Targeting Sphingosine 1-Phosphate Metabolism as a Therapeutic Avenue for Prostate Cancer
Abstract
Simple Summary
Abstract
1. Prostate Cancer Disease: An Overview
1.1. Epidemiology of Prostate Cancer
1.2. The Cellular and Molecular Mechanisms in Prostate Cancer: Bone Metastasis
1.2.1. Preparation of the Metastatic Niche
1.2.2. Metastatic Homing
1.2.3. Bone Colonization
1.3. Extracellular Vesicles: Crucial Messengers in Prostate Cancer–Osteoblast Crosstalk
1.4. Summary of Treatments in Prostate Cancer
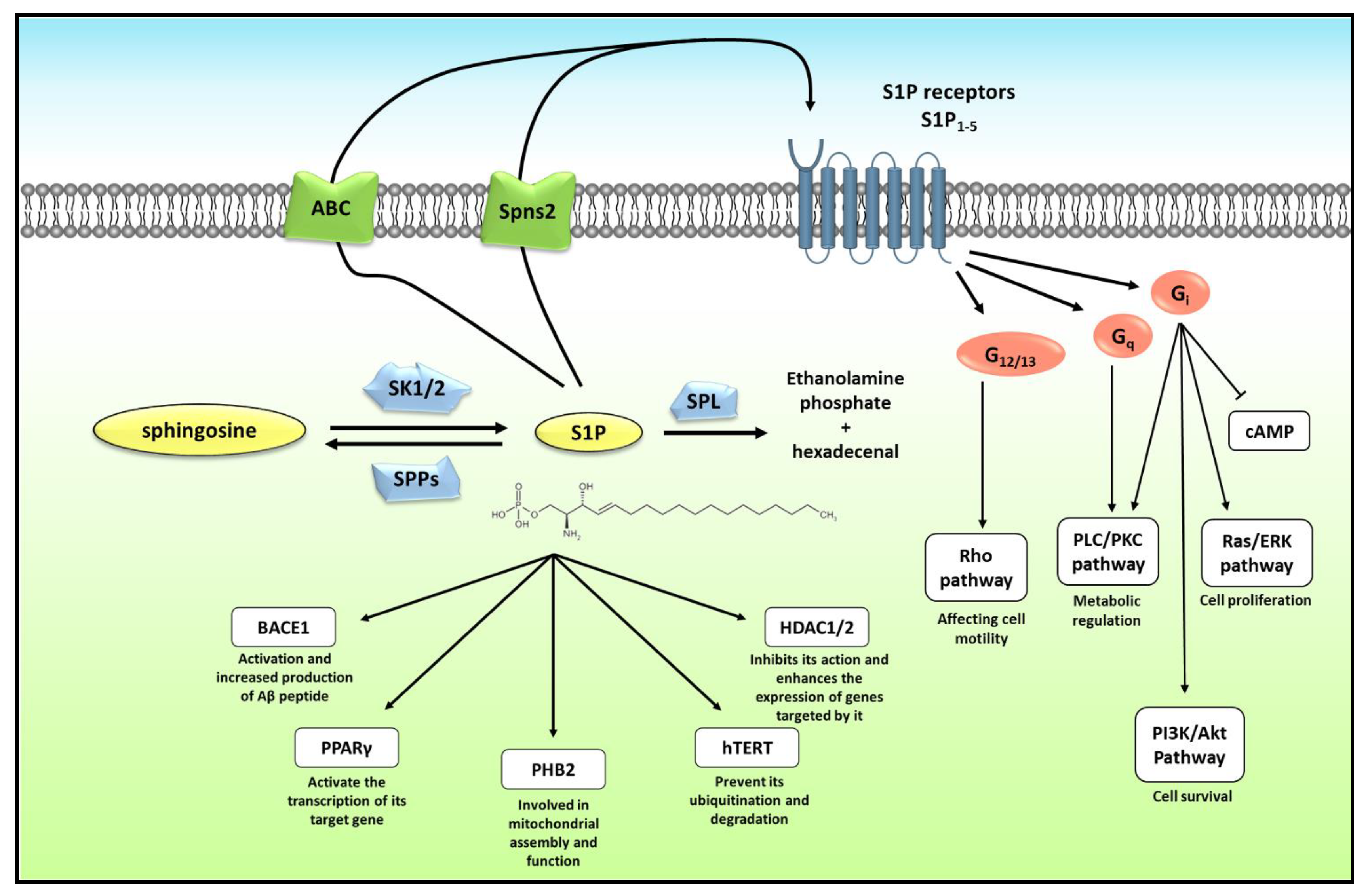
2. Sphingosine 1-Phosphate Metabolism and Mode of Action
3. Role of Sphingosine 1-Phosphate Metabolism in Prostate Cancer
3.1. Role in Cell Death and Apoptosis
3.2. Role in Cell Viability and Proliferation
3.3. Role in Hypoxia
3.4. Role in Radiosensitivity
3.5. Role in Chemosensitivity
3.6. Role in Metastasis
3.7. Role as Biomarker of Disease
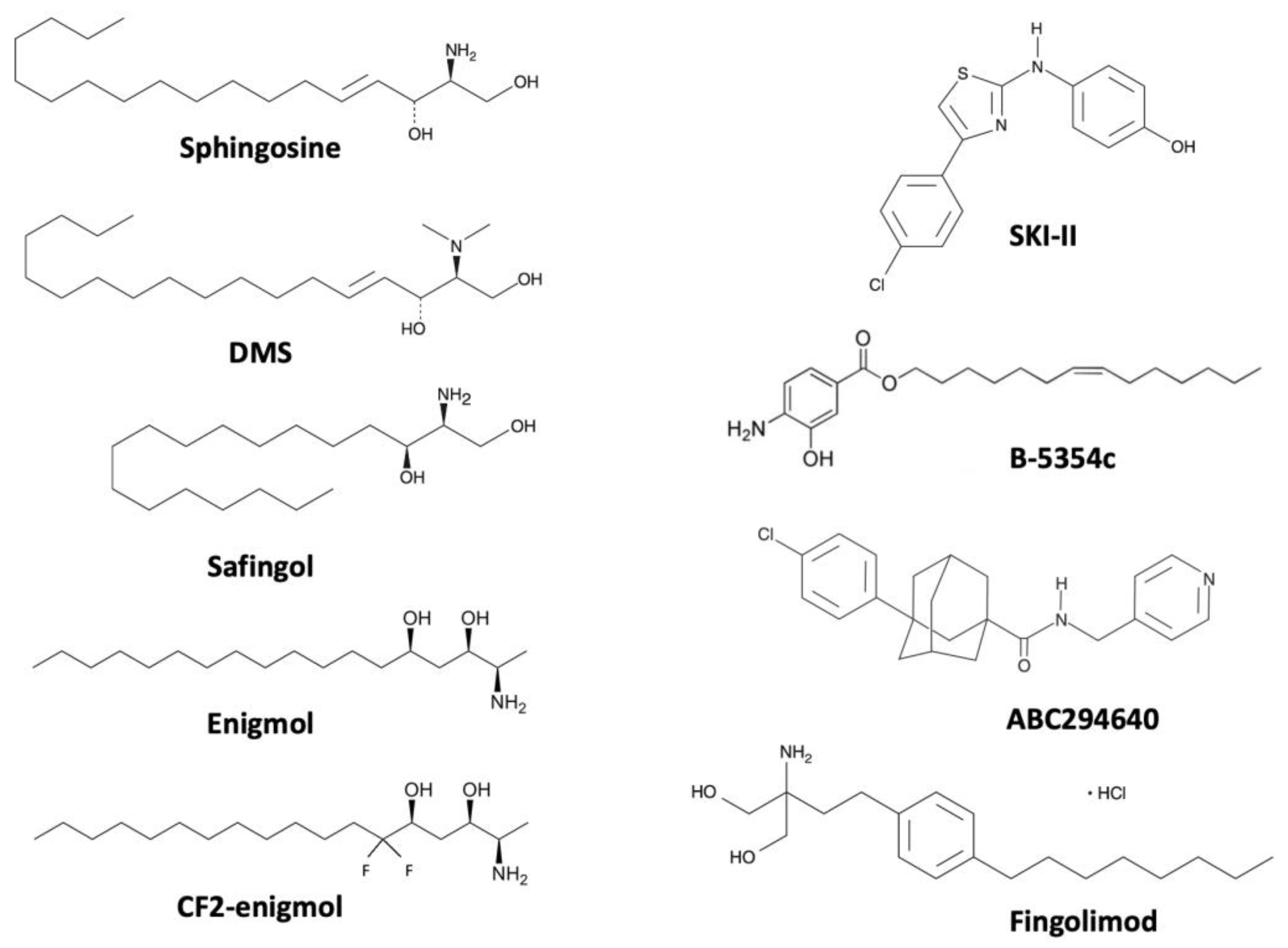
4. Targeting SK/S1P Metabolism as a Therapeutic Option for Prostate Cancer
4.1. N,N-Dimethylsphingosine (DMS)
4.2. Safingol
4.3. Enigmol
4.4. SKI-II
4.5. ABC294640
4.6. Fingolimod
4.7. Sphingomab/Sonepcizumab
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gandaglia, G.; Leni, R.; Bray, F.; Fleshner, N.; Freedland, S.J.; Kibel, A.; Stattin, P.; Van Poppel, H.; La Vecchia, C. Epidemiology and Prevention of Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Saini, S. PSA and beyond: Alternative prostate cancer biomarkers. Cell Oncol. 2016, 39, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Gourdin, T.; Velayati, A. Treatments and challenges in advanced prostate cancer. Curr. Opin. Oncol. 2023, 35, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate cancer. Nat. Rev. Dis. Primers 2021, 7, 9. [Google Scholar] [CrossRef]
- Tsuchiya, N.; Wang, L.; Suzuki, H.; Segawa, T.; Fukuda, H.; Narita, S.; Shimbo, M.; Kamoto, T.; Mitsumori, K.; Ichikawa, T.; et al. Impact of IGF-I and CYP19 gene polymorphisms on the survival of patients with metastatic prostate cancer. J Clin. Oncol. 2006, 24, 1982–1989. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.D.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Owen, K.L.; Parker, B.S. Beyond the vicious cycle: The role of innate osteoimmunity, automimicry and tumor-inherent changes in dictating bone metastasis. Mol. Immunol. 2019, 110, 57–68. [Google Scholar] [CrossRef]
- Lo Bianco, G.; Lanza, E.; Provenzano, S.; Federico, M.; Papa, A.; Imani, F.; Shirkhany, G.; Laudicella, R.; Quartuccio, N. A Multimodal Clinical Approach for the Treatment of Bone Metastases in Solid Tumors. Anesth. Pain Med. 2022, 12, e126333. [Google Scholar] [CrossRef]
- Jiang, J.; Li, J.; Zhou, X.; Zhao, X.; Huang, B.; Qin, Y. Exosomes Regulate the Epithelial–Mesenchymal Transition in Cancer. Front. Oncol. 2022, 12, 864980. [Google Scholar] [CrossRef]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef]
- Kang, J.; La Manna, F.; Bonollo, F.; Sampson, N.; Alberts, I.L.; Mingels, C.; Afshar-Oromieh, A.; Thalmann, G.N.; Karkampouna, S. Tumor microenvironment mechanisms and bone metastatic disease progression of prostate cancer. Cancer Lett. 2022, 530, 156–169. [Google Scholar] [CrossRef]
- Baldessari, C.; Pipitone, S.; Molinaro, E.; Cerma, K.; Fanelli, M.; Nasso, C.; Oltrecolli, M.; Pirola, M.; D’Agostino, E.; Pugliese, G.; et al. Bone Metastases and Health in Prostate Cancer: From Pathophysiology to Clinical Implications. Cancers 2023, 15, 1518. [Google Scholar] [CrossRef]
- Sowder, M.E.; Johnson, R.W. Bone as a Preferential Site for Metastasis. JBMR Plus 2019, 3, e10126. [Google Scholar] [CrossRef]
- Kan, C.; Vargas, G.; Pape, F.L.; Clézardin, P. Cancer Cell Colonisation in the Bone Microenvironment. Int. J. Mol. Sci. 2016, 17, 1674. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Ye, Y.; Li, S.-L.; Ma, Y.-Y.; Diao, Y.-J.; Yang, L.; Su, M.-Q.; Li, Z.; Ji, Y.; Wang, J.; Lei, L.; et al. Exosomal miR-141-3p regulates osteoblast activity to promote the osteoblastic metastasis of prostate cancer. Oncotarget 2017, 8, 94834–94849. [Google Scholar] [CrossRef]
- Lu, Z.; Hou, J.; Li, X.; Zhou, J.; Luo, B.; Liang, S.; Lo, R.K.; Wong, T.M.; Kuang, G.-M. Exosome-Derived miRNAs as Potential Biomarkers for Prostate Bone Metastasis. Int. J. Gen. Med. 2022, 15, 5369–5383. [Google Scholar] [CrossRef]
- Akoto, T.; Saini, S. Role of Exosomes in Prostate Cancer Metastasis. Int. J. Mol. Sci. 2021, 22, 3528. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.-Y.; Gleave, M.E.; Beltran, H. Towards precision oncology in advanced prostate cancer. Nat. Rev. Urol. 2019, 16, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.G.; Oh, W.K. The evolving landscape of immunotherapy in advanced prostate cancer. Immunotherapy 2019, 11, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.A.; Arora, V.K.; Sawyers, C.L. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat. Rev. Cancer 2015, 15, 701–711. [Google Scholar] [CrossRef]
- Marchetti, A.; Tassinari, E.; Rosellini, M.; Rizzo, A.; Massari, F.; Mollica, V. Prostate cancer and novel pharmacological treatment options-what’s new for 2022? Expert Rev. Clin. Pharmacol. 2023, 16, 231–244. [Google Scholar] [CrossRef]
- Hatano, K.; Nonomura, N. Systemic Therapies for Metastatic Castration-Resistant Prostate Cancer: An Updated Review. World J. Mens. Health 2023, 41, e27. [Google Scholar] [CrossRef]
- Hashemi, M.; Zandieh, M.A.; Talebi, Y.; Rahmanian, P.; Shafiee, S.S.; Nejad, M.M.; Babaei, R.; Sadi, F.H.; Rajabi, R.; Abkenar, Z.O.; et al. Paclitaxel and docetaxel resistance in prostate cancer: Molecular mechanisms and possible therapeutic strategies. Biomed. Pharm. 2023, 160, 114392. [Google Scholar] [CrossRef]
- Rastogi, I.; Muralidhar, A.; McNeel, D.G. Vaccines as treatments for prostate cancer. Nat. Rev. Urol. 2023, 1–16. [Google Scholar] [CrossRef]
- Pulkoski-Gross, M.J.; Donaldson, J.C.; Obeid, L.M. Sphingosine-1-phosphate metabolism: A structural perspective. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 298–313. [Google Scholar] [CrossRef]
- Companioni, O.; Mir, C.; Garcia-Mayea, Y.; LLeonart, M.E. Targeting Sphingolipids for Cancer Therapy. Front. Oncol. 2021, 11, 745092. Available online: https://www.frontiersin.org/articles/10.3389/fonc.2021.745092 (accessed on 9 May 2023). [CrossRef]
- Xiong, Y.; Yang, P.; Proia, R.L.; Hla, T. Erythrocyte-derived sphingosine 1-phosphate is essential for vascular development. J. Clin. Investig. 2014, 124, 4823–4828. [Google Scholar] [CrossRef]
- Ishii, M.; Kikuta, J. Sphingosine-1-phosphate signaling controlling osteoclasts and bone homeostasis. Biochim. Biophys. Acta 2013, 1831, 223–227. [Google Scholar] [CrossRef]
- Mizugishi, K.; Li, C.; Olivera, A.; Bielawski, J.; Bielawska, A.; Deng, C.-X.; Proia, R.L. Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J. Clin. Investig. 2007, 117, 2993–3006. [Google Scholar] [CrossRef]
- Green, C.D.; Maceyka, M.; Cowart, L.A.; Spiegel, S. Sphingolipids in metabolic disease: The good, the bad, and the unknown. Cell Metab. 2021, 33, 1293–1306. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- van Echten-Deckert, G. The role of sphingosine 1-phosphate metabolism in brain health and disease. Pharmacol. Ther. 2023, 244, 108381. [Google Scholar] [CrossRef]
- Pyne, S.; Adams, D.R.; Pyne, N.J. Sphingosine 1-phosphate and sphingosine kinases in health and disease: Recent advances. Prog. Lipid. Res. 2016, 62, 93–106. [Google Scholar] [CrossRef]
- Brunkhorst, R.; Vutukuri, R.; Pfeilschifter, W. Fingolimod for the treatment of neurological diseases-state of play and future perspectives. Front. Cell Neurosci. 2014, 8, 283. [Google Scholar] [CrossRef]
- Nagahashi, M.; Abe, M.; Sakimura, K.; Takabe, K.; Wakai, T. The role of sphingosine-1-phosphate in inflammation and cancer progression. Cancer Sci. 2018, 109, 3671–3678. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Watson, D.G.; Tonelli, F.; Alossaimi, M.; Williamson, L.; Chan, E.; Gorshkova, I.; Berdyshev, E.; Bittman, R.; Pyne, N.J.; Pyne, S. The roles of sphingosine kinases 1 and 2 in regulating the Warburg effect in prostate cancer cells. Cell Signal. 2013, 25, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Mitra, P.; Oskeritzian, C.A.; Payne, S.G.; Beaven, M.A.; Milstien, S.; Spiegel, S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc. Natl. Acad. Sci. USA 2006, 103, 16394–16399. [Google Scholar] [CrossRef] [PubMed]
- Takabe, K.; Kim, R.H.; Allegood, J.C.; Mitra, P.; Ramachandran, S.; Nagahashi, M.; Harikumar, K.B.; Hait, N.C.; Milstien, S.; Spiegel, S. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J. Biol. Chem. 2010, 285, 10477–10486. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, A.; Nishi, T.; Hisano, Y.; Fukui, H.; Yamaguchi, A.; Mochizuki, N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science 2009, 323, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Sensken, S.-C.; Peest, U.; Beutel, G.; Thol, F.; Levkau, B.; Li, Z.; Bittman, R.; Huang, T.; Tölle, M.; et al. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. J. Cell Biochem. 2010, 109, 1232–1243. [Google Scholar] [CrossRef]
- Nakajima, M.; Nagahashi, M.; Rashid, O.M.; Takabe, K.; Wakai, T. The role of sphingosine-1-phosphate in the tumor microenvironment and its clinical implications. Tumour. Biol. 2017, 39. [Google Scholar] [CrossRef]
- Kawabori, M.; Kacimi, R.; Karliner, J.S.; Yenari, M.A. Sphingolipids in cardiovascular and cerebrovascular systems: Pathological implications and potential therapeutic targets. World J. Cardiol. 2013, 5, 75–86. [Google Scholar] [CrossRef]
- Takasugi, N.; Sasaki, T.; Suzuki, K.; Osawa, S.; Isshiki, H.; Hori, Y.; Shimada, N.; Higo, T.; Yokoshima, S.; Fukuyama, T.; et al. BACE1 activity is modulated by cell-associated sphingosine-1-phosphate. J. Neurosci. 2011, 31, 6850–6857. [Google Scholar] [CrossRef]
- Parham, K.A.; Zebol, J.R.; Tooley, K.L.; Sun, W.Y.; Moldenhauer, L.M.; Cockshell, M.P.; Gliddon, B.L.; Moretti, P.A.; Tigyi, G.; Pitson, S.M.; et al. Sphingosine 1-phosphate is a ligand for peroxisome proliferator-activated receptor-γ that regulates neoangiogenesis. FASEB J. 2015, 29, 3638–3653. [Google Scholar] [CrossRef]
- Strub, G.M.; Paillard, M.; Liang, J.; Gomez, L.; Allegood, J.C.; Hait, N.C.; Maceyka, M.; Price, M.M.; Chen, Q.; Simpson, D.C.; et al. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2011, 25, 600–612. [Google Scholar] [CrossRef]
- Panneer Selvam, S.; De Palma, R.M.; Oaks, J.J.; Oleinik, N.; Peterson, Y.K.; Stahelin, R.V.; Skordalakes, E.; Ponnusamy, S.; Garrett-Mayer, E.; Smith, C.D.; et al. Binding of the sphingolipid S1P to hTERT stabilizes telomerase at the nuclear periphery by allosterically mimicking protein phosphorylation. Sci. Signal. 2015, 8, ra58. [Google Scholar] [CrossRef]
- Hait, N.C.; Allegood, J.; Maceyka, M.; Strub, G.M.; Harikumar, K.B.; Singh, S.K.; Luo, C.; Marmorstein, R.; Kordula, T.; Milstien, S.; et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 2009, 325, 1254–1257. [Google Scholar] [CrossRef]
- Takabe, K.; Paugh, S.W.; Milstien, S.; Spiegel, S. “Inside-out” signaling of sphingosine-1-phosphate: Therapeutic targets. Pharmacol. Rev. 2008, 60, 181–195. [Google Scholar] [CrossRef]
- Cuvillier, O.; Pirianov, G.; Kleuser, B.; Vanek, P.G.; Coso, O.A.; Gutkind, S.; Spiegel, S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 1996, 381, 800–803. [Google Scholar] [CrossRef]
- Shirahama, T.; Sakakura, C.; Sweeney, E.A.; Ozawa, M.; Takemoto, M.; Nishiyama, K.; Ohi, Y.; Igarashi, Y. Sphingosine induces apoptosis in androgen-independent human prostatic carcinoma DU-145 cells by suppression of bcl-X(L) gene expression. FEBS Lett. 1997, 407, 97–100. [Google Scholar] [CrossRef]
- Herrmann, J.L.; Menter, D.G.; Beham, A.; von Eschenbach, A.; McDonnell, T.J. Regulation of lipid signaling pathways for cell survival and apoptosis by bcl-2 in prostate carcinoma cells. Exp. Cell Res. 1997, 234, 442–451. [Google Scholar] [CrossRef]
- Eto, M.; Bennouna, J.; Hunter, O.C.; Lotze, M.T.; Amoscato, A.A. Importance of C16 ceramide accumulation during apoptosis in prostate cancer cells. Int. J. Urol. 2006, 13, 148–156. [Google Scholar] [CrossRef]
- Leroux, M.E.; Auzenne, E.; Evans, R.; Hail, N.; Spohn, W.; Ghosh, S.C.; Farquhar, D.; McDonnell, T.; Klostergaard, J. Sphingolipids and the sphingosine kinase inhibitor, SKI II, induce BCL-2-independent apoptosis in human prostatic adenocarcinoma cells. Prostate 2007, 67, 1699–1717. [Google Scholar] [CrossRef]
- Pchejetski, D.; Golzio, M.; Bonhoure, E.; Calvet, C.; Doumerc, N.; Garcia, V.; Mazerolles, C.; Rischmann, P.; Teissié, J.; Malavaud, B.; et al. Sphingosine kinase-1 as a chemotherapy sensor in prostate adenocarcinoma cell and mouse models. Cancer Res. 2005, 65, 11667–11675. [Google Scholar] [CrossRef]
- Kono, K.; Tanaka, M.; Ogita, T.; Kohama, T. Characterization of B-5354c, a new sphingosine kinase inhibitor, produced by a marine bacterium. J. Antibiot. 2000, 53, 759–764. [Google Scholar] [CrossRef]
- Pchejetski, D.; Doumerc, N.; Golzio, M.; Naymark, M.; Teissié, J.; Kohama, T.; Waxman, J.; Malavaud, B.; Cuvillier, O. Chemosensitizing effects of sphingosine kinase-1 inhibition in prostate cancer cell and animal models. Mol. Cancer Ther. 2008, 7, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.D.; Takahara, S.; Nonomura, N.; Ichimaru, N.; Toki, K.; Azuma, H.; Matsumiya, K.; Okuyama, A.; Suzuki, S. Early induction of apoptosis in androgen-independent prostate cancer cell line by FTY720 requires caspase-3 activation. Prostate 1999, 40, 50–55. [Google Scholar] [CrossRef]
- Permpongkosol, S.; Wang, J.-D.; Takahara, S.; Matsumiya, K.; Nonomura, N.; Nishimura, K.; Tsujimura, A.; Kongkanand, A.; Okuyama, A. Anticarcinogenic effect of FTY720 in human prostate carcinoma DU145 cells: Modulation of mitogenic signaling, FAK, cell-cycle entry and apoptosis. Int. J. Cancer 2002, 98, 167–172. [Google Scholar] [CrossRef]
- Pchejetski, D.; Bohler, T.; Brizuela, L.; Sauer, L.; Doumerc, N.; Golzio, M.; Salunkhe, V.; Teissié, J.; Malavaud, B.; Waxman, J.; et al. FTY720 (fingolimod) sensitizes prostate cancer cells to radiotherapy by inhibition of sphingosine kinase-1. Cancer Res. 2010, 70, 8651–8661. [Google Scholar] [CrossRef] [PubMed]
- Leu, W.-J.; Swain, S.P.; Chan, S.-H.; Hsu, J.-L.; Liu, S.-P.; Chan, M.-L.; Yu, C.-C.; Hsu, L.-C.; Chou, Y.-L.; Chang, W.-L.; et al. Non-immunosuppressive triazole-based small molecule induces anticancer activity against human hormone-refractory prostate cancers: The role in inhibition of PI3K/AKT/mTOR and c-Myc signaling pathways. Oncotarget 2016, 7, 76995–77009. [Google Scholar] [CrossRef]
- Akao, Y.; Banno, Y.; Nakagawa, Y.; Hasegawa, N.; Kim, T.-J.; Murate, T.; Igarashi, Y.; Nozawa, Y. High expression of sphingosine kinase 1 and S1P receptors in chemotherapy-resistant prostate cancer PC3 cells and their camptothecin-induced up-regulation. Biochem. Biophys. Res. Commun. 2006, 342, 1284–1290. [Google Scholar] [CrossRef]
- Gibbs, T.C.; Rubio, M.V.; Zhang, Z.; Xie, Y.; Kipp, K.R.; Meier, K.E. Signal transduction responses to lysophosphatidic acid and sphingosine 1-phosphate in human prostate cancer cells: Responses to LPA and S1P in Human Prostate Cancer Cells. Prostate 2009, 69, 1493–1506. [Google Scholar] [CrossRef]
- Andrieu, G.; Ledoux, A.; Branka, S.; Bocquet, M.; Gilhodes, J.; Walzer, T.; Kasahara, K.; Inagaki, M.; Sabbadini, R.A.; Cuvillier, O.; et al. Sphingosine 1-phosphate signaling through its receptor S1P5 promotes chromosome segregation and mitotic progression. Sci. Signal 2017, 10, eaah4007. [Google Scholar] [CrossRef]
- Chang, C.-L.; Ho, M.-C.; Lee, P.-H.; Hsu, C.-Y.; Huang, W.-P.; Lee, H. S1P 5 is required for sphingosine 1-phosphate-induced autophagy in human prostate cancer PC-3 cells. Am. J. Physiol.-Cell Physiol. 2009, 297, C451–C458. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Chang, C.-L.; Tang, C.-H.; Lin, Y.-C.; Ju, T.-K.; Huang, W.-P.; Lee, H. Extrinsic sphingosine 1-phosphate activates S1P5 and induces autophagy through generating endoplasmic reticulum stress in human prostate cancer PC-3 cells. Cell Signal. 2014, 26, 611–618. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Guo, K.; Feng, Y.; Zheng, X.; Sun, L.; Wasan, H.S.; Ruan, S.; Shen, M. Resveratrol and Its Analogs: Potent Agents to Reverse Epithelial-to-Mesenchymal Transition in Tumors. Front. Oncol. 2021, 11, 644134. [Google Scholar] [CrossRef]
- Sufianova, G.; Gareev, I.; Beylerli, O.; Wu, J.; Shumadalova, A.; Sufianov, A.; Chen, X.; Zhao, S. Modern aspects of the use of natural polyphenols in tumor prevention and therapy. Front. Cell Dev. Biol. 2022, 10, 1011435. [Google Scholar] [CrossRef]
- Brizuela, L.; Dayon, A.; Doumerc, N.; Ader, I.; Golzio, M.; Izard, J.-C.; Hara, Y.; Malavaud, B.; Cuvillier, O. The sphingosine kinase-1 survival pathway is a molecular target for the tumor-suppressive tea and wine polyphenols in prostate cancer. FASEB J. 2010, 24, 3882–3894. [Google Scholar] [CrossRef]
- Gestaut, M.M.; Antoon, J.W.; Burow, M.E.; Beckman, B.S. Inhibition of sphingosine kinase-2 ablates androgen resistant prostate cancer proliferation and survival. Pharmacol. Rep. 2014, 66, 174–178. [Google Scholar] [CrossRef]
- Schrecengost, R.S.; Keller, S.N.; Schiewer, M.J.; Knudsen, K.E.; Smith, C.D. Downregulation of Critical Oncogenes by the Selective SK2 Inhibitor ABC294640 Hinders Prostate Cancer Progression. Mol. Cancer Res. 2015, 13, 1591–1601. [Google Scholar] [CrossRef]
- Venant, H.; Rahmaniyan, M.; Jones, E.E.; Lu, P.; Lilly, M.B.; Garrett-Mayer, E.; Drake, R.R.; Kraveka, J.M.; Smith, C.D.; Voelkel-Johnson, C. The Sphingosine Kinase 2 Inhibitor ABC294640 Reduces the Growth of Prostate Cancer Cells and Results in Accumulation of Dihydroceramides In Vitro and In Vivo. Mol. Cancer Ther. 2015, 14, 2744–2752. [Google Scholar] [CrossRef]
- Allam, R.M.; Al-Abd, A.M.; Khedr, A.; Sharaf, O.A.; Nofal, S.M.; Khalifa, A.E.; Mosli, H.A.; Abdel-Naim, A.B. Fingolimod interrupts the cross talk between estrogen metabolism and sphingolipid metabolism within prostate cancer cells. Toxicol. Lett. 2018, 291, 77–85. [Google Scholar] [CrossRef]
- Chua, C.-W.; Lee, D.T.-W.; Ling, M.-T.; Zhou, C.; Man, K.; Ho, J.; Chan, F.L.; Wang, X.; Wong, Y.-C. FTY720, a fungus metabolite, inhibits in vivo growth of androgen-independent prostate cancer. Int. J. Cancer 2005, 117, 1039–1048. [Google Scholar] [CrossRef]
- Cristóbal, I.; González-Alonso, P.; Daoud, L.; Solano, E.; Torrejón, B.; Manso, R.; Madoz-Gúrpide, J.; Rojo, F.; García-Foncillas, J. Activation of the Tumor Suppressor PP2A Emerges as a Potential Therapeutic Strategy for Treating Prostate Cancer. Mar. Drugs 2015, 13, 3276–3286. [Google Scholar] [CrossRef]
- Zhou, C.; Ling, M.-T.; Kin-Wah Lee, T.; Man, K.; Wang, X.; Wong, Y.-C. FTY720, a fungus metabolite, inhibits invasion ability of androgen-independent prostate cancer cells through inactivation of RhoA-GTPase. Cancer Lett. 2006, 233, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Nagabhushan, M.; Miller, C.M.; Pretlow, T.P.; Giaconia, J.M.; Edgehouse, N.L.; Schwartz, S.; Kung, H.J.; de Vere White, R.W.; Gumerlock, P.H.; Resnick, M.I.; et al. CWR22: The first human prostate cancer xenograft with strongly androgen-dependent and relapsed strains both in vivo and in soft agar. Cancer Res. 1996, 56, 3042–3046. [Google Scholar] [PubMed]
- Dayon, A.; Brizuela, L.; Martin, C.; Mazerolles, C.; Pirot, N.; Doumerc, N.; Nogueira, L.; Golzio, M.; Teissié, J.; Serre, G.; et al. Sphingosine kinase-1 is central to androgen-regulated prostate cancer growth and survival. PLoS ONE 2009, 4, e8048. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, F.; Alossaimi, M.; Williamson, L.; Tate, R.J.; Watson, D.G.; Chan, E.; Bittman, R.; Pyne, N.J.; Pyne, S. The sphingosine kinase inhibitor 2-(p-hyroxyanilino)-4-(p-chlorophenyl)thiazole reduces androgen receptor expression via an oxidative stress-dependent mechanism. Br. J. Pharmacol. 2013, 168, 1497–1505. [Google Scholar] [CrossRef]
- Lin, H.-M.; Mak, B.; Yeung, N.; Huynh, K.; Meikle, T.G.; Mellett, N.A.; Kwan, E.M.; Fettke, H.; Tran, B.; Davis, I.D.; et al. Overcoming enzalutamide resistance in metastatic prostate cancer by targeting sphingosine kinase. EBioMedicine 2021, 72, 103625. [Google Scholar] [CrossRef]
- Lee, C.-F.; Chen, Y.-A.; Hernandez, E.; Pong, R.-C.; Ma, S.; Hofstad, M.; Kapur, P.; Zhau, H.; Chung, L.W.; Lai, C.-H.; et al. The central role of Sphingosine kinase 1 in the development of neuroendocrine prostate cancer (NEPC): A new targeted therapy of NEPC. Clin. Transl. Med. 2022, 12, e695. [Google Scholar] [CrossRef]
- Ader, I.; Brizuela, L.; Bouquerel, P.; Malavaud, B.; Cuvillier, O. Sphingosine Kinase 1: A New Modulator of Hypoxia Inducible Factor 1α during Hypoxia in Human Cancer Cells. Cancer Res. 2008, 68, 8635–8642. [Google Scholar] [CrossRef]
- Anelli, V.; Gault, C.R.; Cheng, A.B.; Obeid, L.M. Sphingosine kinase 1 is up-regulated during hypoxia in U87MG glioma cells. Role of hypoxia-inducible factors 1 and 2. J. Biol. Chem. 2008, 283, 3365–3375. [Google Scholar] [CrossRef]
- Bouquerel, P.; Gstalder, C.; Müller, D.; Laurent, J.; Brizuela, L.; Sabbadini, R.A.; Malavaud, B.; Pyronnet, S.; Martineau, Y.; Ader, I.; et al. Essential role for SphK1/S1P signaling to regulate hypoxia-inducible factor 2α expression and activity in cancer. Oncogenesis 2016, 5, e209. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Lee, H.-J.; Jeong, S.-J.; Lee, H.-J.; Kim, H.-S.; Chen, C.Y.; Lee, E.-O.; Kim, S.-H. Sphingosine kinase 1 pathway is involved in melatonin-induced HIF-1α inactivation in hypoxic PC-3 prostate cancer cells. J. Pineal. Res. 2011, 51, 87–93. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Cho, S.; Park, E.; Kim, B.; Sohn, E.J.; Oh, B.; Lee, E.-O.; Lee, H.-J.; Kim, S.-H. Coumestrol suppresses hypoxia inducible factor 1α by inhibiting ROS mediated sphingosine kinase 1 in hypoxic PC-3 prostate cancer cells. Bioorg. Med. Chem. Lett. 2014, 24, 2560–2564. [Google Scholar] [CrossRef]
- Lee, S.-O.; Kim, J.-S.; Lee, M.-S.; Lee, H.-J. Anti-cancer effect of pristimerin by inhibition of HIF-1α involves the SPHK-1 pathway in hypoxic prostate cancer cells. BMC Cancer 2016, 16, 701. [Google Scholar] [CrossRef]
- Lee, M.-S.; Lee, S.-O.; Kim, K.-R.; Lee, H.-J. Sphingosine Kinase-1 Involves the Inhibitory Action of HIF-1α by Chlorogenic Acid in Hypoxic DU145 Cells. Int. J. Mol. Sci. 2017, 18, 325. [Google Scholar] [CrossRef]
- Ader, I.; Gstalder, C.; Bouquerel, P.; Golzio, M.; Andrieu, G.; Zalvidea, S.; Richard, S.; Sabbadini, R.A.; Malavaud, B.; Cuvillier, O. Neutralizing S1P inhibits intratumoral hypoxia, induces vascular remodelling and sensitizes to chemotherapy in prostate cancer. Oncotarget 2015, 6, 13803–13821. [Google Scholar] [CrossRef]
- Nava, V.E.; Cuvillier, O.; Edsall, L.C.; Kimura, K.; Milstien, S.; Gelmann, E.P.; Spiegel, S. Sphingosine enhances apoptosis of radiation-resistant prostate cancer cells. Cancer Res. 2000, 60, 4468–4474. [Google Scholar]
- Kimura, K.; Markowski, M.; Edsall, L.C.; Spiegel, S.; Gelmann, E.P. Role of ceramide in mediating apoptosis of irradiated LNCaP prostate cancer cells. Cell Death Differ. 2003, 10, 240–248. [Google Scholar] [CrossRef]
- Brizuela, L.; Ader, I.; Mazerolles, C.; Bocquet, M.; Malavaud, B.; Cuvillier, O. First evidence of sphingosine 1-phosphate lyase protein expression and activity downregulation in human neoplasm: Implication for resistance to therapeutics in prostate cancer. Mol. Cancer Ther. 2012, 11, 1841–1851. [Google Scholar] [CrossRef]
- Sauer, L.; Nunes, J.; Salunkhe, V.; Skalska, L.; Kohama, T.; Cuvillier, O.; Waxman, J.; Pchejetski, D. Sphingosine kinase 1 inhibition sensitizes hormone-resistant prostate cancer to docetaxel. Int. J. Cancer 2009, 125, 2728–2736. [Google Scholar] [CrossRef]
- Aoyama, Y.; Sobue, S.; Mizutani, N.; Inoue, C.; Kawamoto, Y.; Nishizawa, Y.; Ichihara, M.; Kyogashima, M.; Suzuki, M.; Nozawa, Y.; et al. Modulation of the sphingolipid rheostat is involved in paclitaxel resistance of the human prostate cancer cell line PC3-PR. Biochem. Biophys. Res. Commun. 2017, 486, 551–557. [Google Scholar] [CrossRef]
- Ponnusamy, S.; Selvam, S.P.; Mehrotra, S.; Kawamori, T.; Snider, A.J.; Obeid, L.M.; Shao, Y.; Sabbadini, R.; Ogretmen, B. Communication between host organism and cancer cells is transduced by systemic sphingosine kinase 1/sphingosine 1-phosphate signalling to regulate tumour metastasis. EMBO Mol. Med. 2012, 4, 761–775. [Google Scholar] [CrossRef]
- El Jamal, A.; Bougault, C.; Mebarek, S.; Magne, D.; Cuvillier, O.; Brizuela, L. The role of sphingosine 1-phosphate metabolism in bone and joint pathologies and ectopic calcification. Bone 2020, 130, 115087. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.-W.; Chiu, Y.-T.; Yuen, H.-F.; Chan, K.-W.; Man, K.; Wang, X.; Ling, M.-T.; Wong, Y.-C. Suppression of androgen-independent prostate cancer cell aggressiveness by FTY720: Validating Runx2 as a potential antimetastatic drug screening platform. Clin. Cancer Res. 2009, 15, 4322–4335. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, L.; Martin, C.; Jeannot, P.; Ader, I.; Gstalder, C.; Andrieu, G.; Bocquet, M.; Laffosse, J.-M.; Gomez-Brouchet, A.; Malavaud, B.; et al. Osteoblast-derived sphingosine 1-phosphate to induce proliferation and confer resistance to therapeutics to bone metastasis-derived prostate cancer cells. Mol. Oncol. 2014, 8, 1181–1195. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-F.; Dang, A.; Hernandez, E.; Pong, R.-C.; Chen, B.; Sonavane, R.; Raj, G.; Kapur, P.; Lin, H.-Y.; Wu, S.-R.; et al. Activation of sphingosine kinase by lipopolysaccharide promotes prostate cancer cell invasion and metastasis via SphK1/S1PR4/matriptase. Oncogene 2019, 38, 5580–5598. [Google Scholar] [CrossRef]
- Fujita, K.; Matsushita, M.; De Velasco, M.A.; Hatano, K.; Minami, T.; Nonomura, N.; Uemura, H. The Gut-Prostate Axis: A New Perspective of Prostate Cancer Biology through the Gut Microbiome. Cancers 2023, 15, 1375. [Google Scholar] [CrossRef]
- Malavaud, B.; Pchejetski, D.; Mazerolles, C.; de Paiva, G.R.; Calvet, C.; Doumerc, N.; Pitson, S.; Rischmann, P.; Cuvillier, O. Sphingosine kinase-1 activity and expression in human prostate cancer resection specimens. Eur. J. Cancer 2010, 46, 3417–3424. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Haddadi, N.; Zhu, X.; Hatoum, D.; Chen, S.; Nassif, N.T.; Lin, Y.; McGowan, E.M. Expression Profile of Sphingosine Kinase 1 Isoforms in Human Cancer Tissues and Cells: Importance and Clinical Relevance of the Neglected 1b-Isoform. J. Oncol. 2022, 2022, 2250407. [Google Scholar] [CrossRef]
- Nunes, J.; Naymark, M.; Sauer, L.; Muhammad, A.; Keun, H.; Sturge, J.; Stebbing, J.; Waxman, J.; Pchejetski, D. Circulating sphingosine-1-phosphate and erythrocyte sphingosine kinase-1 activity as novel biomarkers for early prostate cancer detection. Br. J. Cancer 2012, 106, 909–915. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Vu, T.M.; Tukijan, F.; Muralidharan, S.; Foo, J.C.; Li Chin, J.F.; Hasan, Z.; Torta, F.; Nguyen, L.N. Erythrocytes efficiently utilize exogenous sphingosines for S1P synthesis and export via Mfsd2b. J. Biol. Chem. 2021, 296, 100201. [Google Scholar] [CrossRef]
- Ohkawa, R.; Nakamura, K.; Okubo, S.; Hosogaya, S.; Ozaki, Y.; Tozuka, M.; Osima, N.; Yokota, H.; Ikeda, H.; Yatomi, Y. Plasma sphingosine-1-phosphate measurement in healthy subjects: Close correlation with red blood cell parameters. Ann. Clin. Biochem. 2008, 45, 356–363. [Google Scholar] [CrossRef]
- Ren, S.; Shao, Y.; Zhao, X.; Hong, C.S.; Wang, F.; Lu, X.; Li, J.; Ye, G.; Yan, M.; Zhuang, Z.; et al. Integration of Metabolomics and Transcriptomics Reveals Major Metabolic Pathways and Potential Biomarker Involved in Prostate Cancer. Mol. Cell Proteom. 2016, 15, 154–163. [Google Scholar] [CrossRef]
- Lin, X.; Shan, S.-K.; Xu, F.; Zhong, J.-Y.; Wu, F.; Duan, J.-Y.; Guo, B.; Li, F.-X.-Z.; Wang, Y.; Zheng, M.-H.; et al. The crosstalk between endothelial cells and vascular smooth muscle cells aggravates high phosphorus-induced arterial calcification. Cell Death Dis. 2022, 13, 650. [Google Scholar] [CrossRef]
- Xu, B.; Chen, Y.; Chen, X.; Gan, L.; Zhang, Y.; Feng, J.; Yu, L. Metabolomics Profiling Discriminates Prostate Cancer From Benign Prostatic Hyperplasia Within the Prostate-Specific Antigen Gray Zone. Front. Oncol. 2021, 11, 730638. [Google Scholar] [CrossRef]
- Edsall, L.C.; Van Brocklyn, J.R.; Cuvillier, O.; Kleuser, B.; Spiegel, S. N,N-Dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: Modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry 1998, 37, 12892–12898. [Google Scholar] [CrossRef]
- Yatomi, Y.; Ruan, F.; Megidish, T.; Toyokuni, T.; Hakomori, S.; Igarashi, Y. N,N-dimethylsphingosine inhibition of sphingosine kinase and sphingosine 1-phosphate activity in human platelets. Biochemistry 1996, 35, 626–633. [Google Scholar] [CrossRef]
- Sweeney, E.A.; Sakakura, C.; Shirahama, T.; Masamune, A.; Ohta, H.; Hakomori, S.; Igarashi, Y. Sphingosine and its methylated derivative N,N-dimethylsphingosine (DMS) induce apoptosis in a variety of human cancer cell lines. Int. J. Cancer 1996, 66, 358–366. [Google Scholar] [CrossRef]
- Endo, K.; Igarashi, Y.; Nisar, M.; Zhou, Q.H.; Hakomori, S. Cell membrane signaling as target in cancer therapy: Inhibitory effect of N,N-dimethyl and N,N,N-trimethyl sphingosine derivatives on in vitro and in vivo growth of human tumor cells in nude mice. Cancer Res. 1991, 51, 1613–1618. [Google Scholar]
- Shirahama, T.; Sweeney, E.A.; Sakakura, C.; Singhal, A.K.; Nishiyama, K.; Akiyama, S.; Hakomori, S.; Igarashi, Y. In vitro and in vivo induction of apoptosis by sphingosine and N, N-dimethylsphingosine in human epidermoid carcinoma KB-3-1 and its multidrug-resistant cells. Clin. Cancer Res. 1997, 3, 257–264. [Google Scholar]
- Buehrer, B.M.; Bell, R.M. Inhibition of sphingosine kinase in vitro and in platelets. Implications for signal transduction pathways. J. Biol. Chem. 1992, 267, 3154–3159. [Google Scholar] [CrossRef]
- Maurer, B.J.; Melton, L.; Billups, C.; Cabot, M.C.; Reynolds, C.P. Synergistic cytotoxicity in solid tumor cell lines between N-(4-hydroxyphenyl)retinamide and modulators of ceramide metabolism. J. Natl. Cancer Inst. 2000, 92, 1897–1909. [Google Scholar] [CrossRef]
- Sosnowski, J.; Stetter-Neel, C.; Cole, D.; Durham, J.P.; Mawhinney, M.G. Protein kinase C mediated anti-proliferative glucocorticoid-sphinganine synergism in cultured Pollard III prostate tumor cells. J. Urol. 1997, 158, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.R.; Dillehay, D.L.; Moody, S.J.; Pallas, D.C.; Pruett, S.; Allgood, J.C.; Symolon, H.; Merrill, A.H. Safingol toxicology after oral administration to TRAMP mice: Demonstration of safingol uptake and metabolism by N-acylation and N-methylation. Drug Chem. Toxicol. 2007, 30, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.K.; Haimovitz-Friedman, A.; Dhupar, S.K.; Ehleiter, D.; Maslak, P.; Lai, L.; Loganzo, F.; Kelsen, D.P.; Fuks, Z.; Albino, A.P. Potentiation of apoptosis by treatment with the protein kinase C-specific inhibitor safingol in mitomycin C-treated gastric cancer cells. J. Natl. Cancer Inst. 1995, 87, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.-U.; Lin, H.; Tan, K.-B.; Chiu, G.N.C. The role of protein kinase C in the synergistic interaction of safingol and irinotecan in colon cancer cells. Int. J. Oncol. 2009, 35, 1463–1471. [Google Scholar] [CrossRef]
- Ling, L.-U.; Tan, K.-B.; Chiu, G.N.C. Role of reactive oxygen species in the synergistic cytotoxicity of safingol-based combination regimens with conventional chemotherapeutics. Oncol. Lett. 2011, 2, 905–910. [Google Scholar] [CrossRef]
- Schwartz, G.K.; Ward, D.; Saltz, L.; Casper, E.S.; Spiess, T.; Mullen, E.; Woodworth, J.; Venuti, R.; Zervos, P.; Storniolo, A.M.; et al. A pilot clinical/pharmacological study of the protein kinase C-specific inhibitor safingol alone and in combination with doxorubicin. Clin. Cancer Res. 1997, 3, 537–543. [Google Scholar]
- Dickson, M.A.; Carvajal, R.D.; Merrill, A.H.; Gonen, M.; Cane, L.M.; Schwartz, G.K. A phase I clinical trial of safingol in combination with cisplatin in advanced solid tumors. Clin. Cancer Res. 2011, 17, 2484–2492. [Google Scholar] [CrossRef]
- Matula, K.; Collie-Duguid, E.; Murray, G.; Parikh, K.; Grabsch, H.; Tan, P.; Lalwani, S.; Garau, R.; Ong, Y.; Bain, G.; et al. Regulation of cellular sphingosine-1-phosphate by sphingosine kinase 1 and sphingosine-1-phopshate lyase determines chemotherapy resistance in gastroesophageal cancer. BMC Cancer 2015, 15, 762. [Google Scholar] [CrossRef]
- Humpf, H.U.; Schmelz, E.M.; Meredith, F.I.; Vesper, H.; Vales, T.R.; Wang, E.; Menaldino, D.S.; Liotta, D.C.; Merrill, A.H. Acylation of naturally occurring and synthetic 1-deoxysphinganines by ceramide synthase. Formation of N-palmitoyl-aminopentol produces a toxic metabolite of hydrolyzed fumonisin, AP1, and a new category of ceramide synthase inhibitor. J. Biol. Chem. 1998, 273, 19060–19064. [Google Scholar] [CrossRef]
- Garnier-Amblard, E.C.; Mays, S.G.; Arrendale, R.F.; Baillie, M.T.; Bushnev, A.S.; Culver, D.G.; Evers, T.J.; Holt, J.J.; Howard, R.B.; Liebeskind, L.S.; et al. Novel synthesis and biological evaluation of enigmols as therapeutic agents for treating prostate cancer. ACS Med. Chem. Lett. 2011, 2, 438–443. [Google Scholar] [CrossRef]
- Symolon, H.; Bushnev, A.; Peng, Q.; Ramaraju, H.; Mays, S.G.; Allegood, J.C.; Pruett, S.T.; Sullards, M.C.; Dillehay, D.L.; Liotta, D.C.; et al. Enigmol: A novel sphingolipid analogue with anticancer activity against cancer cell lines and in vivo models for intestinal and prostate cancer. Mol. Cancer Ther. 2011, 10, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Menaldino, D.S.; Bushnev, A.; Sun, A.; Liotta, D.C.; Symolon, H.; Desai, K.; Dillehay, D.L.; Peng, Q.; Wang, E.; Allegood, J.; et al. Sphingoid bases and de novo ceramide synthesis: Enzymes involved, pharmacology and mechanisms of action. Pharmacol. Res. 2003, 47, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.J.; Mays, S.G.; Baillie, M.T.; Howard, R.B.; Culver, D.G.; Saindane, M.; Pruett, S.T.; Holt, J.J.; Menaldino, D.S.; Evers, T.J.; et al. Discovery of a Fluorinated Enigmol Analog with Enhanced in Vivo Pharmacokinetic and Anti-Tumor Properties. ACS Med. Chem. Lett. 2016, 7, 537–542. [Google Scholar] [CrossRef]
- French, K.J.; Schrecengost, R.S.; Lee, B.D.; Zhuang, Y.; Smith, S.N.; Eberly, J.L.; Yun, J.K.; Smith, C.D. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003, 63, 5962–5969. [Google Scholar] [PubMed]
- French, K.J.; Upson, J.J.; Keller, S.N.; Zhuang, Y.; Yun, J.K.; Smith, C.D. Antitumor activity of sphingosine kinase inhibitors. J. Pharmacol. Exp. Ther. 2006, 318, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, L.; Shi, X.; Song, Y.; Chen, X.-Y.; Chen, M.-B.; Yao, J.; Zhang, Z.-Q.; Cai, S. The sphingosine kinase inhibitor SKI-V suppresses cervical cancer cell growth. Int. J. Biol. Sci. 2022, 18, 2994–3005. [Google Scholar] [CrossRef]
- Loveridge, C.; Tonelli, F.; Leclercq, T.; Lim, K.G.; Long, J.S.; Berdyshev, E.; Tate, R.J.; Natarajan, V.; Pitson, S.M.; Pyne, N.J.; et al. The sphingosine kinase 1 inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole induces proteasomal degradation of sphingosine kinase 1 in mammalian cells. J. Biol. Chem. 2010, 285, 38841–38852. [Google Scholar] [CrossRef]
- McNaughton, M.; Pitman, M.; Pitson, S.M.; Pyne, N.J.; Pyne, S. Proteasomal degradation of sphingosine kinase 1 and inhibition of dihydroceramide desaturase by the sphingosine kinase inhibitors, SKI or ABC294640, induces growth arrest in androgen-independent LNCaP-AI prostate cancer cells. Oncotarget 2016, 7, 16663–16675. [Google Scholar] [CrossRef]
- French, K.J.; Zhuang, Y.; Maines, L.W.; Gao, P.; Wang, W.; Beljanski, V.; Upson, J.J.; Green, C.L.; Keller, S.N.; Smith, C.D. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J. Pharmacol. Exp. Ther. 2010, 333, 129–139. [Google Scholar] [CrossRef]
- Beljanski, V.; Knaak, C.; Smith, C.D. A novel sphingosine kinase inhibitor induces autophagy in tumor cells. J. Pharmacol. Exp. Ther. 2010, 333, 454–464. [Google Scholar] [CrossRef]
- Britten, C.D.; Garrett-Mayer, E.; Chin, S.H.; Shirai, K.; Ogretmen, B.; Bentz, T.A.; Brisendine, A.; Anderton, K.; Cusack, S.L.; Maines, L.W.; et al. A Phase I Study of ABC294640, a First-in-Class Sphingosine Kinase-2 Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 4642–4650. [Google Scholar] [CrossRef]
- Kang, Y.; Sundaramoorthy, P.; Gasparetto, C.; Feinberg, D.; Fan, S.; Long, G.; Sellars, E.; Garrett, A.; Tuchman, S.A.; Reeves, B.N.; et al. Phase I study of opaganib, an oral sphingosine kinase 2-specific inhibitor, in relapsed and/or refractory multiple myeloma. Ann. Hematol. 2023, 102, 369–383. [Google Scholar] [CrossRef]
- Cohen, J.A.; Barkhof, F.; Comi, G.; Hartung, H.-P.; Khatri, B.O.; Montalban, X.; Pelletier, J.; Capra, R.; Gallo, P.; Izquierdo, G.; et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 402–415. [Google Scholar] [CrossRef]
- Kappos, L.; Radue, E.-W.; O’Connor, P.; Polman, C.; Hohlfeld, R.; Calabresi, P.; Selmaj, K.; Agoropoulou, C.; Leyk, M.; Zhang-Auberson, L.; et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 387–401. [Google Scholar] [CrossRef]
- Chitnis, T.; Arnold, D.L.; Banwell, B.; Brück, W.; Ghezzi, A.; Giovannoni, G.; Greenberg, B.; Krupp, L.; Rostásy, K.; Tardieu, M.; et al. Trial of Fingolimod versus Interferon Beta-1a in Pediatric Multiple Sclerosis. N. Engl. J. Med. 2018, 379, 1017–1027. [Google Scholar] [CrossRef]
- Kharel, Y.; Lee, S.; Snyder, A.H.; Sheasley-O’neill, S.L.; Morris, M.A.; Setiady, Y.; Zhu, R.; Zigler, M.A.; Burcin, T.L.; Ley, K.; et al. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J. Biol. Chem. 2005, 280, 36865–36872. [Google Scholar] [CrossRef]
- Gräler, M.H.; Goetzl, E.J. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004, 18, 551–553. [Google Scholar] [CrossRef]
- Bravo, G.Á.; Cedeño, R.R.; Casadevall, M.P.; Ramió-Torrentà, L. Sphingosine-1-Phosphate (S1P) and S1P Signaling Pathway Modulators, from Current Insights to Future Perspectives. Cells 2022, 11, 2058. [Google Scholar] [CrossRef]
- Constantinescu, V.; Akgün, K.; Ziemssen, T. Current status and new developments in sphingosine-1-phosphate receptor antagonism: Fingolimod and more. Expert Opin. Drug Metab. Toxicol. 2022, 18, 675–693. [Google Scholar] [CrossRef]
- Dumitrescu, L.; Papathanasiou, A.; Coclitu, C.; Garjani, A.; Evangelou, N.; Constantinescu, C.S.; Popescu, B.O.; Tanasescu, R. An update on the use of sphingosine 1-phosphate receptor modulators for the treatment of relapsing multiple sclerosis. Expert Opin. Pharmacother. 2023, 24, 495–509. [Google Scholar] [CrossRef]
- El Jamal, A.; Briolay, A.; Mebarek, S.; Le Goff, B.; Blanchard, F.; Magne, D.; Brizuela, L.; Bougault, C. Cytokine-Induced and Stretch-Induced Sphingosine 1-Phosphate Production by Enthesis Cells Could Favor Abnormal Ossification in Spondyloarthritis. J. Bone Miner. Res. 2019, 34, 2264–2276. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, F.; Lim, K.G.; Loveridge, C.; Long, J.; Pitson, S.M.; Tigyi, G.; Bittman, R.; Pyne, S.; Pyne, N.J. FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteasomal degradation in human pulmonary artery smooth muscle, breast cancer and androgen-independent prostate cancer cells. Cell Signal. 2010, 22, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, K.; Kusumoto, K.; Ogawa, M.; Ando, H.; Shimizu, T.; Ishima, Y.; Ishida, T.; Okuhira, K. FTY720 Reduces Lipid Accumulation by Upregulating ABCA1 through Liver X Receptor and Sphingosine Kinase 2 Signaling in Macrophages. Int. J. Mol. Sci. 2022, 23, 14617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Y.; Zug, C.; Schluesener, H.J. Sphingosine 1-phosphate receptor modulator FTY720 suppresses rat experimental autoimmune prostatitis. Scand. J. Immunol. 2011, 73, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Alshaker, H.; Böhler, T.; Srivats, S.; Chao, Y.; Cooper, C.; Pchejetski, D. Core shell lipid-polymer hybrid nanoparticles with combined docetaxel and molecular targeted therapy for the treatment of metastatic prostate cancer. Sci. Rep. 2017, 7, 5901. [Google Scholar] [CrossRef]
- Gonzalez, P.; Debnath, S.; Chen, Y.-A.; Hernandez, E.; Jha, P.; Dakanali, M.; Hsieh, J.-T.; Sun, X. A Theranostic Small-Molecule Prodrug Conjugate for Neuroendocrine Prostate Cancer. Pharmaceutics 2023, 15, 481. [Google Scholar] [CrossRef]
- Visentin, B.; Vekich, J.A.; Sibbald, B.J.; Cavalli, A.L.; Moreno, K.M.; Matteo, R.G.; Garland, W.A.; Lu, Y.; Yu, S.; Hall, H.S.; et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell 2006, 9, 225–238. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Bullock, A.J.; Callea, M.; Shah, H.; Song, J.; Moreno, K.; Visentin, B.; Deutschman, D.; Alsop, D.C.; et al. Anti-S1P Antibody as a Novel Therapeutic Strategy for VEGFR TKI-Resistant Renal Cancer. Clin. Cancer Res. 2015, 21, 1925–1934. [Google Scholar] [CrossRef]
- Wojciak, J.M.; Zhu, N.; Schuerenberg, K.T.; Moreno, K.; Shestowsky, W.S.; Hiraiwa, M.; Sabbadini, R.; Huxford, T. The crystal structure of sphingosine-1-phosphate in complex with a Fab fragment reveals metal bridging of an antibody and its antigen. Proc. Natl. Acad. Sci. USA 2009, 106, 17717–17722. [Google Scholar] [CrossRef]
- O’Brien, N.; Jones, S.T.; Williams, D.G.; Cunningham, H.B.; Moreno, K.; Visentin, B.; Gentile, A.; Vekich, J.; Shestowsky, W.; Hiraiwa, M.; et al. Production and characterization of monoclonal anti-sphingosine-1-phosphate antibodies. J. Lipid. Res. 2009, 50, 2245–2257. [Google Scholar] [CrossRef]
- Pal, S.K.; Drabkin, H.A.; Reeves, J.A.; Hainsworth, J.D.; Hazel, S.E.; Paggiarino, D.A.; Wojciak, J.; Woodnutt, G.; Bhatt, R.S. A phase 2 study of the sphingosine-1-phosphate antibody sonepcizumab in patients with metastatic renal cell carcinoma. Cancer 2017, 123, 576–582. [Google Scholar] [CrossRef]
- Li, H.; Sibley, C.D.; Kharel, Y.; Huang, T.; Brown, A.M.; Wonilowicz, L.G.; Bevan, D.R.; Lynch, K.R.; Santos, W.L. Lipophilic tail modifications of 2-(hydroxymethyl)pyrrolidine scaffold reveal dual sphingosine kinase 1 and 2 inhibitors. Bioorg. Med. Chem. 2021, 30, 115941. [Google Scholar] [CrossRef]
- Xue, Y.; Jiang, K.; Ou, L.; Shen, M.; Yang, Y.; Lu, J.; Xu, W. Targeting sphingosine kinase 1/2 by a novel dual inhibitor SKI-349 suppresses non-small cell lung cancer cell growth. Cell Death Dis. 2022, 13, 602. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mebarek, S.; Skafi, N.; Brizuela, L. Targeting Sphingosine 1-Phosphate Metabolism as a Therapeutic Avenue for Prostate Cancer. Cancers 2023, 15, 2732. https://doi.org/10.3390/cancers15102732
Mebarek S, Skafi N, Brizuela L. Targeting Sphingosine 1-Phosphate Metabolism as a Therapeutic Avenue for Prostate Cancer. Cancers. 2023; 15(10):2732. https://doi.org/10.3390/cancers15102732
Chicago/Turabian StyleMebarek, Saida, Najwa Skafi, and Leyre Brizuela. 2023. "Targeting Sphingosine 1-Phosphate Metabolism as a Therapeutic Avenue for Prostate Cancer" Cancers 15, no. 10: 2732. https://doi.org/10.3390/cancers15102732
APA StyleMebarek, S., Skafi, N., & Brizuela, L. (2023). Targeting Sphingosine 1-Phosphate Metabolism as a Therapeutic Avenue for Prostate Cancer. Cancers, 15(10), 2732. https://doi.org/10.3390/cancers15102732







