Number of Local Regional Therapies for Hepatocellular Carcinoma and Peri-Operative Outcomes after Liver Transplantation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Cohort
2.3. Liver-Directed Therapies
2.4. Peri-Operative Outcomes
2.5. Transplant Eligibility
2.6. Statistical Analyses
2.7. Propensity Score Matched Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Heimbach, J.; Lee, D.; Dodge, J.L.; Harnois, D.; Burns, J.; Sanchez, W.; Roberts, J.P.; Yao, F.Y. Wait Time of Less Than 6 and Greater Than 18 Months Predicts Hepatocellular Carcinoma Recurrence After Liver Transplantation. Transplantation 2017, 101, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Aoki, T.; Inoue, Y.; Kaneko, J.; Sakamoto, Y.; Sugawara, Y.; Hasegawa, K.; Kokudo, N. Outcome of salvage hepatic resection for recurrent hepatocellular carcinoma after radiofrequency ablation therapy. Surgery 2015, 157, 463–472. [Google Scholar] [CrossRef]
- de Haas, R.J.; Lim, C.; Ricci, C.; Lahat, E.; Fuentes, L.; Salloum, C.; Azoulay, D. Local Ablation Does Not Worsen Perioperative Outcomes After Liver Transplant for Hepatocellular Carcinoma. Am. J. Roentgenol. 2019, 213, 702–709. [Google Scholar] [CrossRef]
- Richard, H.M.; Silberzweig, J.E.; Mitty, H.A.; Lou, W.Y.W.; Ahn, J.; Cooper, J.M. Hepatic Arterial Complications in Liver Transplant Recipients Treated with Pretransplantation Chemoembolization for Hepatocellular Carcinoma. Radiology 2000, 214, 775–779. [Google Scholar] [CrossRef]
- Maeda, N.; Osuga, K.; Mikami, K.; Higashihara, H.; Onishi, H.; Nakaya, Y.; Tatsumi, M.; Hori, M.; Kim, T.; Tomoda, K.; et al. Angiographic evaluation of hepatic arterial damage after transarterial chemoembolization for hepatocellular carcinoma. Radiat. Med. 2008, 26, 206–212. [Google Scholar] [CrossRef]
- Goel, A.; Mehta, N.; Guy, J.; Fidelman, N.; Yao, F.; Roberts, J.; Terrault, N. Hepatic artery and biliary complications in liver transplant recipients undergoing pretransplant transarterial chemoembolization. Liver Transplant. 2014, 20, 1221–1228. [Google Scholar] [CrossRef]
- Sneiders, D.; Boteon, A.P.C.S.; Lerut, J.; Iesari, S.; Gilbo, N.; Blasi, F.; Laureiro, Z.L.; Orlacchio, A.; Tisone, G.; Lai, Q.; et al. Transarterial chemoembolization of hepatocellular carcinoma before liver transplantation and risk of post-transplant vascular complications: A multicentre observational cohort and propensity score-matched analysis. Br. J. Surg. 2021, 108, 1323–1331. [Google Scholar] [CrossRef]
- Sneiders, D.; Houwen, T.; Pengel, L.H.; Polak, W.G.; Dor, F.J.M.F.; Hartog, H. Systematic Review and Meta-Analysis of Posttransplant Hepatic Artery and Biliary Complications in Patients Treated With Transarterial Chemoembolization Before Liver Transplantation. Transplantation 2018, 102, 88–96. [Google Scholar] [CrossRef]
- Kim, H.K.; Chung, Y.H.; Song, B.C.; Yang, S.H.; Yoon, H.K.; Yu, E.; Sung, K.-B.; Lee, Y.S.; Lee, S.G.; Suh, D.J. Ischemic Bile Duct Injury as a Serious Complication after Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma. J. Clin. Gastroenterol. 2001, 32, 423–427. [Google Scholar] [CrossRef]
- Clark, T.W.I. Complications of Hepatic Chemoembolization. Semin. Interv. Radiol. 2006, 23, 119–125. [Google Scholar] [CrossRef]
- Sun, Z.; Li, G.; Ai, X.; Luo, B.; Wen, Y.; Zhao, Z.; Dong, S.; Guan, J. Hepatic and biliary damage after transarterial chemoembolization for malignant hepatic tumors: Incidence, diagnosis, treatment, outcome and mechanism. Crit. Rev. Oncol. 2011, 79, 164–174. [Google Scholar] [CrossRef]
- Shiani, A.; Rodriguez, A.; Lipka, S.; Nehaul, R.; Sethi, S.; Snyder, M.; Chirumamilla, S.; Strauss, A.; Valenzuela, M.; Lai, A.; et al. Incidence of Biliary Stricture After Transarterial Chemoembolization for Hepatocellular Carcinoma in Orthotopic Liver Transplant Patients. Am. J. Gastroenterol. 2015, 110, S907–S908. [Google Scholar] [CrossRef]
- Razafindratsira, T.; Isambert, M.; Evrard, S. Complications of intraoperative radiofrequency ablation of liver metastases. Hpb 2011, 13, 15–23. [Google Scholar] [CrossRef]
- Elsayed, M.; Ermentrout, R.M.; Sethi, I.; Bercu, Z.L.; Galt, J.R.; Whitmore, M.; Brandon, D.C.; Schuster, D.M.; Kokabi, N.; Elsayed, M.; et al. Incidence of Radioembolization-Induced Liver Disease and Liver Toxicity Following Repeat 90Y-Radioembolization. Clin. Nucl. Med. 2020, 45, 100–104. [Google Scholar] [CrossRef]
- Mascarenhas, N.B.; Mulcahy, M.F.; Lewandowski, R.J.; Salem, R.; Ryu, R.K. Hepatic Abscess After Yttrium-90 Radioembolization for Islet-Cell Tumor Hepatic Metastasis. Cardiovasc. Interv. Radiol. 2010, 33, 650–653. [Google Scholar] [CrossRef]
- Radunz, S.; Saner, F.H.; Treckmann, J.; Rekowski, J.; Theysohn, J.M.; Müller, S.; Best, J.; Sotiropoulos, G.C.; Paul, A.; Benkö, T. Hepatic artery and biliary complications in liver transplant recipients with radioembolization bridging treatment for hepatocellular carcinoma. Clin. Transplant. 2017, 31, e13096. [Google Scholar] [CrossRef]
- Agopian, V.G.; Harlander-Locke, M.P.; Ruiz, R.M.; Klintmalm, G.B.; Senguttuvan, S.; Florman, S.S.; Haydel, B.; Hoteit, M.; Levine, M.H.; Lee, D.D.; et al. Impact of Pretransplant Bridging Locoregional Therapy for Patients With Hepatocellular Carcinoma Within Milan Criteria Undergoing Liver Transplantation. Ann. Surg. 2017, 266, 525–535. [Google Scholar] [CrossRef]
- Salem, R.; Gordon, A.C.; Mouli, S.; Hickey, R.; Kallini, J.; Gabr, A.; Mulcahy, M.F.; Baker, T.; Abecassis, M.; Miller, F.H.; et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology 2016, 151, 1155–1163.e2. [Google Scholar] [CrossRef]
- Pardo, F.; Sangro, B.; Lee, R.-C.; Manas, D.; Jeyarajah, R.; Donckier, V.; Maleux, G.; Pinna, A.D.; Bester, L.; Morris, D.L.; et al. The Post-SIR-Spheres Surgery Study (P4S): Retrospective Analysis of Safety Following Hepatic Resection or Transplantation in Patients Previously Treated with Selective Internal Radiation Therapy with Yttrium-90 Resin Microspheres. Ann. Surg. Oncol. 2017, 24, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Forde, J.J.; Bhamidimarri, K.R. Management of Biliary Complications in Liver Transplant Recipients. Clin. Liver Dis. 2022, 26, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.-H.; Ikegami, T.; Balci, D.; Bhangui, P. Biliary reconstruction and complications in living donor liver transplantation. Int. J. Surg. 2020, 82, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Seehofer, D.; Eurich, D.; Veltzke-Schlieker, W.; Neuhaus, P. Biliary Complications After Liver Transplanation: Old Problems and New Challenges. Am. J. Transplant. 2013, 13, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Teh, C.S.C.; Ishizawa, T.; Aoki, T.; Cavallucci, D.; Lee, S.-Y.; Panganiban, K.M.; Perini, M.V.; Shah, S.R.; Wang, H.; et al. Consensus Guidelines for the Use of Fluorescence Imaging in Hepatobiliary Surgery. Ann. Surg. 2021, 274, 97–106. [Google Scholar]
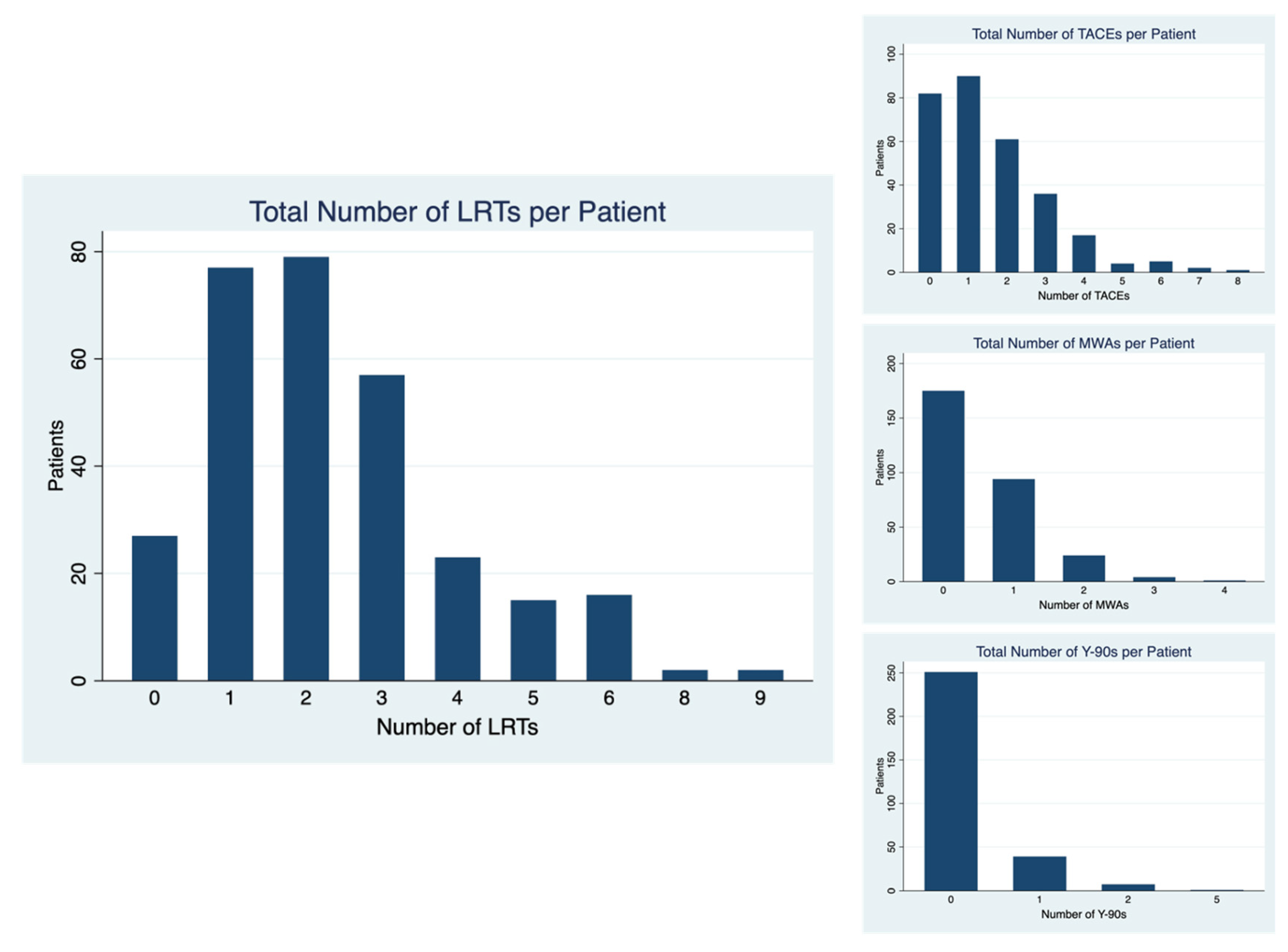
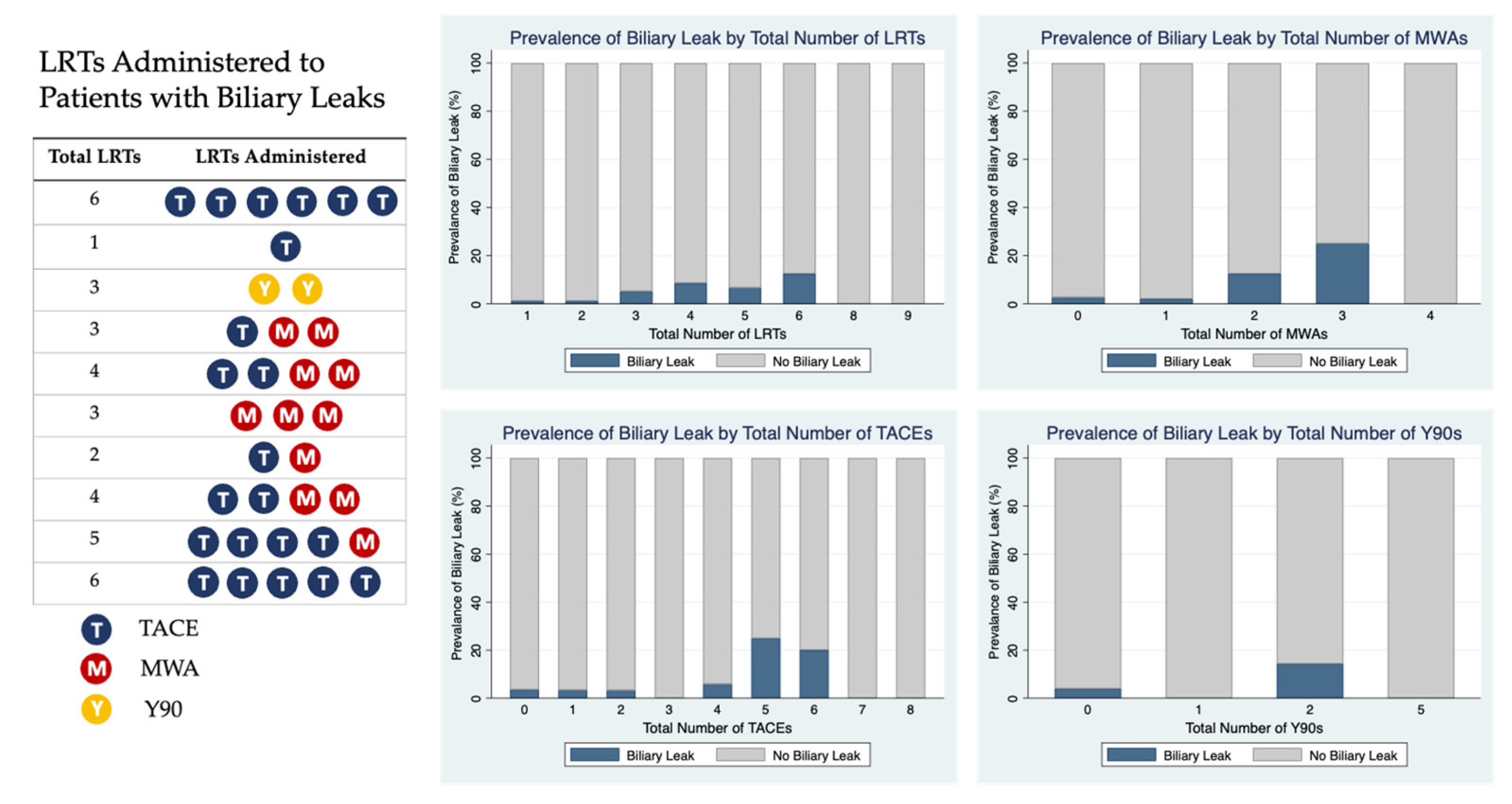
| Number of LRTs | 0 (n = 27) | 1–2 (n = 156) | 3+ (n = 115) | p-Value |
|---|---|---|---|---|
| Age (median, IQR) | 59 (56, 64) | 63 (58, 67) | 64 (61, 68) | 0.007 * |
| Gender | ||||
| Male (%) | 19 (70.4%) | 125 (80.1%) | 85 (73.9%) | 0.34 |
| Female (%) | 8 (29.6%) | 31 (19.9%) | 30 (26.1%) | |
| Etiology of Liver Disease | ||||
| HCV (%) | 6 (22.2%) | 76 (48.7%) | 68 (59.1%) | 0.002 * |
| HBV (%) | 3 (11.1%) | 25 (16.0%) | 18 (15.7%) | 0.81 |
| ETOH (%) | 9 (33.3%) | 28 (17.9%) | 16 (13.9%) | 0.06 |
| NASH (%) | 8 (29.6%) | 25 (16.0%) | 17 (14.8%) | 0.17 |
| Other | 3 (11.1%) | 13 (8.3%) | 10 (8.7%) | 0.89 |
| HCC at Diagnosis | ||||
| Number of tumors (median, IQR) | 1 (0, 1) | 1 (1, 1) | 1 (1, 2) | 0.0003 * |
| Size of largest tumor (cm) (median, IQR) | 2 (0, 2.7) | 2.4 (2.1, 3) | 2.7 (2, 3.9) | 0.0004 * |
| HCC Diagnostic Criteria | ||||
| No HCC diagnosed prior to LT (%) | 8 (28.6%) | 0 (0%) | 1 (0.9%) | <0.0001 * |
| Within Milan (%) | 17 (63.0%) | 149 (95.5%) | 82 (71.3%) | |
| Downstaging Criteria (%) | 1 (3.7%) | 5 (3.2%) | 23 (20.0%) | |
| All-Comers (%) | 1 (3.7%) | 2 (1.3%) | 9 (7.8%) | |
| BMI (median, IQR) | 28.0 (25.1, 31.4) | 28.6 (25.5, 32.1) | 26.6 (23.7, 31.0) | 0.09 |
| Labs at Time of Listing | ||||
| MELD (median, IQR) | 21 (17, 28) | 11 (8, 14) | 10 (7, 12) | 0.0001 * |
| Total Bilirubin (median, IQR) | 4.7 (2.4, 5.8) | 1.4 (0.8, 2.5) | 1.2 (0.8, 1.7) | 0.0001 * |
| Albumin (median, IQR) | 2.6 (2.2, 3.0) | 3.5 (3.4, 3.6) | 3.5 (3.3, 3.6) | 0.16 |
| Platelets (median, IQR) | 66 (54, 79) | 91 (59, 138) | 86 (60.5, 153.5) | 0.20 |
| AFP (median, IQR) | 6.8 (3.3, 31.4) | 5.1 (2.9, 10.9) | 9.6 (4.7, 28.4) | 0.0007 * |
| Graft Type | ||||
| OLT (%) | 21 (77.8%) | 141 (90.4%) | 108 (93.9%) | 0.04 |
| LDLT (%) | 6 (22.2%) | 15 (9.6%) | 7 (6.1%) |
| Number of LRTs | 0 (n = 27) | 1–2 (n = 156) | 3+ (n = 115) | p-Value |
|---|---|---|---|---|
| EBL (mL) (median, IQR) | 5500 (2500, 9400) | 2000 (1000, 4050) | 2000 (1000, 3250) | 0.0001 * |
| pRBCs (units) (median, IQR) | 7 (3, 10) | 1 (0, 4) | 2 (0, 4) | 0.0001 * |
| FFP (units) (median, IQR) | 14 (7, 21) | 4.5 (1.5, 13) | 5 (2, 9) | 0.0001 * |
| Platelets (units) (median, IQR) | 3 (1, 4) | 1 (0, 3) | 1 (0, 2) | 0.0001 * |
| Crystalloid (mean, 95% CI) | 1478 (1167, 1788) | 1585 (1463, 1708) | 1565 (1417, 1713) | 0.81 |
| Albumin (ml) (median, IQR) | 1000 (0, 1500) | 1500 (625, 2000) | 1576 (1405, 1746) | 0.04 * |
| Urine Output (ml) (median, IQR) | 800 (250, 1200) | 705 (450, 1075) | 650 (375, 1100) | 0.79 |
| Extubation in the OR (%) | 16 (59.3%) | 115 (73.7%) | 85 (73.9%) | 0.15 |
| Case Duration (minutes) (median, IQR) | 539 (480, 622) | 457 (398, 535) | 447 (386, 537) | 0.02 * |
| Cold Ischemia Time (hours) (median, IQR) | 6.2 (4.0, 8.0) | 7.4 (5.8, 9.0) | 7.0 (6.0, 8.8) | 0.16 |
| Biliary Anastomosis (% Complex or Roux-en-Y HJ) | 7 (25.9%) | 12 (7.7%) | 6 (5.2%) | 0.02 * |
| Venous Outflow (% Bicaval) | 3 (11.1%) | 13 (8.3%) | 13 (11.3%) | 0.69 |
| Number of LRTs | 0 (n = 27) | 1–2 (n = 156) | 3+ (n = 115) | p-Value |
|---|---|---|---|---|
| Hospital Stay | ||||
| Overall LOS (days) (median, IQR) | 9.5 (6, 13) | 7 (5, 9) | 7 (5, 9) | 0.01 * |
| ICU LOS (days) (median, IQR) | 2 (1, 4) | 2 (1, 3) | 2 (1, 2) | 0.14 |
| pRBCs within 24 h (units) (median, IQR) | 7 (3, 10) | 1 (0, 4.5) | 1 (0, 3) | 0.0001 * |
| Need for reoperation (same hospital stay) | 4 (14.8%) | 13 (8.3%) | 11 (9.6%) | 0.57 |
| Labs at time of Discharge | ||||
| Final INR (median, IQR) | 1.9 (1.3, 2.8) | 1.4 (1.2, 1.8) | 1.3 (1.1, 1.6) | 0.0001 * |
| Final Bilirubin (median, IQR) | 5.6 (2.8, 19.6) | 1. (0.9, 2.9) | 1.5 (0.8, 2.1) | 0.0001 * |
| Final Sodium (median, IQR) | 134 (131, 137) | 137 (137, 138) | 137 (136, 139) | 0.0009 * |
| Final Creatinine (median, IQR) | 1.1 (0.9, 1.4) | 0.9 (0.8, 1.1) | 0.9 (0.7, 1.1) | 0.03* |
| Complications | ||||
| Organ Space SSI | 1 (3.7%) | 2 (1.9%) | 3 (2.6%) | 0.83 |
| Wound Dehiscence | 0 | 0 | 1 (0.9%) | 0.45 |
| Number of Readmissions (median, IQR) | 1 (0, 3) | 0 (0, 1) | 0 (0, 1) | 0.13 |
| Reoperation (overall) | 8 (29.6%) | 16 (10.3%) | 14 (12.2%) | 0.02 * |
| Biliary Stricture | 8 (29.6%) | 30 (19.2%) | 20 (17.4%) | 0.35 |
| Biliary Leak | 3 (11.1%) | 2 (1.3%) | 8 (7.0%) | 0.02 * |
| Biliary Complication requiring ERCP | 9 (33.3%) | 30 (19.2%) | 24 (20.9%) | 0.25 |
| Biliary Leak requiring IR Drain | 2 (7.4%) | 2 (1.3%) | 6 (5.2%) | 0.10 |
| Arterial Stenosis | 0 | 11 (7.1%) | 3 (2.6%) | 0.11 |
| Arterial Stenosis requiring Angioplasty | 0 | 9 (5.8%) | 3 (2.6%) | 0.23 |
| Venous Stenosis | 2 (7.4%) | 7 (4.5%) | 3 (2.6%) | 0.48 |
| Venous Stenosis requiring Angioplasty | 1 (3.7%) | 7 (4.5%) | 2 (1.7%) | 0.46 |
| Linear or Median Regression Outcome | Coefficient | 95% CI | p-Value |
|---|---|---|---|
| Peri-operative | |||
| EBL (ml) * | 83.3 | (−71.4, 238.0) | 0.29 |
| pRBCs (units) * | 0.25 | (−0.12, 0.62) | 0.19 |
| FFP (units) * | 0 | (−0.57, 0.57) | >0.99 |
| Platelets (units) * | 1.74 | (−2.51, 5.99) | 0.42 |
| Case Duration (minutes) * | −2.03 | (−16.6, 12.6) | 0.79 |
| Cold Ischemia Time (hours) * | −0.78 | (−2.5, 0.9) | 0.37 |
| Hospital Stay | |||
| Overall Length of Stay (days) * | 0 | (−0.26, 0.26) | >0.99 |
| ICU Length of Stay (days) | 0 | (−0.12, 0.12) | >0.99 |
| pRBCs within 24 h (units) | 0 | (−0.25, 0.25) | >0.99 |
| Complications | |||
| Number of Readmissions | −0.04 | (−0.13, 0.04) | 0.34 |
| Total Subsequent Operations & Procedures | 0.08 | (−0.08, 0.24) | 0.30 |
| Logistic Regression Outcome | OR | 95% CI | p-Value |
| Anatomic | |||
| Biliary Anastomosis (Main Duct-choledocholedochostomy vs. Complex or Roux-en-Y HJ) | 1.01 | (0.75, 1.36) | 0.96 |
| Venous Outflow (Piggyback vs. Bicaval) | 1.20 | (0.96, 1.50) | 0.11 |
| Complications | |||
| Reoperation (overall) | 1.06 | (0.84, 1.33) | 0.61 |
| Biliary Stricture | 1.04 | (0.86, 1.26) | 0.67 |
| Biliary Leak | 1.4 | (1.03, 1.90) | 0.03 ** |
| Biliary Complication requiring ERCP | 1.11 | (0.93, 1.32) | 0.26 |
| Biliary Leak requiring IR Drain | 1.45 | (1.04, 2.02) | 0.03 ** |
| Arterial Stenosis | 0.72 | (0.46, 1.14) | 0.16 |
| Arterial Stenosis requiring angioplasty | 0.72 | (0.44, 1.18) | 0.19 |
| Venous Stenosis | 0.92 | (0.60, 1.41) | 0.70 |
| Venous Stenosis requiring angioplasty | 0.72 | (0.41, 1.27) | 0.26 |
| Unadjusted Logistic Regression Models for Biliary Leak vs. Risk Factor | |||
| Risk Factor | OR | 95% CI | p-Value |
| Total Number of LRTs | 1.40 | (1.03, 1.90) | 0.03 * |
| Total TACEs | 1.22 | (0.85, 1.76) | 0.28 |
| Total MWAs | 2.06 | (1.06, 3.99) | 0.03 * |
| Total Y-90s | 0.95 | (0.28, 3.23) | 0.93 |
| Age | 1.00 | (0.91, 1.10) | 0.93 |
| Donor Age | 0.95 | (0.91, 0.99) | 0.03 * |
| MELD | 1.04 | (0.94, 1.15) | 0.42 |
| Cold Ischemia Time | 0.95 | (0.75, 1.21) | 0.69 |
| Case Duration | 1.00 | (0.99, 1.00) | 0.07 |
| Estimated Blood Loss | 1.00 | (1.00, 1.00) | 0.70 |
| Intra-op pRBCs (units) | 1.03 | (0.96, 1.11) | 0.37 |
| Arterial Stenosis | 2.12 | (0.25, 18.0) | 0.49 |
| LDLT | 5.46 | (1.31, 22.8) | 0.02 * |
| Biliary Anastomosis (Main Duct-choledochole-dochostomy vs. Complex or Roux-en-Y HJ) | 3.83 | (0.75, 19.5) | 0.11 |
| Logistic Regression Models for Biliary Leak vs. Total Number of LRTs, Controlling for Potential Confounders | |||
| Risk Factor | OR | 95% CI | p-Value |
| Total Number of LRTs | 1.43 | (1.04, 1.96) | 0.03 * |
| Biliary Anastomosis (Main Duct-choledochole-dochostomy vs. Complex or Roux-en-Y HJ) | 4.23 | (0.80, 22.3) | 0.09 |
| Total Number of LRTs | 1.44 | (1.05, 1.98) | 0.02 * |
| LDLT status | 6.41 | (1.46, 28.2) | 0.01 * |
| Total Number of LRTs | 1.43 | (1.04, 1.96) | 0.03 * |
| Arterial Stenosis | 3.07 | (0.34, 27.8) | 0.32 |
| Total Number of LRTs | 1.46 | (1.06, 2.02) | 0.02 * |
| MELD | 1.07 | (0.96, 1.19) | 0.22 |
| Total Number of LRTs | 1.36 | (1.00, 1.86) | 0.05 * |
| Donor Age | 0.96 | (0.92, 1.00) | 0.04 * |
| Total Number of LRTs | 1.44 | (1.04, 1.97) | 0.03 * |
| Cold Ischemia Time | 0.93 | (0.73, 1.19) | 0.57 |
| Total Number of LRTs | 1.36 | (1.00, 1.86) | 0.02 * |
| Case Duration | 1.00 | (1.00, 1.00) | 0.05 * |
| Unadjusted Logistic Regression Models for Any Biliary Complications vs. Risk Factor | |||
| Risk Factor | OR | 95% CI | p-Value |
| Total Number of LRTs | 1.10 | (0.93, 1.32) | 0.25 |
| Total TACEs | 1.06 | (0.87, 1.29) | 0.56 |
| Total MWAs | 1.46 | (1.00, 2.12) | 0.05 * |
| Total Y-90s | 0.86 | (0.47, 1.57) | 0.62 |
| Age | 0.99 | (0.95, 1.03) | 0.59 |
| Donor Age | 0.99 | (0.97, 1.01) | 0.19 |
| MELD | 1.08 | (1.03, 1.14) | 0.003 * |
| Cold Ischemia Time | 0.99 | (0.90, 1.08) | 0.75 |
| Case Duration | 1.00 | (1.00, 1.00) | 0.43 |
| Estimated Blood Loss | 1.00 | (1.00, 1.00) | 0.42 |
| Intra-op pRBCs (units) | 1.03 | (0.99, 1.08) | 0.19 |
| Arterial Stenosis | 1.62 | (0.49, 5.36) | 0.43 |
| LDLT | 2.46 | (0.97, 6.19) | 0.06 |
| Biliary Anastomosis (Main Duct-choledochole-dochostomy vs. Complex or Roux-en-Y HJ) | 4.50 | (1.69, 11.96) | 0.003 * |
| Multivariable Logistic Regression Models for Any Biliary Complications | |||
| Risk Factor | OR | 95% CI | p-Value |
| Total Number of LRTs | 1.17 | (0.97, 1.42) | 0.09 |
| Biliary Anastomosis (Main Duct-choledochole-dochostomy vs. Complex or Roux-en-Y HJ) | 4.38 | (1.42, 13.5) | 0.01 * |
| MELD | 1.09 | (1.04, 1.16) | 0.001 * |
| LDLT | 1.33 | (0.44, 4.06) | 0.62 |
| Donor Age | 0.99 | (0.97, 1.01) | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, A.E.; Shui, A.M.; Adelmann, D.; Mehta, N.; Roll, G.R.; Hirose, R.; Syed, S.M. Number of Local Regional Therapies for Hepatocellular Carcinoma and Peri-Operative Outcomes after Liver Transplantation. Cancers 2023, 15, 620. https://doi.org/10.3390/cancers15030620
Brown AE, Shui AM, Adelmann D, Mehta N, Roll GR, Hirose R, Syed SM. Number of Local Regional Therapies for Hepatocellular Carcinoma and Peri-Operative Outcomes after Liver Transplantation. Cancers. 2023; 15(3):620. https://doi.org/10.3390/cancers15030620
Chicago/Turabian StyleBrown, Audrey E., Amy M. Shui, Dieter Adelmann, Neil Mehta, Garrett R. Roll, Ryutaro Hirose, and Shareef M. Syed. 2023. "Number of Local Regional Therapies for Hepatocellular Carcinoma and Peri-Operative Outcomes after Liver Transplantation" Cancers 15, no. 3: 620. https://doi.org/10.3390/cancers15030620
APA StyleBrown, A. E., Shui, A. M., Adelmann, D., Mehta, N., Roll, G. R., Hirose, R., & Syed, S. M. (2023). Number of Local Regional Therapies for Hepatocellular Carcinoma and Peri-Operative Outcomes after Liver Transplantation. Cancers, 15(3), 620. https://doi.org/10.3390/cancers15030620






