Increased Prevalence of EBV Infection in Nasopharyngeal Carcinoma Patients: A Six-Year Cross-Sectional Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Design
2.3. Patients’ Data Collection
2.4. FFPE Collection and Histopathological Examination
2.5. DNA Extraction and Identification of EBV Genotype
2.6. Statistical Analysis
3. Results
3.1. NPC Characteristics of the Studied Population
3.2. EBV Infection and NPC Patients’ Baseline Characteristics
3.3. EBV Infection and NPC Patients’ Clinical Characteristics
3.4. EBV Infection and NPC Patients’ DFS and Overall Survival
3.5. EBV Genotypic Analysis
4. Discussion
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hau, P.M.; Lung, H.L.; Wu, M.; Tsang, C.M.; Wong, K.-L.; Mak, N.K.; Lo, K.W. Targeting Epstein-Barr virus in nasopharyngeal carcinoma. Front. Oncol. 2020, 10, 600. [Google Scholar] [CrossRef]
- Fierti, A.O.; Yakass, M.B.; Okertchiri, E.A.; Adadey, S.M.; Quaye, O. The Role of Epstein-Barr Virus in Modulating Key Tumor Suppressor Genes in Associated Malignancies: Epigenetics, Transcriptional, and Post-Translational Modifications. Biomolecules 2022, 12, 127. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bazarbashi, S.; Al Eid, H.; Minguet, J. Cancer incidence in Saudi Arabia: 2012 data from the Saudi cancer registry. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 2437. [Google Scholar] [PubMed]
- Alotaibi, A.D.; Ahmed, H.G.; Elasbali, A.M. Nasopharyngeal cancer in Saudi Arabia: Epidemiology and possible risk factors. J. Oncol. Sci. 2019, 5, 23–30. [Google Scholar] [CrossRef]
- Stelow, E.B.; Wenig, B.M. Update from the 4th edition of the World Health Organization classification of head and neck tumours: Nasopharynx. Head Neck Pathol. 2017, 11, 16–22. [Google Scholar] [CrossRef]
- Wu, L.; Li, C.; Pan, L. Nasopharyngeal carcinoma: A review of current updates. Exp. Ther. Med. 2018, 15, 3687–3692. [Google Scholar] [CrossRef]
- Wang, L.; Song, Y.; Huang, S.; Tao, H.; Zhao, Y.; Yan, N.; Xu, D. The clinical significance of EBV DNA analysis in nasopharyngeal carcinoma screening. J. Clin. Otorhinolaryngol. Head Neck Surg. 2018, 32, 298–301. [Google Scholar]
- Smatti, M.K.; Al-Sadeq, D.W.; Ali, N.H.; Pintus, G.; Abou-Saleh, H.; Nasrallah, G.K. Epstein–Barr virus epidemiology, serology, and genetic variability of LMP-1 oncogene among healthy population: An update. Front. Oncol. 2018, 8, 211. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, Y.; Fu, M.; Zheng, B.; Qiu, Q.; Huang, Z. Implication of viral microRNAs in the genesis and diagnosis of Epstein-Barr virus-associated tumors. Oncol. Lett. 2019, 18, 3433–3442. [Google Scholar] [CrossRef]
- Abbott, R.J.; Pachnio, A.; Pedroza-Pacheco, I.; Leese, A.M.; Begum, J.; Long, H.M.; Croom-Carter, D.; Stacey, A.; Moss, P.A.; Hislop, A.D. Asymptomatic primary infection with Epstein-Barr virus: Observations on young adult cases. J. Virol. 2017, 91, e00382-17. [Google Scholar] [CrossRef]
- Jean-Pierre, V.; Lupo, J.; Buisson, M.; Morand, P.; Germi, R. Main Targets of Interest for the Development of a Prophylactic or Therapeutic Epstein-Barr Virus Vaccine. Front. Microbiol. 2021, 12, 701611. [Google Scholar] [CrossRef]
- Santpere, G.; Darre, F.; Blanco, S.; Alcami, A.; Villoslada, P.; Mar Alba, M.; Navarro, A. Genome-wide analysis of wild-type Epstein–Barr virus genomes derived from healthy individuals of the 1000 Genomes Project. Genome Biol. Evol. 2014, 6, 846–860. [Google Scholar] [CrossRef]
- Arvin, G.; Campadelli-Fiume, G.; Mocarski, E.; Moore, P.S.; Roizman, B.; Whitley, R.; Yamanishi, K. (Eds.) Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Walling, D.M.; Brown, A.L.; Etienne, W.; Keitel, W.A.; Ling, P.D. Multiple Epstein-Barr virus infections in healthy individuals. J. Virol. 2003, 77, 6546–6550. [Google Scholar] [CrossRef]
- Farrell, P.J. Epstein–Barr virus strain variation. In Epstein Barr Virus Volume 1; Springer: Cham, Switzerland, 2015; pp. 45–69. [Google Scholar]
- Shuja, M.; Abanamy, A.; Khaleel, M.; Ghazal, S.; Salman, H.; Cherian, M.; Azim, M.A. The spectrum of acute Epstein-Barr virus infection in Saudi children. Ann. Saudi Med. 1992, 12, 446–448. [Google Scholar] [CrossRef]
- Zaki, A. Primary Epstein–Barr Virus Infection in Healthy Children in Saudi Arabia: A Single Hospital-Based Study. J. Trop. Pediatr. 2021, 67, fmaa121. [Google Scholar] [CrossRef]
- Nasrin, N.; Taiba, K.; Hannan, N.; Hannan, M.; Al-Sedairy, S. A molecular study of EBV DNA and p53 mutations in nasopharyngeal carcinoma of Saudi Arab patients. Cancer Lett. 1994, 82, 189–198. [Google Scholar] [CrossRef]
- Al-Kuraya, K.; Narayanappa, R.; Al-Dayel, F.; El-Solh, H.; Ezzat, A.; Ismail, H.; Belgaumi, A.; Bavi, P.; Atizado, V.; Sauter, G. Epstein–Barr virus infection is not the sole cause of high prevalence for Hodgkin’s lymphoma in Saudi Arabia. Leuk. Lymphoma 2006, 47, 707–713. [Google Scholar] [CrossRef]
- AlDhahri, S.; Alshareef, R.; Fatani, H.; Chentoufi, A.A. Potential significance of epstein barr virus-positive mucosa in patients with nasopharyngeal carcinoma. Otolaryngol. Head Neck Surg. 2016, 1, 86–88. [Google Scholar] [CrossRef]
- Habibian, A.; Makvandi, M.; Samarbaf-Zadeh, A.; Neisi, N.; Soleimani-Jelodar, R.; Makvandi, K.; Izadi, S. Detection and Genotyping of Epstein-Bar Virus Among Paraffin Embedded Tissues of Hodgkin and Non-Hodgkin’s Lymphoma Patients in Ahvaz, Iran. Acta Med. Iran. 2018, 56, 434–440. [Google Scholar]
- Yu, H.; Yin, X.; Mao, Y.; Chen, M.; Tang, Q.; Yan, S. The global burden of nasopharyngeal carcinoma from 2009 to 2019: An observational study based on the Global Burden of Disease Study 2019. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 1519–1533. [Google Scholar] [CrossRef] [PubMed]
- Mankowski, N.L.; Bordoni, B. Anatomy, Head and Neck, Nasopharynx; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Thompson, M.P.; Kurzrock, R. Epstein-Barr virus and cancer. Clin. Cancer Res. 2004, 10, 803–821. [Google Scholar] [CrossRef]
- Bakkalci, D.; Jia, Y.; Winter, J.R.; Lewis, J.E.; Taylor, G.S.; Stagg, H.R. Risk factors for Epstein Barr virus-associated cancers: A systematic review, critical appraisal, and mapping of the epidemiological evidence. J. Glob. Health 2020, 10, 010405. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.; Meehan, M.T.; Burrows, S.R.; Doolan, D.L.; Miles, J.J. Estimating the global burden of Epstein-Barr virus-related cancers. J. Cancer Res. Clin. Oncol. 2022, 148, 31–46. [Google Scholar] [CrossRef] [PubMed]
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Ruuskanen, M.; Irjala, H.; Minn, H.; Vahlberg, T.; Randen-Brady, R.; Hagström, J.; Syrjänen, S.; Leivo, I. Epstein-Barr virus and human papillomaviruses as favorable prognostic factors in nasopharyngeal carcinoma: A nationwide study in Finland. Head Neck 2019, 41, 349–357. [Google Scholar] [CrossRef]
- Ahmed, H.G.; Suliman, R.S.A.G.; El Aziz, M.S.A.; Alshammari, F.D. Molecular screening for Epstein Barr virus (EBV) among Sudanese patients with nasopharyngeal carcinoma (NPC). Infect. Agents Cancer 2015, 10, 1–6. [Google Scholar] [CrossRef]
- Saito, Y.; Ushiku, T.; Omura, G.; Yasuhara, K.; Yoshida, M.; Takahashi, W.; Ando, M.; Fukayama, M.; Yamasoba, T. Clinical value of the Epstein-Barr virus and p16 status in patients with nasopharyngeal carcinoma: A single-centre study in Japan. ORL 2016, 78, 334–343. [Google Scholar] [CrossRef]
- Doğan, H.T.; Kılıçarslan, A.; Doğan, M.; Süngü, N.; Tezel, G.G.; Güler, G. Retrospective analysis of oncogenic human papilloma virus and Epstein-Barr virus prevalence in Turkish nasopharyngeal cancer patients. Pathol. Res. Pract. 2016, 212, 1021–1026. [Google Scholar] [CrossRef]
- Tay, J.K.; Chan, S.H.; Lim, C.M.; Siow, C.H.; Goh, H.L.; Loh, K.S. The role of Epstein-Barr virus DNA load and serology as screening tools for nasopharyngeal carcinoma. Otolaryngol. Head Neck Surg. 2016, 155, 274–280. [Google Scholar] [CrossRef]
- Salano, V.E.; Mwakigonja, A.R.; Abdulshakoor, A.; Kahinga, A.A.; Richard, E.M. Epstein-Barr Virus Latent Membrane Protein-1 Expression in Nasopharyngeal Carcinoma. JCO Glob. Oncol. 2021, 7, 1406–1412. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, L.; Liu, Q.; Sun, J.; Adami, H.-O.; Ye, W.; Zhang, Z.; Fang, F. Hospital-Treated Infections and Increased Risk of Two EBV-Related Malignancies: A Nested Case-Control Study. Cancers 2022, 14, 3804. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Hubner, R.A.; Siveke, J.T.; Von Hoff, D.D.; Belanger, B.; de Jong, F.A.; Mirakhur, B.; Chen, L.-T. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur. J. Cancer 2019, 108, 78–87. [Google Scholar] [CrossRef]
- Chen, C.; Wu, J.-B.; Jiang, H.; Gao, J.; Chen, J.-X.; Pan, C.-C.; Shen, L.-J.; Chen, Y.; Chang, H.; Tao, Y.-L. A prognostic score for nasopharyngeal carcinoma with bone metastasis: Development and validation from multicenter. J. Cancer 2018, 9, 797. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Zhou, P.; Deng, X.; Wang, Y.; Dang, Q.; Zheng, Y.; Yang, D. Survival Effects of Radiotherapy on Patients Newly Diagnosed with Distant Metastatic Nasopharyngeal Carcinoma in Non-High-Incidence Areas. Cancer Manag. Res. 2021, 13, 8169. [Google Scholar] [CrossRef]
- Lu, X.; Kang, Y. Organotropism of breast cancer metastasis. J. Mammary Gland Biol. Neoplasia 2007, 12, 153–162. [Google Scholar] [CrossRef]
- Chen, W.; Hoffmann, A.D.; Liu, H.; Liu, X. Organotropism: New insights into molecular mechanisms of breast cancer metastasis. NPJ Precis Oncol. 2018, 2, 4. [Google Scholar] [CrossRef]
- Ayee, R.; Ofori, M.E.O.; Tagoe, E.A.; Languon, S.; Searyoh, K.; Armooh, L.; Bilson-Amoah, E.; Baidoo, K.; Kitcher, E.; Wright, E.; et al. Genotypic Characterization of Epstein Barr Virus in Blood of Patients with Suspected Nasopharyngeal Carcinoma in Ghana. Viruses 2020, 12, 766. [Google Scholar] [CrossRef]
- Banko, A.V.; Lazarevic, I.B.; Folic, M.M.; Djukic, V.B.; Cirkovic, A.M.; Karalic, D.Z.; Cupic, M.D.; Jovanovic, T.P. Characterization of the Variability of Epstein-Barr Virus Genes in Nasopharyngeal Biopsies: Potential Predictors for Carcinoma Progression. PLoS ONE 2016, 11, e0153498. [Google Scholar] [CrossRef]
- Smatti, M.K.; Yassine, H.M.; AbuOdeh, R.; AlMarawani, A.; Taleb, S.A.; Althani, A.A.; Nasrallah, G.K. Prevalence and molecular profiling of Epstein Barr virus (EBV) among healthy blood donors from different nationalities in Qatar. PLoS ONE 2017, 12, e0189033. [Google Scholar] [CrossRef]
- Sarnecka, A.K.; Nawrat, D.; Piwowar, M.; Ligęza, J.; Swadźba, J.; Wójcik, P. DNA extraction from FFPE tissue samples—A comparison of three procedures. Contemp. Oncol. 2019, 23, 52–58. [Google Scholar] [CrossRef] [PubMed]
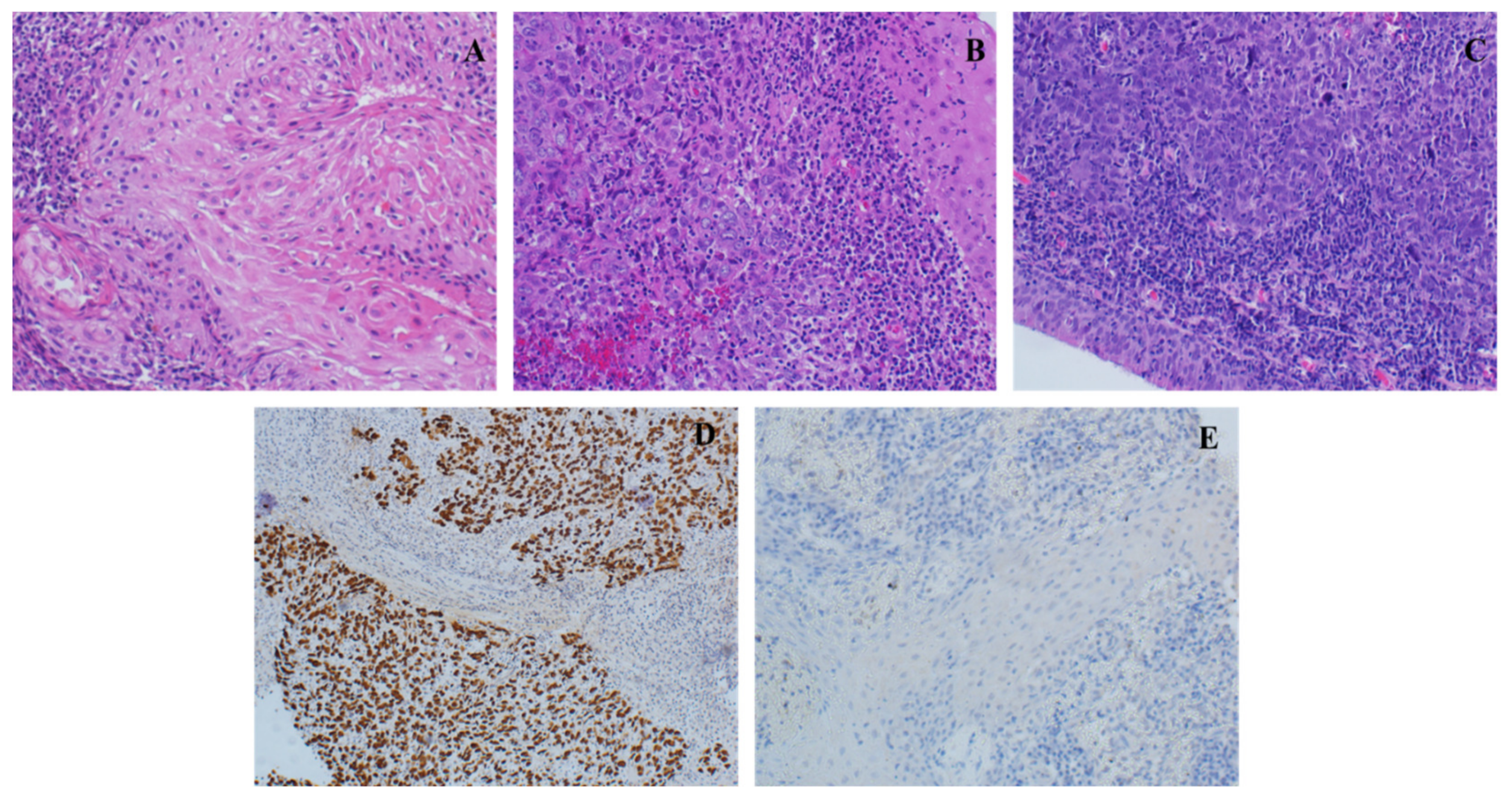
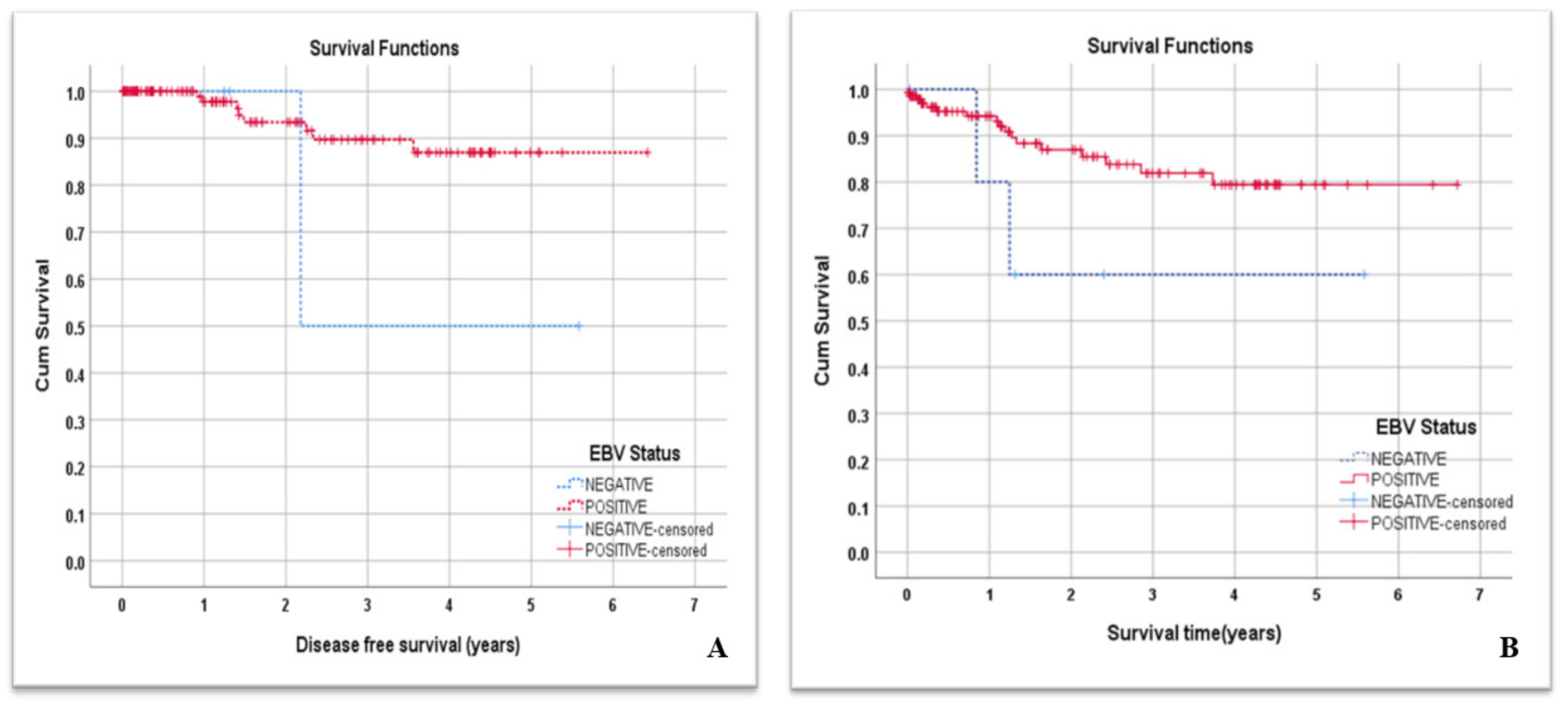
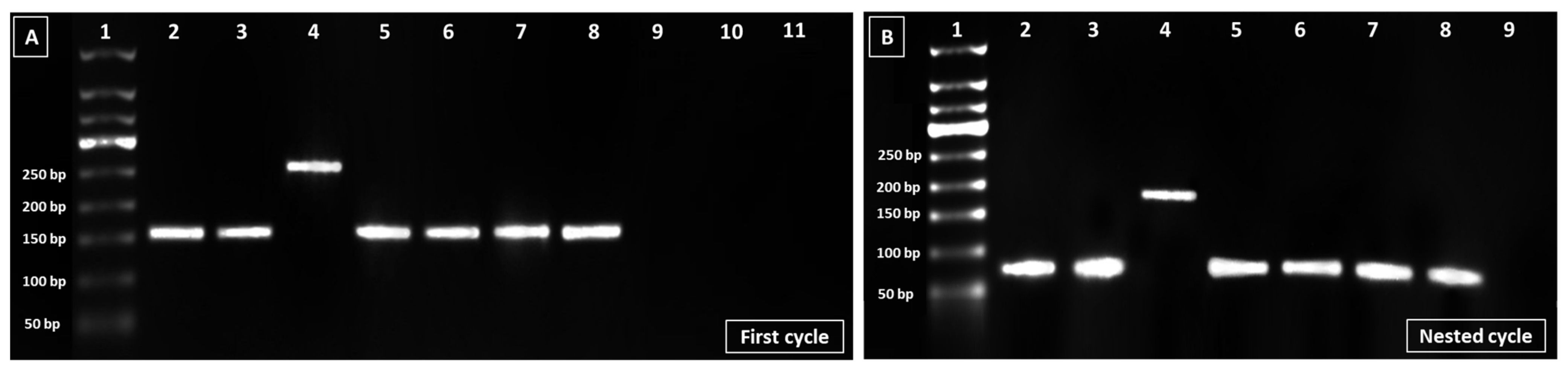
| Patients’ Characteristics | Variables | Number | Percentage |
|---|---|---|---|
| Age (years) | ≤30 | 20 | 13.7% |
| 31–40 | 25 | 17.1% | |
| 41–50 | 40 | 27.4% | |
| 51–60 | 37 | 25.3% | |
| ≥61 | 24 | 16.4% | |
| Gender | Male | 108 | 74.0% |
| Female | 38 | 26.0% | |
| Nationality | Saudi Arabian | 116 | 79.5% |
| Non-Saudi Arabian | 30 | 20.5% | |
| Comorbidities | Hypertension | 23 | 15.7% |
| Diabetes mellitus | 25 | 17% | |
| Other diseases | 25 | 17% | |
| EBV Status | Negative | 6 | 4% |
| Positive | 140 | 96% | |
| Patient’s Status | Alive | 127 | 87.0% |
| Deceased | 19 | 13.0% | |
| Cause of death | NPC | 12 | 63.2% |
| Not NPC | 7 | 36.8% |
| Patients’ Characteristics | Variables | Number | Percentage |
|---|---|---|---|
| Stage of cancer | I | 2 | 1.4% |
| II | 10 | 6.8% | |
| III | 56 | 38.4% | |
| IV-A | 55 | 37.7% | |
| IV-B | 23 | 15.8% | |
| Morphology | Keratinized, squamous cell carcinoma | 1 | 0.7% |
| Differentiated, non-keratinizing Squamous cell carcinoma | 11 | 7.5% | |
| Undifferentiated, non-keratinizing squamous cell carcinoma | 134 | 91.8% | |
| TNM staging (T) | T0 | 2 | 1.4% |
| T1 | 35 | 24% | |
| T2 | 24 | 16.4% | |
| T3 | 37 | 25.3% | |
| T4 | 48 | 32.9% | |
| TNM staging (N) | N0 | 12 | 8.2% |
| N1 | 25 | 17.1% | |
| N2 | 68 | 46.6% | |
| N3 | 41 | 28.1% | |
| TNM staging (M) | M0 | 123 | 84% |
| M1 | 23 | 16% | |
| Metastasis | Yes | 23 | 15.8% |
| No | 123 | 84.2% | |
| Recurrent cancer | No | 137 | 93.8% |
| Yes | 9 | 6.2% | |
| Treatment protocol | No treatment | 9 | 6.2% |
| RT | 2 | 1.4% | |
| CCRT | 35 | 24.0% | |
| Induction + CCRT | 88 | 60.3% | |
| Palliative | 12 | 8.2% | |
| Treatment Response | No treatment | 9 | 6.2% |
| Good response | 123 | 84.2% | |
| Poor response | 14 | 9.6% | |
| Cause of 19 deaths | NPC | 12 | 63.2% |
| Not NPC | 7 | 36.8% |
| Variable | Categories | EBV Status | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | OR | 95%CI for OR | ||||||
| N | % | N | % | Lower | Upper | p-Value | |||
| Age (years) | ≤30 | 1 | 5.0 | 19 | 95.0 | 1.73 | 0.15 | 20.58 | 0.665 |
| 31–40 | 1 | 4.0 | 24 | 96.0 | 2.18 | 0.19 | 25.77 | 0.536 | |
| 41–50 | 1 | 2.5 | 39 | 97.5 | 3.55 | 0.30 | 41.36 | 0.313 | |
| 51–60 | 1 | 2.7 | 36 | 97.3 | 3.27 | 0.28 | 38.24 | 0.345 | |
| ≥61 | 2 | 8.3 | 22 | 91.7 | 1.00 | ||||
| Gender | Male | 1 | 0.9 | 107 | 99.1 | 16.21 | 1.83 | 143.74 | 0.012 * |
| Female | 5 | 13.2 | 33 | 86.8 | 1.00 | ||||
| Nationality | Saudi | 6 | 5.2 | 110 | 94.8 | 0.28 | 0.02 | 5.07 | 0.389 |
| Non-Saudi | 0 | 0.0 | 30 | 100.0 | 1.00 | ||||
| DM | No | 6 | 5.0 | 115 | 95.0 | 1 | 0.48 | ||
| Yes | 0 | 0.0 | 25 | 100.0 | 2.78 | 0.16 | 52.60 | ||
| HTN | No | 5 | 4.1 | 118 | 95.9 | 1 | 0.95 | ||
| Yes | 1 | 4.3 | 22 | 95.7 | 0.93 | 0.10 | 8.37 | ||
| Other diseases | No | 5 | 4.1 | 116 | 95.9 | 1 | 0.98 | ||
| Yes | 1 | 4.0 | 24 | 96.0 | 1.03 | 0.12 | 9.25 | ||
| Treatment protocol | RT | 0 | 0.0 | 2 | 100.0 | 0.88 | 0.03 | 29.14 | 0.944 |
| CCRT | 2 | 5.7 | 33 | 94.3 | 2.06 | 0.17 | 25.68 | 0.574 | |
| Induction + CCRT | 3 | 3.4 | 85 | 96.6 | 3.54 | 0.33 | 38.13 | 0.297 | |
| Palliative | 0 | 0.0 | 12 | 100.0 | 4.41 | 0.16 | 121.70 | 0.381 | |
| No treatment | 1 | 11.1 | 8 | 88.9 | 1.00 | ||||
| Variable | Categories | EBV Status | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | OR | 95%CI for OR | ||||||
| N | % | N | % | Lower | Upper | p-Value | |||
| Stage group | I | 1 | 50.0 | 1 | 50.0 | 0.10 | 0.01 | 2.18 | 0.141 |
| II | 0 | 0.0 | 10 | 100.0 | 2.44 | 0.11 | 55.56 | 0.576 | |
| III | 2 | 3.6 | 54 | 96.4 | 2.57 | 0.34 | 19.46 | 0.360 | |
| IV-A | 1 | 1.8 | 54 | 98.2 | 5.14 | 0.44 | 59.77 | 0.191 | |
| IV-B | 2 | 8.7 | 21 | 91.3 | 1.00 | ||||
| Bone metastasis | No | 5 | 3.7 | 129 | 96.3 | 1 | 0.45 | ||
| Yes | 1 | 8.3 | 11 | 91.7 | 0.43 | 0.05 | 3.97 | ||
| Liver metastasis | No | 5 | 3.6 | 133 | 96.4 | 1 | 0.25 | ||
| Yes | 1 | 12.5 | 7 | 87.5 | 0.263 | 0.03 | 2.57 | ||
| Lung metastasis | No | 4 | 2.9 | 135 | 97.1 | 1 | 0.008 * | ||
| Yes | 2 | 28.6 | 5 | 71.4 | 0.07 | 0.01 | 0.51 | ||
| Other sites of Metastasis | No | 6 | 4.4 | 131 | 95.6 | 1 | 0.97 | ||
| Yes | 0 | 0.0 | 9 | 100.0 | 0.94 | 0.05 | 17.95 | ||
| Morphology | Keratinized, squamous cell carcinoma | 1 | 100.0 | 0 | 0.0 | 0.01 | 0.00 | 0.32 | 0.009 * |
| Differentiated, non-keratinizing squamous cell carcinoma | 1 | 9.1 | 10 | 90.9 | 0.308 | 0.031 | 3.02 | 0.312 | |
| Undifferentiated, non-keratinizing squamous cell carcinoma | 4 | 3.0 | 130 | 97.0 | 1.00 | - | - | - | |
| Treatment Response | Good response | 3 | 2.4 | 120 | 97.6 | 5.00 | 0.47 | 53.70 | 0.184 |
| Poor response | 2 | 14.3 | 12 | 85.7 | 0.75 | 0.06 | 9.72 | 0.826 | |
| No treatment | 1 | 11.1 | 8 | 88.9 | 1.00 | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Anazi, A.E.; Alanazi, B.S.; Alshanbari, H.M.; Masuadi, E.; Hamed, M.E.; Dandachi, I.; Alkathiri, A.; Hanif, A.; Nour, I.; Fatani, H.; et al. Increased Prevalence of EBV Infection in Nasopharyngeal Carcinoma Patients: A Six-Year Cross-Sectional Study. Cancers 2023, 15, 643. https://doi.org/10.3390/cancers15030643
Al-Anazi AE, Alanazi BS, Alshanbari HM, Masuadi E, Hamed ME, Dandachi I, Alkathiri A, Hanif A, Nour I, Fatani H, et al. Increased Prevalence of EBV Infection in Nasopharyngeal Carcinoma Patients: A Six-Year Cross-Sectional Study. Cancers. 2023; 15(3):643. https://doi.org/10.3390/cancers15030643
Chicago/Turabian StyleAl-Anazi, Abdullah E., Bader S. Alanazi, Huda M. Alshanbari, Emad Masuadi, Maaweya E. Hamed, Iman Dandachi, Abdulrahman Alkathiri, Atif Hanif, Islam Nour, Hanadi Fatani, and et al. 2023. "Increased Prevalence of EBV Infection in Nasopharyngeal Carcinoma Patients: A Six-Year Cross-Sectional Study" Cancers 15, no. 3: 643. https://doi.org/10.3390/cancers15030643
APA StyleAl-Anazi, A. E., Alanazi, B. S., Alshanbari, H. M., Masuadi, E., Hamed, M. E., Dandachi, I., Alkathiri, A., Hanif, A., Nour, I., Fatani, H., Alsaran, H., AlKhareeb, F., Al Zahrani, A., Alsharm, A. A., Eifan, S., & Alosaimi, B. (2023). Increased Prevalence of EBV Infection in Nasopharyngeal Carcinoma Patients: A Six-Year Cross-Sectional Study. Cancers, 15(3), 643. https://doi.org/10.3390/cancers15030643








