Geographic Variation and Risk Factor Association of Early Versus Late Onset Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Sources
2.2. Variables of Interest
2.3. Analytic Approach
2.3.1. Composite Counties
2.3.2. Random Forest and Variable Importance
3. Results
3.1. Patient Characteristics of EOCRC and LOCRC
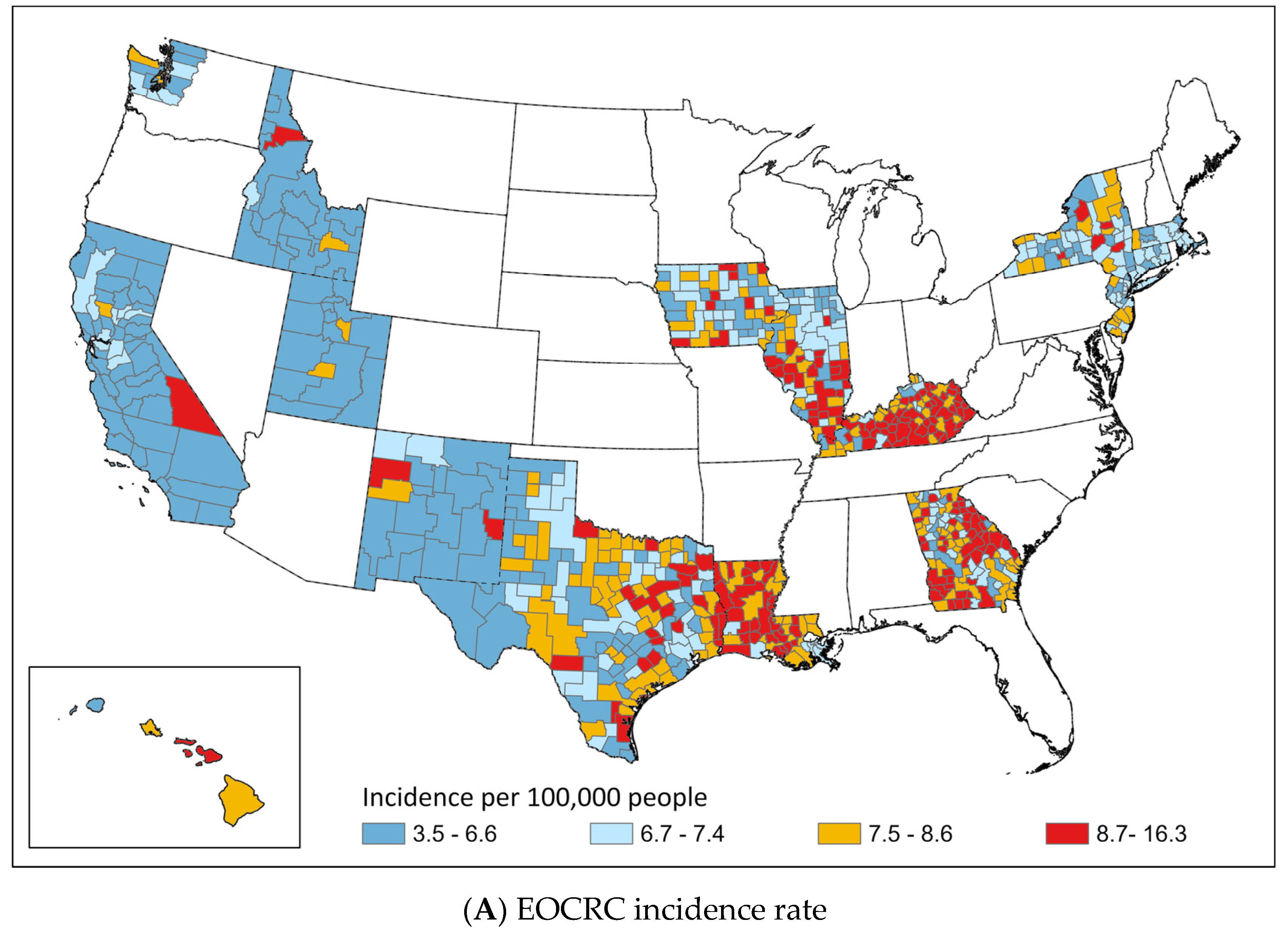
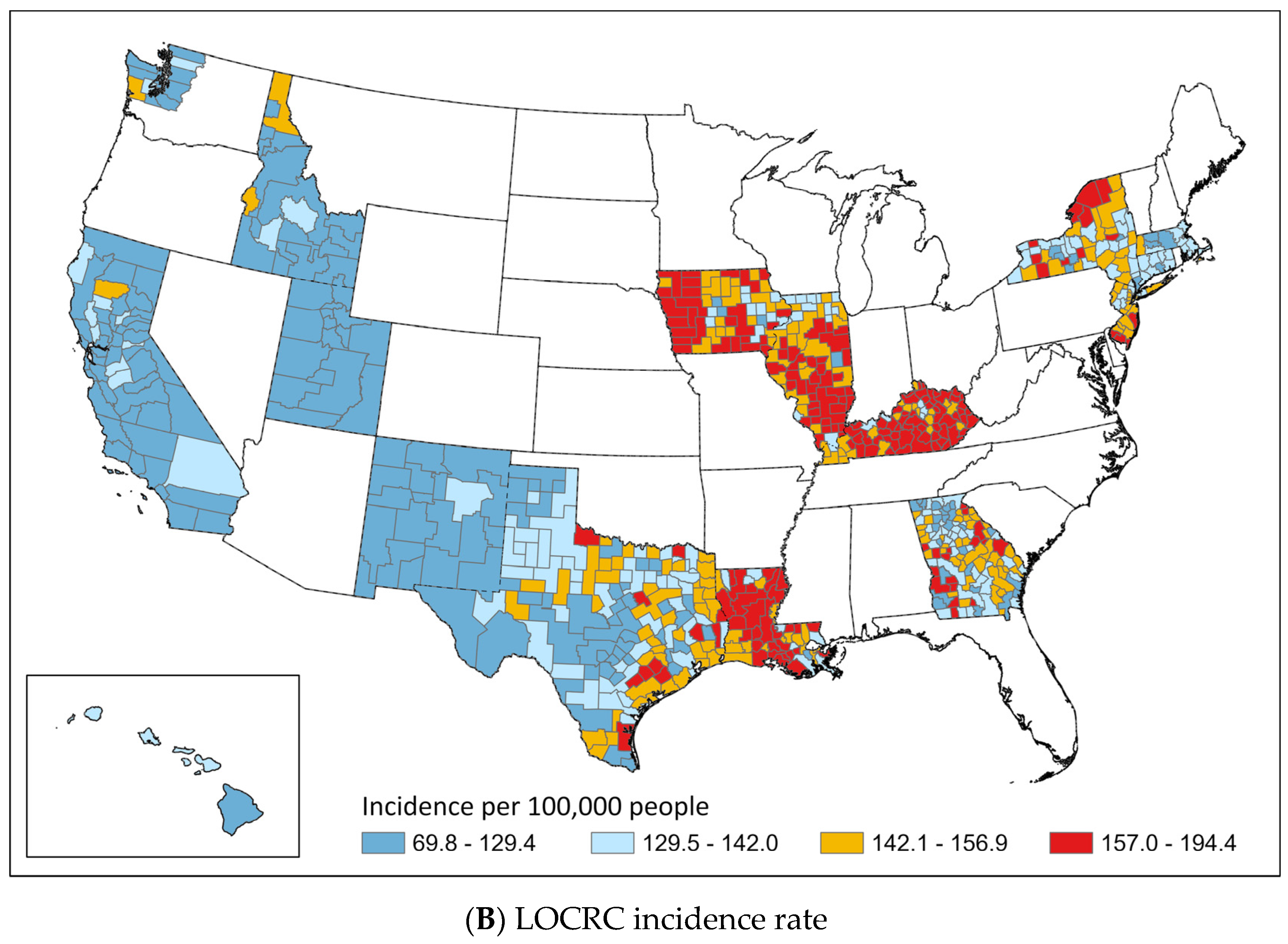
3.2. Geographic Distribution of EOCRC and LOCRC
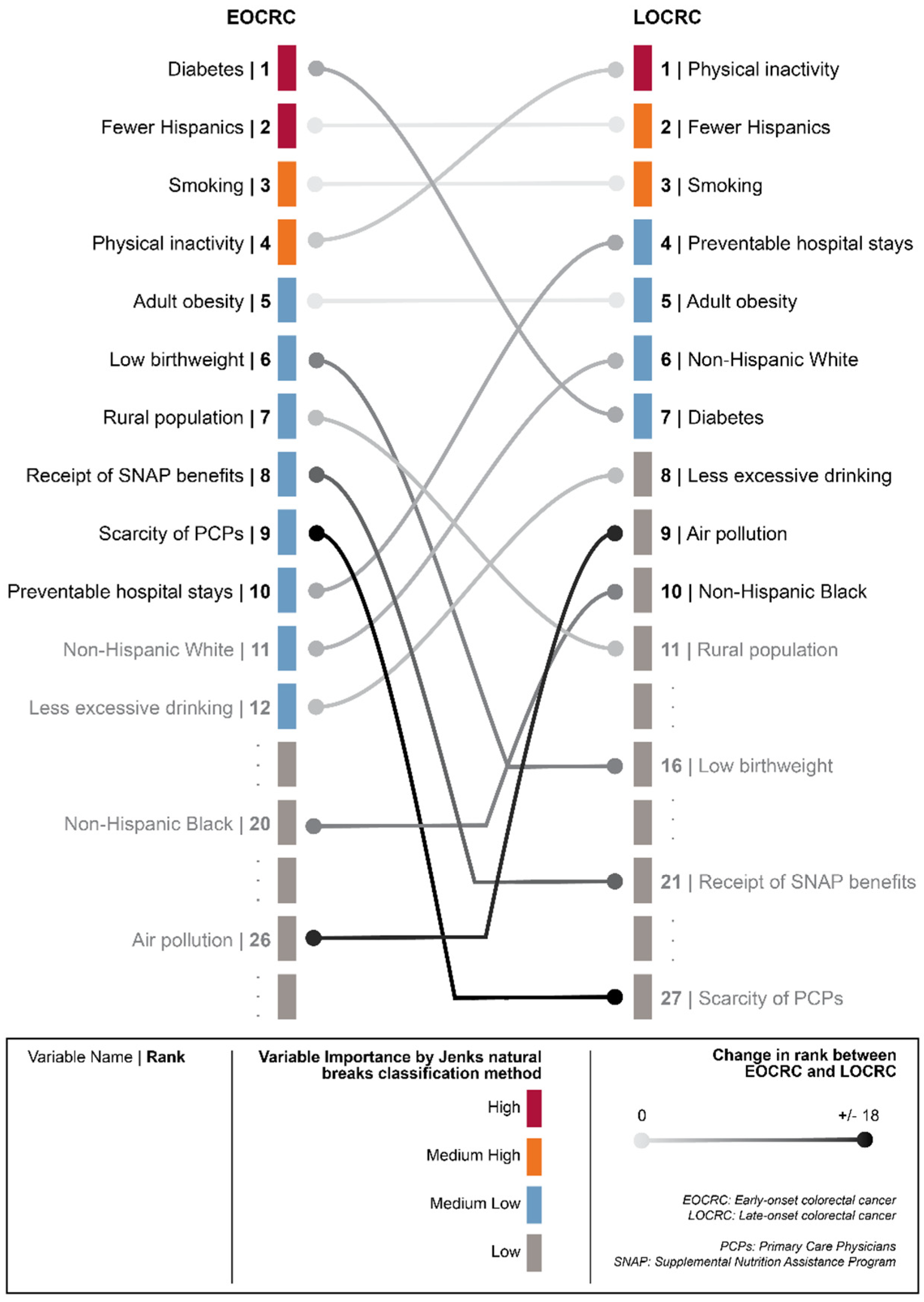
3.3. Importance of Risk Factors in Predicting EOCRC and LOCRC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Wang, W.; Chen, W.; Lin, J.; Shen, Q.; Zhou, X.; Lin, C. Incidence and characteristics of young-onset colorectal cancer in the United States: An analysis of SEER data collected from 1988 to 2013. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 208–215. [Google Scholar] [CrossRef]
- Archambault, A.N.; Lin, Y.; Jeon, J.; Harrison, T.A.; Bishop, D.T.; Brenner, H.; Casey, G.; Chan, A.T.; Chang-Claude, J.; Figueiredo, J.C.; et al. Nongenetic Determinants of Risk for Early-Onset Colorectal Cancer. JNCI Cancer Spectr. 2021, 5, pkab029. [Google Scholar] [CrossRef]
- Hayes, R.B. Advances in Understanding Early-Onset Colorectal Cancer. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1775–1777. [Google Scholar] [CrossRef]
- Sinicrope, F.A. Increasing Incidence of Early-Onset Colorectal Cancer. N. Engl. J. Med. 2022, 386, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Mauri, G.; Sartore-Bianchi, A.; Russo, A.G.; Marsoni, S.; Bardelli, A.; Siena, S. Early-onset colorectal cancer in young individuals. Mol. Oncol. 2019, 13, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Doubeni, C.A.; Laiyemo, A.O.; Major, J.M.; Schootman, M.; Lian, M.; Park, Y.; Graubard, B.I.; Hollenbeck, A.R.; Sinha, R. Socioeconomic status and the risk of colorectal cancer: An analysis of more than a half million adults in the National Institutes of Health-AARP Diet and Health Study. Cancer 2012, 118, 3636–3644. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program, SEER*Stat Database: Incidence—SEER Research Plus Limited-Field Data, 22 Registries, November 2021 Sub (2000–2019)—Linked To County Attributes—Total U.S. 1969-2020 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2022, based on the November 2021 Submission. Available online: www.seer.cancer.gov (accessed on 28 November 2022).
- Surveillance, Epidemiology, and End Results (SEER) Program. Overview of the SEER Program. Available online: https://seer.cancer.gov/about/overview.html (accessed on 27 January 2023).
- County Health Rankings & Roadmaps. Available online: https://www.countyhealthrankings.org (accessed on 27 January 2023).
- Health Resources and Services Administration—Area Health Resources Files. Available online: https://data.hrsa.gov/topics/health-workforce/ahrf (accessed on 27 January 2023).
- Duque, J.C.; Anselin, L.; Rey, S.J. The Max-P-Regions Problem*. J. Reg. Sci. 2012, 52, 397–419. [Google Scholar] [CrossRef]
- Singh, G.K. Area deprivation and widening inequalities in US mortality, 1969–1998. Am. J. Public Health 2003, 93, 1137–1143. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Dong, W.; Bensken, W.P.; Kim, U.; Rose, J.; Berger, N.A.; Koroukian, S.M. Phenotype Discovery and Geographic Disparities of Late-Stage Breast Cancer Diagnosis across U.S. Counties: A Machine Learning Approach. Cancer Epidemiol. Biomark. Prev. 2022, 31, 66–76. [Google Scholar] [CrossRef]
- Jenks, G.F. The data model concept in statistical mapping. Int. Yearb. Cartogr. 1967, 7, 186–190. [Google Scholar]
- Ma, Y.; Yang, W.; Song, M.; Smith-Warner, S.A.; Yang, J.; Li, Y.; Ma, W.; Hu, Y.; Ogino, S.; Hu, F.B.; et al. Type 2 diabetes and risk of colorectal cancer in two large U.S. prospective cohorts. Br. J. Cancer 2018, 119, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- de Kort, S.; Masclee, A.A.; Sanduleanu, S.; Weijenberg, M.P.; van Herk-Sukel, M.P.; Oldenhof, N.J.; van den Bergh, J.P.; Haak, H.R.; Janssen-Heijnen, M.L. Higher risk of colorectal cancer in patients with newly diagnosed diabetes mellitus before the age of colorectal cancer screening initiation. Sci. Rep. 2017, 7, 46527. [Google Scholar] [CrossRef]
- Ali Khan, U.; Fallah, M.; Tian, Y.; Sundquist, K.; Sundquist, J.; Brenner, H.; Kharazmi, E. Personal History of Diabetes as Important as Family History of Colorectal Cancer for Risk of Colorectal Cancer: A Nationwide Cohort Study. Am. J. Gastroenterol. 2020, 115, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Barker, L.E.; Kirtland, K.A.; Gregg, E.W.; Geiss, L.S.; Thompson, T.J. Geographic distribution of diagnosed diabetes in the U.S.: A diabetes belt. Am. J. Prev. Med. 2011, 40, 434–439. [Google Scholar] [CrossRef]
- Myers, C.A.; Slack, T.; Broyles, S.T.; Heymsfield, S.B.; Church, T.S.; Martin, C.K. Diabetes prevalence is associated with different community factors in the diabetes belt versus the rest of the United States. Obesity 2017, 25, 452–459. [Google Scholar] [CrossRef]
- Lascar, N.; Brown, J.; Pattison, H.; Barnett, A.H.; Bailey, C.J.; Bellary, S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018, 6, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Wang, M.C.; Shah, N.S.; Carnethon, M.R.; O’Brien, M.J.; Khan, S.S. Age at Diagnosis of Diabetes by Race and Ethnicity in the United States From 2011 to 2018. JAMA Intern. Med. 2021, 181, 1537–1539. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Potter, J.; Caan, B.; Edwards, S.; Coates, A.; Ma, K.N.; Berry, T.D. Energy balance and colon cancer—beyond physical activity. Cancer Res. 1997, 57, 75–80. Available online: https://aacrjournals.org/cancerres/article/57/1/75/503178/Energy-Balance-and-Colon-Cancer-beyond-Physical (accessed on 27 January 2023). [PubMed]
- Hu, F.B.; Li, T.Y.; Colditz, G.A.; Willett, W.C.; Manson, J.E. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA 2003, 289, 1785–1791. [Google Scholar] [CrossRef]
- Qi, Q.; Li, Y.; Chomistek, A.K.; Kang, J.H.; Curhan, G.C.; Pasquale, L.R.; Willett, W.C.; Rimm, E.B.; Hu, F.B.; Qi, L. Television watching, leisure time physical activity, and the genetic predisposition in relation to body mass index in women and men. Circulation 2012, 126, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Izzicupo, P.; Moscucci, F.; Sciomer, S.; Maffei, S.; Di Baldassarre, A.; Mattioli, A.V.; Gallina, S. Recommendations for Physical Inactivity and Sedentary Behavior During the Coronavirus Disease (COVID-19) Pandemic. Front. Public Health 2020, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Wilms, P.; Schröder, J.; Reer, R.; Scheit, L. The Impact of “Home Office” Work on Physical Activity and Sedentary Behavior during the COVID-19 Pandemic: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 12344. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Liu, P.H.; Zheng, X.; Keum, N.; Zong, X.; Li, X.; Wu, K.; Fuchs, C.S.; Ogino, S.; Ng, K.; et al. Sedentary Behaviors, TV Viewing Time, and Risk of Young-Onset Colorectal Cancer. JNCI Cancer Spectr. 2018, 2, pky073. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.R.; Moore, J.X.; Qeadan, F.; Gu, L.Y.; Huntington, M.S.; Holowatyj, A.N. Examining factors underlying geographic disparities in early-onset colorectal cancer survival among men in the United States. Am. J. Cancer Res. 2020, 10, 1592–1607. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7269786 (accessed on 27 January 2023).
- Schmid, D.; Leitzmann, M.F. Television viewing and time spent sedentary in relation to cancer risk: A meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju098. [Google Scholar] [CrossRef]
- Steele, C.B.; Thomas, C.C.; Henley, S.J.; Massetti, G.M.; Galuska, D.A.; Agurs-Collins, T.; Puckett, M.; Richardson, L.C. Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity—United States, 2005–2014. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1052–1058. [Google Scholar] [CrossRef]
- Koroukian, S.M.; Dong, W.; Berger, N.A. Changes in Age Distribution of Obesity-Associated Cancers. JAMA Netw. Open 2019, 2, e199261. [Google Scholar] [CrossRef]
- Liu, P.H.; Wu, K.; Ng, K.; Zauber, A.G.; Nguyen, L.H.; Song, M.; He, X.; Fuchs, C.S.; Ogino, S.; Willett, W.C.; et al. Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol. 2019, 5, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Fedirko, V.; Tramacere, I.; Bagnardi, V.; Rota, M.; Scotti, L.; Islami, F.; Negri, E.; Straif, K.; Romieu, I.; La Vecchia, C.; et al. Alcohol drinking and colorectal cancer risk: An overall and dose-response meta-analysis of published studies. Ann. Oncol. 2011, 22, 1958–1972. [Google Scholar] [CrossRef]
- Moskal, A.; Norat, T.; Ferrari, P.; Riboli, E. Alcohol intake and colorectal cancer risk: A dose-response meta-analysis of published cohort studies. Int. J. Cancer 2007, 120, 664–671. [Google Scholar] [CrossRef]
- Pedersen, A.; Johansen, C.; Grønbaek, M. Relations between amount and type of alcohol and colon and rectal cancer in a Danish population based cohort study. Gut 2003, 52, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Klarich, D.S.; Brasser, S.M.; Hong, M.Y. Moderate Alcohol Consumption and Colorectal Cancer Risk. Alcohol Clin. Exp. Res. 2015, 39, 1280–1291. [Google Scholar] [CrossRef]
- Ellis, L.; Abrahão, R.; McKinley, M.; Yang, J.; Somsouk, M.; Marchand, L.L.; Cheng, I.; Gomez, S.L.; Shariff-Marco, S. Colorectal Cancer Incidence Trends by Age, Stage, and Racial/Ethnic Group in California, 1990–2014. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Patten, E. The Nation’s Latino Population Is Defined by Its Youth. Pew Research Center 2016. Available online: https://www.pewresearch.org/hispanic/2016/04/20/the-nations-latino-population-is-defined-by-its-youth/ (accessed on 27 January 2023).
- Bermudez, O.I.; Falcon, L.M.; Tucker, K.L. Intake and food sources of macronutrients among older Hispanic adults: Association with ethnicity, acculturation, and length of residence in the United States. J. Am. Diet. Assoc. 2000, 100, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Pulvers, K.; Cupertino, A.P.; Scheuermann, T.S.; Cox, L.S.; Ho, Y.Y.; Nollen, N.L.; Cuellar, R.; Ahluwalia, J.S. Daily and Nondaily Smoking Varies by Acculturation among English-Speaking, US Latino Men and Women. Ethn. Dis. 2018, 28, 105–114. [Google Scholar] [CrossRef]
- Joseph, R.P.; Benitez, T.J.; Ainsworth, B.E.; Todd, M.; Keller, C. Acculturation and Physical Activity among Latinas Enrolled in a 12-Month Walking Intervention. West. J. Nurs. Res. 2018, 40, 942–960. [Google Scholar] [CrossRef]
- Lara, M.; Gamboa, C.; Kahramanian, M.I.; Morales, L.S.; Hayes Bautista, D.E. Acculturation and Latino health in the United States: A review of the literature and its sociopolitical context. Annu. Rev. Public Health 2005, 26, 367–397. [Google Scholar] [CrossRef]
- Chen, H.; Wu, A.H.; Wang, S.; Bookstein, A.; Le Marchand, L.; Wilkens, L.R.; Haiman, C.A.; Cheng, I.; Monroe, K.R.; Setiawan, V.W. Cancer Mortality Patterns by Birthplace and Generation Status of Mexican Latinos: The Multiethnic Cohort. J. Natl. Cancer Inst. 2022, 114, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, P.S.; Callahan, K.E.; Gomez, S.L.; Marcos-Gragera, R.; Cobb, T.R.; Roca-Barcelo, A.; Ramirez, A.G. High cancer mortality for US-born Latinos: Evidence from California and Texas. BMC Cancer 2017, 17, 478. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Barrera, A. More Mexicans Leaving Than Coming to the US. Pew Research Center 2015. Available online: https://www.pewresearch.org/hispanic/2015/11/19/more-mexicans-leaving-than-coming-to-the-u-s/ (accessed on 27 January 2023).
- Pinheiro, P.S.; Callahan, K.E.; Stern, M.C.; de Vries, E. Migration from Mexico to the United States: A high-speed cancer transition. Int. J. Cancer 2018, 142, 477–488. [Google Scholar] [CrossRef]
- Dong, W.; Bensken, W.P.; Kim, U.; Rose, J.; Fan, Q.; Schiltz, N.K.; Berger, N.A.; Koroukian, S.M. Variation in and Factors Associated With US County-Level Cancer Mortality, 2008–2019. JAMA Netw. Open 2022, 5, e2230925. [Google Scholar] [CrossRef] [PubMed]

| Variable | Description | Year | Original Source |
|---|---|---|---|
| Ethnicity/Race | |||
| Non-Hispanic Black | Percentage of Non-Hispanic African American population | 2011 | Census—PE |
| Non-Hispanic White | Percentage of Non-Hispanic White population | ||
| Hispanic | Percentage of population identifying as Hispanics | ||
| Health Status | |||
| Diabetes | Percentage of adults (age 20+) with diagnosed diabetes (age-adjusted) | 2009 | CDC—DIA |
| Low birthweight | Percentage of live births with low birthweight (<2500 g) | 2004–2010 | CDC—NCHS |
| Poor or fair health | Percentage of adults reporting fair or poor health (age-adjusted) | 2014 | CDC—BRFSS |
| Health Behaviors | |||
| Adult obesity | Percentage of the people (age 18+) that report a body mass index ≥ 30 (age-adjusted) | 2009 | CDC—DIA |
| Physical inactivity | Percentage of adults (age 18+) reporting no leisure-time physical activity (age-adjusted) | 2009 | |
| Excessive drinking | Percentage of adults reporting binge or heavy drinking (age-adjusted) | 2014 | CDC—BRFSS |
| Insufficient sleep | Percentage of adults who report fewer than 7 h of sleep on average (age-adjusted) | 2014 | |
| Smoking | Percentage of adults who are current smokers (age-adjusted) | 2014 | |
| Access to recreational facilities | Number of recreational facilities (engaged in fitness and recreational sports) per 100,000 persons | 2010 | USDA FEA and Census CBP |
| Food insecurity | Percentage of population who lack adequate access to food | 2013 | MMP |
| Teen birth | Number of births per 1000 females ages 15–19 | 2004–2010 | CDC—NCHS |
| Clinical Care | |||
| Uninsured adults | Percentage of adults under age 65 without health insurance | 2010 | Census—SAHIE |
| Health care costs | Per capita spending of Medicare enrollees | 2009 | DAHC |
| Preventable hospital stays | Rate of hospital stays for ambulatory-care sensitive conditions per 100,000 Medicare enrollees | 2010 | |
| Primary care physicians | Primary care physicians in patient care per 100,000 persons | 2010 | HRSA |
| Community health centers | Community health centers per 100,000 persons | 2013 | |
| Physical Environment | |||
| Air pollution | Total days when the fine particulate matter (PM2.5) exceeded the 24 h primary standard established by the US EPA. | 2008 | CDC WONDER |
| Driving alone to work | Percentage of the workforce that drives alone to work (indicators of the transit system and physical inactivity) | 2007–2011 | Census—ACS |
| Rural population | Percentage of people living in rural areas | 2010 | Census—PE |
| Socioeconomic Status | |||
| Area deprivation index | A composite measure of 17 census variables designed to describe social deprivation | 2008–2012 | Census—ACS |
| Median household income | The income where half of households in a county earn more and half of households earn less | 2011 | Census—SAIPE |
| Poverty | Percentage of people in poverty | 2010 | |
| Receipt of SNAP benefits | Percentage of people who were food stamp recipients | 2010 | Census—SNAP |
| Some college | Percentage of people (age 25–44) with some post-secondary education | 2007–2011 | Census—ACS |
| Female-headed households | Percentage of female-headed households | 2010 | Census (Decennial) |
| Unemployment | Percentage of population (age 16+) unemployed but seeking work | 2011 | BLS |
| Violent crime | Number of reported violent crime offenses per 100,000 persons | 2008–2010 | FBI—UCR |
| EOCRC (Age < 50) | LOCRC (Age 50+) | p-Value | |
|---|---|---|---|
| N | 136,065 | 1,141,775 | |
| Incidence rate * | 6.9 | 134.7 | |
| Sex (%) | <0.0001 | ||
| Male | 71,127 (52.3) | 588,623 (51.6) | |
| Female | 64,938 (47.7) | 553,152 (48.4) | |
| Race/Ethnicity (%) | <0.0001 | ||
| Non-Hispanic White | 79,807 (58.7) | 824,210 (72.2) | |
| Non-Hispanic Black | 18,953 (13.9) | 127,910 (11.2) | |
| Non-Hispanic American Indian/Alaska Native | 711 (0.5) | 3632 (0.3) | |
| Non-Hispanic Asian or Pacific Islander | 10,252 (7.5) | 62,769 (5.5) | |
| Hispanic (All Races) | 25,240 (18.5) | 118,234 (10.4) | |
| Non-Hispanic Unknown Race | 1102 (0.8) | 5020 (0.4) | |
| Year of Diagnosis (%) | <0.0001 | ||
| 2000–2004 | 29,908 (22.0) | 306,441 (26.8) | |
| 2005–2009 | 32,582 (23.9) | 287,722 (25.2) | |
| 2010–2014 | 34,203 (25.1) | 271,980 (23.8) | |
| 2015–2019 | 39,372 (28.9) | 275,632 (24.1) | |
| Tumor Site (%) | <0.0001 | ||
| Colon excluding Sigmoid Colon | 55,063 (40.6) | 606,845 (53.1) | |
| Sigmoid Colon | 28,722 (21.1) | 216,986 (19.0) | |
| Rectum and Rectosigmoid Junction | 52,280 (38.4) | 317,944 (27.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, W.; Kim, U.; Rose, J.; Hoehn, R.S.; Kucmanic, M.; Eom, K.; Li, S.; Berger, N.A.; Koroukian, S.M. Geographic Variation and Risk Factor Association of Early Versus Late Onset Colorectal Cancer. Cancers 2023, 15, 1006. https://doi.org/10.3390/cancers15041006
Dong W, Kim U, Rose J, Hoehn RS, Kucmanic M, Eom K, Li S, Berger NA, Koroukian SM. Geographic Variation and Risk Factor Association of Early Versus Late Onset Colorectal Cancer. Cancers. 2023; 15(4):1006. https://doi.org/10.3390/cancers15041006
Chicago/Turabian StyleDong, Weichuan, Uriel Kim, Johnie Rose, Richard S. Hoehn, Matthew Kucmanic, Kirsten Eom, Shu Li, Nathan A. Berger, and Siran M. Koroukian. 2023. "Geographic Variation and Risk Factor Association of Early Versus Late Onset Colorectal Cancer" Cancers 15, no. 4: 1006. https://doi.org/10.3390/cancers15041006
APA StyleDong, W., Kim, U., Rose, J., Hoehn, R. S., Kucmanic, M., Eom, K., Li, S., Berger, N. A., & Koroukian, S. M. (2023). Geographic Variation and Risk Factor Association of Early Versus Late Onset Colorectal Cancer. Cancers, 15(4), 1006. https://doi.org/10.3390/cancers15041006







