Magnetic Resonance Imaging of Macrophage Response to Radiation Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Perfluorocarbon (PFC) Nanoemulsion
2.2. Cell Lines
2.3. Cytotoxicity and Cell Apoptosis Assays
2.4. Mouse Model
2.5. MRI Acquisition
2.6. Radiation Therapy
2.7. Data Analysis
2.8. Statistical Analysis
3. Results
3.1. F MRI Allows for Longitudinal Tracking of Macrophages in Tumors following RT
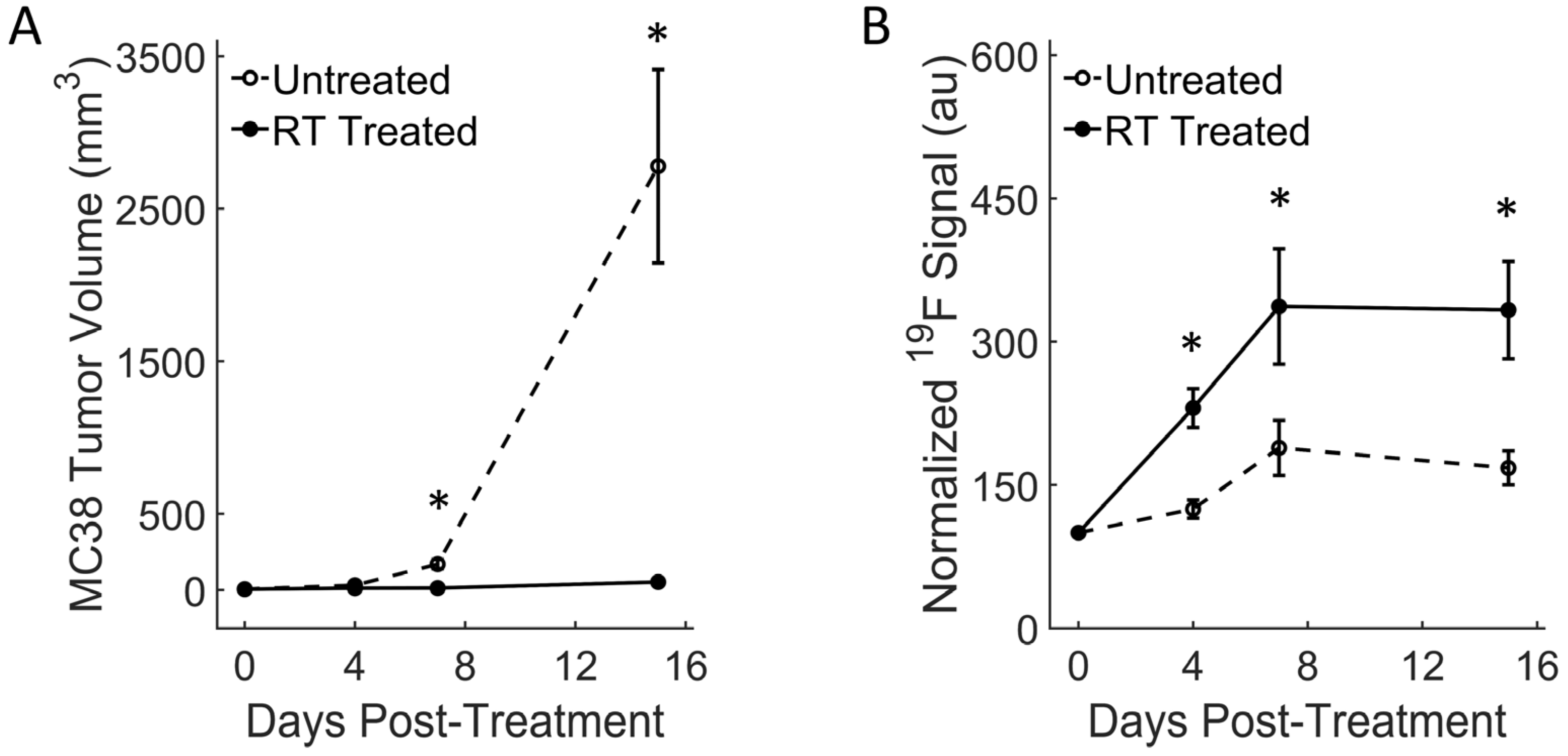
3.2. Colon RT-Treated Tumors Show Strong Macrophage Influx and Growth Arrest
3.3. Breast RT-Treated Tumors Show Moderate Fluorine Signal Increase and Growth Reduction
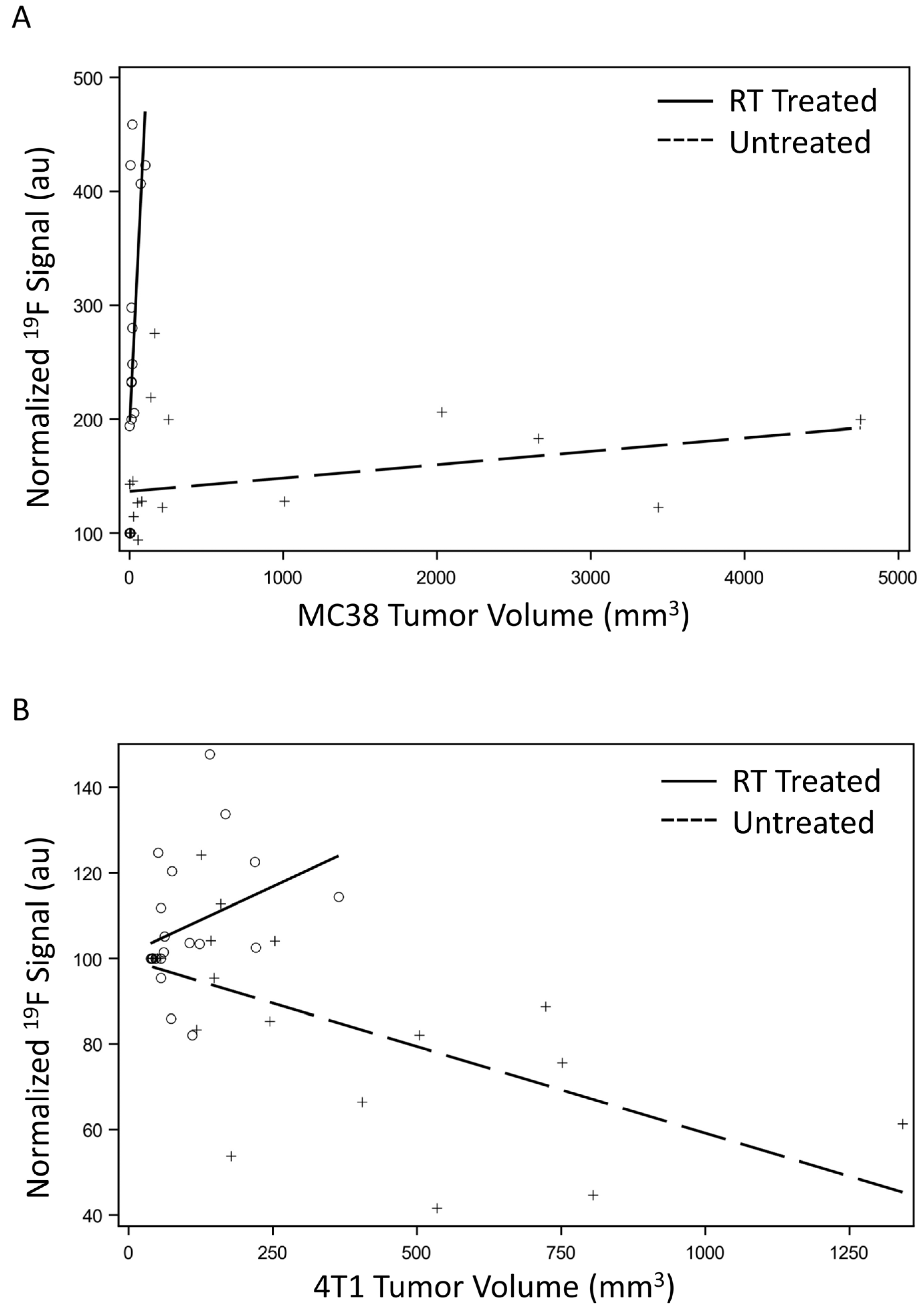
3.4. Macrophage Signal Correlates to Tumor Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Vakkila, J.; Lotze, M.T. Inflammation and necrosis promote tumour growth. Nat. Rev. Immunol. 2004, 4, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.S.; Brown, J.M. The irradiated tumor microenvironment: Role of tumor-associated macrophages in vascular recovery. Front. Physiol. 2013, 4, 157. [Google Scholar] [CrossRef] [PubMed]
- Baidoun, F.; Elshiwy, K.; Elkeraie, Y.; Merjaneh, Z.; Khoudari, G.; Sarmini, M.T.; Gad, M.; Al-Husseini, M.; Saad, A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr. Drug Targets 2021, 22, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Chen, H.-D.; Yu, Y.-W.; Li, N.; Chen, W.-Q. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin. Med. J. 2021, 134, 783–791. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Peto, R.; Davies, C.; Godwin, J.; Gray, R.; Pan, H.C.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Lancet 2012, 379, 432–444. [Google Scholar] [CrossRef]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.-H.; Wolmark, N. Twenty-Year Follow-up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy plus Irradiation for the Treatment of Invasive Breast Cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef]
- Hughes, K.S.; Schnaper, L.A.; Bellon, J.R.; Cirrincione, C.T.; Berry, D.A.; McCormick, B.; Muss, H.B.; Smith, B.L.; Hudis, C.A.; Winer, E.P.; et al. Lumpectomy Plus Tamoxifen with or without Irradiation in Women Age 70 Years or Older with Early Breast Cancer: Long-Term Follow-Up of CALGB 9343. J. Clin. Oncol. 2013, 31, 2382–2387. [Google Scholar] [CrossRef] [PubMed]
- Litière, S.; Werutsky, G.; Fentiman, I.S.; Rutgers, E.; Christiaens, M.-R.; Van Limbergen, E.; Baaijens, M.H.; Bogaerts, J.; Bartelink, H. Breast conserving therapy versus mastectomy for stage I–II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol. 2012, 13, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Polgár, C.; Kahán, Z.; Ivanov, O.; Chorváth, M.; Ligačová, A.; Csejtei, A.; Gábor, G.; Landherr, L.; Mangel, L.; Mayer, A.; et al. Radiotherapy of Breast Cancer—Professional Guideline 1st Central-Eastern European Professional Consensus Statement on Breast Cancer. Pathol. Oncol. Res. 2022, 28, 1610378. [Google Scholar] [CrossRef] [PubMed]
- Greenup, R.A.; Blitzblau, R.C.; Houck, K.L.; Sosa, J.A.; Horton, J.; Peppercorn, J.M.; Taghian, A.G.; Smith, B.L.; Hwang, E.S. Cost Implications of an Evidence-Based Approach to Radiation Treatment After Lumpectomy for Early-Stage Breast Cancer. J. Oncol. Pract. 2017, 13, e283–e290. [Google Scholar] [CrossRef]
- Waheed, A.; Cason, F.D. Adjuvant Radiation Survival Benefits in Patients with Stage 1B Rectal Cancer: A Population-based Study from the Surveillance Epidemiology and End Result Database (1973–2010). Cureus 2019, 11, e6299. [Google Scholar] [CrossRef]
- Okubo, M.; Kioi, M.; Nakashima, H.; Sugiura, K.; Mitsudo, K.; Aoki, I.; Taniguchi, H.; Tohnai, I. M2-polarized macrophages contribute to neovasculogenesis, leading to relapse of oral cancer following radiation. Sci. Rep. 2016, 6, 27548. [Google Scholar] [CrossRef]
- Balermpas, P.; Rödel, F.; Liberz, R.; Oppermann, J.; Wagenblast, J.; Ghanaati, S.; Harter, P.N.; Mittelbronn, M.; Weiss, C.; Rödel, C.; et al. Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences. Br. J. Cancer 2014, 111, 1509–1518. [Google Scholar] [CrossRef]
- Kozin, S.V.; Kamoun, W.S.; Huang, Y.; Dawson, M.R.; Jain, R.K.; Duda, D.G. Recruitment of Myeloid but not Endothelial Precursor Cells Facilitates Tumor Regrowth after Local Irradiation. Cancer Res 2010, 70, 5679–5685. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Pham, T.V.; Goodman, A.M.; Sivakumar, S.; Frampton, G.; Kurzrock, R. Intra-patient stability of tumor mutational burden from tissue biopsies at different time points in advanced cancers. Genome Med. 2021, 13, 159. [Google Scholar] [CrossRef]
- Yamamoto, K.; Makino, T.; Sato, E.; Noma, T.; Urakawa, S.; Takeoka, T.; Yamashita, K.; Saito, T.; Tanaka, K.; Takahashi, T.; et al. Tumor-infiltrating M2 macrophage in pretreatment biopsy sample predicts response to chemotherapy and survival in esophageal cancer. Cancer Sci. 2020, 111, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Engers, R. Reproducibility and reliability of tumor grading in urological neoplasms. World J. Urol. 2007, 25, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.V.; E McDunn, J.; Gunst, P.R.; Smith, E.M.; Milburn, M.V.; A Troyer, D.; A Lawton, K. Cancer detection and biopsy classification using concurrent histopathological and metabolomic analysis of core biopsies. Genome Med. 2012, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Daldrup-Link, H.E.; Chan, C.; Lenkov, O.; Taghavigarmestani, S.; Nazekati, T.; Nejadnik, H.; Chapelin, F.; Khurana, A.; Tong, X.; Yang, F.; et al. Detection of Stem Cell Transplant Rejection with Ferumoxytol MR Imaging: Correlation of MR Imaging Findings with Those at Intravital Microscopy. Radiology 2017, 284, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Makela, A.V.; Foster, P.J. Imaging macrophage distribution and density in mammary tumors and lung metastases using fluorine-19 MRI cell tracking. Magn. Reson. Med. 2018, 80, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Makela, A.V.; Gaudet, J.M.; Foster, P.J. Quantifying tumor associated macrophages in breast cancer: A comparison of iron and fluorine-based MRI cell tracking. Sci. Rep. 2017, 7, srep42109. [Google Scholar] [CrossRef]
- Khurana, A.; Chapelin, F.; Xu, H.; Acevedo, J.R.; Molinolo, A.; Nguyen, Q.; Ahrens, E.T. Visualization of macrophage recruitment in head and neck carcinoma model using fluorine-19 magnetic resonance imaging. Magn. Reson. Med. 2018, 79, 1972–1980. [Google Scholar] [CrossRef]
- Hingorani, D.V.; Chapelin, F.; Stares, E.; Adams, S.R.; Okada, H.; Ahrens, E.T. Cell penetrating peptide functionalized perfluorocarbon nanoemulsions for targeted cell labeling and enhanced fluorine-19 MRI detection. Magn. Reson. Med. 2020, 83, 974–987. [Google Scholar] [CrossRef]
- Chapelin, F.; Leach, B.I.; Chen, R.; Lister, D.; Messer, K.; Okada, H.; Ahrens, E.T. Assessing Oximetry Response to Chimeric Antigen Receptor T-cell Therapy against Glioma with 19F MRI in a Murine Model. Radiol. Imaging Cancer 2021, 3, e200062. [Google Scholar] [CrossRef]
- Gonzales, C.; Yoshihara, H.A.I.; Dilek, N.; Leignadier, J.; Irving, M.; Mieville, P.; Helm, L.; Michielin, O.; Schwitter, J. In-Vivo Detection and Tracking of T Cells in Various Organs in a Melanoma Tumor Model by 19F-Fluorine MRS/MRI. PLoS ONE 2016, 11, e0164557. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Fu, Y.; Shea, S.M.; Hegde, S.S.; Kraitchman, D.L. Quantitative CT and 19F-MRI tracking of perfluorinated encapsulated mesenchymal stem cells to assess graft immunorejection. Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, B.E.; Blakey, I.; Squires, O.; Peng, H.; Boase, N.R.B.; Alexander, C.; Parsons, P.G.; Boyle, G.M.; Whittaker, A.K.; Thurecht, K.J. Multimodal Polymer Nanoparticles with Combined 19F Magnetic Resonance and Optical Detection for Tunable, Targeted, Multimodal Imaging in vivo. J. Am. Chem. Soc. 2014, 136, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Mills, P.H.; Hitchens, T.K.; Ahrens, E.T. Accelerated fluorine-19 MRI cell tracking using compressed sensing. Magn. Reson. Med. 2013, 69, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Janjic, J.M.; Ahrens, E.T. Fluorine-containing nanoemulsions for MRI cell tracking. WIREs Nanomed. Nanobiotechnol. 2009, 1, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Partlow, K.C.; Chen, J.; Brant, J.A.; Neubauer, A.M.; Meyerrose, T.E.; Creer, M.H.; Nolta, J.A.; Caruthers, S.D.; Lanza, G.M.; Wicldine, S.A. 19F magnetic resonance imaging for stem/progenitor cell tracking with multiple unique perfluorocarbon nanobeacons. FASEB J. 2007, 21, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, M.; Morel, P.; Ernst, L.A.; Laidlaw, D.H.; Ahrens, E.T. Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn. Reson. Med. 2007, 58, 725–734. [Google Scholar] [CrossRef]
- Ahrens, E.T.; Flores, R.; Xu, H.; Morel, P. In vivo imaging platform for tracking immunotherapeutic cells. Nat. Biotechnol. 2005, 23, 983–987. [Google Scholar] [CrossRef]
- Croci, D.; Méndez, R.S.; Temme, S.; Soukup, K.; Fournier, N.; Zomer, A.; Colotti, R.; Wischnewski, V.; Flögel, U.; van Heeswijk, R.B.; et al. Multispectral fluorine-19 MRI enables longitudinal and noninvasive monitoring of tumor-associated macrophages. Sci. Transl. Med. 2022, 14, eabo2952. [Google Scholar] [CrossRef]
- Ahrens, E.T.; Helfer, B.M.; O’Hanlon, C.F.; Schirda, C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn. Reson. Med. 2014, 72, 1696–1701. [Google Scholar] [CrossRef]
- Ahrens, E.T.; Helfer, B.M.; O’hanlon, C.F.; Lister, D.R.; Bykowski, J.L.; Messer, K.; I Leach, B.; Chen, J.; Xu, H.; A Daniels, G.; et al. Method for estimation of apoptotic cell fraction of cytotherapy using in vivo fluorine-19 magnetic resonance: Pilot study in a patient with head and neck carcinoma receiving tumor-infiltrating lymphocytes labeled with perfluorocarbon nanoemulsion. J. Immunother. Cancer 2023, 11, e007015. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Shi, L.; Kidder, K.; Zen, K.; Garnett-Benson, C.; Liu, Y. Intratumoral SIRPα-deficient macrophages activate tumor antigen-specific cytotoxic T cells under radiotherapy. Nat. Commun. 2021, 12, 3229. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.S.; Gaudet, J.M.; Foster, P.J. Fluorine-19 Mri Contrast Agents for Cell Tracking and Lung Imaging. Magn. Reson. Insights 2015, 8, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Halaweish, A.F.; Charles, H.C. Physiorack: An integrated MRI safe/conditional, Gas delivery, respiratory gating, and subject monitoring solution for structural and functional assessments of pulmonary function. J. Magn. Reson. Imaging 2014, 39, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Halaweish, A.F.; Moon, R.E.; Foster, W.M.; Soher, B.J.; McAdams, H.P.; MacFall, J.R.; Ainslie, M.D.; MacIntyre, N.R.; Charles, H.C. Perfluoropropane Gas as a Magnetic Resonance Lung Imaging Contrast Agent in Humans. Chest 2013, 144, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Couch, M.J.; Ball, I.K.; Li, T.; Fox, M.S.; Littlefield, S.L.; Biman, B.; Albert, M.S. Pulmonary Ultrashort Echo Time19F MR Imaging with Inhaled Fluorinated Gas Mixtures in Healthy Volunteers: Feasibility. Radiology 2013, 269, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Sarkar, S.; Yong, V.W.; Dunn, J.F. In Vivo MR Imaging of Tumor-Associated Macrophages: The Next Frontier in Cancer Imaging. Magn. Reson. Insights 2018, 11, 1178623X1877197. [Google Scholar] [CrossRef]
- Daldrup-Link, H.E.; Golovko, D.; Ruffell, B.; DeNardo, D.G.; Castaneda, R.; Ansari, C.; Rao, J.; Tikhomirov, G.A.; Wendland, M.F.; Corot, C.; et al. MRI of Tumor-Associated Macrophages with Clinically Applicable Iron Oxide Nanoparticles. Clin. Cancer Res. 2011, 17, 5695–5704. [Google Scholar] [CrossRef]
- Ahn, G.-O.; Tseng, D.; Liao, C.-H.; Dorie, M.J.; Czechowicz, A.; Brown, J.M. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc. Natl. Acad. Sci. USA 2010, 107, 8363–8368. [Google Scholar] [CrossRef]
- Filatenkov, A.; Baker, J.; Mueller, A.M.; Kenkel, J.; Ahn, G.-O.; Dutt, S.; Zhang, N.; Kohrt, H.; Jensen, K.; Dejbakhsh-Jones, S.; et al. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin. Cancer Res. 2015, 21, 3727–3739. [Google Scholar] [CrossRef]
- Filatenkov, A.; Baker, J.; Müller, A.M.; Ahn, G.-O.; Kohrt, H.; Dutt, S.; Jensen, K.; Dejbakhsh-Jones, S.; Negrin, R.S.; A Shizuru, J.; et al. Treatment of 4T1 Metastatic Breast Cancer with Combined Hypofractionated Irradiation and Autologous T-Cell Infusion. Radiat. Res. 2014, 182, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Pilones, K.A.; Kawashima, N.; Yang, A.M.; Babb, J.S.; Formenti, S.C.; Demaria, S. Invariant Natural Killer T Cells Regulate Breast Cancer Response to Radiation and CTLA-4 Blockade. Clin. Cancer Res. 2009, 15, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Chapelin, F. 19F Magnetic Resonance Imaging Platform to Quantify T Cell Therapy Biodistribution, Persistence and Efficacy. Ph.D. Thesis, UC San Diego, La Jolla, CA, USA, 2019. [Google Scholar]
- Khatik, R.; Wang, Z.; Zhi, D.; Kiran, S.; Dwivedi, P.; Liang, G.; Qiu, B.; Yang, Q. Integrin αvβ3 Receptor Overexpressing on Tumor-Targeted Positive MRI-Guided Chemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Molloy, J.; Izumi, T.; Sterpin, E. Impact of backscatter material thickness on the depth dose of orthovoltage irradiators for radiobiology research. Phys. Med. Biol. 2019, 64, 055001. [Google Scholar] [CrossRef] [PubMed]
- Sukati, S.; Ho, J.; Chaiswing, L.; Sompol, P.; Pandit, H.; Wei, W.; Izumi, T.; Chen, Q.; Weiss, H.; Noel, T.; et al. Extracellular vesicles released after cranial radiation: An insight into an early mechanism of brain injury. Brain Res. 2022, 1782, 147840. [Google Scholar] [CrossRef] [PubMed]
- Darçot, E.; Colotti, R.; Pellegrin, M.; Wilson, A.; Siegert, S.; Bouzourene, K.; Yerly, J.; Mazzolai, L.; Stuber, M.; van Heeswijk, R.B. Towards Quantification of Inflammation in Atherosclerotic Plaque in the Clinic—Characterization and Optimization of Fluorine-19 MRI in Mice at 3 T. Sci. Rep. 2019, 9, 17488. [Google Scholar] [CrossRef] [PubMed]
- Chapelin, F.; Capitini, C.M.; Ahrens, E.T. Fluorine-19 MRI for detection and quantification of immune cell therapy for cancer. J. Immunother. Cancer 2018, 6, 105. [Google Scholar] [CrossRef]
- Perez, A.S.; Zhou, J.; Leach, B.; Xu, H.; Lister, D.; Adams, S.R.; Ahrens, E.T.; Louie, A.Y. Click-Ready Perfluorocarbon Nanoemulsion for 19F MRI and Multimodal Cellular Detection. ACS Nanosci. Au 2022, 2, 102–110. [Google Scholar] [CrossRef]
- Beach, C.; MacLean, D.; Majorova, D.; Arnold, J.N.; Olcina, M.M. The effects of radiation therapy on the macrophage response in cancer. Front. Oncol. 2022, 12, 1020606. [Google Scholar] [CrossRef]
- Fortin, D.; Mestrovic, A.; Alexander, A. Stereotactic ablative radiation therapy with volumetric modulated arc therapy in flattening filter-free mode for low-, intermediate-, and high-risk prostate cancer patients: Are 2 arcs better than 1? Pract. Radiat. Oncol. 2015, 5, e489–e497. [Google Scholar] [CrossRef]
- Song, H.-N.; Jin, H.; Kim, J.-H.; Ha, I.-B.; Kang, K.-M.; Choi, H.-S.; Jeong, H.-J.; Kim, M.-Y.; Kim, H.-J.; Jeong, B.-K. Abscopal Effect of Radiotherapy Enhanced with Immune Checkpoint Inhibitors of Triple Negative Breast Cancer in 4T1 Mammary Carcinoma Model. Int. J. Mol. Sci. 2021, 22, 10476. [Google Scholar] [CrossRef] [PubMed]
- Fite, B.Z.; Hinostroza, V.; States, L.; Hicks-Nelson, A.; Baratto, L.; Kallianos, K.; Codari, M.; Yu, B.; Jha, P.; Shams, M.; et al. Increasing Diversity in Radiology and Molecular Imaging: Current Challenges. Mol. Imaging Biol. 2021, 23, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Mishima, H.; Kobayashi, T.; Shimizu, M.; Tamaki, Y.; Baba, M.; Shimano, T.; Itoh, S.; Yamazaki, M.; Iriguchi, N.; Takahashi, M.; et al. In vivo F-19 chemical shift imaging with FTPA and antibody-coupled FMIQ. J. Magn. Reson. Imaging 1991, 1, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P. Non-Invasive Physiology:19F NMR of Perfluorocarbons. Artif. Cells Blood Substit. Biotechnol. 1994, 22, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Dardzinski, B.J.; Sotak, C.H. Rapid tissue oxygen tension mapping using 19F inversion-recovery echo-planar imaging of P erfluoro-15 -crown-5-ether. Magn. Reson. Med. 1994, 32, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Chapelin, F.; Beck, G.; Lenkov, O.D.; Donig, J.; Nejadnik, H.; Messing, S.; Derugin, N.; Chan, R.C.-F.; Gaur, A.; et al. Iron Administration before Stem Cell Harvest Enables MR Imaging Tracking after Transplantation. Radiology 2013, 269, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, E.T.; Balducci, A.; Helfer, B.; Wesa, A.; O’Hanlon, C.; Schirda, C.; Bartlett, D.; Kalinski, P. First Clinical Experience Using Fluorine-19 MRI to Track Immunotherapeutic Dendritic Cells in Colorectal Cancer Patients (Ed)^(Eds) ISMRM; International Society for Magnetic Resonance in Medicine: Milan, Italy, 2014; p. 0474. [Google Scholar]
- Mauri, G.; Vitiello, P.P.; Sogari, A.; Crisafulli, G.; Sartore-Bianchi, A.; Marsoni, S.; Siena, S.; Bardelli, A. Liquid biopsies to monitor and direct cancer treatment in colorectal cancer. Br. J. Cancer 2022, 127, 394–407. [Google Scholar] [CrossRef]
- Parvez, K.; Parvez, A.; Zadeh, G. The Diagnosis and Treatment of Pseudoprogression, Radiation Necrosis and Brain Tumor Recurrence. Int. J. Mol. Sci. 2014, 15, 11832–11846. [Google Scholar] [CrossRef]
- Egeblad, M.; Nakasone, E.S.; Werb, Z. Tumors as Organs: Complex Tissues that Interface with the Entire Organism. Dev. Cell 2010, 18, 884–901. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Liu, L.; Wang, J.; Wu, J.; Sun, C. Role of macrophages in tumor progression and therapy (Review). Int. J. Oncol. 2022, 60, 57. [Google Scholar] [CrossRef]
- Netea-Maier, R.T.; Smit, J.W.; Netea, M.G. Metabolic changes in tumor cells and tumor-associated macrophages: A mutual relationship. Cancer Lett. 2018, 413, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Perri, F.; Pacelli, R.; Della Vittoria Scarpati, G.; Cella, L.; Giuliano, M.; Caponigro, F.; Pepe, S. Radioresistance in head and neck squamous cell carcinoma: Biological bases and therapeutic implications. Head Neck 2015, 37, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-Dose Irradiation Programs Macrophage Differentiation to an iNOS+/M1 Phenotype that Orchestrates Effective T Cell Immunotherapy. Cancer Cell 2013, 24, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Becherini, C.; Lancia, A.; Detti, B.; Lucidi, S.; Scartoni, D.; Ingrosso, G.; Carnevale, M.G.; Roghi, M.; Bertini, N.; Orsatti, C.; et al. Modulation of tumor-associated macrophage activity with radiation therapy: A systematic review. Strahlenther. Onkol. 2023, 199, 1173–1190. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tian, T.; Zhang, J. Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): From Mechanism to Therapy and Prognosis. Int. J. Mol. Sci. 2021, 22, 8470. [Google Scholar] [CrossRef]
- Rahal, O.M.; Wolfe, A.R.; Mandal, P.K.; Larson, R.; Tin, S.; Jimenez, C.; Zhang, D.; Horton, J.; Reuben, J.M.; McMurray, J.S.; et al. Blocking Interleukin (IL)4- and IL13-Mediated Phosphorylation of STAT6 (Tyr641) Decreases M2 Polarization of Macrophages and Protects Against Macrophage-Mediated Radioresistance of Inflammatory Breast Cancer. Int. J. Radiat. Oncol. 2018, 100, 1034–1043. [Google Scholar] [CrossRef]
- Shi, J.-J.; Xing, H.; Wang, Y.-X.; Zhang, X.; Zhan, Q.-M.; Geng, M.-Y.; Ding, J.; Meng, L.-H. PI3Kα inhibitors sensitize esophageal squamous cell carcinoma to radiation by abrogating survival signals in tumor cells and tumor microenvironment. Cancer Lett. 2019, 459, 145–155. [Google Scholar] [CrossRef]
- Dijkstra, E.A.; Mul, V.E.M.; Hemmer, P.H.J.; Havenga, K.; Hospers, G.A.P.; Muijs, C.T.; van Etten, B. Re-Irradiation in Patients with Recurrent Rectal Cancer is Safe and Feasible. Ann. Surg. Oncol. 2021, 28, 5194–5204. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Zhang, Q.; Sioud, M. Tumor-Associated Macrophage Subsets: Shaping Polarization and Targeting. Int. J. Mol. Sci. 2023, 24, 7493. [Google Scholar] [CrossRef]




| Batch | Size (nm) | PDI |
|---|---|---|
| 1 | 179.4 | 0.158 |
| 2 | 175.7 | 0.144 |
| 3 | 139.0 | 0.010 |
| 4 | 179.8 | 0.142 |
| 5 | 173.2 | 0.150 |
| 6 | 149.6 | 0.013 |
| Standard Deviation | ±17.4 | ±0.071 |
| Correlation Coefficient (p-Value) | MC38 | 4T1 | ||
|---|---|---|---|---|
| Treated | Untreated | Treated | Untreated | |
| Spearman | 0.710 (0.002) | 0.575 (0.008) | 0.474 (0.035) | −0.644 (0.002) |
| Kendall Tau | 0.528 (0.006) | 0.405 (0.015) | 0.292 (0.077) | −0.454 (0.006) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Howerton, B.; Brown, L.; Izumi, T.; Cheek, D.; Brandon, J.A.; Marti, F.; Gedaly, R.; Adatorwovor, R.; Chapelin, F. Magnetic Resonance Imaging of Macrophage Response to Radiation Therapy. Cancers 2023, 15, 5874. https://doi.org/10.3390/cancers15245874
Yang H, Howerton B, Brown L, Izumi T, Cheek D, Brandon JA, Marti F, Gedaly R, Adatorwovor R, Chapelin F. Magnetic Resonance Imaging of Macrophage Response to Radiation Therapy. Cancers. 2023; 15(24):5874. https://doi.org/10.3390/cancers15245874
Chicago/Turabian StyleYang, Harrison, Brock Howerton, Logan Brown, Tadahide Izumi, Dennis Cheek, J. Anthony Brandon, Francesc Marti, Roberto Gedaly, Reuben Adatorwovor, and Fanny Chapelin. 2023. "Magnetic Resonance Imaging of Macrophage Response to Radiation Therapy" Cancers 15, no. 24: 5874. https://doi.org/10.3390/cancers15245874
APA StyleYang, H., Howerton, B., Brown, L., Izumi, T., Cheek, D., Brandon, J. A., Marti, F., Gedaly, R., Adatorwovor, R., & Chapelin, F. (2023). Magnetic Resonance Imaging of Macrophage Response to Radiation Therapy. Cancers, 15(24), 5874. https://doi.org/10.3390/cancers15245874







