg. 1999, 117, 54–63. [Google Scholar] [CrossRef]
- Rusch, V.W.; Piantadosi, S.; Holmes, E.C. The role of extrapleural pneumonectomy in malignant pleural mesothelioma. A Lung Cancer Study Group trial. J. Thorac. Cardiovasc. Surg. 1991, 102, 1–9. [Google Scholar] [CrossRef]
- MacLeod, N.; Chalmers, A.; O’Rourke, N.; Moore, K.; Sheridan, J.; McMahon, L.; Bray, C.; Stobo, J.; Price, A.; Fallon, M.; et al. Is Radiotherapy Useful for Treating Pain in Mesothelioma?: A Phase II Trial. J. Thorac. Oncol. 2015, 10, 944–950. [Google Scholar] [CrossRef]
- Baldini, E.H.; Recht, A.; Strauss, G.M.; DeCamp, M.M., Jr.; Swanson, S.J.; Liptay, M.J.; Mentzer, S.J.; Sugarbaker, D.J. Patterns of failure after trimodality therapy for malignant pleural mesothelioma. Ann. Thorac. Surg. 1997, 63, 334–338. [Google Scholar] [CrossRef]
- Miles, E.F.; Larrier, N.A.; Kelsey, C.R.; Hubbs, J.L.; Ma, J.; Yoo, S.; Marks, L.B. Intensity-modulated radiotherapy for resected mesothelioma: The Duke experience. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1143–1150. [Google Scholar] [CrossRef]
- Krug, L.M.; Pass, H.I.; Rusch, V.W.; Kindler, H.L.; Sugarbaker, D.J.; Rosenzweig, K.E.; Flores, R.; Friedberg, J.S.; Pisters, K.; Monberg, M.; et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J. Clin. Oncol. 2009, 27, 3007–3013. [Google Scholar] [CrossRef]
- Sterzing, F.; Sroka-Perez, G.; Schubert, K.; Münter, M.W.; Thieke, C.; Huber, P.; Debus, J.; Herfarth, K.K. Evaluating target coverage and normal tissue sparing in the adjuvant radiotherapy of malignant pleural mesothelioma: Helical tomotherapy compared with step-and-shoot IMRT. Radiother. Oncol. 2008, 86, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Sylvestre, A.; Mahé, M.A.; Lisbona, A.; Zefkili, S.; Savignoni, A.; Bonnette, P.; Barthes Fle, P.; Paris, E.; Perigaud, C.; Yassa, M.; et al. Mesothelioma at era of helical tomotherapy: Results of two institutions in combining chemotherapy, surgery and radiotherapy. Lung Cancer 2011, 74, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Treasure, T.; Lang-Lazdunski, L.; Waller, D.; Bliss, J.M.; Tan, C.; Entwisle, J.; Snee, M.; O’Brien, M.; Thomas, G.; Senan, S.; et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: Clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011, 12, 763–772. [Google Scholar] [CrossRef]
- Flores, R.M.; Pass, H.I.; Seshan, V.E.; Dycoco, J.; Zakowski, M.; Carbone, M.; Bains, M.S.; Rusch, V.W. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: Results in 663 patients. J. Thorac. Cardiovasc. Surg. 2008, 135, 620–626. [Google Scholar] [CrossRef]
- Cao, C.; Tian, D.; Park, J.; Allan, J.; Pataky, K.A.; Yan, T.D. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014, 83, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Pembrolizumab and Hypofractionated Stereotactic Radiotherapy in Patients With Malignant Pleural Mesothelioma (MESO-PRIME). ClinicalTrials.gov Identifier: NCT03269227. Available online: https://clinicaltrials.gov/ct2/show/NCT04166734 (accessed on 26 May 2016).
- Accelerated Hypofractionated Radiotherapy in the Treatment of Malignant Pleural Mesothelioma (MesoRT). ClinicalTrials.gov Identifier: NCT04166734. Available online: https://clinicaltrials.gov/ct2/show/NCT03269227 (accessed on 31 August 2017).
- Parisi, E.; Romeo, A.; Sarnelli, A.; Ghigi, G.; Bellia, S.R.; Neri, E.; Micheletti, S.; Dipalma, B.; Arpa, D.; Furini, G.; et al. High dose irradiation after pleurectomy/decortication or biopsy for pleural mesothelioma treatment. Cancer Radiother. 2017, 21, 766–773. [Google Scholar] [CrossRef]
- Parisi, E.; Genestreti, G.; Sarnelli, A.; Ghigi, G.; Arpa, D.; Burgio, M.A.; Gavelli, G.; Rossi, A.; Scarpi, E.; Monti, M.; et al. Accelerated hypofractionated radiotherapy plus chemotherapy for inoperable locally advanced non-small-cell lung cancer: Final results of a prospective phase-II trial with a long-term follow-up. Radiat. Oncol. 2019, 14, 112. [Google Scholar] [CrossRef]
- Forster, K.M.; Smythe, W.R.; Starkschall, G.; Liao, Z.; Takanaka, T.; Kelly, J.F.; Vaporciyan, A.; Ahamad, A.; Dong, L.; Salehpour, M.; et al. Intensity-modulated radiotherapy following extrapleural pneumonectomy for the treatment of malignant mesothelioma: Clinical implementation. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 606–616. [Google Scholar] [CrossRef]
- Byrne, M.J.; Nowak, A.K. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann. Oncol. 2004, 15, 257–260. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- de Perrot, M.; Feld, R.; Leighl, N.B.; Hope, A.; Waddell, T.K.; Keshavjee, S.; Cho, B.C. Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma. J. Thorac. Cardiovasc. Surg. 2016, 151, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Lang-Lazdunski, L.; Bille, A.; Lal, R.; Cane, P.; McLean, E.; Landau, D.; Steele, J.; Spicer, J. Pleurectomy/decortication is superior to extrapleural pneumonectomy in the multimodality management of patients with malignant pleural mesothelioma. J. Thorac. Oncol. 2012, 7, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; Czerminska, M.; Jänne, P.A.; Sugarbaker, D.J.; Bueno, R.; Harris, J.R.; Court, L.; Baldini, E.H. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Krug, L.M.; Laser, B.; Hudka, K.; Flores, R.; Rusch, V.W.; Rosenzweig, K.E. Patterns of local and nodal failure in malignant pleural mesothelioma after extrapleural pneumonectomy and photon-electron radiotherapy. J. Thorac. Oncol. 2009, 4, 746–750. [Google Scholar] [CrossRef]
- Rosenzweig, K.E.; Zauderer, M.G.; Laser, B.; Krug, L.M.; Yorke, E.; Sima, C.S.; Rimner, A.; Flores, R.; Rusch, V. Pleural intensity-modulated radiotherapy for malignant pleural mesothelioma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1278–1283. [Google Scholar] [CrossRef]
- Rimner, A.; Zauderer, M.G.; Gomez, D.R.; Adusumilli, P.S.; Parhar, P.K.; Wu, A.J.; Woo, K.M.; Shen, R.; Ginsberg, M.S.; Yorke, E.D.; et al. Phase II Study of Hemithoracic Intensity-Modulated Pleural Radiation Therapy (IMPRINT) As Part of Lung-Sparing Multimodality Therapy in Patients With Malignant Pleural Mesothelioma. J. Clin. Oncol. 2016, 34, 2761–2768. [Google Scholar] [CrossRef]
- Minatel, E.; Trovo, M.; Polesel, J.; Baresic, T.; Bearz, A.; Franchin, G.; Gobitti, C.; Rumeileh, I.A.; Drigo, A.; Fontana, P.; et al. Radical pleurectomy/decortication followed by high dose of radiation therapy for malignant pleural mesothelioma. Final results with long-term follow-up. Lung Cancer 2014, 83, 78–82. [Google Scholar] [CrossRef]
- Patel, R.; Ludmir, E.B.; Miccio, J.A.; Menon, H.; Barsky, A.R.; Mesko, S.M.; Kodali, M.; Lautenschlaeger, T.; Adeberg, S.; Simone, C.B., 2nd; et al. Disease-Related Outcomes and Toxicities of Intensity Modulated Radiation Therapy After Lung-Sparing Pleurectomy for Malignant Pleural Mesothelioma: A Systematic Review. Pract. Radiat. Oncol. 2020, 10, 423–433. [Google Scholar] [CrossRef]
| (A) Patients’ Characteristics | |
| Gender | n (%) |
| Male | 16 (80%) |
| Female | 4 (20%) |
| Age | Years (%) |
| Median (range) | 72.1 (44–82.5) |
| Asbestos exposure | n (%) |
| Yes | 13 (76.5%) |
| No | 4 (23.5%) |
| Unknown | 3 |
| Smoking | n (%) |
| Yes | 9 (64.3%) |
| No | 5 (35.7%) |
| Unknown | 6 |
| Laterality | n (%) |
| Right | 12 (60%) |
| Left | 8 (40%) |
| (B) Tumor and Treatment Characteristics | |
| Histology | n (%) |
| Epithelioid | 19 (95%) |
| Sarcomatoid | 1 (5%) |
| T,N Stage | n (%) |
| T1b | 1 (5%) |
| T2 | 8 (40%) |
| T3 | 7 (35%) |
| T4 | 3 (15%) |
| Tx | 1 (5%) |
| N0 | 14 (70%) |
| N1 | 1 (15%) |
| N2 | 3 (15%) |
| Nx | 2 (10%) |
| Previous surgery | n |
| Yes | 14 |
| No | 6 |
| Previous chemotherapy | n (%) |
| Pemetrexed | 2 (10%) |
| CBCDA/Pem | 6 (30%) |
| CDDP/Pem | 11 (55%) |
| CDDP | 1 (5%) |
| Acute n of pts (%) | Late n of pts (%) | |||||
|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G1 | G2 | G3 | |
| Pleural effusion | 2 (10%) | 2 (10%) | 0 (0%) | 3 (15%) | 0 (0%) | 0 (0%) |
| Pain | 8 (27%) | 0 (0%) | 0 (0%) | 2 (10%) | 0 (0%) | 0 (0%) |
| Vomiting | 1 (5%) | 1 (5%) | 0 (0%) | 1 (5%) | 0 (0%) | 0 (0%) |
| Erythema/Rush | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Nausea | 3 (15%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Fever | 3 (15%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Tachycardia | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%)) | 0 (0%) |
| Dyspnoea | 13 (65%) | 3 (15%) | 0 (0%) | 2 (10%) | 0 (0%) | 0 (0%) |
| Asthenia/Fatigue | 5 (25%) | 3 (15%) | 0 (0%) | 2 (10%) | 1 (5%) | 0 (0%) |
| Pneumonitis | 13 (65%) | 2 (10%) | 0 (0%) | 2 (10%) | 0 (0%) | 0 (0%) |
| Myalgia | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Cough | 7 (35%) | 3 (15%) | 0 (0%) | 3 (15%) | 2 (10%) | 0 (0%) |
| Skin toxicity | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Pericardial effusion | 3 (15%) | 0 (0%) | 0 (0%) | 2 (10%) | 0 (0%) | 0 (0%) |
| Loss of appetite | 1 (5%) | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Atelectasis | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Dyspepsia | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Dysphagia | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Emoftoe | 1 (5%) | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Herpes zoster | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Temporary disorientation | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Asymptomatic pulmonary embolism | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (5%) |
| Contact dermatitis | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (5%) | 0 (0%) |
| Mastitis | 0 (0%) | 0 (0%) | 0 (0%) | 1 (5%) | 1 (5%) | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisi, E.; Arpa, D.; Ghigi, G.; Fabbri, L.; Foca, F.; Tontini, L.; Neri, E.; Pieri, M.; Cima, S.; Burgio, M.A.; et al. Malignant Pleural Mesothelioma: Preliminary Toxicity Results of Adjuvant Radiotherapy Hypofractionation in a Prospective Trial (MESO-RT). Cancers 2023, 15, 1057. https://doi.org/10.3390/cancers15041057
Parisi E, Arpa D, Ghigi G, Fabbri L, Foca F, Tontini L, Neri E, Pieri M, Cima S, Burgio MA, et al. Malignant Pleural Mesothelioma: Preliminary Toxicity Results of Adjuvant Radiotherapy Hypofractionation in a Prospective Trial (MESO-RT). Cancers. 2023; 15(4):1057. https://doi.org/10.3390/cancers15041057
Chicago/Turabian StyleParisi, Elisabetta, Donatella Arpa, Giulia Ghigi, Lucia Fabbri, Flavia Foca, Luca Tontini, Elisa Neri, Martina Pieri, Simona Cima, Marco Angelo Burgio, and et al. 2023. "Malignant Pleural Mesothelioma: Preliminary Toxicity Results of Adjuvant Radiotherapy Hypofractionation in a Prospective Trial (MESO-RT)" Cancers 15, no. 4: 1057. https://doi.org/10.3390/cancers15041057
APA StyleParisi, E., Arpa, D., Ghigi, G., Fabbri, L., Foca, F., Tontini, L., Neri, E., Pieri, M., Cima, S., Burgio, M. A., Belli, M. L., Luzzi, L., & Romeo, A. (2023). Malignant Pleural Mesothelioma: Preliminary Toxicity Results of Adjuvant Radiotherapy Hypofractionation in a Prospective Trial (MESO-RT). Cancers, 15(4), 1057. https://doi.org/10.3390/cancers15041057





