Different Outcomes According to Needling Point Location Used in Sham Acupuncture for Cancer-Related Pain: A Systematic Review and Network Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Study Selection and Data Collection
2.4. Risk of Bias Assessment
2.5. Data Analysis and Synthesis
2.6. Certainty of Evidence Assessment
3. Results
3.1. Study Selection and Characteristics
3.2. Risk of Bias Assessment
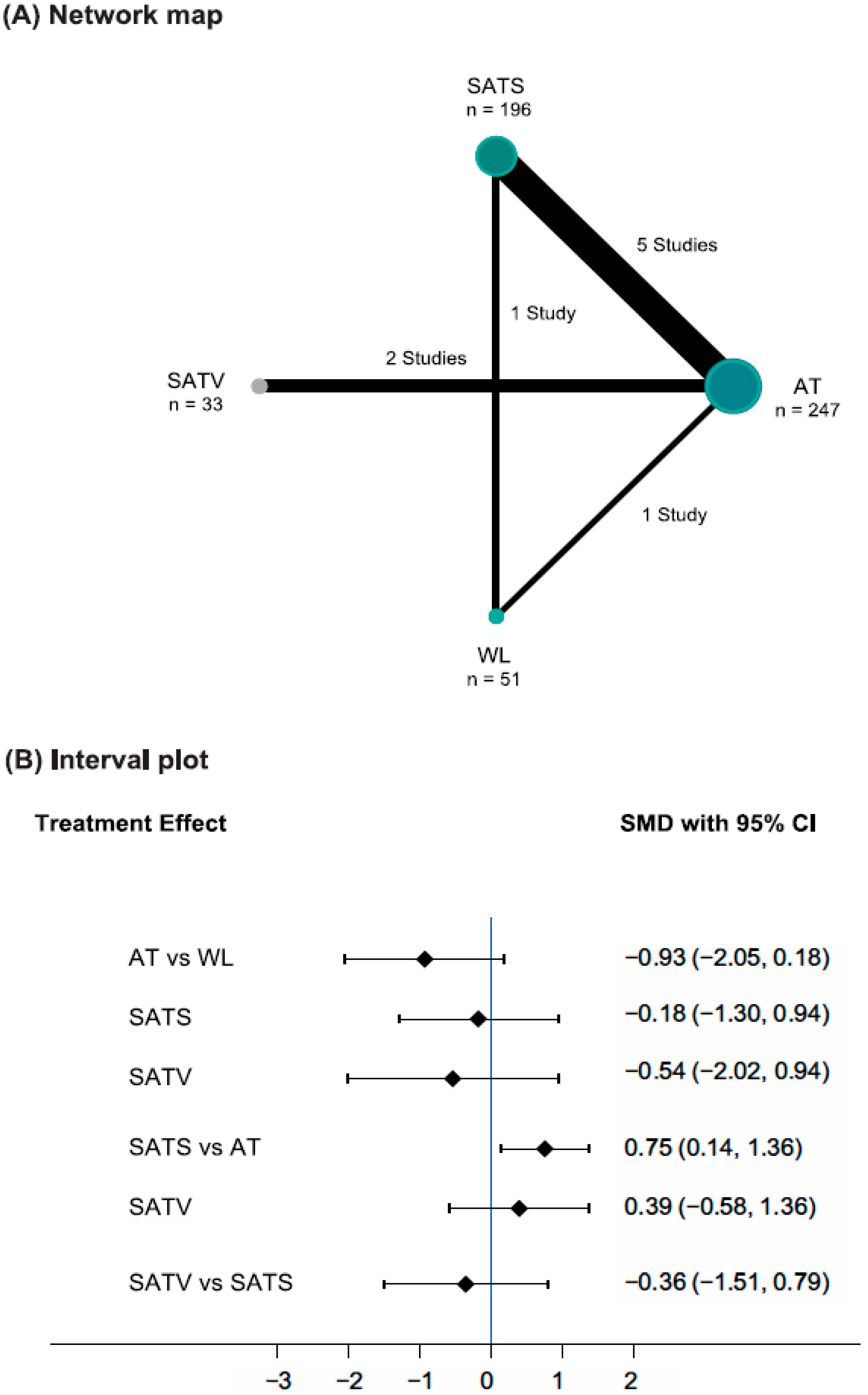
3.3. Data Analysis: Pain Severity
3.4. Certainty of the Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Birch, S.; Lee, M.S.; Kim, T.H.; Alraek, T. Historical perspectives on using sham acupuncture in acupuncture clinical trials. Integr. Med. Res. 2022, 11, 100725. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, M.S.; Lee, H. Sham Acupuncture Is Not Just a Placebo. J. Acupunct. Meridian Stud. 2022, 15, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; White, A.; Lee, H.; Ernst, E. Development of a new sham needle. Acupunct. Med. 1999, 17, 110–112. [Google Scholar] [CrossRef]
- Choi, E.M.; Jiang, F.; Longhurst, J.C. Point specificity in acupuncture. Chin. Med. 2012, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.J.; Zeng, B.Y.; Li, J.; Zhuang, Y.; Liang, F.R. Acupuncture point specificity. Int. Rev. Neurobiol. 2013, 111, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Birch, S.; Alraek, T.; Kim, K.H.; Lee, M.S. Placebo-controlled trials in acupuncture: Problems and solutions. In Evidence-Based Research Methods for Chinese Medicine; Springer: Singapore, 2016; pp. 55–64. [Google Scholar]
- Shi, G.X.; Yang, X.M.; Liu, C.Z.; Wang, L.P. Factors contributing to therapeutic effects evaluated in acupuncture clinical trials. Trials 2012, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Ots, T.; Kandirian, A.; Szilagyi, I.; DiGiacomo, S.M.; Sandner-Kiesling, A. The selection of dermatomes for sham (placebo) acupuncture points is relevant for the outcome of acupuncture studies: A systematic review of sham (placebo)-controlled randomized acupuncture trials. Acupunct. Med. 2020, 38, 211–226. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wang, X.M.; McAlonan, G.M. Neural acupuncture unit: A new concept for interpreting effects and mechanisms of acupuncture. Evid. Based Complement. Alternat. Med. 2012, 2012, 429412. [Google Scholar] [CrossRef]
- Lee, B.; Kwon, C.-Y.; Lee, H.W.; Nielsen, A.; Wieland, L.S.; Kim, T.-H.; Birch, S.; Alraek, T.; Lee, M.S. Needling Point Location used in Sham Acupuncture for Chronic Nonspecific Low Back Pain: A Systematic Review and Network Meta-Analysis. JAMA Network Open 2023, 6, e2332452. [Google Scholar] [CrossRef]
- Lee, B.; Kwon, C.-Y.; Lee, H.W.; Nielsen, A.; Wieland, L.S.; Kim, T.-H.; Birch, S.; Alraek, T.; Lee, M.S. The effect of sham acupuncture can be different according to the points needled in knee osteoarthritis: A systematic review and network meta-analysis. In Proceedings of the 2nd World Congress of Integrative Medicine and Health, Rome, Italy, 20–23 September 2023. in review. [Google Scholar]
- Snijders, R.A.H.; Brom, L.; Theunissen, M.; van den Beuken-van Everdingen, M.H.J. Update on Prevalence of Pain in Patients with Cancer 2022: A Systematic Literature Review and Meta-Analysis. Cancers 2023, 15, 591. [Google Scholar] [CrossRef]
- Höxtermann, M.D.; Haller, H.; Aboudamaah, S.; Bachemir, A.; Dobos, G.; Cramer, H.; Voiss, P. Safety of acupuncture in oncology: A systematic review and meta-analysis of randomized controlled trials. Cancer 2022, 128, 2159–2173. [Google Scholar] [CrossRef] [PubMed]
- Chahin, M.; Matosz, S.; Khalel, I.; Day, S.; Keruakous, A. Pain Management in Oncology Patients Amidst the Opioid Epidemic: How To Minimize Non-Medical Opioid Use. Cureus 2021, 13, e19500. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Wang, Q.; He, Y.; Wu, D.; Zhou, Q.; Xu, N.; Yang, K.; Chen, Y.; Zhang, A.L.; Hua, H.; et al. Acupuncture for cancer pain: An evidence-based clinical practice guideline. Chin. Med. 2022, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Sharfstein, J.M.; Olsen, Y. Lessons Learned From the Opioid Epidemic. JAMA 2019, 322, 809–810. [Google Scholar] [CrossRef] [PubMed]
- Swarm, R.A.; Paice, J.A.; Anghelescu, D.L.; Are, M.; Bruce, J.Y.; Buga, S.; Chwistek, M.; Cleeland, C.; Craig, D.; Gafford, E.; et al. Adult Cancer Pain, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 977–1007. [Google Scholar] [CrossRef] [PubMed]
- Tick, H.; Nielsen, A.; Pelletier, K.R.; Bonakdar, R.; Simmons, S.; Glick, R.; Ratner, E.; Lemmon, R.L.; Wayne, P.; Zador, V. Evidence-Based Nonpharmacologic Strategies for Comprehensive Pain Care: The Consortium Pain Task Force White Paper. Explore 2018, 14, 177–211. [Google Scholar] [CrossRef] [PubMed]
- Wengström, Y.; Geerling, J.; Rustøen, T. European Oncology Nursing Society breakthrough cancer pain guidelines. Eur. J. Oncol. Nurs. 2014, 18, 127–131. [Google Scholar] [CrossRef]
- Mao, J.J.; Ismaila, N.; Bao, T.; Barton, D.; Ben-Arye, E.; Garland, E.L.; Greenlee, H.; Leblanc, T.; Lee, R.T.; Lopez, A.M.; et al. Integrative Medicine for Pain Management in Oncology: Society for Integrative Oncology-ASCO Guideline. J. Clin. Oncol. 2022, 40, 3998–4024. [Google Scholar] [CrossRef]
- Simone, C.B., II; Vapiwala, N.; Hampshire, M.K.; Metz, J.M. Cancer patient attitudes toward analgesic usage and pain intervention. Clin. J. Pain 2012, 28, 157–162. [Google Scholar] [CrossRef]
- Wode, K.; Henriksson, R.; Sharp, L.; Stoltenberg, A.; Hök Nordberg, J. Cancer patients’ use of complementary and alternative medicine in Sweden: A cross-sectional study. BMC Complement. Altern. Med. 2019, 19, 62. [Google Scholar] [CrossRef]
- He, Y.; Guo, X.; May, B.H.; Zhang, A.L.; Liu, Y.; Lu, C.; Mao, J.J.; Xue, C.C.; Zhang, H. Clinical Evidence for Association of Acupuncture and Acupressure with Improved Cancer Pain: A Systematic Review and Meta-Analysis. JAMA Oncol. 2020, 6, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, H.; Wu, W.; Yu, W.; Li, Y.; Bai, J.; Luo, B.; Li, S. Acupuncture for Pain Management in Cancer: A Systematic Review and Meta-Analysis. Evid. Based Complement. Alternat. Med. 2016, 2016, 1720239. [Google Scholar] [CrossRef] [PubMed]
- Paley, C.A.; Johnson, M.I.; Tashani, O.A.; Bagnall, A.M. Acupuncture for cancer pain in adults. Cochrane Database Syst. Rev. 2015, 2015, cd007753. [Google Scholar] [CrossRef] [PubMed]
- Zia, F.Z.; Olaku, O.; Bao, T.; Berger, A.; Deng, G.; Fan, A.Y.; Garcia, M.K.; Herman, P.M.; Kaptchuk, T.J.; Ladas, E.J.; et al. The National Cancer Institute’s Conference on Acupuncture for Symptom Management in Oncology: State of the Science, Evidence, and Research Gaps. J. Natl. Cancer Inst. Monogr. 2017, 2017, lgx005. [Google Scholar] [CrossRef] [PubMed]
- Katta, M.R.; Valisekka, S.S.; Agarwal, P.; Hameed, M.; Shivam, S.; Kaur, J.; Prasad, S.; Bethineedi, L.D.; Lavu, D.V.; Katamreddy, Y. Non-pharmacological integrative therapies for chronic cancer pain. J. Oncol. Pharm. Pract. 2022, 28, 1859–1868. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Caraceni, A.; Shkodra, M. Cancer Pain Assessment and Classification. Cancers 2019, 11, 510. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Hsieh, Y.J.; Tsai, P.S. Systematic review and meta-analysis of acupuncture to reduce cancer-related pain. Eur. J. Cancer Care 2017, 26, e12457. [Google Scholar] [CrossRef]
- Dincer, F.; Linde, K. Sham interventions in randomized clinical trials of acupuncture—A review. Complement. Ther. Med. 2003, 11, 235–242. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Brignardello-Petersen, R.; Bonner, A.; Alexander, P.E.; Siemieniuk, R.A.; Furukawa, T.A.; Rochwerg, B.; Hazlewood, G.S.; Alhazzani, W.; Mustafa, R.A.; Murad, M.H.; et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J. Clin. Epidemiol. 2018, 93, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Alimi, D.; Rubino, C.; Pichard-Léandri, E.; Fermand-Brulé, S.; Dubreuil-Lemaire, M.L.; Hill, C. Analgesic effect of auricular acupuncture for cancer pain: A randomized, blinded, controlled trial. J. Clin. Oncol. 2003, 21, 4120–4126. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Cai, L.; Giles, J.T.; Gould, J.; Tarpinian, K.; Betts, K.; Medeiros, M.; Jeter, S.; Tait, N.; Chumsri, S.; et al. A dual-center randomized controlled double blind trial assessing the effect of acupuncture in reducing musculoskeletal symptoms in breast cancer patients taking aromatase inhibitors. Breast Cancer Res. Treat. 2013, 138, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Crew, K.D.; Capodice, J.L.; Greenlee, H.; Brafman, L.; Fuentes, D.; Awad, D.; Tsai, W.Y.; Hershman, D.L. Randomized, blinded, sham-controlled trial of acupuncture for the management of aromatase inhibitor-associated joint symptoms in women with early-stage breast cancer. J. Clin. Oncol. 2010, 28, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Rusch, V.; Vickers, A.; Malhotra, V.; Ginex, P.; Downey, R.; Bains, M.; Park, B.; Rizk, N.; Flores, R.; et al. Randomized controlled trial of a special acupuncture technique for pain after thoracotomy. J. Thorac. Cardiovasc. Surg. 2008, 136, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.L.; Unger, J.M.; Greenlee, H.; Capodice, J.L.; Lew, D.L.; Darke, A.K.; Melnik, M.K.; Jorgensen, C.W.; Kreisle, W.H.; Minasian, L.M. Effect of acupuncture vs. sham acupuncture or waitlist control on joint pain related to aromatase inhibitors among women with early-stage breast cancer—A randomized clinical trial. J. Am. Med. Assoc. 2018, 320, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, S. Intradermal Acupuncture Along with Analgesics for Pain Control in Advanced Cancer Cases: A Pilot, Randomized, Patient-Assessor-Blinded, Controlled Trial. Integr. Cancer Ther. 2018, 17, 1137–1143. [Google Scholar] [CrossRef]
- Ruela, L.O.; Iunes, D.H.; Nogueira, D.A.; Stefanello, J.; Gradim, C.V.C. Effectiveness of auricular acupuncture in the treatment of cancer pain: Randomized clinical trial. Rev. Esc. Enferm. USP 2018, 52, e03402. [Google Scholar] [CrossRef]
- Wang, X.Q.; Xiao, L.; Duan, P.B.; Xu, Q.; Yang, L.H.; Wang, A.Q.; Wang, Y. The feasibility and efficacy of perioperative auricular acupuncture technique via intradermal needle buried for postoperative movement-evoked pain after open radical gastrectomy: A randomized controlled pilot trial. Explore 2021, 18, 36–43. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Booker, S.Q.; Herr, K.A.; Horgas, A.L. A Paradigm Shift for Movement-Based Pain Assessment in Older Adults: Practice, Policy and Regulatory Drivers. Pain Manag. Nurs. 2021, 22, 21–27. [Google Scholar] [CrossRef] [PubMed]
- So, E.W.; Ng, E.H.; Wong, Y.Y.; Lau, E.Y.; Yeung, W.S.; Ho, P.C. A randomized double blind comparison of real and placebo acupuncture in IVF treatment. Hum. Reprod. 2009, 24, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.S.; Tan, H.Y.; Zhang, G.S.; Zhang, A.L.; Xue, C.C.; Xie, Y.M. Placebo Devices as Effective Control Methods in Acupuncture Clinical Trials: A Systematic Review. PLoS ONE 2015, 10, e0140825. [Google Scholar] [CrossRef] [PubMed]
- Society, A.C. Facts about Cancer Pain. Available online: https://www.cancer.org/treatment/treatments-and-side-effects/physical-side-effects/pain/facts-about-cancer-pain.html (accessed on 16 August 2023).
- Kim, T.H.; Lee, M.S.; Alraek, T.; Birch, S. Acupuncture in sham device controlled trials may not be as effective as acupuncture in the real world: A preliminary network meta-analysis of studies of acupuncture for hot flashes in menopausal women. Acupunct. Med. 2020, 38, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kim, T.H.; Birch, S.; Alraek, T.; Lee, H.W.; Nielsen, A.; Wieland, L.S.; Lee, M.S. Comparative effectiveness of acupuncture in sham-controlled trials for knee osteoarthritis: A systematic review and network meta-analysis. Front. Med. 2022, 9, 1061878. [Google Scholar] [CrossRef] [PubMed]
- Lund, I.; Näslund, J.; Lundeberg, T. Minimal acupuncture is not a valid placebo control in randomised controlled trials of acupuncture: A physiologist’s perspective. Chin. Med. 2009, 4, 1. [Google Scholar] [CrossRef]
- MacPherson, H.; Vertosick, E.; Lewith, G.; Linde, K.; Sherman, K.J.; Witt, C.M.; Vickers, A.J. Influence of control group on effect size in trials of acupuncture for chronic pain: A secondary analysis of an individual patient data meta-analysis. PLoS ONE 2014, 9, e93739. [Google Scholar] [CrossRef]
- Langevin, H.M.; Wayne, P.M.; Macpherson, H.; Schnyer, R.; Milley, R.M.; Napadow, V.; Lao, L.; Park, J.; Harris, R.E.; Cohen, M.; et al. Paradoxes in acupuncture research: Strategies for moving forward. Evid. Based Complement. Alternat. Med. 2011, 2011, 180805. [Google Scholar] [CrossRef]
- MacPherson, H.; Hammerschlag, R.; Coeytaux, R.R.; Davis, R.T.; Harris, R.E.; Kong, J.T.; Langevin, H.M.; Lao, L.; Milley, R.J.; Napadow, V.; et al. Unanticipated Insights into Biomedicine from the Study of Acupuncture. J. Altern. Complement. Med. 2016, 22, 101–107. [Google Scholar] [CrossRef]
- NICE. NICE Real-World Evidence Framework. Corporate Document [ECD9]. Available online: www.nice.org.uk/corporate/ecd9 (accessed on 21 August 2023).
- NIH. NIH Introduction to Pragmatic Clinical Trials. NIH Collaboratory. Available online: https://dcricollab.dcri.duke.edu/sites/NIHKR/KR/Introduction%20to%20pragmatic%20clinical%20trials.pdf (accessed on 13 August 2023).

| Study ID (Country) | Study Design | Population | Total Sample Size (AT/SAT/WL) | Mean Age (y) | Sex (Male/Female) | Details of SAT | Outcome (Pain Severity) | Treatment Duration | Timepoint for Analysis |
|---|---|---|---|---|---|---|---|---|---|
| Alimi 2003 (France) [34] | a parallel-group RCT (AT vs. SAT) | - Chronic peripheral or central neuropathic pain arising after cancer treatment, prolonged for at least 1 month - 0–100 pain VAS ≥ 30 mm | 87 (29/58/–) | 57 (range: 37–84) | 17/70 | SATS, Group 1 (28 participants): steel implants at non-acupuncture points; Group 2 (30 participants): auricular seeds fixed at non-acupuncture points | 0–100 VAS | 2 months | 2 months |
| Bao 2013 (United States) [35] | a parallel-group RCT (AT vs. SAT) | - Postmenopausal women with early stage breast cancer, experiencing aromatase inhibitor-associated musculoskeletal symptoms - 0–100 pain VAS ≥ 20 mm | 47 (23/24/–) | AT: median 61 (range: 45–85), SAT: median 61 (range: 44–82) | 0/47 | SATS, nonpenetrating Park sham needle at non-acupuncture points | 0–100 VAS | 8 weeks | 8 weeks (not analyzed in meta-analysis) |
| Crew 2010 (United States) [36] | a parallel-group RCT (AT vs. SAT) | - Postmenopausal women with breast cancer, experiencing aromatase inhibitor-associated musculoskeletal pain - BPI–SF worst pain ≥ 3 points | 38 (20/18/–) | Median 58 (range: 37–77) | 0/38 | SATS, superficial needling at non-acupuncture points | BPI–SF pain severity | 6 weeks | 6 weeks |
| Deng 2008 (United States) [37] | a parallel-group RCT (AT vs. SAT) | - Patients with cancer scheduled for unilateral thoracotomy | 106 (52/54/–) | AT: median 65 (IQR: 58–72), SAT: median 63 (IQR: 57–70) | 52/54 | SATS, dummy studs without needle at non-acupuncture points | BPI pain severity, 0–10 NRS | ST36, shenmen: 1 week, others: 4 weeks | 30 days |
| Hershman 2018 (United States) [38] | a parallel-group RCT (AT vs. SAT vs. WL) | - Postmenopausal or premenopausal women with early-stage breast cancer who were taking an aromatase inhibitor - BPI–SF worst pain ≥ 3 points | 206 (101/54/51) | 60.7 ± SD 8.6 | 0/206 | SATS, shallow needling (body), or ear pellet with pellets removed (ear) at non-acupuncture points | BPI–SF pain severity | 12 weeks | 12 weeks |
| Kim 2018 (Republic of Korea) [39] | a parallel-group RCT (AT vs. SAT) | - Patients with advanced cancer who were being administered analgesics for cancer pain | 27 (14/13/–) | 56 (range: 42–73) | 11/16 | SATV, nonpenetrating bent needle at acupuncture points | 0–10 NRS | 3 weeks | 3 weeks |
| Ruela 2018 (Brazil) [40] | a parallel-group RCT (AT vs. SAT) | - Patients with cancer receiving chemotherapy - 0–10 pain NRS ≥ 4 points | 23 (11/12/–) | AT: 58.27 ± SD 10.09, SAT: 52.08 ± SD 7.99 | 5/18 | SATS, penetrating auricular needle at irrelevant acupuncture points | 0–10 NRS | 8 weeks | 9 weeks |
| Wang 2022 (China) [41] | a parallel-group RCT (AT vs. SAT) | - Diagnosed with gastric cancer by pathology and underwent open radical gastrectomy (operation time ≤ 3 h) | 40 (20/20/–) | AT: 63.10 ± 8.30, SAT: 65.60 ± 6.10 | 21/19 | SATV, nonpenetrating adhesive tape without needle at acupuncture points | 0–10 NRS | 6 days (embedded into the skin 24 h before the surgery, and was replaced once every 3 days) | 5 days post-operation |
| WL | −0.71 (−1.05, −0.36) | −0.42 (−0.80, −0.03) | - |
| −0.93 (−2.05, 0.18) | AT | 0.73 (0.19, 1.28) | 0.42 (−0.07, 0.92) |
| −0.18 (−1.30, 0.94) | 0.75 (0.14, 1.36) | SATS | - |
| −0.54 (−2.02, 0.94) | 0.39 (−0.58, 1.36) | −0.36 (−1.51, 0.79) | SATV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.; Kwon, C.-Y.; Lee, H.W.; Nielsen, A.; Wieland, L.S.; Kim, T.-H.; Birch, S.; Alraek, T.; Lee, M.S. Different Outcomes According to Needling Point Location Used in Sham Acupuncture for Cancer-Related Pain: A Systematic Review and Network Meta-Analysis. Cancers 2023, 15, 5875. https://doi.org/10.3390/cancers15245875
Lee B, Kwon C-Y, Lee HW, Nielsen A, Wieland LS, Kim T-H, Birch S, Alraek T, Lee MS. Different Outcomes According to Needling Point Location Used in Sham Acupuncture for Cancer-Related Pain: A Systematic Review and Network Meta-Analysis. Cancers. 2023; 15(24):5875. https://doi.org/10.3390/cancers15245875
Chicago/Turabian StyleLee, Boram, Chan-Young Kwon, Hye Won Lee, Arya Nielsen, L. Susan Wieland, Tae-Hun Kim, Stephen Birch, Terje Alraek, and Myeong Soo Lee. 2023. "Different Outcomes According to Needling Point Location Used in Sham Acupuncture for Cancer-Related Pain: A Systematic Review and Network Meta-Analysis" Cancers 15, no. 24: 5875. https://doi.org/10.3390/cancers15245875
APA StyleLee, B., Kwon, C.-Y., Lee, H. W., Nielsen, A., Wieland, L. S., Kim, T.-H., Birch, S., Alraek, T., & Lee, M. S. (2023). Different Outcomes According to Needling Point Location Used in Sham Acupuncture for Cancer-Related Pain: A Systematic Review and Network Meta-Analysis. Cancers, 15(24), 5875. https://doi.org/10.3390/cancers15245875









