Longitudinal Evaluation of Brain Plasticity in Low-Grade Gliomas: fMRI and Graph-Theory Provide Insights on Language Reorganization
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Awake Surgery Mapping
2.3. fMRI Acquisition
2.4. fMRI Analysis
2.5. Functional Connectivity and Graph Theory
2.6. Statistical Analysis
3. Results
3.1. Cohort Clinical Description
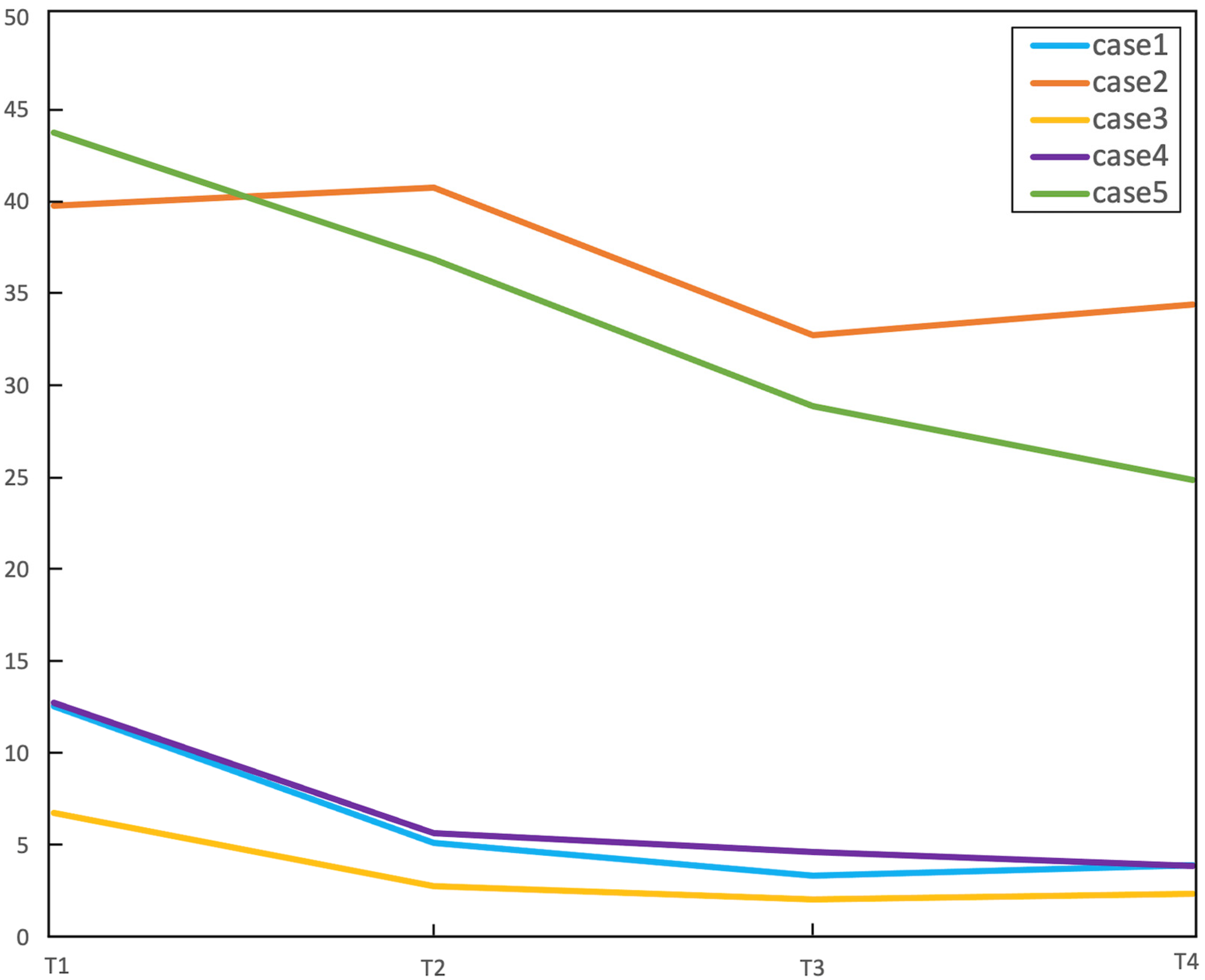
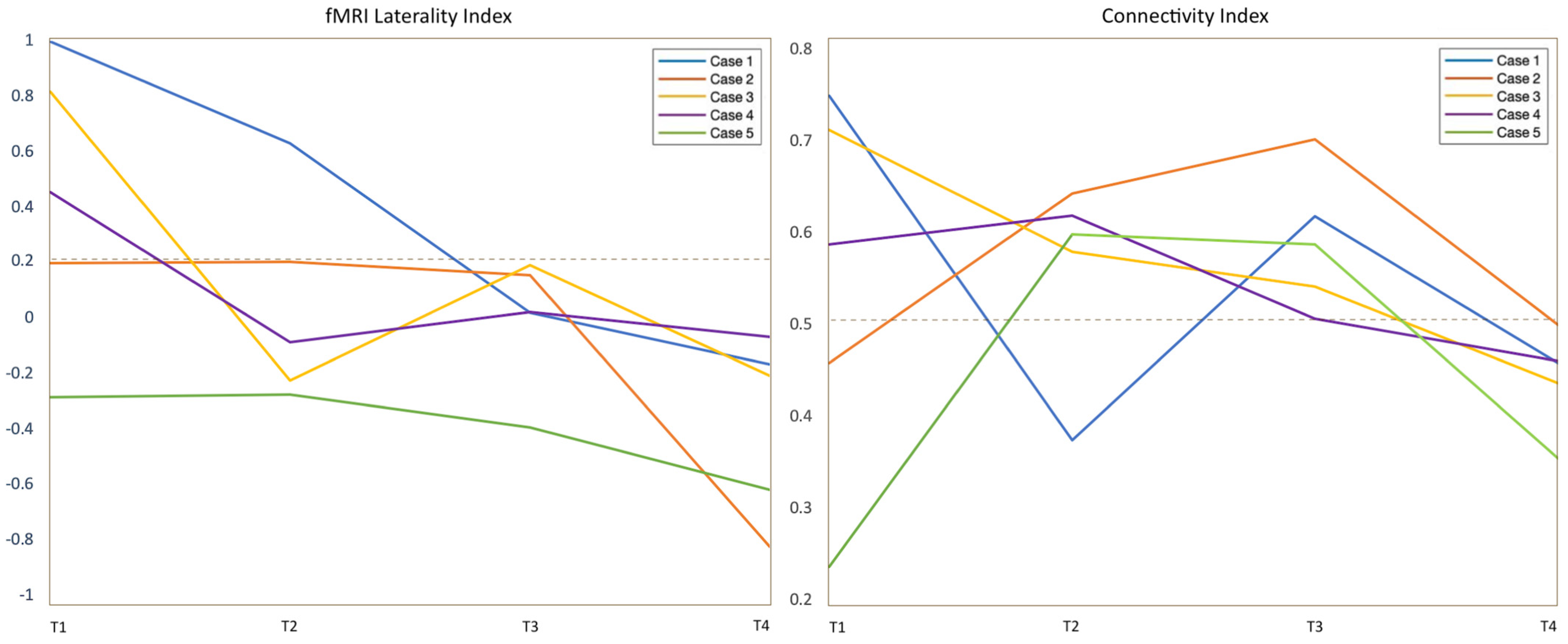
3.2. fMRI Activation Maps and Laterality Analysis
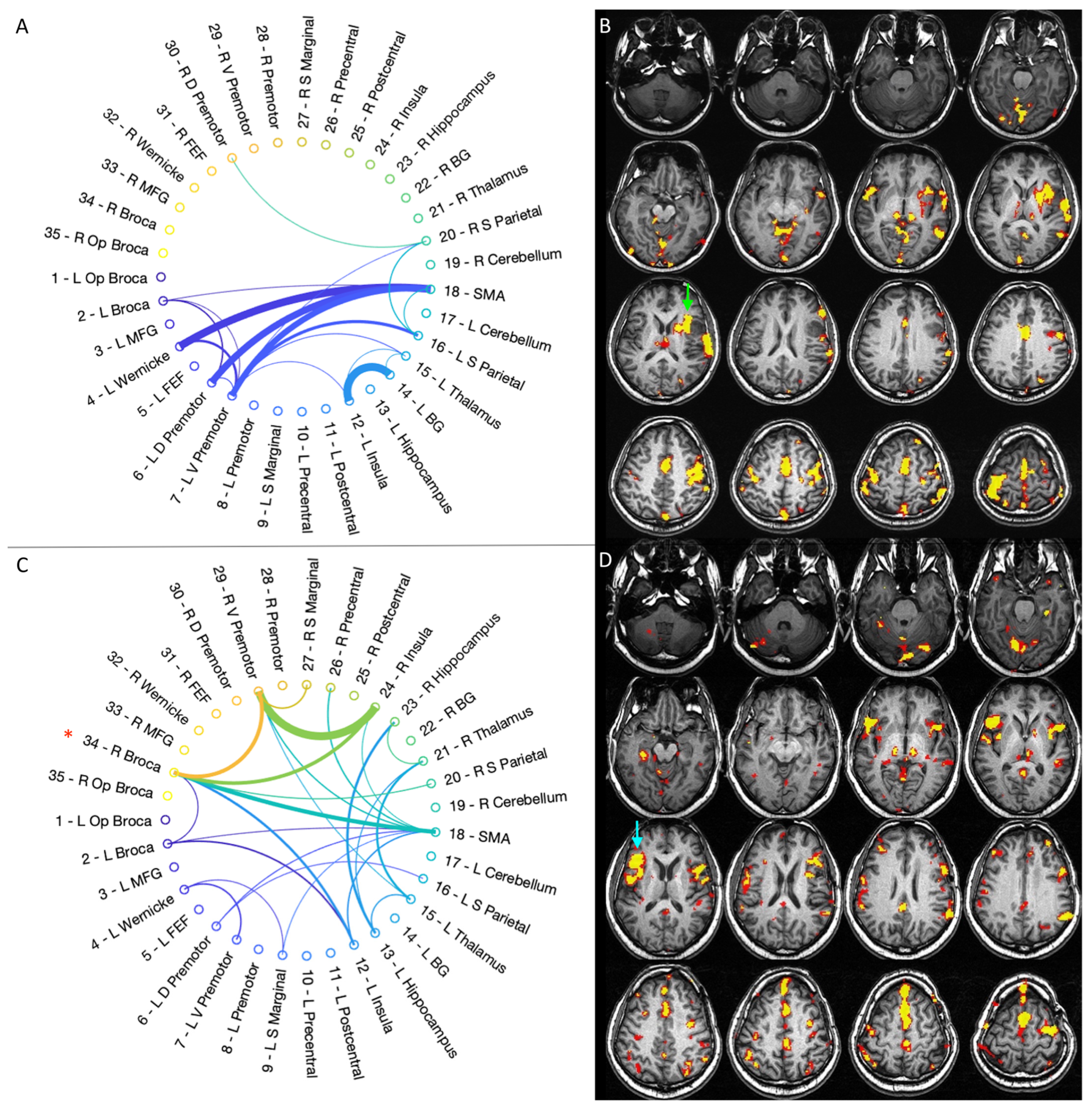
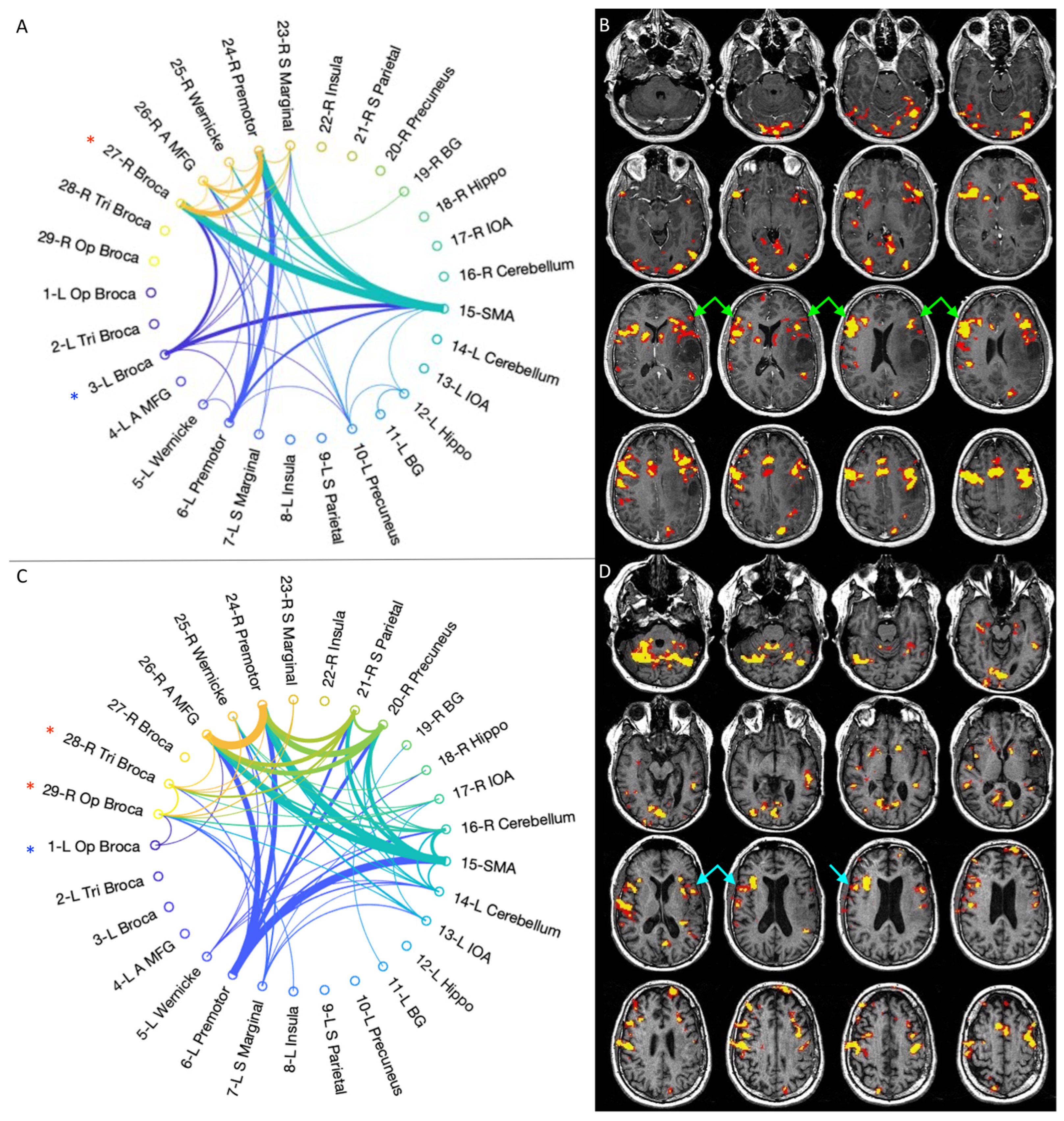
3.3. Graph-Theory Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Isaacs, K.L.; Barr, W.B.; Nelson, P.K.; Devinsky, O. Degree of Handedness and Cerebral Dominance. Neurology 2006, 66, 1855–1858. [Google Scholar] [CrossRef]
- Amoruso, L.; Geng, S.; Molinaro, N.; Timofeeva, P.; Gisbert-Muñoz, S.; Gil-Robles, S.; Pomposo, I.; Quiñones, I.; Carreiras, M. Oscillatory and Structural Signatures of Language Plasticity in Brain Tumor Patients: A Longitudinal Study. Hum. Brain Mapp. 2021, 42, 1777–1793. [Google Scholar] [CrossRef]
- Fisicaro, R.A.; Jost, E.; Shaw, K.; Brennan, N.P.; Peck, K.K.; Holodny, A.I. Cortical Plasticity in the Setting of Brain Tumors. Top. Magn. Reson. Imaging 2016, 25, 25–30. [Google Scholar] [CrossRef]
- Pasquini, L.; Di Napoli, A.; Rossi-Espagnet, M.C.; Visconti, E.; Napolitano, A.; Romano, A.; Bozzao, A.; Peck, K.K.; Holodny, A.I. Understanding Language Reorganization with Neuroimaging: How Language Adapts to Different Focal Lesions. Insights into Clinical Applications. Front. Hum. Neurosci. 2022, 16, 747215. [Google Scholar] [CrossRef]
- Pasquini, L.; Jenabi, M.; Yildirim, O.; Silveira, P.; Peck, K.K.; Holodny, A.I. Brain Functional Connectivity in Low- and High-Grade Gliomas: Differences in Network Dynamics Associated with Tumor Grade and Location. Cancers 2022, 14, 3327. [Google Scholar] [CrossRef]
- Cargnelutti, E.; Ius, T.; Skrap, M.; Tomasino, B. NeuroImage: Clinical What Do We Know about Pre- and Postoperative Plasticity in Patients with Glioma ? A Review of Neuroimaging and Intraoperative Mapping Studies. Neuroimage Clin. 2020, 28, 102435. [Google Scholar] [CrossRef] [PubMed]
- Desmurget, M.; Bonnetblanc, F.; Duffau, H. Contrasting Acute and Slow-Growing Lesions: A New Door to Brain Plasticity. Brain 2007, 130, 898–914. [Google Scholar] [CrossRef]
- Duffau, H. Diffuse Low-Grade Gliomas and Neuroplasticity. Diagn. Interv. Imaging 2014, 95, 945–955. [Google Scholar] [CrossRef]
- Picart, T.; Herbet, G.; Moritz-Gasser, S.; Duffau, H. Iterative Surgical Resections of Diffuse Glioma with Awake Mapping: How to Deal with Cortical Plasticity and Connectomal Constraints? Clin. Neurosurg. 2019, 85, 105–116. [Google Scholar] [CrossRef]
- Robles, S.G.; Gatignol, P.; Lehéricy, S.; Duffau, H. Long-Term Brain Plasticity Allowing a Multistage Surgical Approach to World Health Organization Grade II Gliomas in Eloquent Areas: Report of 2 Cases. J. Neurosurg. 2008, 109, 615–624. [Google Scholar] [CrossRef]
- Rivera-Rivera, P.A.; Rios-Lago, M.; Sanchez-Casarrubios, S.; Salazar, O.; Yus, M.; González-Hidalgo, M.; Sanz, A.; Avecillas-Chasin, J.; Alvarez-Linera, J.; Pascual-Leone, A.; et al. Cortical Plasticity Catalyzed by Prehabilitation Enables Extensive Resection of Brain Tumors in Eloquent Areas. J. Neurosurg. 2017, 126, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Luna, L.P.; Sherbaf, F.G.; Sair, H.I.; Mukherjee, D.; Oliveira, I.B.; Köhler, C.A. Can Preoperative Mapping with Functional MRI Reduce Morbidity in Brain Tumor Resection? A Systematic Review and Meta-Analysis of 68 Observational Studies. Radiology 2021, 300, 338–349. [Google Scholar] [CrossRef]
- Herbet, G.; Duffau, H. Revisiting the Functional Anatomy of the Human Brain: Toward a Meta-Networking Theory of Cerebral Functions. Physiol. Rev. 2020, 100, 1181–1228. [Google Scholar] [CrossRef]
- Bottino, F.; Lucignani, M.; Pasquini, L.; Mastrogiovanni, M.; Gazzellini, S.; Ritrovato, M.; Longo, D.; Figà-Talamanca, L.; Rossi Espagnet, M.C.; Napolitano, A. Spatial Stability of Functional Networks: A Measure to Assess the Robustness of Graph-Theoretical Metrics to Spatial Errors Related to Brain Parcellation. Front. Neurosci. 2022, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Del Ferraro, G.; Pasquini, L.; Peck, K.K.; Makse, H.A.; Holodny, A.I. Core Language Brain Network for FMRI Language Task Used in Clinical Applications. Netw. Neurosci. 2020, 4, 134–154. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Pasquini, L.; Del Ferraro, G.; Gene, M.; Peck, K.K.; Makse, H.A.; Holodny, A.I. Monolingual and Bilingual Language Networks in Healthy Subjects Using Functional MRI and Graph Theory. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.J.; Wefel, J.S.; Schiff, D.; Taphoorn, M.J.B.; Jaeckle, K.; Junck, L.; Armstrong, T.; Choucair, A.; Waldman, A.D.; Gorlia, T.; et al. Response Assessment in Neuro-Oncology (a Report of the RANO Group): Assessment of Outcome in Trials of Diffuse Low-Grade Gliomas. Lancet Oncol. 2011, 12, 583–593. [Google Scholar] [CrossRef]
- Kaplan, E.; Goodglass, H.; Weintraub, S. Boston Naming Test, 2nd ed.; Lea & Febiger: Philadelphia, PA, USA, 1983. [Google Scholar]
- Benton, A.L.; des Kerry, H.; Sivan, A.B. Multilingual Aphasia Examination; University of Iowa: Iowa City, IA, USA, 1976. [Google Scholar]
- Oldfield, R.C. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Duffau, H. Functional Mapping before and after Low-Grade Glioma Surgery: A New Way to Decipher Various Spatiotemporal Patterns of Individual Neuroplastic Potential in Brain Tumor Patients. Cancers 2020, 12, 2611. [Google Scholar] [CrossRef]
- Cox, R.W. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput. Biomed. Res. 1996, 29, 162–173. [Google Scholar] [CrossRef]
- Bandettini, P.A.; Jesmanowicz, A.; Wong, E.C.; Hyde, J.S. Processing Strategies for Time-course Data Sets in Functional Mri of the Human Brain. Magn. Reson. Med. 1993, 30, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Seghier, M.L. Laterality Index in Functional MRI: Methodological Issues. Magn. Reson. Imaging 2008, 26, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, W.D.; Balsamo, L.; Xu, B.; Grandin, C.B.; Braniecki, S.H.; Papero, P.H.; Weinstein, S.; Conry, J.; Pearl, P.L.; Sachs, B.; et al. Language Dominance in Partial Epilepsy Patients Identified with an FMRI Reading Task. Neurology 2002, 59, 256–265. [Google Scholar] [CrossRef]
- Del Ferraro, G.; Moreno, A.; Min, B.; Morone, F.; Pérez-Ramírez, Ú.; Pérez-Cervera, L.; Parra, L.C.; Holodny, A.; Canals, S.; Makse, H.A. Finding Influential Nodes for Integration in Brain Networks Using Optimal Percolation Theory. Nat. Commun. 2018, 9, 2274. [Google Scholar] [CrossRef]
- Arese Lucini, F.; Del Ferraro, G.; Sigman, M.; Makse, H.A. How the Brain Transitions from Conscious to Subliminal Perception. Neuroscience 2019, 411, 280–290. [Google Scholar] [CrossRef]
- Kristo, G.; Raemaekers, M.; Rutten, G.J.; de Gelder, B.; Ramsey, N.F. Inter-Hemispheric Language Functional Reorganization in Low-Grade Glioma Patients after Tumour Surgery. Cortex 2015, 64, 235–248. [Google Scholar] [CrossRef]
- Sarubbo, S.; le Bars, E.; Sylvie, M.G.; Duffau, H.; Sarubbo, S. Complete Recovery after Surgical Resection of Left Wernicke’s Area in Awake Patient: A Brain Stimulation and Functional MRI Study. Neurosurg. Rev. 2012, 35, 287–292. [Google Scholar] [CrossRef]
- Duffau, H. The “Frontal Syndrome” Revisited: Lessons from Electrostimulation Mapping Studies. Cortex 2012, 48, 120–131. [Google Scholar] [CrossRef]
- Quinones, A.; Jenabi, M.; Pasquini, L.; Peck, K.; Moss, N.S.; Brennan, C.; Tabar, V.; Holodny, A. Longitudinal Functional MRI Demonstrates Translocation of Language Function in Patients with Brain Tumors. J. Neurosurg. 2022, 1–9. [Google Scholar] [CrossRef]
- Wigmore, P. The Effect of Systemic Chemotherapy on Neurogenesis, Plasticity and Memory. In Neurogenesis and Neural Plasticity; Springer: Berlin/Heidelberg, Germany, 2012; pp. 211–240. [Google Scholar] [CrossRef]
- Holodny, A.I.; Schulder, M.; Ybasco, A.; Liu, W.C. Translocation of Broca’s Area to the Contralateral Hemisphere as the Result of the Growth of a Left Inferior Frontal Glioma. J. Comput. Assist. Tomogr. 2002, 26, 941–943. [Google Scholar] [CrossRef]
- Petrovich, N.M.; Holodny, A.I.; Brennan, C.W.; Gutin, P.H. Isolated Translocation of Wernicke’s Area to the Right Hemisphere in a 62-Year-Man with a Temporo-Parietal Glioma. Am. J. Neuroradiol. 2004, 25, 130–133. [Google Scholar]
- Li, Q.; Dong, J.W.; Del Ferraro, G.; Brennan, N.P.; Peck, K.K.; Tabar, V.; Makse, H.A.; Holodny, A.I. Functional Translocation of Broca’s Area in a Low-Grade Left Frontal Glioma: Graph Theory Reveals the Novel, Adaptive Network Connectivity. Front. Neurol. 2019, 10, 1–6. [Google Scholar] [CrossRef]
- Pasquini, L.; Jenabi, M.; Peck, K.K.; Holodny, A. Language Reorganization in Patients with Left-Hemispheric Gliomas Is Associated with Increased Cortical Volume in Language-Related Areas and in the Default Mode Network. Cortex 2022, 157, 245–255. [Google Scholar] [CrossRef]
- Rosenberg, K.; Liebling, R.; Avidan, G.; Perry, D.; Siman-Tov, T.; Andelman, F.; Ram, Z.; Fried, I.; Hendler, T. Language Related Reorganization in Adult Brain with Slow Growing Glioma: FMRI Prospective Case-Study. Neurocase 2008, 14, 465–473. [Google Scholar] [CrossRef]
- Altamura, C.; Reinhard, M.; Vry, M.S.; Kaller, C.P.; Hamzei, F.; Vernieri, F.; Rossini, P.M.; Hetzel, A.; Weiller, C.; Saur, D. The Longitudinal Changes of BOLD Response and Cerebral Hemodynamics from Acute to Subacute Stroke. A FMRI and TCD Study. BMC Neurosci. 2009, 10, 151. [Google Scholar] [CrossRef]
- Tantillo, G.; Peck, K.K.; Arevalo-Perez, J.; Lyo, J.K.; Chou, J.F.; Young, R.J.; Petrovich-Brennan, N.M.; Holodny, A.I. Corpus Callosum Diffusion and Language Lateralization in Patients with Brain Tumors: A DTI and FMRI Study. J. Neuroimaging 2016, 26, 224–231. [Google Scholar] [CrossRef]
- Nahmani, M.; Turrigiano, G.G. Adult Cortical Plasticity Following Injury: Recapitulation of Critical Period Mechanisms? Neuroscience 2014, 283, 4–16. [Google Scholar] [CrossRef]
- Kardan, O.; Reuter-Lorenz, P.A.; Peltier, S.; Churchill, N.W.; Misic, B.; Askren, M.K.; Jung, M.S.; Cimprich, B.; Berman, M.G. Brain Connectivity Tracks Effects of Chemotherapy Separately from Behavioral Measures. Neuroimage Clin. 2019, 21, 101654. [Google Scholar] [CrossRef]
- Mitchell, T.J.; Seitzman, B.A.; Ballard, N.; Petersen, S.E.; Shimony, J.S.; Leuthardt, E.C. Human Brain Functional Network Organization Is Disrupted after Whole-Brain Radiation Therapy. Brain Connect. 2020, 10, 29–38. [Google Scholar] [CrossRef]
- Jehna, M.; Becker, J.; Zaar, K.; von Campe, G.; Ali, K.M.; Reishofer, G.; Payer, F.; Synowitz, M.; Fazekas, F.; Enzinger, C.; et al. Symmetry of the Arcuate Fasciculus and Its Impact on Language Performance of Patients with Brain Tumors in the Language-Dominant Hemisphere. J. Neurosurg. 2017, 127, 1407–1416. [Google Scholar] [CrossRef]

| Case | Hand | Sex | T1 (D) | T2 (M) | T3 (M) | T4 (M) | Tumor Diagnosis | Location | Eloquent Areas | IOS |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | R | F | 60 | 8 | 6 | 9 | astro WHO2 | 3 | 1 | |
| 2 | R | M | 1 | 4 | 6 | 7 | astro WHO2 | 2,3,4 | 2 | 1 |
| 3 | R | M | 30 | 7 | 4 | 6 | oligo WHO2 | 1 | 1 | 1 |
| 4 | R | M | 1 | 4 | 7 | 6 | oligo WHO2 | 1,3 | 1 | 1 |
| 5 | R | M | 2 | 4 | 6 | 6 | astro WHO2 | 1,3 | 1 | 2 |
| RT Follow-Up Interval | RT Fractions | RT Total Adjuvant Dose/Energy | CT Follow-Up Interval | CT Regime | |
|---|---|---|---|---|---|
| Case 1 | |||||
| T1–T2 | 30 | Total 5400 cGy, 180 cGy/fraction, 6 MV | / | / | |
| Case 2 | |||||
| T1–T2 | 33 | Total 5940 cGy, 180 cGy/fraction, 6 MV | T2–T3 | Temozolomide, 5 cycles | |
| Case 3 | |||||
| T1–T2 | 30 | Total 5400 cGy, 180 cGy/fraction, 6 MV | / | / | |
| Case 4 | |||||
| / | / | / | / | / | |
| Case 5 | |||||
| T1–T2 | 33 | Total 5940 cGy, 180 cGy/fraction, 6 MV | T3–T4 | Temozolomide, 6 cycles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasquini, L.; Peck, K.K.; Tao, A.; Del Ferraro, G.; Correa, D.D.; Jenabi, M.; Kobylarz, E.; Zhang, Z.; Brennan, C.; Tabar, V.; et al. Longitudinal Evaluation of Brain Plasticity in Low-Grade Gliomas: fMRI and Graph-Theory Provide Insights on Language Reorganization. Cancers 2023, 15, 836. https://doi.org/10.3390/cancers15030836
Pasquini L, Peck KK, Tao A, Del Ferraro G, Correa DD, Jenabi M, Kobylarz E, Zhang Z, Brennan C, Tabar V, et al. Longitudinal Evaluation of Brain Plasticity in Low-Grade Gliomas: fMRI and Graph-Theory Provide Insights on Language Reorganization. Cancers. 2023; 15(3):836. https://doi.org/10.3390/cancers15030836
Chicago/Turabian StylePasquini, Luca, Kyung K. Peck, Alice Tao, Gino Del Ferraro, Denise D. Correa, Mehrnaz Jenabi, Erik Kobylarz, Zhigang Zhang, Cameron Brennan, Viviane Tabar, and et al. 2023. "Longitudinal Evaluation of Brain Plasticity in Low-Grade Gliomas: fMRI and Graph-Theory Provide Insights on Language Reorganization" Cancers 15, no. 3: 836. https://doi.org/10.3390/cancers15030836
APA StylePasquini, L., Peck, K. K., Tao, A., Del Ferraro, G., Correa, D. D., Jenabi, M., Kobylarz, E., Zhang, Z., Brennan, C., Tabar, V., Makse, H., & Holodny, A. I. (2023). Longitudinal Evaluation of Brain Plasticity in Low-Grade Gliomas: fMRI and Graph-Theory Provide Insights on Language Reorganization. Cancers, 15(3), 836. https://doi.org/10.3390/cancers15030836





