Immune Microenvironment in Sporadic Early-Onset versus Average-Onset Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. DNA and RNA Isolation
2.3. MSI Analysis
2.4. Mutation Analysis
2.5. Methylation Analysis
2.6. Immunofluorescence
2.7. Gene Expression Analysis
2.8. Statistical Analyses
3. Results
3.1. Clinical, Pathological and Molecular Characteristics
3.2. T-Cell Infiltration in Early and Average-Onset CRC
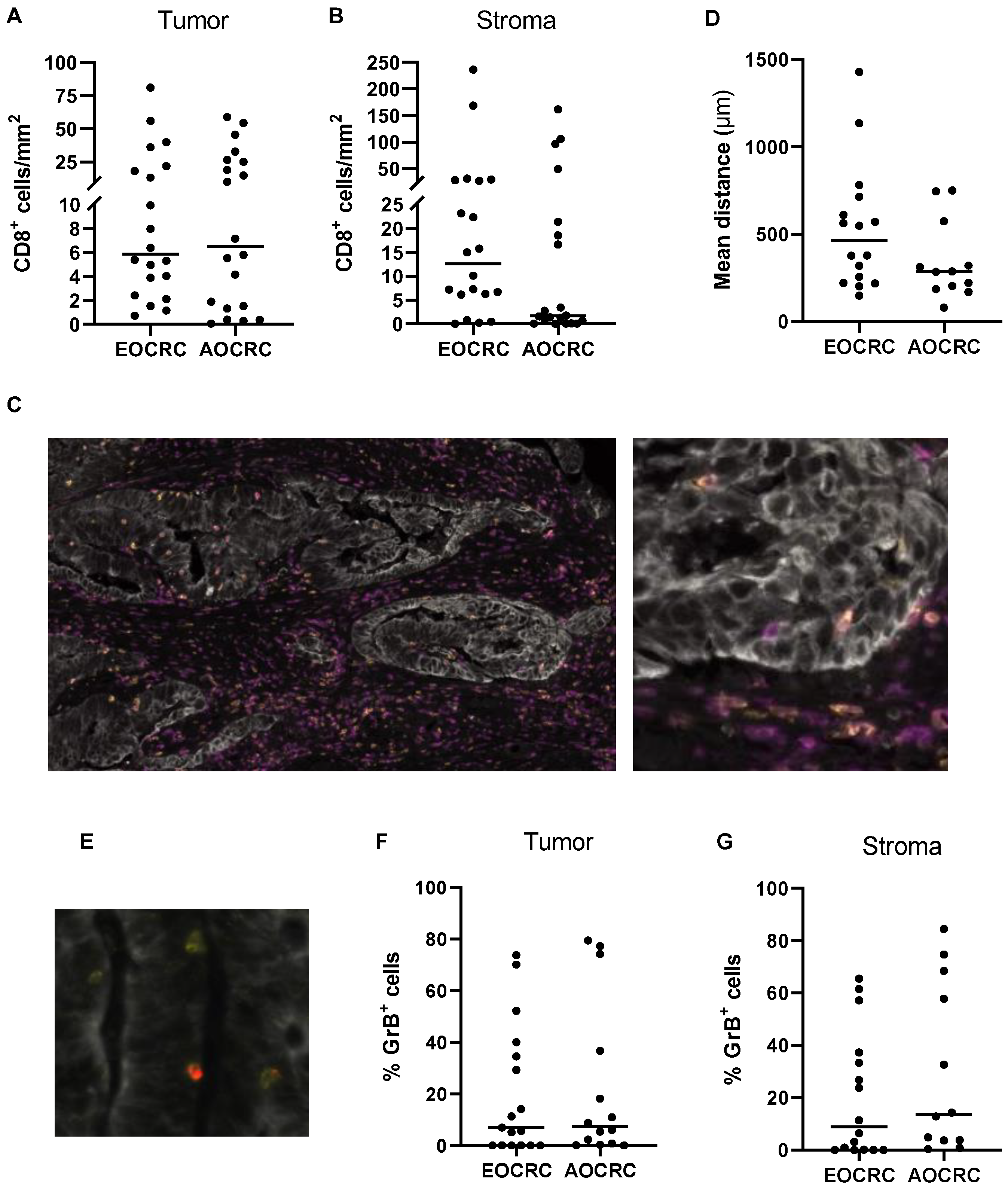
3.3. Distribution of CD8+ T Cells in Early and Average-Onset CRC Tumors
3.4. CD4+ T Helper Cell and Treg Infiltration in Early and Average-Onset CRC Tumors
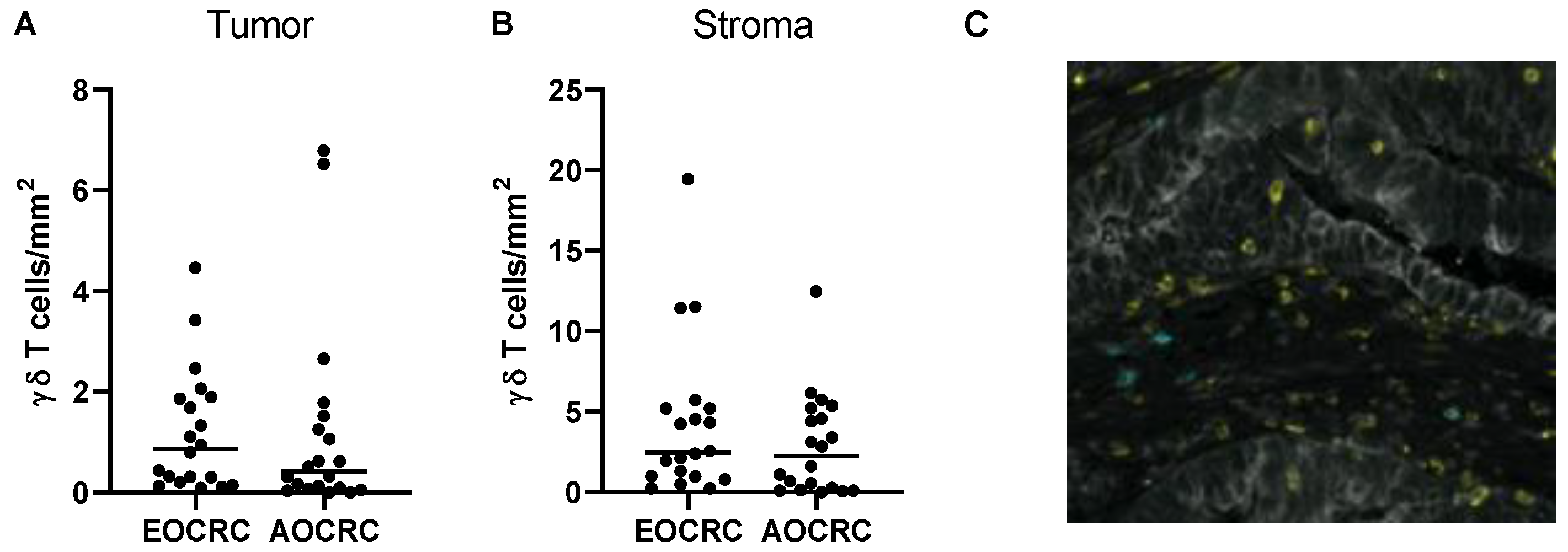
3.5. γδ T Cells in Early and Average-Onset CRC Tumors
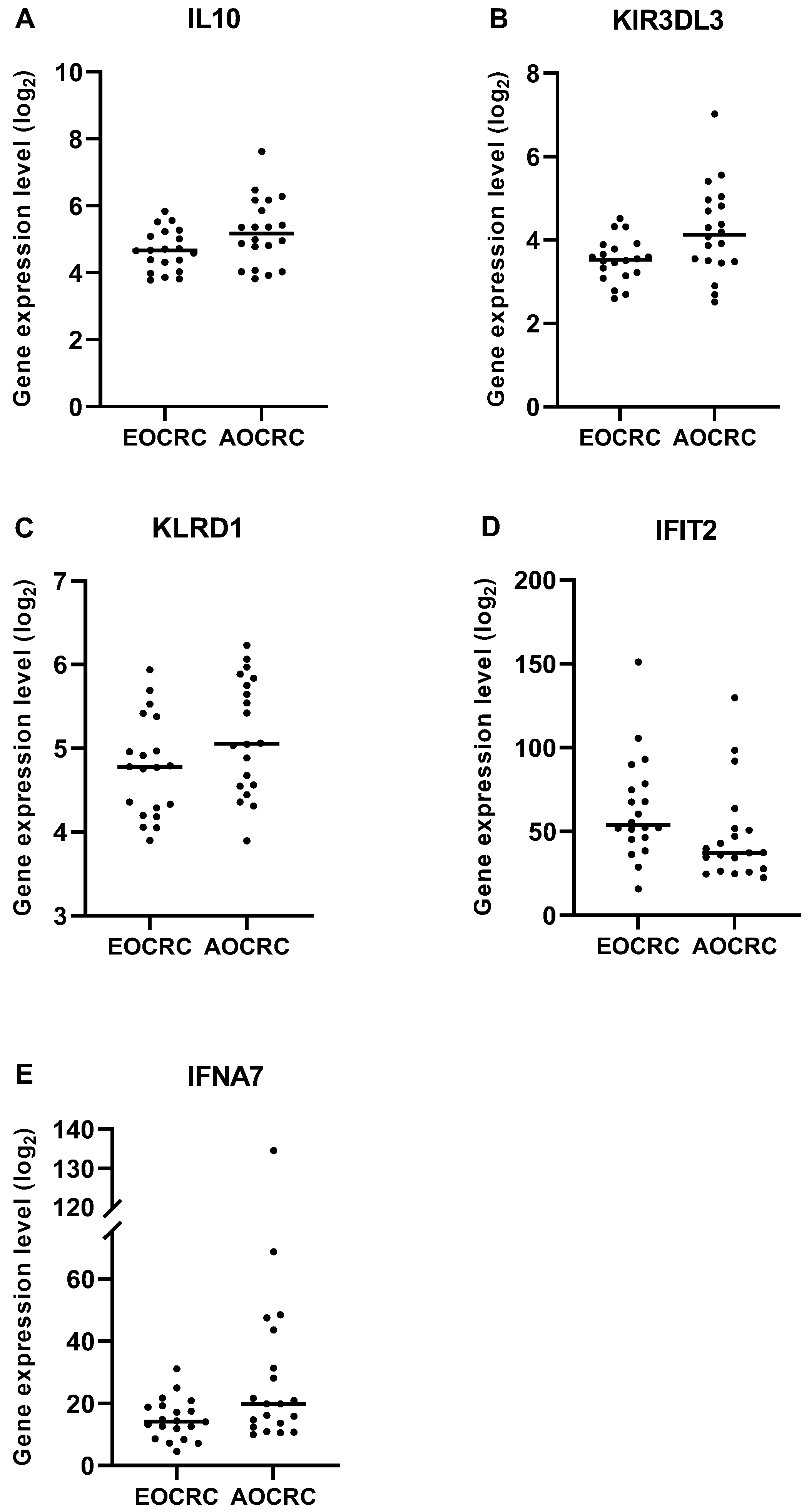
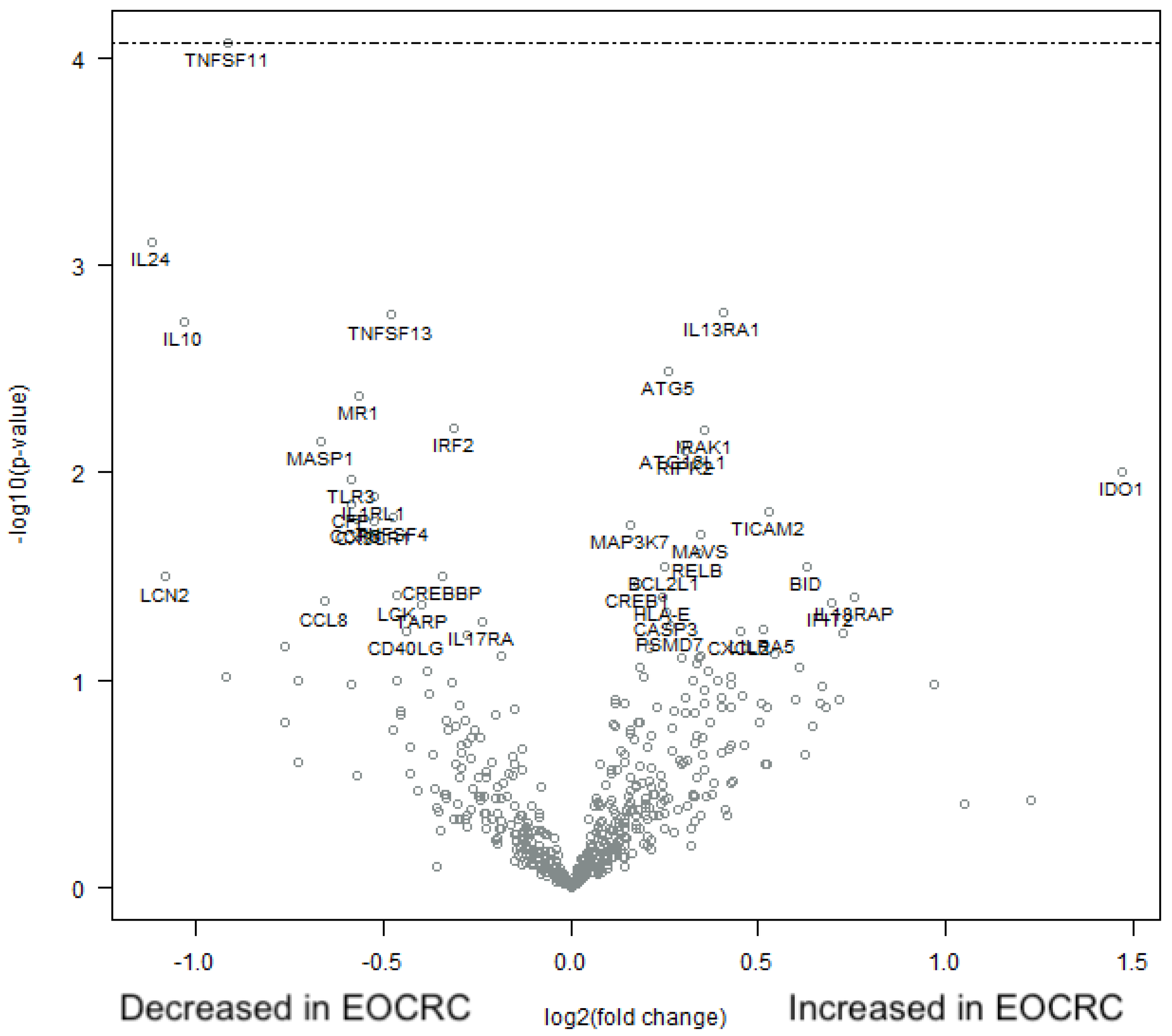
3.6. Immune Profile in Early and Average-Onset CRC Tumors
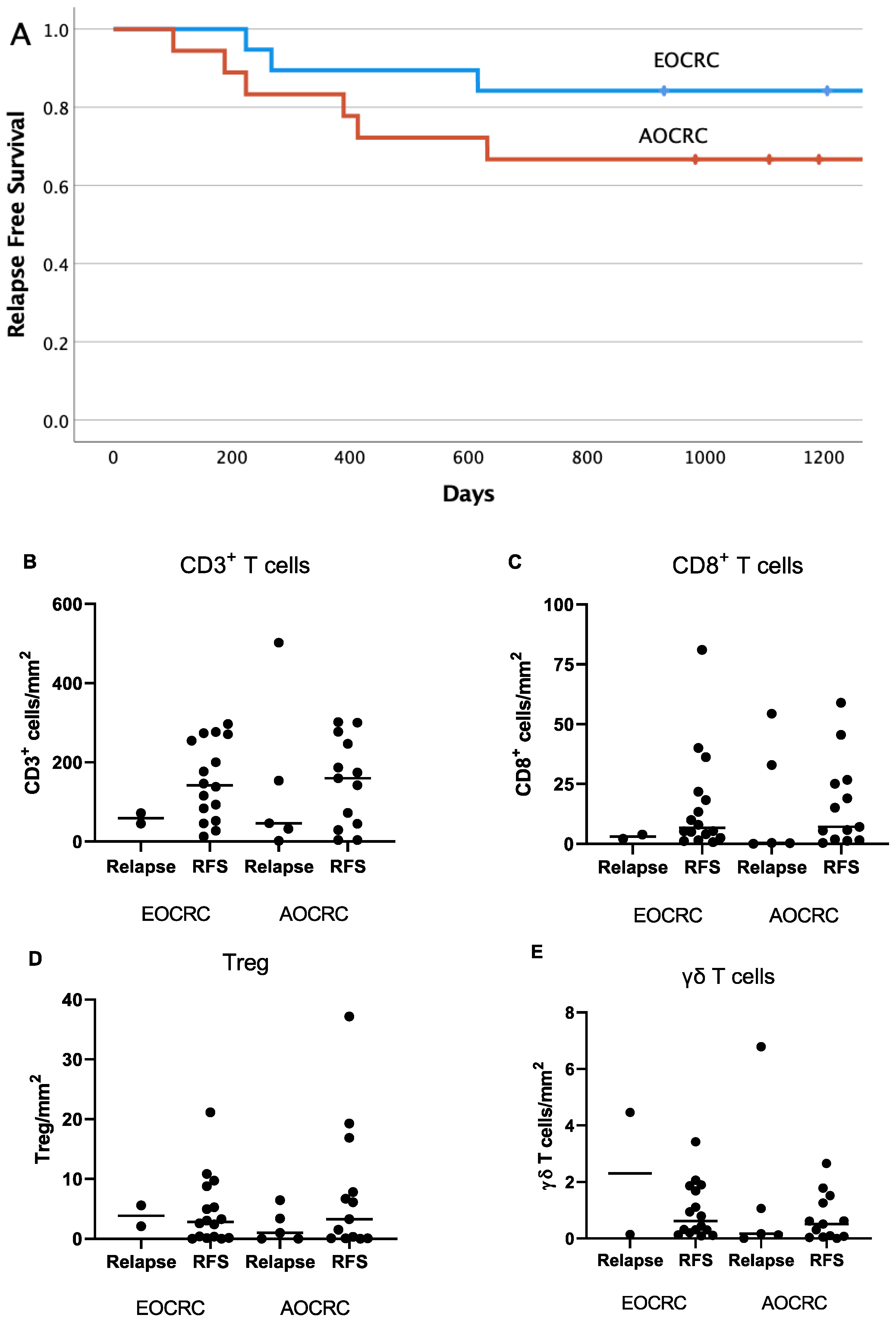
3.7. Correlation between Immune Parameters and Patient Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Saad El Din, K.; Loree, J.M.; Sayre, E.C.; Gill, S.; Brown, C.J.; Dau, H.; De Vera, M.A. Trends in the epidemiology of young-onset colorectal cancer: A worldwide systematic review. BMC Cancer 2020, 20, 288. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.E.; Hu, C.Y.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015, 150, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef]
- Patel, S.G.; Karlitz, J.J.; Yen, T.; Lieu, C.H.; Boland, C.R. The rising tide of early-onset colorectal cancer: A comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol. Hepatol. 2022, 7, 262–274. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pages, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Ugai, T.; Vayrynen, J.P.; Lau, M.C.; Borowsky, J.; Akimoto, N.; Vayrynen, S.A.; Zhao, M.; Zhong, R.; Haruki, K.; Dias Costa, A.; et al. Immune cell profiles in the tumor microenvironment of early-onset, intermediate-onset, and later-onset colorectal cancer. Cancer Immunol. Immunother. 2022, 71, 933–942. [Google Scholar] [CrossRef]
- Pages, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Yantiss, R.K.; Goodarzi, M.; Zhou, X.K.; Rennert, H.; Pirog, E.C.; Banner, B.F.; Chen, Y.-T. Clinical, pathologic, and molecular features of early-onset colorectal carcinoma. Am. J. Surg. Pathol. 2009, 33, 572–582. [Google Scholar] [CrossRef]
- Koi, M.; Carethers, J.M. The colorectal cancer immune microenvironment and approach to immunotherapies. Future Oncol. 2017, 13, 1633–1647. [Google Scholar] [CrossRef]
- Saito, T.; Nishikawa, H.; Wada, H.; Nagano, Y.; Sugiyama, D.; Atarashi, K.; Maeda, Y.; Hamaguchi, M.; Ohkura, N.; Sato, E.; et al. Two FOXP3+CD4+ T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat. Med. 2016, 22, 679–684. [Google Scholar] [CrossRef]
- Betts, G.; Jones, E.; Junaid, S.; El-Shanawany, T.; Scurr, M.; Mizen, P.; Kumar, M.; Jones, S.; Rees, B.; Williams, G. Suppression of tumour-specific CD4+ T cells by regulatory T cells is associated with progression of human colorectal cancer. Gut 2012, 61, 1163–1171. [Google Scholar] [CrossRef]
- Brudvik, K.W.; Henjum, K.; Aandahl, E.M.; Bjørnbeth, B.A.; Taskén, K. Regulatory T-cell-mediated inhibition of antitumor immune responses is associated with clinical outcome in patients with liver metastasis from colorectal cancer. Cancer Immunol. Immunother. 2012, 61, 1045–1053. [Google Scholar] [CrossRef]
- Strasser, K.; Birnleitner, H.; Beer, A.; Pils, D.; Gerner, M.C.; Schmetterer, K.G.; Bachleitner-Hofmann, T.; Stift, A.; Bergmann, M.; Oehler, R. Immunological differences between colorectal cancer and normal mucosa uncover a prognostically relevant immune cell profile. Oncoimmunology 2019, 8, e1537693. [Google Scholar] [CrossRef]
- Chaput, N.; Louafi, S.; Bardier, A.; Charlotte, F.; Vaillant, J.-C.; Ménégaux, F.; Rosenzwajg, M.; Lemoine, F.; Klatzmann, D.; Taieb, J. Identification of CD8+ CD25+ Foxp3+ suppressive T cells in colorectal cancer tissue. Gut 2009, 58, 520–529. [Google Scholar] [CrossRef]
- Salama, P.; Phillips, M.; Grieu, F.; Morris, M.; Zeps, N.; Joseph, D.; Platell, C.; Iacopetta, B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J. Clin. Oncol. 2009, 27, 186–192. [Google Scholar] [CrossRef]
- Mlecnik, B.; Bindea, G.; Angell, H.K.; Maby, P.; Angelova, M.; Tougeron, D.; Church, S.E.; Lafontaine, L.; Fischer, M.; Fredriksen, T. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity 2016, 44, 698–711. [Google Scholar] [CrossRef]
- Haruki, K.; Kosumi, K.; Li, P.; Arima, K.; Väyrynen, J.P.; Lau, M.C.; Twombly, T.S.; Hamada, T.; Glickman, J.N.; Fujiyoshi, K. An integrated analysis of lymphocytic reaction, tumour molecular characteristics and patient survival in colorectal cancer. Br. J. Cancer 2020, 122, 1367–1377. [Google Scholar] [CrossRef]
- Reissfelder, C.; Stamova, S.; Gossmann, C.; Braun, M.; Bonertz, A.; Walliczek, U.; Grimm, M.; Rahbari, N.N.; Koch, M.; Saadati, M. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis. J. Clin. Investig. 2015, 125, 739–751. [Google Scholar] [CrossRef]
- Li, Y.; Li, G.; Zhang, J.; Wu, X.; Chen, X. The dual roles of human γδ T cells: Anti-tumor or tumor-promoting. Front. Immunol. 2021, 11, 619954. [Google Scholar] [CrossRef] [PubMed]
- Wing, J.B.; Tanaka, A.; Sakaguchi, S. Human FOXP3+ regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity 2019, 50, 302–316. [Google Scholar] [CrossRef]
- Frey, D.M.; Droeser, R.A.; Viehl, C.T.; Zlobec, I.; Lugli, A.; Zingg, U.; Oertli, D.; Kettelhack, C.; Terracciano, L.; Tornillo, L. High frequency of tumor-infiltrating FOXP3+ regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int. J. Cancer 2010, 126, 2635–2643. [Google Scholar] [CrossRef]
- Ling, A.; Edin, S.; Wikberg, M.L.; Oberg, A.; Palmqvist, R. The intratumoural subsite and relation of CD8(+) and FOXP3(+) T lymphocytes in colorectal cancer provide important prognostic clues. Br. J. Cancer 2014, 110, 2551–2559. [Google Scholar] [CrossRef] [PubMed]
- Perea, J.; García, J.L.; Pérez, J.; Rueda, D.; Arriba, M.; Rodríguez, Y.; Urioste, M.; González-Sarmiento, R. NOMO-1 gene is deleted in early-onset colorectal cancer. Oncotarget 2017, 8, 24429. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, A.; Carethers, J.M. Epidemiology and biology of early onset colorectal cancer. EXCLI J. 2022, 21, 162. [Google Scholar] [PubMed]
- Gardner, I.H.; Siddharthan, R.; Watson, K.; Dewey, E.; Ruhl, R.; Khou, S.; Guan, X.; Xia, Z.; Tsikitis, V.L.; Anand, S. A Distinct Innate Immune Signature of Early Onset Colorectal Cancer. Immunohorizons 2021, 5, 489–499. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reynies, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Wang, L.; Hirano, Y.; Heng, G.; Ishii, T.; Kondo, H.; Hara, K.; Obara, N.; Asari, M.; Kato, T.; Yamaguchi, S. Does signet ring cell carcinoma component signify worse outcomes for patients with colorectal cancer? Asian J. Surg. 2021, 44, 105–110. [Google Scholar] [CrossRef]
- Hashimoto, S.; Hamada, K.; Sumida, Y.; Araki, M.; Wakata, K.; Kugiyama, T.; Shibuya, A.; Nishimuta, M.; Morino, S.; Baba, M.; et al. Short- and long-term survival after curative resection for colorectal cancer in nonagenarian patients. Asian J. Surg. 2022, 45, 208–212. [Google Scholar] [CrossRef]


| Variable | EOCRC n = 20 | AOCRC n = 20 | p Value | Total n = 40 |
|---|---|---|---|---|
| Sex n (%) | ||||
| Male | 10 (50%) | 10 (50%) | 1.00 | 20 (50%) |
| Female | 10 (50%) | 10 (50%) | 20 (50%) | |
| Tumor localization n (%) | ||||
| Left-sided Colon | 13 (65%) | 13 (65%) | 0.63 | 26 (65%) |
| Rectum | 7 (35%) | 7 (35%) | 14 (35%) | |
| Tumor differentiation (%) | ||||
| High grade | 5 (25%) | 1 (5%) | 0.34 | 6 (15%) |
| Low grade | 13 (65%) | 17 (85%) | 30 (76%) | |
| Mucinous | 2 (10%) | 2 (10%) | 4 (10%) | |
| Tumor stage n (%) | ||||
| Stage I | 2 (10%) | 2 (10%) | 1.00 | 4 (10%) |
| Stage II | 7 (35%) | 7 (35%) | 14 (35%) | |
| Stage III | 9 (45%) | 9 (45%) | 18 (45%) | |
| Stage IV | 2 (10%) | 2 (10%) | 4 (10%) | |
| Tumor | ||||
| pT1 | 0 (0%) | 2 (10%) | 0.38 | 2 (5%) |
| pT2 | 3 (15%) | 4 (20%) | 7 (18%) | |
| pT3 | 16 (80%) | 12 (60%) | 28 (70%) | |
| pT4 | 1 (5%) | 2 (10%) | 3 (8%) | |
| Nodes | ||||
| pN0 | 10 (50%) | 10 (50%) | 1.00 | 20 (50%) |
| pN1 | 5 (25%) | 5 (25%) | 10 (25%) | |
| pN2 | 5 (25%) | 5 (25%) | 10 (25%) | |
| Number of examined lymph nodes; mean (SD) | 27 (11) | 24 (13) | 0.04 | 25.5 (12) |
| Number of positive lymph nodes; mean (SD) | 2 (3) | 3 (4) | 0.87 | 2 (3) |
| Lymph node ratio; mean % (SD) | 6 (11) | 10 (15) | 0.76 | 8 (13) |
| dMMR **, n | 1 (5%) | 1 (5%) | 1.00 | 2 (5%) |
| BRAF V600E mutation, n | 5 (25%) | 2 (10%) | 0.20 | 7 (18%) |
| KRAS mutation, n | 10 (50%) | 9 (45%) | 0.75 | 19 (48%) |
| MLH1 methylation, n | 0 (0%) | 1 (5%) | 1.00 | 1 (3%) |
| MGMT methylation (SD) | 16 (24) | 16 (25) | 0.89 | 16 (24) |
| P16INK4a methylation (SD) | 4 (5) | 13 (19) | 0.97 | 9 (15) |
| LINE1 methylation (SD) | 68 (8) | 70 (7) | 0.44 | 69(7) |
| Adjuvant chemotherapy | ||||
| 5-FU | 5 (25%) | 7 (35%) | 0.20 | 12 (30%) |
| 5 + FU + Oxaliplatin | 5 (25%) | 1 (5%) | 6 (15%) | |
| No chemotherapy | 10 (50%) | 12 (60%) | 22 (55%) | |
| Relapse at three years n | 0.56 | |||
| Yes | 3 (16%) | 5 (28%) | 8 (22%) | |
| No | 16 (84%) | 13 (72%) | 29 (78%) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andric, F.; Al-Fairouzi, A.; Wettergren, Y.; Szeponik, L.; Bexe-Lindskog, E.; Cusack, J.C., Jr.; Tumusiime, G.; Quiding-Järbrink, M.; Ljungman, D. Immune Microenvironment in Sporadic Early-Onset versus Average-Onset Colorectal Cancer. Cancers 2023, 15, 1457. https://doi.org/10.3390/cancers15051457
Andric F, Al-Fairouzi A, Wettergren Y, Szeponik L, Bexe-Lindskog E, Cusack JC Jr., Tumusiime G, Quiding-Järbrink M, Ljungman D. Immune Microenvironment in Sporadic Early-Onset versus Average-Onset Colorectal Cancer. Cancers. 2023; 15(5):1457. https://doi.org/10.3390/cancers15051457
Chicago/Turabian StyleAndric, Fanny, Ala Al-Fairouzi, Yvonne Wettergren, Louis Szeponik, Elinor Bexe-Lindskog, James C. Cusack, Jr., Gerald Tumusiime, Marianne Quiding-Järbrink, and David Ljungman. 2023. "Immune Microenvironment in Sporadic Early-Onset versus Average-Onset Colorectal Cancer" Cancers 15, no. 5: 1457. https://doi.org/10.3390/cancers15051457
APA StyleAndric, F., Al-Fairouzi, A., Wettergren, Y., Szeponik, L., Bexe-Lindskog, E., Cusack, J. C., Jr., Tumusiime, G., Quiding-Järbrink, M., & Ljungman, D. (2023). Immune Microenvironment in Sporadic Early-Onset versus Average-Onset Colorectal Cancer. Cancers, 15(5), 1457. https://doi.org/10.3390/cancers15051457







