Simple Summary
Risk factors of hemorrhage in brain metastases from lung adenocarcinoma has been unknown and how hemorrhage in brain metastases affects patients’ prognosis has not been clarified. We performed a retrospective analysis on 159 BMs and found that hemorrhage in BMs from lung adenocarcinomas may be associated with BM tumor size and a combination of TKI and intracranial radiotherapy. However, BM hemorrhage did not affect OSBM.
Abstract
Background: Hemorrhage in brain metastases (BMs) from lung cancer is common and associated with a poor prognosis. Research on associated factors of spontaneous hemorrhage in patients with BMs is limited. This study aimed to investigate the predictive risk factors for BM hemorrhage and assess whether hemorrhage affects patient survival. Methods: We retrospectively evaluated 159 BMs from 80 patients with lung adenocarcinoma from January 2017 to May 2022. Patients were classified into hemorrhagic and non-hemorrhagic groups. Patient demographics, lung cancer molecular subtype, treatment type, and tumor–node–metastasis stage were compared between the groups. Multivariate generalized estimating equation (GEE) analysis and gradient boosting were performed. To determine whether BM hemorrhage can stratify overall survival after BM (OSBM), univariate survival analysis was performed. Results: In the univariate analysis, hemorrhagic BMs were significantly larger and had a history of receiving combination therapy with tyrosine kinase inhibitor (TKI) and intracranial radiation (p < 0.05). Multivariate GEE showed that tumor size and combination therapy were independent risk factors for BM hemorrhage (p < 0.05). Gradient boosting demonstrated that the strongest predictor of BM hemorrhage was tumor size (variable importance: 49.83), followed by age (16.65) and TKI combined with intracranial radiation (13.81). There was no significant difference in OSBM between the two groups (p = 0.33). Conclusions: Hemorrhage in BMs from lung adenocarcinomas may be associated with BM tumor size and a combination of TKI and intracranial radiotherapy. BM hemorrhage did not affect OSBM.
1. Introduction
Brain metastases (BMs) are the most common intracranial tumors in adult patients and typically arise from lung cancer [1,2,3]. Non-small cell lung cancer (NSCLC) constitutes approximately 85% of all lung cancer cases [4]. The prevalence of BMs at initial presentation is 15–20%, and up to 40% of patients eventually develop BMs during the course of NSCLC [5,6]. In particular, patients with adenocarcinoma have a higher risk of BM development than those with other histologic subtypes [7]. Although advanced therapies have been developed that have substantially improved the survival of patients with NSCLC [8], BMs remain an important cause of lung cancer morbidities and are associated with progressive neurologic deficits [9]. BM hemorrhage in particular may lead to serious neurologic deterioration [10] and has been frequently reported in cases of thyroid cancer, melanoma, and renal cell carcinoma [11,12,13]. We anecdotally noted that hemorrhage was a common finding in lung cancer BMs, but its associated factors and clinical significance have not been meticulously investigated.
Hypertension and antithrombogenic medications are known to be significant risk factors for intracranial hemorrhage [14,15]. If these factors lead to BM hemorrhage, hypertension should be controlled and antithrombogenic agents should be avoided. Moreover, with the use of next-generation TKIs, with improved central nervous system penetration, immune check point inhibitors, and sophisticated radiation therapy, it remains to be determined whether BM hemorrhage is a therapeutic response and may affect patients’ survival or not [16,17].
The aims of the study were twofold: (1) to explore any risk factors associated with hemorrhage in BMs from lung adenocarcinomas and (2) to determine whether BM hemorrhage can predict patients’ overall survival.
2. Materials and Methods
2.1. Participants
This retrospective study was approved by the institutional review board, which waived the requirement for informed patient consent (3-2021-0418). We retrospectively searched electronic medical records to identify magnetic resonance imaging (MRI) sets of patients with histologically confirmed lung cancer and BM from January 2017 to May 2022. After identifying 1090 potential participants, the following exclusion criteria were applied: (1) histologic subtypes of primary lung cancer other than adenocarcinoma (n = 456), (2) history of neurosurgery (n = 4), (3) lack of susceptibility-weighted angiography (SWAN) MRI of the brain for evaluation of the hemorrhage (n = 356), (4) leptomeningeal or dural metastasis (n = 41), (5) absence of “evaluable BM” for the hemorrhage (n = 61), and (6) more than five BMs in a patient, as clustered data may lead a statistical bias (n = 92). “Evaluable BM” was defined as BM where the longest diameter was >5 mm. After the exclusion criteria were applied, a total of 80 patients with 144 MRI scans and 159 BMs were included in this study (Figure 1).
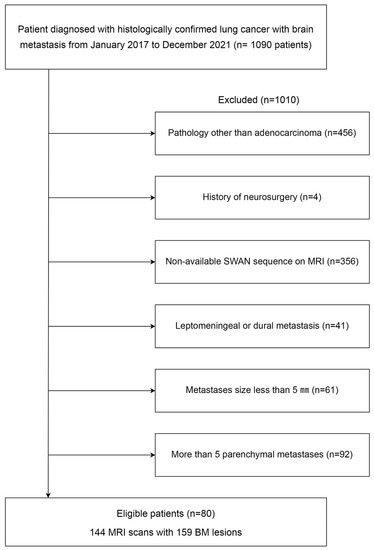
Figure 1.
Flow chart of patient enrollment. BM: brain metastasis; MRI: magnetic resonance imaging; SWAN: susceptibility-weighted angiography.
Histopathological diagnoses of lung cancer were obtained using bronchoscopic, percutaneous needle-guided, or surgical biopsies for all patients. To determine EGFR mutation status, DNA was extracted from formalin-fixed paraffin-embedded tissues using a DNeasy isolation kit (Qiagen, Valencia, CA, USA), according to the manufacturer’s instructions. For EGFR, direct DNA sequencing of exons 18–21 was performed, or the PNAClampTM EGFR Mutation Detection Kit (PANAGENE, Daejeon, Korea) was used. Each case was classified as positive or negative for a mutation by comparing with the wild-type sequence. Anaplastic lymphoma kinase (ALK) fusion was identified using the Ventana ALK (D5F3) CDx Assay. ROS1 fusion was screened using immunohistochemistry staining and confirmed using an AmoyDx ROS1 Gene Fusion Detection Kit [18]. All data were anonymized, and all experiments were conducted in accordance with approved guidelines.
NSCLC staging was performed in accordance with the 8th edition of the American Joint Committee on Cancer guidelines [19]. Tumor–node–metastasis (TNM) staging of lung cancer at diagnosis was based on computed tomography (CT) scans of the chest and abdomen, whole-bone scanning, and positron emission tomography–CT, which were performed during the initial evaluation of NSCLC.
2.2. MRI and Image Analysis
Routine MRI scans for evaluating BMs were performed using a GE 3T Discovery MR750 (GE Healthcare, Milwaukee, WI, USA) scanner. The brain MRI protocol used in this study included three-dimensional (3D) T1-weighted imaging, T2-weighted imaging, fluid-attenuated inversion recovery, SWAN, and subsequent contrast-enhanced 3D T1-weighted imaging. The sequence parameters for SWAN were as follows: repetition time (TR) = 31 ms, echo time (TE) = 3 echoes centered around 23 ms, flip angle = 10°, slice thickness = 2 mm, intersection gap = 0 mm, field of view = 210 mm, matrix number = 320 × 224, and bandwidth = 62.50 kHz (Figure 2).
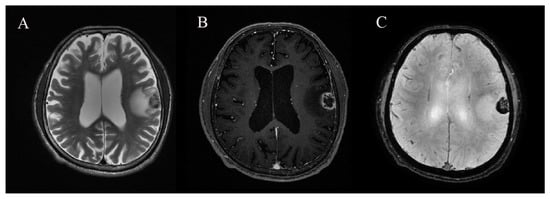
Figure 2.
A 67-year-old man with hemorrhagic brain metastasis (BM) in left frontoparietal lobe. (A) T2-weighted imaging, (B) contrast-enhanced 3D T1-weighted imaging, and (C) susceptibility-weighted angiography (SWAN).
Two radiologists, who were blinded to the clinical and histopathologic findings, independently evaluated the MR images on the picture archiving and communication system workstation monitors for the following characteristics: number of metastatic lesions, size of the metastatic lesions, and presence of intratumoral hemorrhage. The lesion size was defined as the lesion’s largest dimension in any plane on the MR image. Intratumoral hemorrhage was considered to be present if the lesion contained dark signals on the phase map of SWAN [20]. All disagreements were resolved by consensus.
2.3. Statistical Analyses
The cohort was divided into two groups: non-hemorrhagic and hemorrhagic BMs. Each patient’s age, sex, smoking history (never smoker vs. ever smoker), hypertension, antithrombogenic agents, time interval to BM, TNM classification, cancer stage, molecular subtype (EGFR, ALK, and ROS1), treatment type (including radiotherapy and target agents), and lesion size were compared between both groups using a generalized estimating equation (GEE). The advantage of using GEE is that it accounts for possible dependence among clinical variables and MRI measures within the patients who tend to be alike [21]. Multivariate GEE analysis was also performed to adjust for age, sex, tumor size, EGFR mutation, and treatment options, which were statistically significant in the univariate analysis or were basic clinical factors. To determine the influence of variables in predicting hemorrhage within BMs, variable importance scores were calculated using a gradient boosting algorithm (GBM).
To explore the predictability of BM hemorrhage for overall survival after BM (OSBM), Kaplan–Meier analysis was performed. Log-rank tests were used to compare non-hemorrhagic and hemorrhagic BMs. For subgroup analysis, we split our cohort into patients with single BM and multiple BMs. Then, survival analyses were also performed according to presence of intracranial hemorrhage in each subgroup. Interrater reliability was assessed using the intraclass correlation coefficient with a two-way random model of absolute agreement. Statistical significance was set at p-value < 0.05. All data analyses were performed using R version 3.5.3.
3. Results
3.1. Hemorrhagic Versus Non-Hemorrhagic BMs from Lung Adenocarcinoma
Clinical characteristics were compared between the two groups (Table 1). The mean size of hemorrhagic BMs was significantly larger than that of non-hemorrhagic BMs (16.1 ± 11.7 vs. 11.7 ± 9.7 mm, p = 0.03). Radiation or TKI therapy was marginally associated with hemorrhage in BMs (p = 0.06). Moreover, patients with hemorrhagic BMs were more likely to have received a combination of TKI and radiation therapy than those with non-hemorrhagic BMs (40.9% vs. 19.0%, p = 0.03). No significant differences were observed in the type of TKI, brain radiotherapy, and immunotherapy; TNM staging of lung cancer at the initial diagnosis; time interval from the initial diagnosis of lung cancer to BM surveillance; EGFR, ALK, and ROS1 mutations; age; hypertension; antithrombogenic agent; and sex of patients.

Table 1.
Clinical characteristics of patients with brain metastases (BMs) from lung adenocarcinoma.
3.2. Most Influential Variable Predicting BM Hemorrhage
Multivariate GEE analysis found that hemorrhage in BMs was independently associated with combined TKI and radiation therapies (odds ratio (OR) = 2.31, 95% confidence interval (CI): 1.13, 4.71, p = 0.02) and BM size (OR = 1.07, 95% CI: 1, 1.13, p = 0.03, Table 2). In the GBM, BM size demonstrated the highest predictive power for hemorrhage in BM, followed by age, combination therapy, TKI therapy, radiation therapy, sex, time interval, and EGFR mutation (Table 3).

Table 2.
Multivariate generalized estimating equation (GEE) analysis for hemorrhage occurrence in brain metastases (BMs).

Table 3.
Relative influence of variables for prediction of hemorrhagic brain metastases (BMs) using gradient boosted model (GBM).
3.3. Effect of BM Hemorrhage on Overall Survival after BM of Patients
The median OSBM was 23.0 months, and no significant difference in OSBM was observed between those with non-hemorrhagic and hemorrhagic BMs (p = 0.6, Figure 3). In subgroup analyses, there was no significant difference in OSBM depending on intracranial hemorrhage in patients with single BM (p = 0.42) as well as multiple BMs (p = 0.8, Supplemental Figure S1).
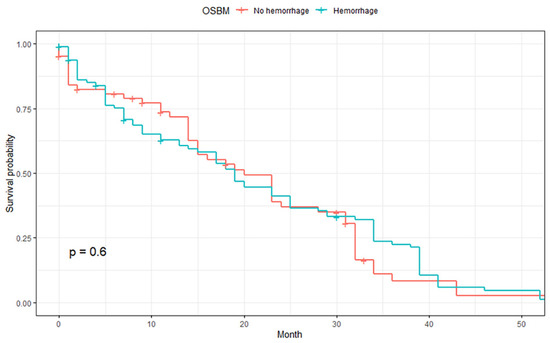
Figure 3.
Comparison of overall survival after brain metastasis (OSBM) between hemorrhagic and non-hemorrhagic BMs.
4. Discussion
In this study, we explored the risk factors for spontaneous hemorrhage in BM from lung adenocarcinoma and assessed whether hemorrhage in BM may affect patient survival. The results indicated that hemorrhage was more likely to occur in larger BMs, and a combination of TKI and radiation therapies increased the risk of hemorrhage in BMs. However, hemorrhage in BMs was not significantly associated with OSBM. These results may contribute to elucidating the pathophysiology of hemorrhage in BMs from lung adenocarcinomas. These results may also have a clinical impact as neurologic deterioration could be prevented by identifying patients with a high risk of developing BM hemorrhage.
The pathophysiology of hemorrhage in BMs remains unclear. One theory is that abnormal tumor vascularization may play an important role as newly formed vessels within the tumor mass are characterized by numerous structural abnormalities and may be thin-walled, poorly formed, or dilated, leading to their dysfunction [22]. In metastatic brain tumors, vascular endothelial growth factor (VEGF) and metalloproteinase 2-mediated hypoxic signaling pathway may result in the loss of vascular integrity, leading to tumor-associated hemorrhage and necrosis [23,24]. The results of a previous study are similar to our finding that bleeding occurrence may increase with tumor size [25]. We infer that after increasing to a certain size, the tumor experiences hypoxia, driving the production of angiogenic factors such as VEGF. Therefore, larger BMs may be more vulnerable to hemorrhage [26].
There have been contradictory findings on the relationship between radiation therapy and the risk of hemorrhage. A study identified hemorrhage in 7.4% of BMs before radiosurgery and in 18.5% of BMs after radiosurgery, suggesting that stereotactic radiosurgery (SRS) may increase the risk of hemorrhage in BMs [27]. Moreover, intratumor hemorrhage has been reported after radiosurgery in patients with BMs [28,29]. In contrast, other studies have shown that radiosurgery is not significantly related with hemorrhage in BMs [30,31]. Furthermore, whole-brain radiation therapy (WBRT) alone has been found to decrease hemorrhagic events [32,33]. The results of our study suggest that patients undergoing prior radiation therapy were more likely to have a hemorrhage in BMs than patients who did not undergo prior radiation therapy, although this result was not statistically significant. The tendency for hemorrhage after radiation therapy could be due to endothelial cell damage caused by the irradiation, resulting in the release of various cytokines, which may produce thrombotic lesions or disrupt the blood–brain barrier, leading to hemorrhage [34]. Additionally, an increase in the intravascular outflow resistance, resulting from radiation-induced venous obliteration, could promote the occurrence of hemorrhage [35].
We also found that EGFR-TKI therapy was associated with hemorrhage in BMs from lung adenocarcinomas, particularly when combined with radiation therapy. In a case report of two patients with lung cancer, hemorrhagic BMs developed 1 month after a combination of EGFR-TKI and radiation therapies [36]. Thus, the combination of EGFR-TKI and radiation therapies may be the cause of hemorrhage in BMs. Increasing evidence has demonstrated that EGFR-TKIs are a radiation sensitizer in the treatment of NSCLC, head and neck, breast, and colorectal cancers [37,38]. We believe that EGFR-TKI may reinforce the role of radiation-induced endothelial injury and subsequent hemorrhage. In addition, although statistically not significant, more patients with hemorrhagic BMs received 3rd generation TKI compared with those with non-hemorrhagic BMs (24.5% vs. 0.0%, p = 0.14, Supplemental Table S1). This finding is noticeable as 3rd generation TKI has a better brain penetrance than 1st and 2nd generation TKIs [39]. However, this hypothesis requires further validation in future studies with larger population.
Bleeding risk varies depending on histologic diagnosis. Hemorrhage is most commonly observed in melanoma BM, with the hemorrhage rate ranging from 9% to 30% [30,40]. BMs from renal carcinoma, choriocarcinoma, and papillary carcinoma of the thyroid also frequently exhibit intratumoral hemorrhage [11,13,41]. A few studies have reported on hemorrhage in lung cancer BMs. A previous study reported that the incidence of hemorrhage in NSCLC BM is low (approximately 1.2%); however, the true incidence of hemorrhage is likely higher than that reported [42]. Our study demonstrated that the incidence of hemorrhage was 60.1%. There are a number of explanations for the large discrepancy between the two studies. First, this study used the SWAN sequence for the detection of hemorrhage, which is sensitive to bleeding. The results of another study using the susceptibility-weighted imaging sequence also support these findings; 68.9% of patients with lung cancer BM showed intratumoral hemorrhage [43]. Second, the size of BM used in this study was larger than that of the previous study as we excluded small BMs (with the longest diameter less than 5 mm). Third, it is unlikely that the previous study accounted for the effect of a combination of EGFR-TKI and radiation therapies because EGFR-TKIs have been recently implemented.
Hypertension, current smoking and antithrombogenic medication are significant risk factors for intracranial hemorrhage [14,15,44,45,46]. However, our study demonstrated that these clinical factors are not associated with intratumor hemorrhage of BM. Previous studies also reported that there is no additional risk for intracranial hemorrhage attributed to the use of anticoagulation in patients with BMs [47,48]. We may speculate that this phenomenon can be explained by different vasculature of BMs from that of normal tissue as well as primary lung cancer [49].
Intratumor hemorrhage could be an after effect of rapid tumor growth, but it could also be a response to therapy. The effect of BM hemorrhage on patient prognosis is clinically important. However, according to the results of this study, there was no significant difference in OSBM between the hemorrhagic and non-hemorrhagic groups.
This study had limitations. First, it was a retrospective case-control study and might not be sufficient to relate BM hemorrhage to particular exposures. A prospective longitudinal study is required to support our results. Second, our results apply to “Evaluable BM” with the longest diameter >5 mm. Thus, the relationship between hemorrhage and small BMs is still unresolved. Third, to avoid bias from clustered data, we used generalized estimating equation and excluded patients with more than five BMs. Thus, our results might not be applied in patients with more than five BMs. Fourth, although our results indicated that hemorrhage was not associated with OSBM, this should be carefully interpreted because we did not exclude hemorrhage as the cause of death with post-mortem analysis. Finally, the results were obtained from a single center and may have limited generalizability.
5. Conclusions
We demonstrated that hemorrhage in BMs from NSCLC adenocarcinomas may be associated with larger BM size and combination therapy involving intracranial radiation exposure and TKI use. However, hemorrhage was not associated with patient prognosis. These findings may help explain the mechanism by which hemorrhage occurs in lung adenocarcinoma BM and aid clinicians in preventing BM hemorrhage or its aggravation. Particularly, a clinician is required to closely monitor patient with large BMs of if they have received combination therapy of EGFR-TKI and radiation therapy. However, stopping the antithrombotic agent may not be necessary to prevent BM hemorrhage.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15030619/s1, Table S1: Comparison of TKI subtypes between non-hemorrhagic and hemorrhagic BMs; Figure S1: Overall survival after brain metastasis (OSBM) depending on intracranial hemorrhage in patients with single BM (A) and multiple BMs (B).
Author Contributions
Conceptualization, S.J.A.; methodology, S.S.K.; software, M.P.; validation, B.J.; formal analysis, S.S.K.; investigation, S.S.K. and S.L.; resources, S.L.; data curation, S.L.; writing—original draft preparation, S.S.K. and S.L.; writing—review and editing, S.J.A. and S.H.S.; supervision, S.J.A.; funding acquisition, S.J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1F1A1056512), a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI20C2125), and a grant from the Central Medical Service (CMS) Research Fund to S.J.A.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by Gangnam Severance Hospital (3-2021-0418).
Informed Consent Statement
Patient consent was waived by the Institutional Review Board due to the retrospective nature of the study.
Data Availability Statement
The data can be shared up on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gavrilovic, I.T.; Posner, J.B. Brain metastases: Epidemiology and pathophysiology. J. Neuro-Oncol. 2005, 75, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Villano, J.L.; Durbin, E.B.; Normandeau, C.; Thakkar, J.P.; Moirangthem, V.; Davis, F.G. Incidence of brain metastasis at initial presentation of lung cancer. Neuro-Oncology 2015, 17, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Hochstenbag, M.M.; Twijnstra, A.; Hofman, P.; Wouters, E.F.; ten Velde, G.P. Mr-imaging of the brain of neurologic asymptomatic patients with large cell or adenocarcinoma of the lung. Does it influence prognosis and treatment? Lung Cancer 2003, 42, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Moro-Sibilot, D.; Smit, E.; de Castro Carpeno, J.; Lesniewski-Kmak, K.; Aerts, J.G.; Villatoro, R.; Kraaij, K.; Nacerddine, K.; Dyachkova, Y.; Smith, K.T.; et al. Non-small cell lung cancer patients with brain metastases treated with first-line platinum-doublet chemotherapy: Analysis from the european frame study. Lung Cancer 2015, 90, 427–432. [Google Scholar] [CrossRef]
- Waqar, S.N.; Samson, P.P.; Robinson, C.G.; Bradley, J.; Devarakonda, S.; Du, L.; Govindan, R.; Gao, F.; Puri, V.; Morgensztern, D. Non-small-cell lung cancer with brain metastasis at presentation. Clin. Lung Cancer 2018, 19, e373–e379. [Google Scholar] [CrossRef]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The effect of advances in lung-cancer treatment on population mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Besse, B.; Le Moulec, S.; Mazieres, J.; Senellart, H.; Barlesi, F.; Chouaid, C.; Dansin, E.; Berard, H.; Falchero, L.; Gervais, R.; et al. Bevacizumab in patients with nonsquamous non-small cell lung cancer and asymptomatic, untreated brain metastases (brain): A nonrandomized, phase ii study. Clin. Cancer Res. 2015, 21, 1896–1903. [Google Scholar] [CrossRef]
- Maiuri, F.; D'Andrea, F.; Gallicchio, B.; Carandente, M. Intracranial hemorrhages in metastatic brain tumors. J. Neurosurg. Sci. 1985, 29, 37–41. [Google Scholar]
- Kim, S.S.; Kim, S.M.; Park, M.; Suh, S.H.; Ahn, S.J. Clinico-radiological features of brain metastases from thyroid cancer. Medicine 2021, 100, e28069. [Google Scholar] [CrossRef] [PubMed]
- Zoga, E.; Wolff, R.; Ackermann, H.; Meissner, M.; Rodel, C.; Tselis, N.; Chatzikonstantinou, G. Factors associated with hemorrhage of melanoma brain metastases after stereotactic radiosurgery in the era of targeted/immune checkpoint inhibitor therapies. Cancers 2022, 14, 2391. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, J.W.; Chung, H.T.; Paek, S.H.; Kim, D.G.; Jung, H.W. Brain metastasis from renal cell carcinoma. Prog. Neurol. Surg. 2012, 25, 163–175. [Google Scholar]
- Ariesen, M.J.; Claus, S.P.; Rinkel, G.J.; Algra, A. Risk factors for intracerebral hemorrhage in the general population: A systematic review. Stroke 2003, 34, 2060–2065. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Whelton, P.K.; Vu, B.; Klag, M.J. Aspirin and risk of hemorrhagic stroke: A meta-analysis of randomized controlled trials. JAMA 1998, 280, 1930–1935. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Rajan, A.; Giaccone, G. Tyrosine kinase inhibitors in lung cancer. Hematol./Oncol. Clin. North Am. 2012, 26, 589–605. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Perez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, pd-l1-positive, advanced non-small-cell lung cancer (keynote-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Rangachari, D.; Yamaguchi, N.; VanderLaan, P.A.; Folch, E.; Mahadevan, A.; Floyd, S.R.; Uhlmann, E.J.; Wong, E.T.; Dahlberg, S.E.; Huberman, M.S.; et al. Brain metastases in patients with egfr-mutated or alk-rearranged non-small-cell lung cancers. Lung Cancer 2015, 88, 108–111. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The eighth edition ajcc cancer staging manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Weng, C.L.; Jeng, Y.; Li, Y.T.; Chen, C.J.; Chen, D.Y. Black dipole or white dipole: Using susceptibility phase imaging to differentiate cerebral microbleeds from intracranial calcifications. AJNR Am. J. Neuroradiol. 2020, 41, 1405–1413. [Google Scholar] [CrossRef]
- Højsgaard, S.; Halekoh, U.; Yan, J. The r package geepack for generalized estimating equations. J. Stat. Softw. 2006, 15, 1–11. [Google Scholar]
- Guyon, J.; Chapouly, C.; Andrique, L.; Bikfalvi, A.; Daubon, T. The normal and brain tumor vasculature: Morphological and functional characteristics and therapeutic targeting. Front. Physiol. 2021, 12, 622615. [Google Scholar] [CrossRef] [PubMed]
- Suri, M.F.; Vazquez, G.; Ezzeddine, M.A.; Qureshi, A.I. A multicenter comparison of outcomes associated with intravenous nitroprusside and nicardipine treatment among patients with intracerebral hemorrhage. Neurocrit. Care 2009, 11, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Moon, K.S.; Jung, T.Y.; Kim, I.Y.; Lee, Y.H.; Rhu, H.H.; Sun, H.S.; Jeong, Y.I.; Kim, K.K.; Kang, S.S. Possible pathophysiological role of vascular endothelial growth factor (vegf) and matrix metalloproteinases (mmps) in metastatic brain tumor-associated intracerebral hemorrhage. J. Neuro-Oncol. 2006, 76, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Schafer, N.; Bode, C.; Borger, V.; Potthoff, A.L.; Eichhorn, L.; Giordano, F.A.; Guresir, E.; Heimann, M.; Ko, Y.D.; et al. Preoperative metastatic brain tumor-associated intracerebral hemorrhage is associated with dismal prognosis. Front. Oncol. 2021, 11, 699860. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Toyoda, S.; Muramatsu, M.; Shimizu, T.; Kojima, T.; Taki, W. Spontaneous haemorrhage into metastatic brain tumours after stereotactic radiosurgery using a linear accelerator. J. Neurol. Neurosurg. Psychiatry 2003, 74, 908–912. [Google Scholar] [CrossRef]
- Suzuki, S.; Omagari, J.; Nishio, S.; Nishiye, E.; Fukui, M. Gamma knife radiosurgery for simultaneous multiple metastatic brain tumors. J. Neurosurg. 2000, 93 (Suppl. S3), 30–31. [Google Scholar] [CrossRef]
- Kim, D.G.; Chung, H.T.; Gwak, H.S.; Paek, S.H.; Jung, H.W.; Han, D.H. Gamma knife radiosurgery for brain metastases: Prognostic factors for survival and local control. J. Neurosurg. 2000, 93 (Suppl. S3), 23–29. [Google Scholar] [CrossRef]
- Redmond, A.J.; Diluna, M.L.; Hebert, R.; Moliterno, J.A.; Desai, R.; Knisely, J.P.; Chiang, V.L. Gamma knife surgery for the treatment of melanoma metastases: The effect of intratumoral hemorrhage on survival. J. Neurosurg. 2008, 109, 99–105. [Google Scholar] [CrossRef]
- Mathieu, D.; Kondziolka, D.; Cooper, P.B.; Flickinger, J.C.; Niranjan, A.; Agarwala, S.; Kirkwood, J.; Lunsford, L.D. Gamma knife radiosurgery for malignant melanoma brain metastases. Clin. Neurosurg. 2007, 54, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Raben, D.; Helfrich, B. Angiogenesis inhibitors: A rational strategy for radiosensitization in the treatment of non-small-cell lung cancer? Clin. Lung Cancer 2004, 6, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Andres, M.L.; Timiryasova, T.M.; Fodor, I.; Slater, J.M.; Gridley, D.S. Radiation-enhanced endostatin gene expression and effects of combination treatment. Technol. Cancer Res. Treat. 2005, 4, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Rubin, P.; Gash, D.M.; Hansen, J.T.; Nelson, D.F.; Williams, J.P. Disruption of the blood-brain barrier as the primary effect of cns irradiation. Radiother. Oncol. 1994, 31, 51–60. [Google Scholar] [CrossRef]
- Park, C.-K.; Kim, D.G.; Gwak, H.-S.; Chung, H.-T.; Paek, S.H. Case report: Intracerebral hemorrhage after γ-knife surgery for metastatic brain tumor. J. Radiosurg. 2000, 3, 17–20. [Google Scholar] [CrossRef]
- Yan, D.F.; Yan, S.X.; Yang, J.S.; Wang, Y.X.; Sun, X.L.; Liao, X.B.; Liu, J.Q. Hemorrhage of brain metastasis from non-small cell lung cancer post gefitinib therapy: Two case reports and review of the literature. BMC Cancer 2010, 10, 49. [Google Scholar] [CrossRef]
- Ochs, J.S. Rationale and clinical basis for combining gefitinib (iressa, zd1839) with radiation therapy for solid tumors. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 941–949. [Google Scholar] [CrossRef]
- Bianco, C.; Tortora, G.; Bianco, R.; Caputo, R.; Veneziani, B.M.; Caputo, R.; Damiano, V.; Troiani, T.; Fontanini, G.; Raben, D.; et al. Enhancement of antitumor activity of ionizing radiation by combined treatment with the selective epidermal growth factor receptor-tyrosine kinase inhibitor zd1839 (iressa). Clin. Cancer Res. 2002, 8, 3250–3258. [Google Scholar]
- Colclough, N.; Chen, K.; Johnstrom, P.; Strittmatter, N.; Yan, Y.; Wrigley, G.L.; Schou, M.; Goodwin, R.; Varnas, K.; Adua, S.J.; et al. Preclinical comparison of the blood-brain barrier permeability of osimertinib with other egfr tkis. Clin. Cancer Res. 2021, 27, 189–201. [Google Scholar] [CrossRef]
- Liew, D.N.; Kano, H.; Kondziolka, D.; Mathieu, D.; Niranjan, A.; Flickinger, J.C.; Kirkwood, J.M.; Tarhini, A.; Moschos, S.; Lunsford, L.D. Outcome predictors of gamma knife surgery for melanoma brain metastases. Clinical article. J. Neurosurg. 2011, 114, 769–779. [Google Scholar] [CrossRef]
- Kidd, D.; Plant, G.T.; Scaravilli, F.; McCartney, A.C.; Stanford, M.; Graham, E.M. Metastatic choriocarcinoma presenting as multiple intracerebral haemorrhages: The role of imaging in the elucidation of the pathology. J. Neurol. Neurosurg. Psychiatry 1998, 65, 939–941. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.; Rana, V.; Wallace, S.; Taylor, S.; Debnam, M.; Feng, L.; Suki, D.; Karp, D.; Stewart, D.; Oh, Y. Risk of intracranial hemorrhage and cerebrovascular accidents in non-small cell lung cancer brain metastasis patients. J. Thorac. Oncol. 2009, 4, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, X.X.; Ji, Y.M.; Kang, X.S.; Li, C.F. Haemorrhage detection in brain metastases of lung cancer patients using magnetic resonance imaging. J. Int. Med. Res. 2009, 37, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Preusser, M. Anti-angiogenic therapies in brain metastases. memo-Mag. Eur. Med. Oncol. 2018, 11, 14–17. [Google Scholar] [CrossRef]
- Martini, S.R.; Flaherty, M.L.; Brown, W.M.; Haverbusch, M.; Comeau, M.E.; Sauerbeck, L.R.; Kissela, B.M.; Deka, R.; Kleindorfer, D.O.; Moomaw, C.J.; et al. Risk factors for intracerebral hemorrhage differ according to hemorrhage location. Neurology 2012, 79, 2275–2282. [Google Scholar] [CrossRef]
- Flaherty, M.L.; Tao, H.; Haverbusch, M.; Sekar, P.; Kleindorfer, D.; Kissela, B.; Khatri, P.; Stettler, B.; Adeoye, O.; Moomaw, C.J.; et al. Warfarin use leads to larger intracerebral hematomas. Neurology 2008, 71, 1084–1089. [Google Scholar] [CrossRef]
- Donato, J.; Campigotto, F.; Uhlmann, E.J.; Coletti, E.; Neuberg, D.; Weber, G.M.; Zwicker, J.I. Intracranial hemorrhage in patients with brain metastases treated with therapeutic enoxaparin: A matched cohort study. Blood 2015, 126, 494–499. [Google Scholar] [CrossRef]
- Alvarado, G.; Noor, R.; Bassett, R.; Papadopoulos, N.E.; Kim, K.B.; Hwu, W.J.; Bedikian, A.; Patel, S.; Hwu, P.; Davies, M.A. Risk of intracranial hemorrhage with anticoagulation therapy in melanoma patients with brain metastases. Melanoma Res. 2012, 22, 310–315. [Google Scholar] [CrossRef]
- Gerstner, E.R.; Fine, R.L. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: Establishing a treatment paradigm. J. Clin. Oncol. 2007, 25, 2306–2312. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).