ATX-LPA-Dependent Nuclear Translocation of Endonuclease G in Respiratory Epithelial Cells: A New Mode Action for DNA Damage Induced by Crystalline Silica Particles
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Particles and Reagents
2.3. Animal Treatment
2.4. RNA Interference
2.5. Nuclear Extraction
2.6. TUNEL Assay
2.7. Comet Assay
2.8. Immunocytochemistry
2.9. Immunohistochemistry
2.10. Western Blotting
2.11. Statistical Analysis
3. Results
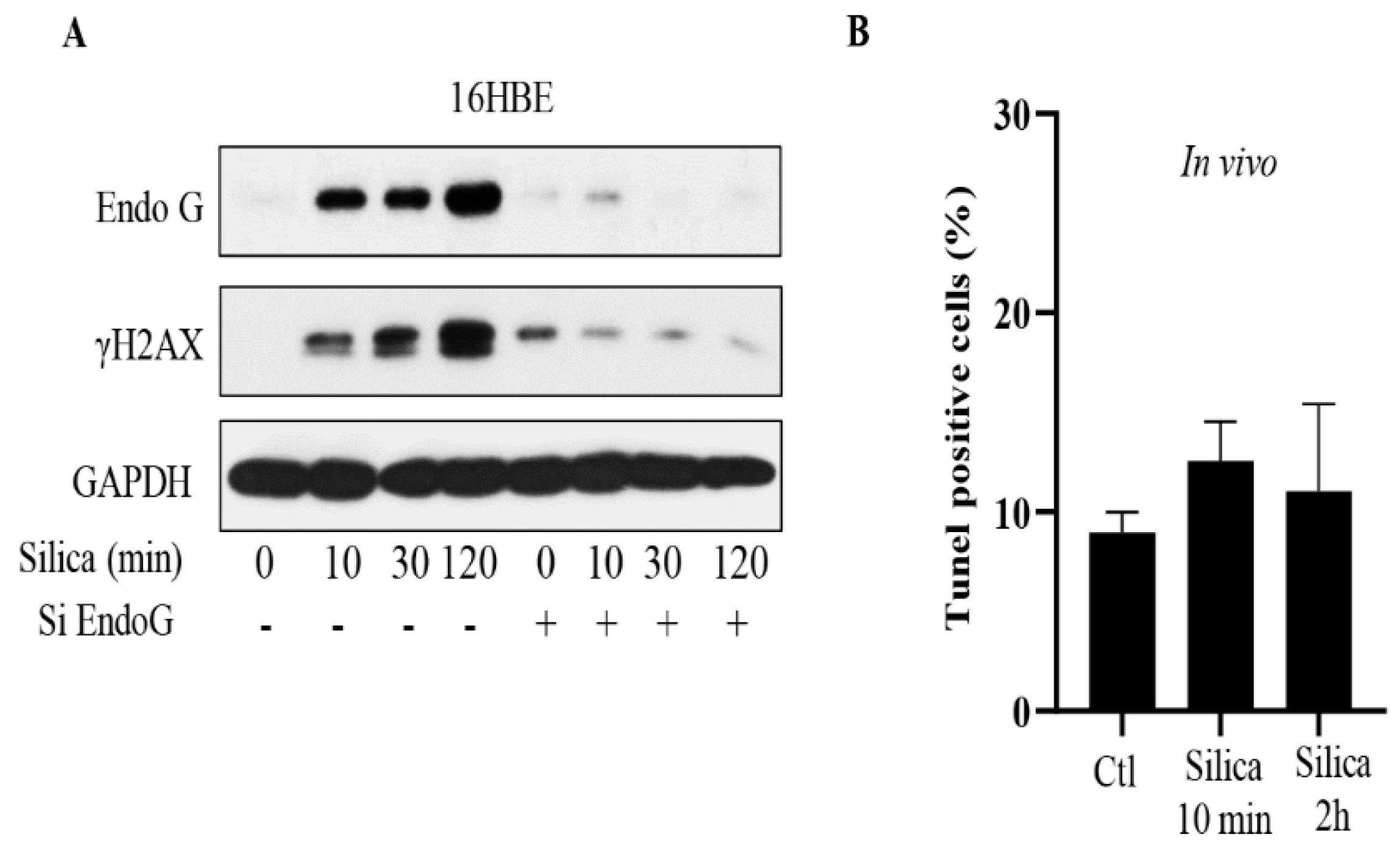
3.1. Silica-Induced Translocation of EndoG in Respiratory Epithelial Cells In Vitro
3.2. Translocation of EndoG In Vivo
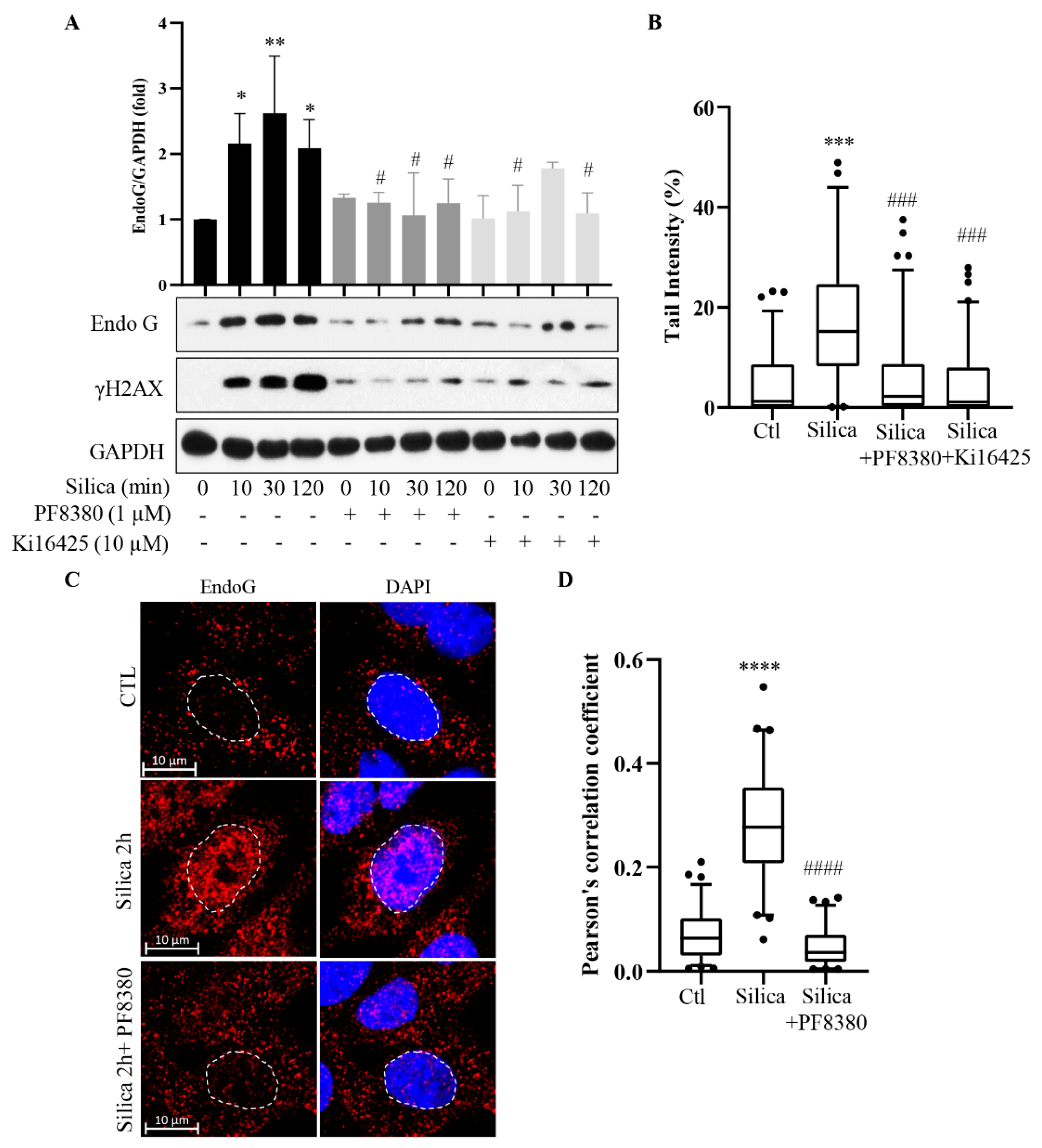
3.3. Inhibition of EndoG Prevents DNA Damage and CSi Does Not Induce Apoptosis
3.4. Inhibition of ATX Inhibits the EndoG Translocation and DNA Damage
3.5. Inhibition of Mitochondrial Depolarization Prevents Mitochondrial Depletion of EndoG and Its Nuclear Translocation
3.6. Common Signaling Pathway for EndoG Translocation and DNA Damage
3.7. CSi-Induced Micronuclei Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IARC Monographs. A Review on the Evaluation of Carcinogenic Risks to Humans. In Arsenic, Metals, Fibres, and Dusts; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans: Lyon, France, 2012; Volume 100C. [Google Scholar]
- Borm, P.J.A.; Fowler, P.; Kirkland, D. An updated review of the genotoxicity of respirable crystalline silica. Part. Fibre Toxicol. 2018, 15, 23. [Google Scholar] [CrossRef]
- Wu, R.; Högberg, J.; Adner, M.; Ramos-Ramírez, P.; Stenius, U.; Zheng, H. Crystalline silica particles cause rapid NLRP3-dependent mitochondrial depolarization and DNA damage in airway epithelial cells. Part. Fibre Toxicol. 2020, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Hogberg, J.; Adner, M.; Stenius, U.; Zheng, H. Crystalline silica particles induce DNA damage in respiratory epithelium by ATX secretion and Rac1 activation. Biochem. Biophys. Res. Commun. 2021, 548, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Hogberg, J.; Stenius, U. ATM-activated autotaxin (ATX) propagates inflammation and DNA damage in lung epithelial cells: A new mode of action for silica-induced DNA damage? Carcinogenesis 2017, 38, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.L.; Mak, K.H.; Hu, S.; Wang, S.S.; Wong, K.M.; Wong, C.S.T.; Wu, H.Y.; Law, H.T.; Liu, K.; Talbot, C.C.; et al. Cell survival, DNA damage, and oncogenic transformation after a transient and reversible apoptotic response. Mol. Biol. Cell 2012, 23, 2240–2252. [Google Scholar] [CrossRef]
- Ichim, G.; Lopez, J.; Ahmed, S.U.; Muthalagu, N.; Giampazolias, E.; Delgado, M.E.; Haller, M.; Riley, J.S.; Mason, S.M.; Athineos, D.; et al. Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Mol. Cell 2015, 57, 860–872. [Google Scholar] [CrossRef]
- Liu, X.; He, Y.; Li, F.; Huang, Q.; Kato, T.A.; Hall, R.P.; Li, C.-Y. Caspase-3 promotes genetic instability and carcinogenesis. Mol. Cell 2015, 58, 284–296. [Google Scholar] [CrossRef]
- Liu, X.; Li, F.; Huang, Q.; Zhang, Z.; Zhou, L.; Deng, Y.; Zhou, M.; Fleenor, D.E.; Wang, H.; Kastan, M.B.; et al. Self-inflicted DNA double-strand breaks sustain tumorigenicity and stemness of cancer cells. Cell Res. 2017, 27, 764–783. [Google Scholar] [CrossRef] [PubMed]
- Berthenet, K.; Ferrer, C.C.; Fanfone, D.; Popgeorgiev, N.; Neves, D.; Bertolino, P.; Gibert, B.; Hernandez-Vargas, H.; Ichim, G. Failed Apoptosis Enhances Melanoma Cancer Cell Aggressiveness. Cell Rep. 2020, 31, 107731. [Google Scholar] [CrossRef]
- Anlar, H.G.; Taner, G.; Bacanli, M.; Iritas, S.; Kurt, T.; Tutkun, E.; Yilmaz, O.H.; Basaran, N. Assessment of DNA damage in ceramic workers. Mutagenesis 2018, 33, 97–104. [Google Scholar] [CrossRef]
- Wultsch, G.; Nersesyan, A.; Kundi, M.; Al-Serori, H.; Knasmuller, S. Induction of chromosomal damage in exfoliated buccal and nasal cells of road markers. J. Toxicol. Environ. Health A 2019, 82, 969–976. [Google Scholar] [CrossRef]
- Wultsch, G.; Setayesh, T.; Kundi, M.; Kment, M.; Nersesyan, A.; Fenech, M.; Knasmüller, S. Induction of DNA damage as a consequence of occupational exposure to crystalline silica: A review and meta-analysis. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108349. [Google Scholar] [CrossRef]
- Martínez-Lagunas, K.; Yamaguchi, Y.; Becker, C.; Geisen, C.; DeRuiter, M.C.; Miura, M.; Fleischmann, B.K.; Hesse, M. In vivo detection of programmed cell death during mouse heart development. Cell Death Differ. 2020, 27, 1398–1414. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Seo, T.W.; Yi, J.H.; Shin, K.S.; Yoo, S.J. CHIP has a protective role against oxidative stress-induced cell death through specific regulation of endonuclease G. Cell Death Dis. 2013, 4, e666. [Google Scholar] [CrossRef]
- Jang, D.S.; Penthala, N.R.; Apostolov, E.O.; Wang, X.; Crooks, P.A.; Basnakian, A.G. Novel cytoprotective inhibitors for apoptotic endonuclease G. DNA Cell Biol. 2015, 34, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; de Lange, T. 53BP1: Pro choice in DNA repair. Trends Cell Biol. 2014, 24, 108–117. [Google Scholar] [CrossRef]
- Schultz, L.B.; Chehab, N.H.; Malikzay, A.; Halazonetis, T.D. p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J. Cell Biol. 2000, 151, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, J.; Tan, J.; Wang, M.; Yang, J.; Zhang, Z.-M.; Li, C.; Basnakian, A.G.; Tang, H.-W.; Perrimon, N.; et al. Endonuclease G promotes autophagy by suppressing mTOR signaling and activating the DNA damage response. Nat. Commun. 2021, 12, 476. [Google Scholar] [CrossRef]
- Wang, X.; Xu, D.; Liao, Y.; Zhong, S.; Song, H.; Sun, B.; Zhou, B.P.; Deng, J.; Han, B. Epithelial neoplasia coincides with exacerbated injury and fibrotic response in the lungs of Gprc5a-knockout mice following silica exposure. Oncotarget 2015, 6, 39578–39593. [Google Scholar] [CrossRef]
- Cartwright, I.M.; Liu, X.; Zhou, M.; Li, F.; Li, C.Y. Essential roles of Caspase-3 in facilitating Myc-induced genetic instability and carcinogenesis. eLife 2017, 6, e26371. [Google Scholar] [CrossRef]
- Leytin, V.; Allen, D.J.; Mutlu, A.; Gyulkhandanyan, A.V.; Mykhaylov, S.; Freedman, J. Mitochondrial control of platelet apoptosis: Effect of cyclosporin A, an inhibitor of the mitochondrial permeability transition pore. Lab. Investig. 2009, 89, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Lopez, K.E.; Bouchier-Hayes, L. Lethal and Non-Lethal Functions of Caspases in the DNA Damage Response. Cells 2022, 11, 1887. [Google Scholar] [CrossRef]
- Hacker, G.; Haimovici, A. Sub-lethal signals in the mitochondrial apoptosis apparatus: Pernicious by-product or physiological event? Cell Death Differ. 2022, 1–8. [Google Scholar] [CrossRef]
- Bao, X.; Liu, X.; Li, F.; Li, C.Y. Limited MOMP, ATM, and their roles in carcinogenesis and cancer treatment. Cell Biosci. 2020, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, C.C.; Berthenet, K.; Ichim, G. Apoptosis—Fueling the oncogenic fire. FEBS J. 2021, 288, 4445–4463. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Surman, D.R.; Diggs, L.; Xi, S.; Gao, S.; Gurusamy, D.; McLoughlin, K.; Drake, J.; Feingold, P.; Brown, K.; et al. Bile acid-induced “Minority MOMP” promotes esophageal carcinogenesis while maintaining apoptotic resistance via Mcl-1. Oncogene 2020, 39, 877–890. [Google Scholar] [CrossRef]
- Bhattarai, S.; Sharma, S.; Ara, H.; Subedi, U.; Sun, G.; Li, C.; Bhuiyan, S.; Kevil, C.; Armstrong, W.P.; Minvielle, M.T.; et al. Disrupted Blood-Brain Barrier and Mitochondrial Impairment by Autotaxin-Lysophosphatidic Acid Axis in Postischemic Stroke. J. Am. Heart Assoc. 2021, 10, e021511. [Google Scholar] [CrossRef]
- Bhattarai, S.; Sharma, S.; Subedi, U.; Ara, H.; Shum, A.; Milena, M.; Bhuiyan, S.; Kidambi, S.; Sun, H.; Miriyala, S.; et al. The ATX-LPA Axis Regulates Vascular Permeability during Cerebral Ischemic-Reperfusion. Int. J. Mol. Sci. 2022, 23, 4138. [Google Scholar] [CrossRef]
- Brokatzky, D.; Dörflinger, B.; Haimovici, A.; Weber, A.; Kirschnek, S.; Vier, J.; Metz, A.; Henschel, J.; Steinfeldt, T.; Gentle, I.E.; et al. A non-death function of the mitochondrial apoptosis apparatus in immunity. EMBO J. 2019, 38, e100907. [Google Scholar] [CrossRef]
- Dörflinger, B.; Badr, M.T.; Haimovici, A.; Fischer, L.; Vier, J.; Metz, A.; Eisele, B.; Bronsert, P.; Aumann, K.; Höppner, J.; et al. Mitochondria supply sub-lethal signals for cytokine secretion and DNA-damage in H. pylori infection. Cell Death Differ. 2022, 29, 2218–2232. [Google Scholar] [CrossRef]
- Boudigaard, S.H.; Schlünssen, V.; Vestergaard, J.M.; Søndergaard, K.; Torén, K.; Peters, S.; Kromhout, H.; Kolstad, H.A. Occupational exposure to respirable crystalline silica and risk of autoimmune rheumatic diseases: A nationwide cohort study. Int. J. Epidemiol. 2021, 50, 1213–1226. [Google Scholar] [CrossRef]
- Dorak, M.T.; Karpuzoglu, E. Gender differences in cancer susceptibility: An inadequately addressed issue. Front. Genet. 2012, 3, 268. [Google Scholar] [CrossRef]
- Cook, M.B.; Dawsey, S.M.; Freedman, N.D.; Inskip, P.D.; Wichner, S.M.; Quraishi, S.M.; Devesa, S.S.; McGlynn, K.A. Sex disparities in cancer incidence by period and age. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 1174–1182. [Google Scholar] [CrossRef]
- Edgren, G.; Liang, L.; Adami, H.O.; Chang, E.T. Enigmatic sex disparities in cancer incidence. Eur. J. Epidemiol. 2012, 27, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Eker, C.; Bilir, M.U.; Celik, H.G.; Balci, B.K.; Gunel, T. Assessment of the Role of Nuclear ENDOG Gene and mtDNA Variations on Paternal Mitochondrial Elimination (PME) in Infertile Men: An Experimental Study. Reprod. Sci. 2022, 29, 2208–2222. [Google Scholar] [CrossRef] [PubMed]
- Al Smadi, M.A.; Hammadeh, M.E.; Solomayer, E.; Batiha, O.; Altalib, M.M.; Jahmani, M.Y.; Shboul, M.A.; Nusair, B.; Amor, H. Impact of Mitochondrial Genetic Variants in ND1, ND2, ND5, and ND6 Genes on Sperm Motility and Intracytoplasmic Sperm Injection (ICSI) Outcomes. Reprod. Sci. 2021, 28, 1540–1555. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Högberg, J.; Hsieh, J.-H.; Auerbach, S.; Korhonen, A.; Stenius, U.; Silins, I. Gender differences in cancer susceptibility: Role of oxidative stress. Carcinogenesis 2016, 37, 985–992. [Google Scholar] [CrossRef]
- Hartwig, A.; Arand, M.; Epe, B.; Guth, S.; Jahnke, G.; Lampen, A.; Martus, H.-J.; Monien, B.; Rietjens, I.M.C.M.; Schmitz-Spanke, S.; et al. Mode of action-based risk assessment of genotoxic carcinogens. Arch. Toxicol. 2020, 94, 1787–1877. [Google Scholar] [CrossRef]
- Hopf, N.B.; Bolognesi, C.; Danuser, B.; Wild, P. Biological monitoring of workers exposed to carcinogens using the buccal micronucleus approach: A systematic review and meta-analysis. Mutat. Res. Rev. Mutat. Res. 2019, 781, 11–29. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Stenius, U.; Högberg, J. ATX-LPA-Dependent Nuclear Translocation of Endonuclease G in Respiratory Epithelial Cells: A New Mode Action for DNA Damage Induced by Crystalline Silica Particles. Cancers 2023, 15, 865. https://doi.org/10.3390/cancers15030865
Zheng H, Stenius U, Högberg J. ATX-LPA-Dependent Nuclear Translocation of Endonuclease G in Respiratory Epithelial Cells: A New Mode Action for DNA Damage Induced by Crystalline Silica Particles. Cancers. 2023; 15(3):865. https://doi.org/10.3390/cancers15030865
Chicago/Turabian StyleZheng, Huiyuan, Ulla Stenius, and Johan Högberg. 2023. "ATX-LPA-Dependent Nuclear Translocation of Endonuclease G in Respiratory Epithelial Cells: A New Mode Action for DNA Damage Induced by Crystalline Silica Particles" Cancers 15, no. 3: 865. https://doi.org/10.3390/cancers15030865
APA StyleZheng, H., Stenius, U., & Högberg, J. (2023). ATX-LPA-Dependent Nuclear Translocation of Endonuclease G in Respiratory Epithelial Cells: A New Mode Action for DNA Damage Induced by Crystalline Silica Particles. Cancers, 15(3), 865. https://doi.org/10.3390/cancers15030865





