Cumulative UV Exposure or a Modified SCINEXA™-Skin Aging Score Do Not Play a Substantial Role in Predicting the Risk of Developing Keratinocyte Cancers after Solid Organ Transplantation—A Case Control Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Mitochondrial DNA Point Heteroplasmy
2.3. Genetic Analyses
2.4. Statistical Analyses
3. Results
3.1. Participants’ Characteristics
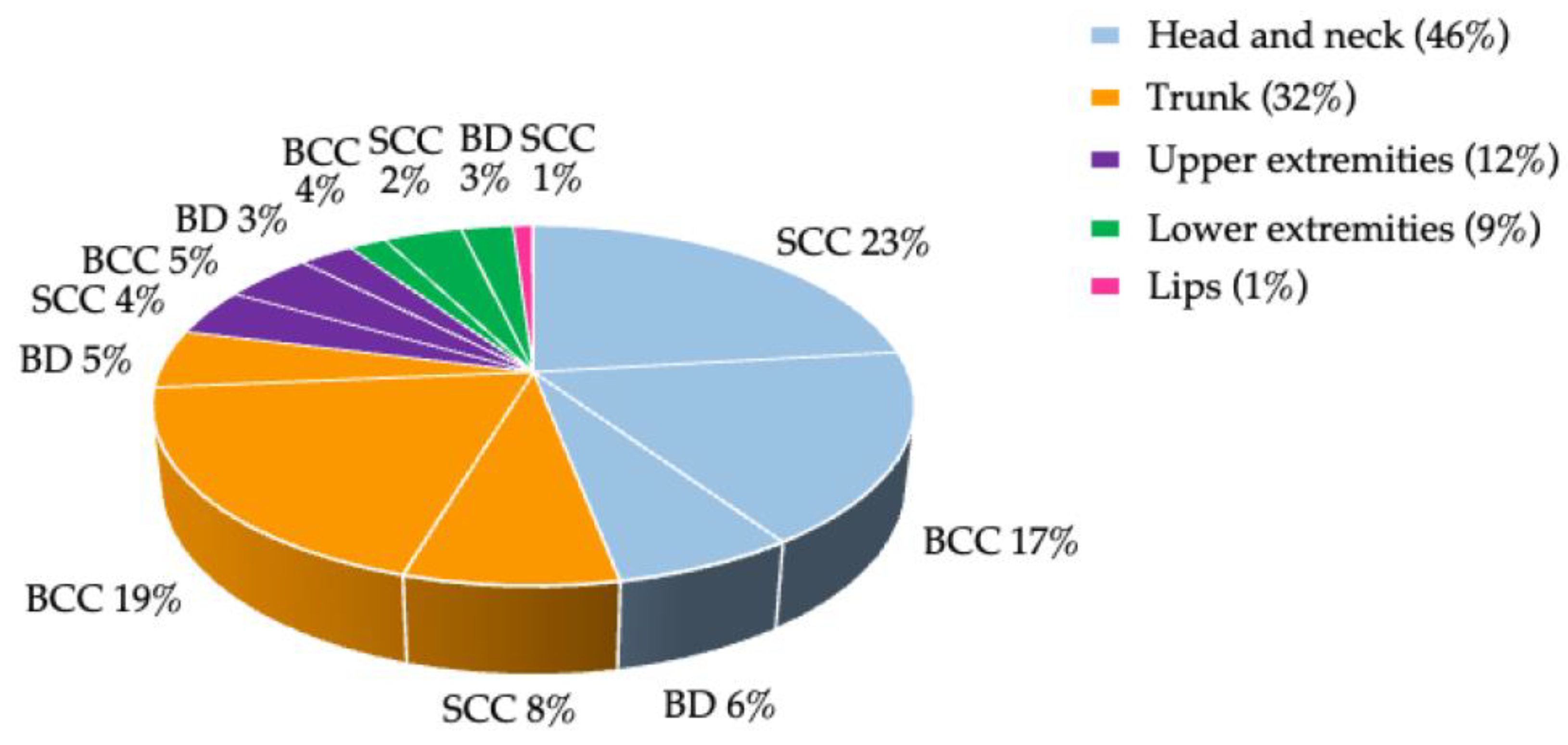
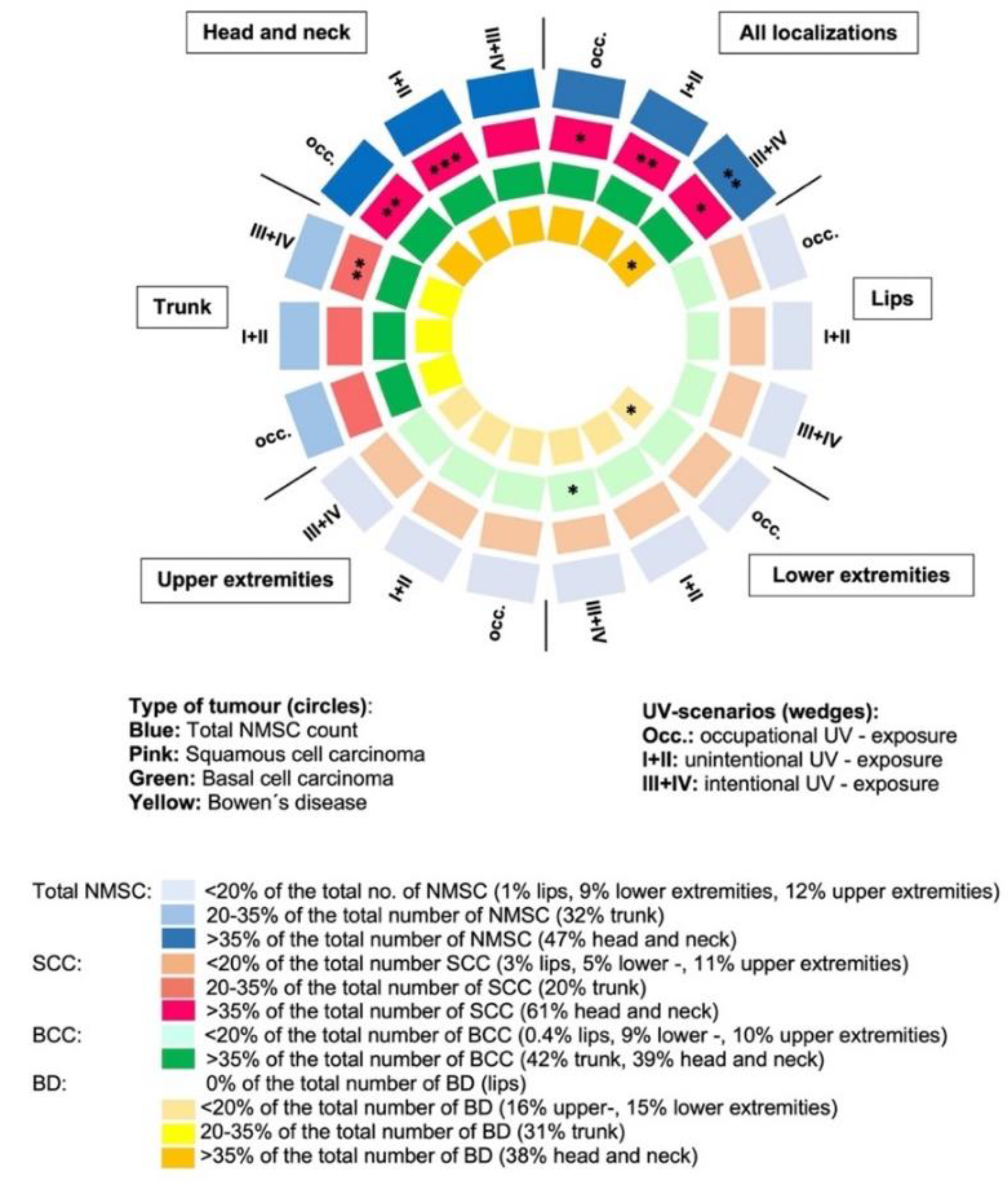
3.2. Non-Melanoma Skin Cancer
3.3. Skin Aging Score
3.4. Mitochondrial DNA Point Heteroplasmy
3.5. Ultraviolet Burden
3.6. Analyses of Cofactors for Non-Melanoma Skin Cancer
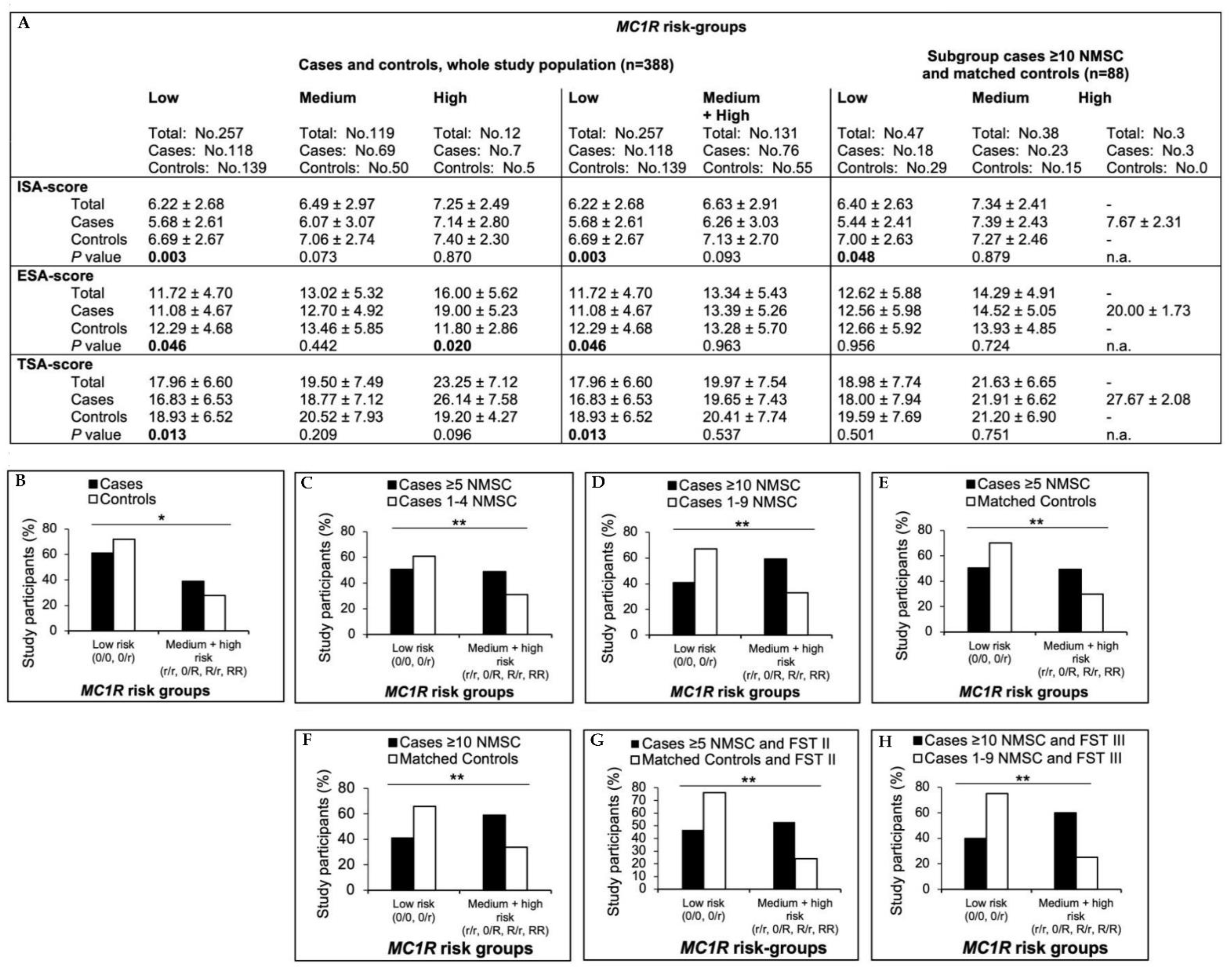
3.7. MC1R Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plasmeijer, E.I.; Sachse, M.M.; Gebhardt, C.; Geusau, A.; Bouwes Bavinck, J.N. Cutaneous squamous cell carcinoma (cSCC) and immunosurveillance—The impact of immunosuppression on frequency of cSCC. J. Eur. Acad. Derm. Venereol. 2019, 33 (Suppl. S8), 33–37. [Google Scholar] [CrossRef] [PubMed]
- Euvrard, S.; Kanitakis, J.; Claudy, A. Skin cancers after organ transplantation. N. Engl. J. Med. 2003, 348, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Grulich, A.E.; van Leeuwen, M.T.; Falster, M.O.; Vajdic, C.M. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet 2007, 370, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Krynitz, B.; Edgren, G.; Lindelof, B.; Baecklund, E.; Brattstrom, C.; Wilczek, H.; Smedby, K.E. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008—A Swedish population-based study. Int. J. Cancer 2013, 132, 1429–1438. [Google Scholar] [CrossRef]
- Billups, K.; Neal, J.; Salyer, J. Immunosuppressant-driven de novo malignant neoplasms after solid-organ transplant. Prog. Transpl. 2015, 25, 182–188. [Google Scholar] [CrossRef]
- Mudigonda, T.; Levender, M.M.; O’Neill, J.L.; West, C.E.; Pearce, D.J.; Feldman, S.R. Incidence, risk factors, and preventative management of skin cancers in organ transplant recipients: A review of single- and multicenter retrospective studies from 2006 to 2010. Derm. Surg. 2013, 39, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Ratushny, V.; Gober, M.D.; Hick, R.; Ridky, T.W.; Seykora, J.T. From keratinocyte to cancer: The pathogenesis and modeling of cutaneous squamous cell carcinoma. J. Clin. Investig. 2012, 122, 464–472. [Google Scholar] [CrossRef]
- Didona, D.; Paolino, G.; Bottoni, U.; Cantisani, C. Non Melanoma Skin Cancer Pathogenesis Overview. Biomedicines 2018, 6, 6. [Google Scholar] [CrossRef]
- Geusau, A.; Dunkler, D.; Messeritsch, E.; Sandor, N.; Heidler, G.; Rodler, S.; Ankersmit, J.; Zuckermann, A.; Tschachler, E. Non-melanoma skin cancer and its risk factors in an Austrian population of heart transplant recipients receiving induction therapy. Int. J. Derm. 2008, 47, 918–925. [Google Scholar] [CrossRef]
- Harwood, C.A.; Toland, A.E.; Proby, C.M.; Euvrard, S.; Hofbauer, G.F.L.; Tommasino, M.; Bouwes Bavinck, J.N.; KeraCon, C. The pathogenesis of cutaneous squamous cell carcinoma in organ transplant recipients. Br. J. Derm. 2017, 177, 1217–1224. [Google Scholar] [CrossRef]
- Hofbauer, G.F.; Bouwes Bavinck, J.N.; Euvrard, S. Organ transplantation and skin cancer: Basic problems and new perspectives. Exp. Derm. 2010, 19, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Derm. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Mancebo, S.E.; Wang, S.Q. Skin cancer: Role of ultraviolet radiation in carcinogenesis. Rev. Environ. Health 2014, 29, 265–273. [Google Scholar] [CrossRef]
- Fuks, K.B.; Huls, A.; Sugiri, D.; Altug, H.; Vierkotter, A.; Abramson, M.J.; Goebel, J.; Wagner, G.G.; Demuth, I.; Krutmann, J.; et al. Tropospheric ozone and skin aging: Results from two German cohort studies. Environ. Int. 2019, 124, 139–144. [Google Scholar] [CrossRef]
- Vierkotter, A.; Ranft, U.; Kramer, U.; Sugiri, D.; Reimann, V.; Krutmann, J. The SCINEXA: A novel, validated score to simultaneously assess and differentiate between intrinsic and extrinsic skin ageing. J. Derm. Sci. 2009, 53, 207–211. [Google Scholar] [CrossRef]
- Yaar, M.; Gilchrest, B.A. Aging versus photoaging: Postulated mechanisms and effectors. J. Investig. Derm. Symp. Proc. 1998, 3, 47–51. [Google Scholar]
- Manganelli, M.; Guida, S.; Ferretta, A.; Pellacani, G.; Porcelli, L.; Azzariti, A.; Guida, G. Behind the Scene: Exploiting MC1R in Skin Cancer Risk and Prevention. Genes 2021, 12, 1093. [Google Scholar] [CrossRef]
- Nasti, T.H.; Timares, L. MC1R, eumelanin and pheomelanin: Their role in determining the susceptibility to skin cancer. Photochem. Photobiol. 2015, 91, 188–200. [Google Scholar] [CrossRef]
- Wendt, J.; Rauscher, S.; Burgstaller-Muehlbacher, S.; Fae, I.; Fischer, G.; Pehamberger, H.; Okamoto, I. Human Determinants and the Role of Melanocortin-1 Receptor Variants in Melanoma Risk Independent of UV Radiation Exposure. JAMA Derm. 2016, 152, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B. The validity and practicality of sun-reactive skin types I through VI. Arch. Derm. 1988, 124, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Sitek, A.; Rosset, I.; Zadzinska, E.; Kasielska-Trojan, A.; Neskoromna-Jedrzejczak, A.; Antoszewski, B. Skin color parameters and Fitzpatrick phototypes in estimating the risk of skin cancer: A case-control study in the Polish population. J. Am. Acad. Derm. 2016, 74, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, T.; Muller, H.K.; Blizzard, L.; Ashbolt, R.; Phillips, G. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol. Biomark. Prev. 1998, 7, 203–206. [Google Scholar] [CrossRef]
- Eilers, S.; Bach, D.Q.; Gaber, R.; Blatt, H.; Guevara, Y.; Nitsche, K.; Kundu, R.V.; Robinson, J.K. Accuracy of self-report in assessing Fitzpatrick skin phototypes I through VI. JAMA Derm. 2013, 149, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Buranasirin, P.; Pongpirul, K.; Meephansan, J. Development of a Global Subjective Skin Aging Assessment score from the perspective of dermatologists. BMC Res. Notes 2019, 12, 364. [Google Scholar] [CrossRef]
- Yu, C.L.; Li, Y.; Freedman, D.M.; Fears, T.R.; Kwok, R.; Chodick, G.; Alexander, B.; Kimlin, M.G.; Kricker, A.; Armstrong, B.K.; et al. Assessment of lifetime cumulative sun exposure using a self-administered questionnaire: Reliability of two approaches. Cancer Epidemiol. Biomark. Prev. 2009, 18, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Borron, J.C.; Sanchez-Laorden, B.L.; Jimenez-Cervantes, C. Melanocortin-1 receptor structure and functional regulation. Pigment. Cell. Res. 2005, 18, 393–410. [Google Scholar] [CrossRef]
- Morita, A. Tobacco smoke causes premature skin aging. J. Derm. Sci. 2007, 48, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Vierkotter, A.; Krutmann, J. Environmental influences on skin aging and ethnic-specific manifestations. Dermato-Endocrinology 2012, 4, 227–231. [Google Scholar] [CrossRef]
- Wigmann, C.; Huls, A.; Krutmann, J.; Schikowski, T. Estimating the Relative Contribution of Environmental and Genetic Risk Factors to Different Aging Traits by Combining Correlated Variables into Weighted Risk Scores. Int. J. Environ. Res. Public Health 2022, 19, 16746. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Yao, A.; Xie, Y.; Lin, J.; Sharifullah, F.; Hong, Y.; Chen, H.; Cheng, F.; Lai, W. Circ_0011129 Encapsulated by the Small Extracellular Vesicles Derived from Human Stem Cells Ameliorate Skin Photoaging. Int. J. Mol. Sci. 2022, 23, 15390. [Google Scholar] [CrossRef]
- Bastiaens, M.T.; ter Huurne, J.A.; Kielich, C.; Gruis, N.A.; Westendorp, R.G.; Vermeer, B.J.; Bavinck, J.N.; Leiden Skin Cancer Study Team. Melanocortin-1 receptor gene variants determine the risk of nonmelanoma skin cancer independently of fair skin and red hair. Am. J. Hum. Genet. 2001, 68, 884–894. [Google Scholar] [CrossRef]
- Beaumont, K.A.; Shekar, S.N.; Newton, R.A.; James, M.R.; Stow, J.L.; Duffy, D.L.; Sturm, R.A. Receptor function, dominant negative activity and phenotype correlations for MC1R variant alleles. Hum. Mol. Genet. 2007, 16, 2249–2260. [Google Scholar] [CrossRef]
- Binstock, M.; Hafeez, F.; Metchnikoff, C.; Arron, S.T. Single-nucleotide polymorphisms in pigment genes and nonmelanoma skin cancer predisposition: A systematic review. Br. J. Derm. 2014, 171, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.L.; Box, N.F.; Chen, W.; Palmer, J.S.; Montgomery, G.W.; James, M.R.; Hayward, N.K.; Martin, N.G.; Sturm, R.A. Interactive effects of MC1R and OCA2 on melanoma risk phenotypes. Hum. Mol. Genet. 2004, 13, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Borron, J.C.; Olivares, C. Melanocortin 1 receptor and skin pathophysiology: Beyond colour, much more than meets the eye. Exp. Derm. 2014, 23, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kraft, P.; Colditz, G.A.; Wong, J.; Hunter, D.J. Melanocortin 1 receptor variants and skin cancer risk. Int. J. Cancer 2006, 119, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Wendt, J.; Rauscher, S.; Burgstaller-Muehlbacher, S.; Sunder-Plassmann, R.; Scheurecker, C.; Richtig, E.; Fae, I.; Fischer, G.; Pehamberger, H.; et al. Characterization of patients at high risk of melanoma in Austria. Br. J. Derm. 2016, 174, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, E.; Garcia-Borron, J.C.; Fargnoli, M.C.; Gandini, S.; Maisonneuve, P.; Bagnardi, V.; Specchia, C.; Liu, F.; Kayser, M.; Nijsten, T.; et al. MC1R variants increased the risk of sporadic cutaneous melanoma in darker-pigmented Caucasians: A pooled-analysis from the M-SKIP project. Int. J. Cancer 2015, 136, 618–631. [Google Scholar] [CrossRef]
- Raimondi, S.; Sera, F.; Gandini, S.; Iodice, S.; Caini, S.; Maisonneuve, P.; Fargnoli, M.C. MC1R variants, melanoma and red hair color phenotype: A meta-analysis. Int. J. Cancer 2008, 122, 2753–2760. [Google Scholar] [CrossRef]
- Scherer, D.; Bermejo, J.L.; Rudnai, P.; Gurzau, E.; Koppova, K.; Hemminki, K.; Kumar, R. MC1R variants associated susceptibility to basal cell carcinoma of skin: Interaction with host factors and XRCC3 polymorphism. Int. J. Cancer 2008, 122, 1787–1793. [Google Scholar] [CrossRef]
- Scherer, D.; Rachakonda, P.S.; Angelini, S.; Mehnert, F.; Sucker, A.; Egberts, F.; Hauschild, A.; Hemminki, K.; Schadendorf, D.; Kumar, R. Association between the germline MC1R variants and somatic BRAF/NRAS mutations in melanoma tumors. J. Investig. Derm. 2010, 130, 2844–2848. [Google Scholar] [CrossRef]
- Tagliabue, E.; Fargnoli, M.C.; Gandini, S.; Maisonneuve, P.; Liu, F.; Kayser, M.; Nijsten, T.; Han, J.; Kumar, R.; Gruis, N.A.; et al. MC1R gene variants and non-melanoma skin cancer: A pooled-analysis from the M-SKIP project. Br. J. Cancer 2015, 113, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, E.; Gandini, S.; Garcia-Borron, J.C.; Maisonneuve, P.; Newton-Bishop, J.; Polsky, D.; Lazovich, D.; Kumar, R.; Ghiorzo, P.; Ferrucci, L.; et al. Association of Melanocortin-1 Receptor Variants with Pigmentary Traits in Humans: A Pooled Analysis from the M-Skip Project. J. Investig. Derm. 2016, 136, 1914–1917. [Google Scholar] [CrossRef] [PubMed]
- Valverde, P.; Healy, E.; Jackson, I.; Rees, J.L.; Thody, A.J. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat. Genet. 1995, 11, 328–330. [Google Scholar] [CrossRef]
- Martens, M.C.; Seebode, C.; Lehmann, J.; Emmert, S. Photocarcinogenesis and Skin Cancer Prevention Strategies: An Update. Anticancer Res. 2018, 38, 1153–1158. [Google Scholar]
- Loney, T.; Paulo, M.S.; Modenese, A.; Gobba, F.; Tenkate, T.; Whiteman, D.C.; Green, A.C.; John, S.M. Global evidence on occupational sun exposure and keratinocyte cancers: A systematic review. Br. J. Derm. 2021, 184, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Diepgen, T.; Bauer, A. Occupational exposure to non-artificial UV-light and non-melanocytic skin cancer—A systematic review concerning a new occupational disease. J. Dtsch. Derm. Ges. 2010, 8, 250–264. [Google Scholar] [CrossRef]
- Paulo, M.S.; Adam, B.; Akagwu, C.; Akparibo, I.; Al-Rifai, R.H.; Bazrafshan, S.; Gobba, F.; Green, A.C.; Ivanov, I.; Kezic, S.; et al. WHO/ILO work-related burden of disease and injury: Protocol for systematic reviews of occupational exposure to solar ultraviolet radiation and of the effect of occupational exposure to solar ultraviolet radiation on melanoma and non-melanoma skin cancer. Environ. Int. 2019, 126, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Derm. 2012, 166, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Goodman, G.D.; Kaufman, J.; Day, D.; Weiss, R.; Kawata, A.K.; Garcia, J.K.; Santangelo, S.; Gallagher, C.J. Impact of Smoking and Alcohol Use on Facial Aging in Women: Results of a Large Multinational, Multiracial, Cross-sectional Survey. J. Clin. Aesthet. Derm. 2019, 12, 28–39. [Google Scholar]
- Rangwala, S.; Tsai, K.Y. Roles of the immune system in skin cancer. Br. J. Derm. 2011, 165, 953–965. [Google Scholar]
- Heidegger, A.; Pisarek, A.; de la Puente, M.; Niederstatter, H.; Pospiech, E.; Wozniak, A.; Schury, N.; Unterlander, M.; Sidstedt, M.; Junker, K.; et al. Development and inter-laboratory validation of the VISAGE enhanced tool for age estimation from semen using quantitative DNA methylation analysis. Forensic Sci. Int. Genet. 2022, 56, 102596. [Google Scholar] [CrossRef]
- Xavier, C.; Eduardoff, M.; Strobl, C.; Parson, W. SD quants-Sensitive detection tetraplex-system for nuclear and mitochondrial DNA quantification and degradation inference. Forensic Sci. Int. Genet. 2019, 42, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Strobl, C.; Churchill Cihlar, J.; Lagace, R.; Wootton, S.; Roth, C.; Huber, N.; Schnaller, L.; Zimmermann, B.; Huber, G.; Lay Hong, S.; et al. Evaluation of mitogenome sequence concordance, heteroplasmy detection, and haplogrouping in a worldwide lineage study using the Precision ID mtDNA Whole Genome Panel. Forensic Sci. Int. Genet. 2019, 42, 244–251. [Google Scholar] [CrossRef]
- Andrews, R.M.; Kubacka, I.; Chinnery, P.F.; Lightowlers, R.N.; Turnbull, D.M.; Howell, N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat. Genet. 1999, 23, 147. [Google Scholar] [CrossRef] [PubMed]
- Thorvaldsdottir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief Bioinform. 2013, 14, 178–192. [Google Scholar] [CrossRef]



| Total | Cases | Controls | p Value # | |
|---|---|---|---|---|
| n = 388 | n = 194 | n = 194 | ||
| Main place of residence (altitude; n (%)) | 0.594 | |||
| <500 m | 370 (95) | 187 (96) | 183 (94) | |
| 500–1000 m | 16 (4) | 6 (3) | 10 (5) | |
| >1000 m | 2 (1) | 1 (1) | 1 (1) | |
| Residence abroad > 2 years (n (%)) | 0.041 | |||
| Northern Europe | 7 (2) | 4 (2) | 3 (2) | |
| Southern Europe | 41 (11) | 15 (8) | 26 (13) | |
| Subtropics | 29 (8) | 10 (5) | 19 (10) | |
| Tropics | 7 (2) | 6 (3) | 1 (1) | |
| Time spent outdoors with unintentional sun exposure(May–August) | ||||
| Outdoor occupation | ||||
| All study participants (hours, mean ± SD) | 12,257 ± 10,355 | 12,246 ± 10,217 | 12,268 ± 10,589 | 1.000 |
| Participants with outdoor occupation (n (%)) | 157 (40) | 72 (37) | 85 (44) | 0.180 |
| Without outdoor occupation (n (%)) | 231 (60) | 122 (63) | 109 (56) | 0.180 |
| Recreation and occupation during the week (UV scenario I; hours, mean ± SD) | ||||
| 17,369 ± 12,868 | 17,080 ± 12,903 | 17,657 ± 12,836 | 0.659 | |
| Ages 10–19 years | 3604 ± 1748 | 3513 ± 1735 | 3703 ± 1761 | 0.286 |
| Ages 20–39 years | 5699 ± 6259 | 5629 ± 7033 | 5769 ± 4939 | 0.820 |
| Ages 40–59 years | 5345 ± 7697 | 5285 ± 8497 | 5377 ± 6897 | 0.908 |
| Ages ≥60 years | 2993 ± 2462 | 2950 ± 2330 | 3110 ± 2593 | 0.550 |
| Recreation on weekends (UV scenario II; hours, mean ± SD) | ||||
| 10,827 ± 3778 | 10,782 ± 3428 | 10,871 ± 4128 | 0.814 | |
| Ages 10–19 years | 2475 ± 607 | 2463 ± 593 | 2487 ± 621 | 0.688 |
| Ages 20–39 years | 3962 ± 1522 | 3995 ± 1461 | 3929 ± 1582 | 0.670 |
| Ages 40–59 years | 3172 ± 1555 | 3132 ± 1494 | 3196 ± 1647 | 0.680 |
| Ages ≥60 years | 1337 ± 1109 | 1316 ± 1039 | 1390 ± 1178 | 0.540 |
| Total time spent outdoors with unintentional sun exposure (UV scenario I + II; total hours mean ± SD) | 28,196 ± 15,163 | 27,862 ± 14,571 | 28,528 ± 15,755 | 0.665 |
| Time spent outdoors with intentional sun exposure(9:00 AM–3:00 PM, May–August; mean ± SD) | ||||
| Central Europe (UV scenario III; hours, mean ± SD) | ||||
| 5678 ± 3946 | 6023 ± 3699 | 5333 ± 4192 | 0.087 | |
| Ages 10–19 years | 1951 ± 1197 | 2087 ± 1180 | 1816 ± 1213 | 0.027 |
| Ages 20–39 years | 1875 ± 1443 | 1949 ± 1396 | 1801 ± 1489 | 0.313 |
| Ages 40–59 years | 1341 ± 1354 | 1454 ± 1371 | 1221 ± 1337 | 0.092 |
| Ages ≥60 years | 598 ± 1455 | 610 ± 1068 | 564 ± 1304 | 0.750 |
| Southern geographic regions (UV scenario IV, Mediterranean, subtropical, tropical regions; hours, mean ± SD) | ||||
| 2774 ± 2610 | 2903 ± 2558 | 2586 ± 2622 | 0.228 | |
| Ages 10–19 years | 288 ± 647 | 253 ± 543 | 323 ± 750 | 0.294 |
| Ages 20–39 years | 1148 ± 1096 | 1189 ± 1158 | 1107 ± 1034 | 0.464 |
| Ages 40–59 years | 995 ± 1054 | 1120 ± 1049 | 863 ± 1058 | 0.017 |
| Ages ≥60 years | 369 ± 718 | 385 ± 663 | 337 ± 772 | 0.590 |
| Total time spent outdoors with intentional sun exposure (UV scenario III + IV; total hours, mean ± SD) | 8423 ± 4645 | 9019 ± 4771 | 8228 ± 4519 | 0.033 |
| Recreational activities independent of vacation (except gardening; hours, mean ± SD) | 3398 ± 4053 | 3136 ± 3471 | 3592 ± 4635 | 0.275 |
| Gardening (hours, mean ± SD) | ||||
| All study participants | 3109 ± 4971 | 2992 ± 4362 | 3227 ± 5579 | 0.645 |
| Gardeners only | 4340 ± 3812 | 4206 ± 3250 | 4471 ± 4375 | 0.700 |
| n (%) | 278 (72) | 138 (71) | 140 (72) | |
| Sunbed use (hours, mean ± SD) | ||||
| All study participants | 6 ± 23 | 7 ± 33 | 5 ± 12 | 0.131 |
| Sunbed users only | 34 ± 10 | 33 ± 12 | 36 ± 8 | 0.392 |
| n (%) | 66 (17) | 40 (21) | 27 (14) |
| Skin Aging Score | ||||
|---|---|---|---|---|
| Intrinsic | Extrinsic | Total | ||
| Time spent outdoors with unintentional sun exposure (May–August; mean lifetime hours) | ||||
| Outdoor occupation | rs | −0.021 | 0.100* | 0.068 |
| p value | 0.678 | 0.048 | 0.182 | |
| Total time spent outdoors with unintentional sun exposure (Recreation and occupation; UV scenario I + II) | rs | 0.129* | 0.231 ** | 0.217 ** |
| p value | 0.011 | <0.001 | <0.001 | |
| Time spent outdoors with intentional sun exposure (9AM–3PM, mean lifetime hours) | ||||
| Central Europe (UV scenario III) | rs | 0.107* | 0.167 ** | 0.166 ** |
| p value | 0.036 | 0.001 | 0.001 | |
| Southern geographic regions (UV scenario IV) | rs | 0.015 | −0.031 | −0.026 |
| p value | 0.765 | 0.538 | 0.611 | |
| Total time spent outdoors with intentional sun exposure (UV scenario III + IV) | rs | 0.110* | 0.132 ** | 0.137 ** |
| p value | 0.030 | 0.009 | 0.007 | |
| Recreational activities during vacation (mean weeks of life) | ||||
| Body covered (mountaineering, hiking, skiing) | rs | 0.073 | 0.083 | 0.091 |
| p value | 0.153 | 0.101 | 0.073 | |
| Wearing swimwear only (watersports, sunbathing) | rs | 0.004 | 0.074 | 0.056 |
| p value | 0.931 | 0.148 | 0.271 | |
| Body uncovered (nudist beach) | rs | 0.041 | 0.046 | 0.042 |
| p value | 0.421 | 0.368 | 0.405 | |
| Sunbed use(mean lifetime hours, sunbed users only) | rs | 0.318 ** | 0.323 ** | 0.362 ** |
| p value | 0.009 | 0.008 | 0.003 | |
| Sunbed use(yes/no) | rs | −0.163 ** | −0.080 | −0.130 * |
| p value | 0.001 | 0.116 | 0.010 | |
| Gardening(mean lifetime hours) | rs | 0.166 ** | 0.195** | 0.213 ** |
| p value | 0.005 | 0.001 | <0.001 | |
| Subgroups of Cases | ||||||
|---|---|---|---|---|---|---|
| 1–4 NMSC | ≥5 NMSC | p Value | 1–9 NMSC | ≥10 NMSC | p Value | |
| n = 107 | n = 87 | n = 150 | n = 44 | |||
| Time spent outdoors with unintentional sun exposure (May–August; hours, mean ± SD) | ||||||
| Outdoor occupation | 4662 ± 8927 | 4402 ± 8178 | 0.834 | 4949 ± 9046 | 3167 ± 6652 | 0.227 |
| Total time spent outside with unintentional sun exposure (UV scenario I + II; total hours, mean ± SD) | 26,082 ± 10,998 | 30,051 ± 17,850 | 0.059 | 27,843 ± 14,225 | 27,927 ± 15,869 | 0.973 |
| Time spent outdoors with intentional sun exposure (May–August; hours, mean ± SD) | ||||||
| Central Europe (UV scenario III) | 5277 ± 3261 | 6940 ± 4010 | 0.002 | 5693 ± 3534 | 7147 ± 4062 | 0.022 |
| Southern geographic regions (UV scenario IV; Mediterranean, subtropical, tropical regions) | 2762 ± 2305 | 3076 ± 2842 | 0.397 | 2658 ± 2287 | 3739 ± 3210 | 0.013 |
| Total time spent outside with intentional sun exposure (UV scenario III + IV; total hours, mean ± SD) | 8040 ± 4040 | 10,017 ± 5464 | 0.004 | 8351 ± 4296 | 10,886 ± 5938 | 0.002 |
| Gardening (hours, mean ± SD) | 4076 ± 4810 | 4386 ± 4467 | 0.701 | 3963 ± 4487 | 5161 ± 5240 | 0.225 |
| Holiday activities (weeks, mean ± SD) | ||||||
| Body covered (mountaineering, hiking, skiing) | 69 ± 107 | 59 ± 73 | 0.472 | 65 ± 97 | 62 ± 78 | 0.808 |
| Wearing swimwear (watersports, sunbathing) | 44 ± 76 | 39 ± 61 | 0.608 | 42 ± 71 | 41 ± 67 | 0.899 |
| Full body uncovered (nudist beach; weeks, mean ± SD) | 3 ± 13 | 8 ± 31 | 0.131 | 5 ± 24 | 6 ± 21 | 0.830 |
| Skin Aging Whole Study Population | NMSC Development Cases Versus Controls | NMSC Numbers Cases | |
|---|---|---|---|
| Demographic data | >60 years Smoking history Male gender | Longer post-TX period Shorter interval from TX to first NMSC Older age at examination | |
| Skin type, hair, and eye color | FST II, lighter hair, and blue and green eyes # | Lighter eye color | |
| Skin aging | + | High aging scores | |
| UVR | Intentional and unintentional high UVR exposure | Vacation-related high intensity UVR * | Sunburns with blistering at age 20–39 Outdoor occupation: cSCC in the head/neck region BD on the lower extremities |
| MC1R | Specific MC1R variants | Specific MC1R variants | |
| Actinic keratosis | AK | AK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borik-Heil, L.; Endler, G.; Parson, W.; Zuckermann, A.; Schnaller, L.; Uyanik-Ünal, K.; Jaksch, P.; Böhmig, G.; Cejka, D.; Staufer, K.; et al. Cumulative UV Exposure or a Modified SCINEXA™-Skin Aging Score Do Not Play a Substantial Role in Predicting the Risk of Developing Keratinocyte Cancers after Solid Organ Transplantation—A Case Control Study. Cancers 2023, 15, 864. https://doi.org/10.3390/cancers15030864
Borik-Heil L, Endler G, Parson W, Zuckermann A, Schnaller L, Uyanik-Ünal K, Jaksch P, Böhmig G, Cejka D, Staufer K, et al. Cumulative UV Exposure or a Modified SCINEXA™-Skin Aging Score Do Not Play a Substantial Role in Predicting the Risk of Developing Keratinocyte Cancers after Solid Organ Transplantation—A Case Control Study. Cancers. 2023; 15(3):864. https://doi.org/10.3390/cancers15030864
Chicago/Turabian StyleBorik-Heil, Liliane, Georg Endler, Walther Parson, Andreas Zuckermann, Lisa Schnaller, Keziban Uyanik-Ünal, Peter Jaksch, Georg Böhmig, Daniel Cejka, Katharina Staufer, and et al. 2023. "Cumulative UV Exposure or a Modified SCINEXA™-Skin Aging Score Do Not Play a Substantial Role in Predicting the Risk of Developing Keratinocyte Cancers after Solid Organ Transplantation—A Case Control Study" Cancers 15, no. 3: 864. https://doi.org/10.3390/cancers15030864
APA StyleBorik-Heil, L., Endler, G., Parson, W., Zuckermann, A., Schnaller, L., Uyanik-Ünal, K., Jaksch, P., Böhmig, G., Cejka, D., Staufer, K., Hielle-Wittmann, E., Rasoul-Rockenschaub, S., Wolf, P., Sunder-Plassmann, R., & Geusau, A. (2023). Cumulative UV Exposure or a Modified SCINEXA™-Skin Aging Score Do Not Play a Substantial Role in Predicting the Risk of Developing Keratinocyte Cancers after Solid Organ Transplantation—A Case Control Study. Cancers, 15(3), 864. https://doi.org/10.3390/cancers15030864








