A Modified Basophil Activation Test for the Clinical Management of Immediate Hypersensitivity Reactions to Paclitaxel: A Proof-of-Concept Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Monoclonal Antibodies
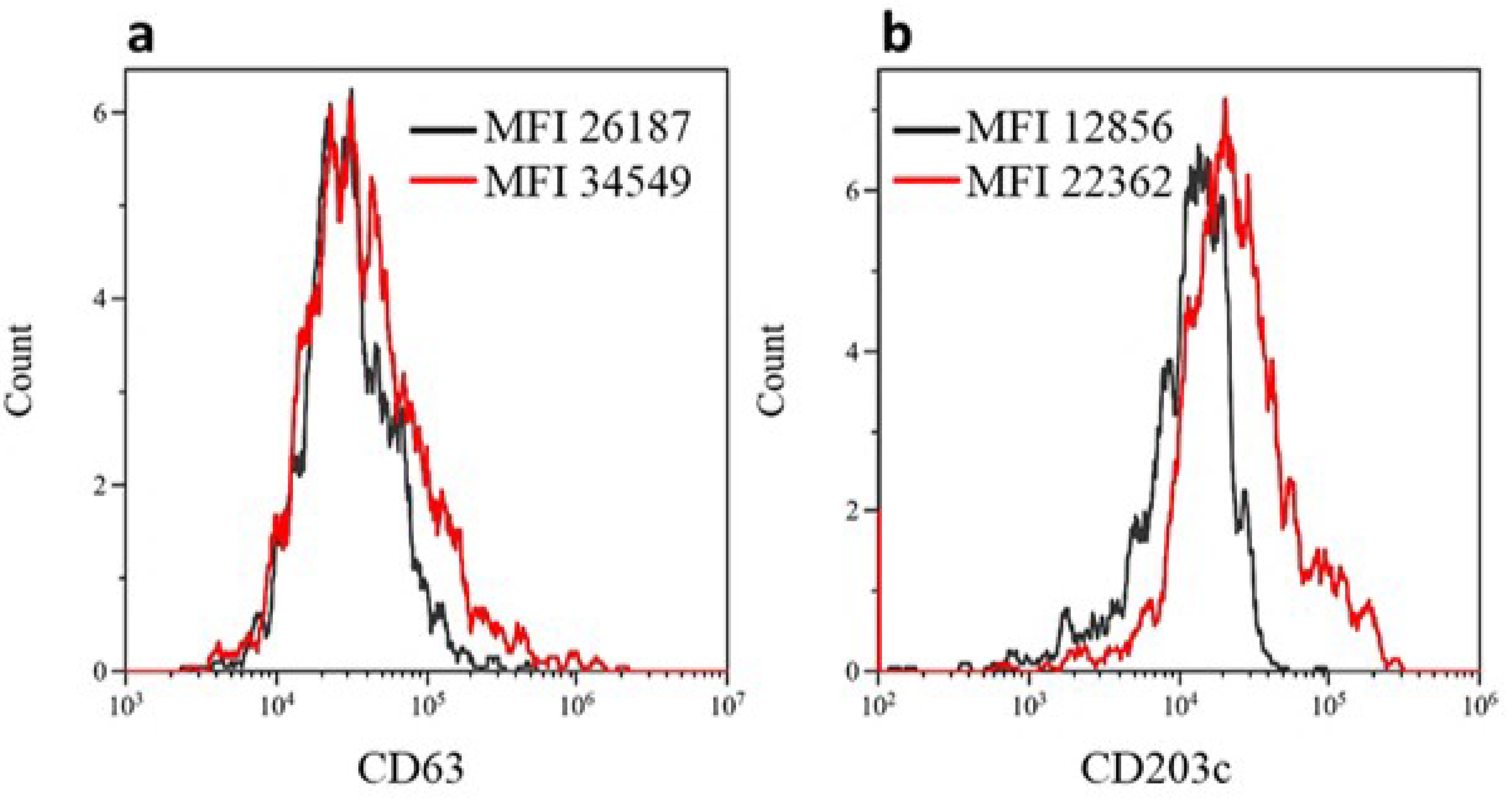
2.3. BAT Procedure
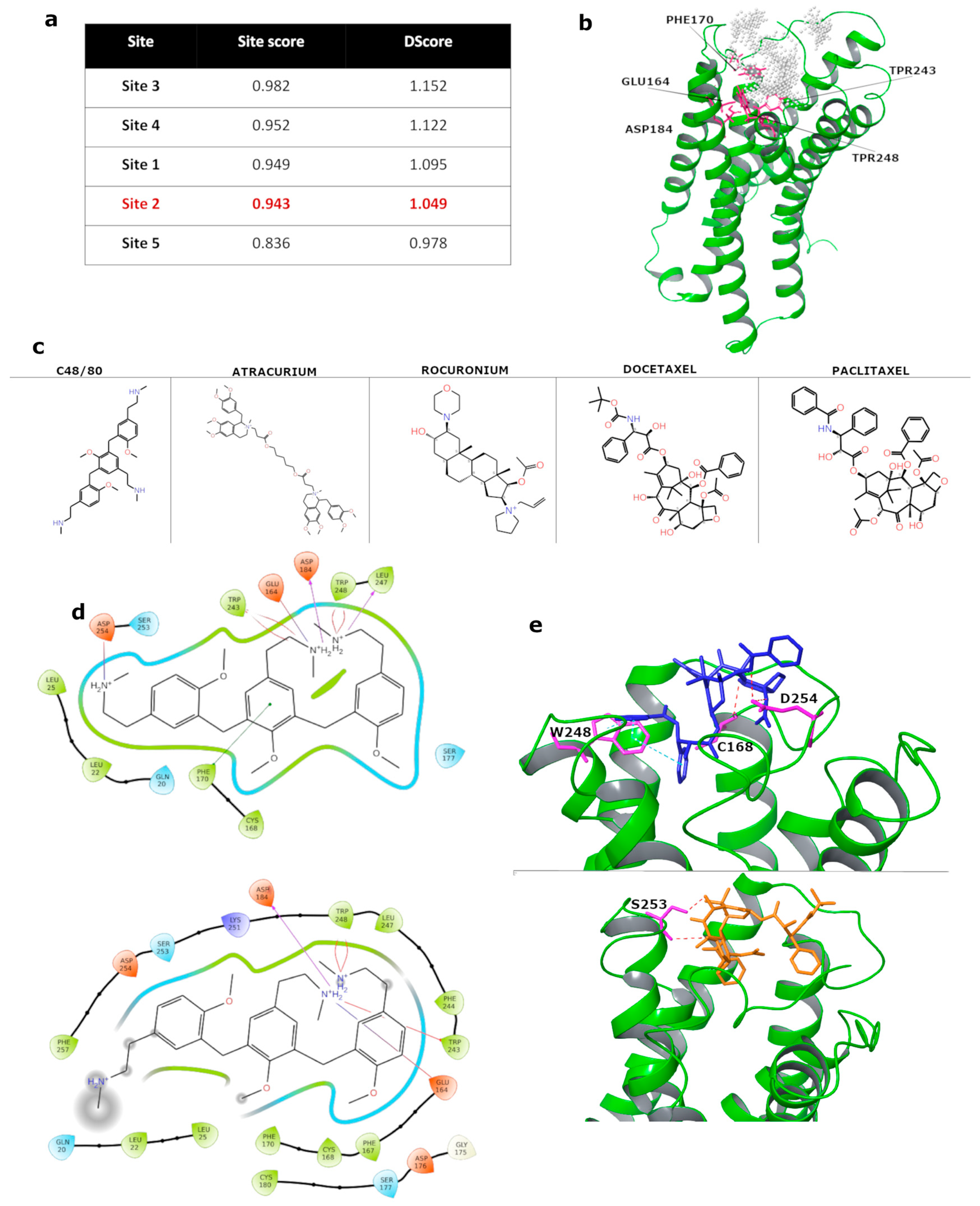
2.4. Molecular Docking Analysis
2.5. Evaluation of BAT Results and Statistical Analysis
- (a)
- (CD63+ basophil percentage increase (SI-CD63%):%CD63+ basophilsstimulated − %CD63+ basophilsnon-stimulated.
- (b)
- CD63 mean fluorescence intensity (MFI) increase (SI-CD63MFI):[(CD63MFIstimulated divided by CD63MFInon-stimulated) − 1].
- (c)
- CD203c+ basophil percentage increase (SI-CD203c%):%CD203c+basophilsstimulated − %CD203c+basophilsnon-stimulated.
- (d)
- CD203cMFI increase (SI-CD203cMFI):[(CD203cMFIstimulated divided by CD203cMFInon-stimulated) − 1].
3. Results
3.1. General Characteristics of the Study Population
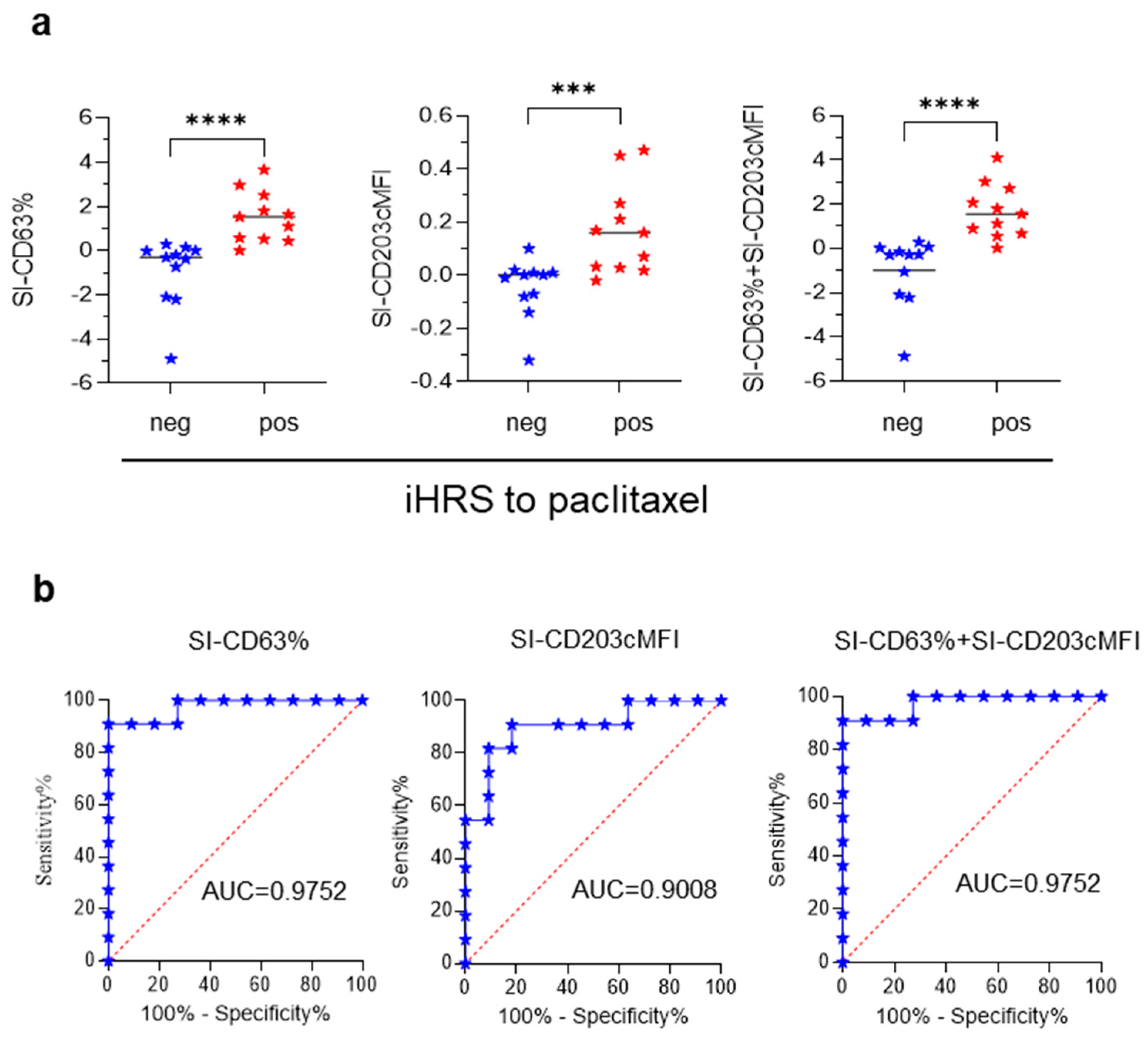
3.2. BAT for Diagnosis of iHSRs to Paclitaxel
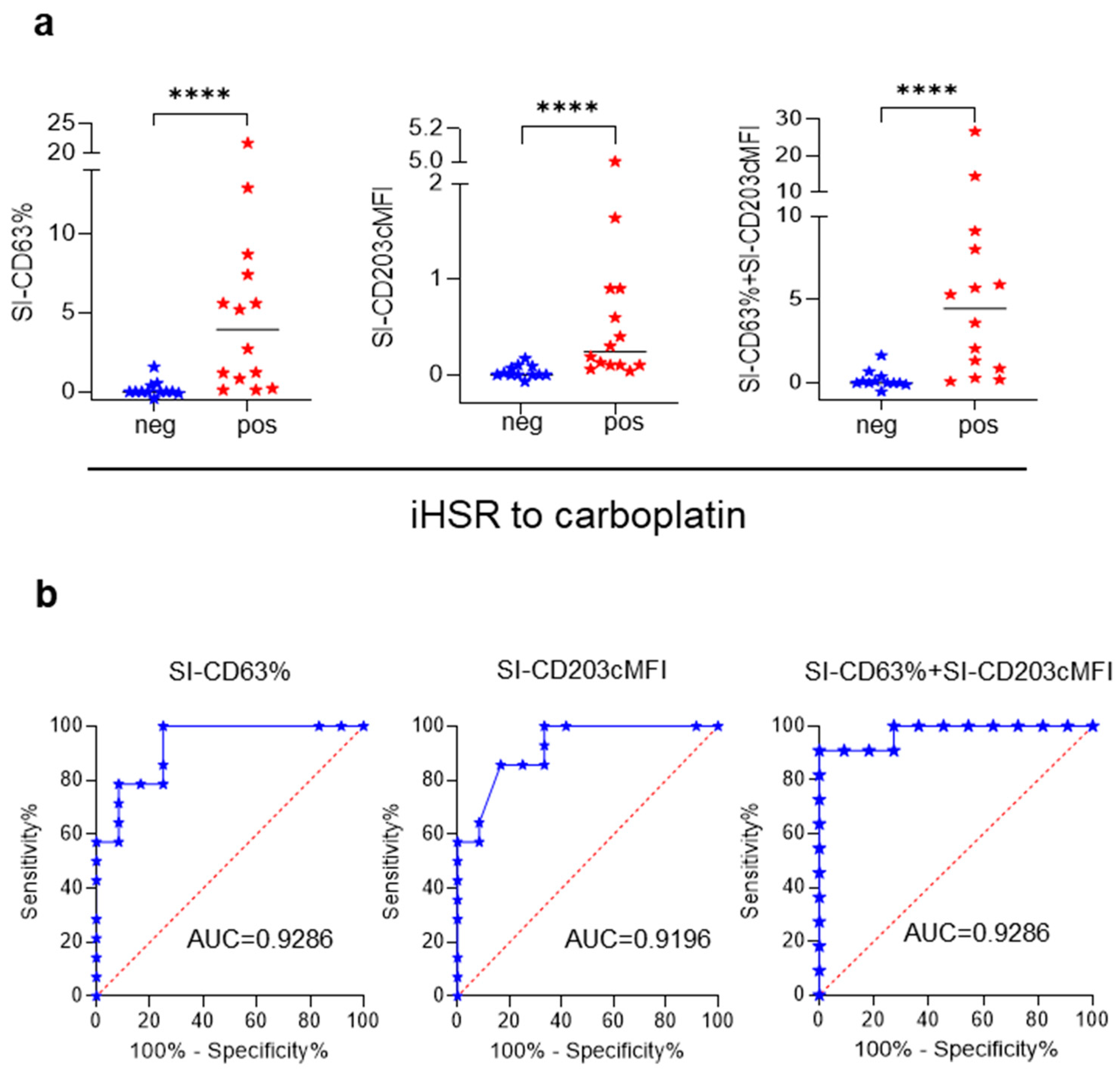
3.3. BAT for Diagnosis of iHSRs to Carboplatin
3.4. Association between BAT Results and iHSR Severity Grading
3.5. Relationship between BAT and ST Results
3.6. In Silico Molecular Docking of Taxanes to MRGPRX2 Receptor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Ledermann, J.A. First-line treatment of ovarian cancer: Questions and controversies to address. Ther. Adv. Med. Oncol. 2018, 10, 1758835918768232. [Google Scholar] [CrossRef] [PubMed]
- Nomura, H.; Aoki, D.; Michimae, H.; Mizuno, M.; Nakai, H.; Arai, M.; Sasagawa, M.; Ushijima, K.; Sugiyama, T.; Saito, M.; et al. Effect of Taxane Plus Platinum Regimens vs Doxorubicin Plus Cisplatin as Adjuvant Chemotherapy for Endometrial Cancer at a High Risk of Progression: A Randomized Clinical Trial. JAMA Oncol. 2019, 5, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Yardley, D.A. Taxanes in the elderly patient with metastatic breast cancer. Breast Cancer 2015, 7, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Caramelo, O.; Silva, C.; Caramelo, F.; Frutuoso, C.; Pinto, L.; Almeida-Santos, T. Efficacy of different neoadjuvant treatment regimens in BRCA-mutated triple negative breast cancer: A systematic review and meta-analysis. Hered. Cancer Clin. Pract. 2022, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.H.; Tai, Y.J.; Hsu, H.C.; Lee, S.P.; Chen, Y.Y.; Chiang, Y.C.; Chen, Y.L.; Chen, C.A.; Cheng, W.F. Risk Factors of Hypersensitivity to Carboplatin in Patients with Gynecologic Malignancies. Front. Pharmacol. 2017, 8, 800. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Iwamoto, T.; Murashima, Y.; Tabata, T.; Sagawa, N.; Okuda, M. Risk factors contributing to the development of carboplatin-related delayed hypersensitivity reactions in Japanese patients with gynecologic cancers. Cancer Chemother. Pharmacol. 2011, 67, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Sliesoraitis, S.; Chikhale, P.J. Carboplatin hypersensitivity. Int. J. Gynecol. Cancer 2005, 15, 13–18. [Google Scholar] [CrossRef]
- Caiado, J.; Castells, M. Presentation and Diagnosis of Hypersensitivity to Platinum Drugs. Curr. Allergy Asthma Rep. 2015, 15, 15. [Google Scholar] [CrossRef]
- Piovano, E.; Pivetta, E.; Modaffari, P.; Martra, F.; Baima Poma, C.; Perotto, S.; Tripodi, E.; Zanfagnin, V.; Zola, P.; Ferrero, A. A search for predictive factors for hypersensitivity reactions to paclitaxel and platinum salts in chemotherapy for gynecologic pelvic neoplasms. Gynecol. Obstet. Investig. 2012, 74, 21–27. [Google Scholar] [CrossRef]
- Picard, M.; Pur, L.; Caiado, J.; Giavina-Bianchi, P.; Galvão, V.R.; Berlin, S.T.; Campos, S.M.; Matulonis, U.A.; Castells, M.C. Risk stratification and skin testing to guide re-exposure in taxane-induced hypersensitivity reactions. J. Allergy Clin. Immunol. 2016, 137, 1154–1164. [Google Scholar] [CrossRef]
- Makrilia, N.; Syrigou, E.; Kaklamanos, I.; Manolopoulos, L.; Saif, M.W. Hypersensitivity reactions associated with platinum antineoplastic agents: A systematic review. Met. Based Drugs 2010, 2010, 207084. [Google Scholar] [CrossRef] [PubMed]
- Tsao, L.R.; Young, F.D.; Otani, I.M.; Castells, M.C. Hypersensitivity Reactions to Platinum Agents and Taxanes. Clin. Rev. Allergy Immunol. 2022, 62, 432–448. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Muñoz, A.; Jiménez, B.; García-Tapiador, A.; Romero-García, G.; Medina, L.; Navarro, V.; González-Sánchez, L.A.; Alba, E. Cross-sensitivity between taxanes in patients with breast cancer. Clin. Transl. Oncol. 2011, 13, 904–906. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Cuesta, E.; Madrigal-Burgaleta, R.; Angel-Pereira, D.; Ureña-Tavera, A.; Zamora-Verduga, M.; Lopez-Gonzalez, P.; Berges-Gimeno, M.P. Delving into cornerstones of hypersensitivity to antineoplastic and biological agents: Value of diagnostic tools prior to desensitization. Allergy 2015, 70, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-González, R.V.; González-Díaz, S.; Vidal-Gutiérrez, O.; Canel-Paredes, A.; de la Cruz-de la Cruz, C.; García-Campa, M.; López-Méndez, A.; Alvarado-Ruiz, S.; Castells, M. Hypersensitivity Reactions to Taxanes: A Comprehensive and Systematic Review of the Efficacy and Safety of Desensitization. Clin. Rev. Allergy Immunol. 2023, 65, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Caraballo, L.; Villa, E.; Ebisawa, M.; Passalacqua, G.; Savi, E.; Ebo, D.; Gómez, R.M.; et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ J. 2020, 13, 100080. [Google Scholar] [CrossRef] [PubMed]
- Castells, M. Drug Hypersensitivity and Anaphylaxis in Cancer and Chronic Inflammatory Diseases: The Role of Desensitizations. Front. Immunol. 2017, 8, 1472. [Google Scholar] [CrossRef]
- Santos, A.F.; Alpan, O.; Hoffmann, H.J. Basophil activation test: Mechanisms and considerations for use in clinical trials and clinical practice. Allergy 2021, 76, 2420–2432. [Google Scholar] [CrossRef]
- Iwamoto, T.; Yuta, A.; Tabata, T.; Sugimoto, H.; Gabazza, E.C.; Hirai, H.; Kojima, S.; Okuda, M. Evaluation of basophil CD203c as a predictor of carboplatin-related hypersensitivity reaction in patients with gynecologic cancer. Biol. Pharm. Bull. 2012, 35, 1487–1495. [Google Scholar] [CrossRef]
- Iwamoto, T.; Hirai, H.; Yamaguchi, N.; Kobayashi, N.; Sugimoto, H.; Tabata, T.; Okuda, M. Carboplatin-induced severe hypersensitivity reaction: Role of IgE-dependent basophil activation and FcεRI. Cancer Sci. 2014, 105, 1472–1479. [Google Scholar] [CrossRef]
- Giavina-Bianchi, P.; Galvão, V.R.; Picard, M.; Caiado, J.; Castells, M.C. Basophil Activation Test is a Relevant Biomarker of the Outcome of Rapid Desensitization in Platinum Compounds-Allergy. J Allergy Clin. Immunol. Pract. 2017, 5, 728–736. [Google Scholar] [CrossRef]
- De Campos, L.; Giavina-Bianchi, P.; Acharya, S.; Lynch, D.M.; Kalil, J.; Castells, M.C. Basophil Activation Test as a Biomarker for Taxanes Anaphylaxis. Front. Allergy 2022, 3, 787749. [Google Scholar] [CrossRef]
- MacGlashan, D., Jr. Expression of CD203c and CD63 in human basophils: Relationship to differential regulation of piecemeal and anaphylactic degranulation processes. Clin. Exp. Allergy 2010, 40, 1365–1377. [Google Scholar] [CrossRef]
- Metcalfe, D.D.; Pawankar, R.; Ackerman, S.J.; Akin, C.; Clayton, F.; Falcone, F.H.; Gleich, G.J.; Irani, A.-M.; Johansson, M.W.; Klion, A.D.; et al. Biomarkers of the involvement of mast cells, basophils and eosinophils in asthma and allergic diseases. World Allergy Organ. J. 2016, 9, 7. [Google Scholar] [CrossRef]
- Ogasawara, H.; Noguchi, M. Therapeutic Potential of MRGPRX2 Inhibitors on Mast Cells. Cells 2021, 10, 2906. [Google Scholar] [CrossRef]
- Kumar, M.; Duraisamy, K.; Chow, B.K. Unlocking the Non-IgE-Mediated Pseudo-Allergic Reaction Puzzle with Mas-Related G-Protein Coupled Receptor Member X2 (MRGPRX2). Cells 2021, 10, 1033. [Google Scholar] [CrossRef]
- Thapaliya, M.; Chompunud Na Ayudhya, C.; Amponnawarat, A.; Roy, S.; Ali, H. Mast Cell-Specific MRGPRX2: A Key Modulator of Neuro-Immune Interaction in Allergic Diseases. Curr. Allergy Asthma Rep. 2021, 21, 3. [Google Scholar] [CrossRef]
- Baldo, B.A.; Pham, N.H. Opioid toxicity: Histamine, hypersensitivity, and MRGPRX2. Arch. Toxicol. 2023, 97, 359–375. [Google Scholar] [CrossRef]
- Roy, S.; Chompunud Na Ayudhya, C.; Thapaliya, M.; Deepak, V.; Ali, H. Multifaceted MRGPRX2: New insight into the role of mast cells in health and disease. J. Allergy Clin. Immunol. 2021, 148, 293–308. [Google Scholar] [CrossRef]
- Sabato, V.; Ebo, D.G.; Van Der Poorten, M.M.; Toscano, A.; Van Gasse, A.L.; Mertens, C.; Van Houdt, M.; Beyens, M.; Elst, J. Allergenic and Mas-Related G Protein-Coupled Receptor X2-Activating Properties of Drugs: Resolving the Two. J. Allergy Clin. Immunol. Pract. 2023, 11, 395–404. [Google Scholar] [CrossRef]
- Elst, J.; Maurer, M.; Sabato, V.; Faber, M.A.; Bridts, C.H.; Mertens, C.; Van Houdt, M.; Van Gasse, A.L.; van der Poorten, M.-L.M.; De Puysseleyr, L.P.; et al. Novel Insights on MRGPRX2-Mediated Hypersensitivity to Neuromuscular Blocking Agents And Fluoroquinolones. Front. Immunol. 2021, 12, 668962. [Google Scholar] [CrossRef]
- Fernandopulle, N.A.; Zhang, S.S.; Soeding, P.F.; Mackay, G.A. MRGPRX2 activation in mast cells by neuromuscular blocking agents and other agonists: Modulation by sugammadex. Clin. Exp. Allergy 2021, 51, 685–695. [Google Scholar] [CrossRef]
- Wedi, B.; Gehring, M.; Kapp, A. The pseudoallergen receptor MRGPRX2 on peripheral blood basophils and eosinophils: Expression and function. Allergy 2020, 75, 2229–2242. [Google Scholar] [CrossRef]
- Sabato, V.; Van Gasse, A.L.; Cop, N.; Claesen, K.; Decuyper, I.I.; Faber, M.A.; Bridts, C.; Mertens, C.; Hagendorens, M.; De Clerck, L.; et al. The mas-related G protein-cou-pled receptor MRGPR X2 is expressed on human basophils and up-reg-ulated upon activation. J. Allergy Clin. Immunol. 2017, 139, AB168. [Google Scholar] [CrossRef]
- Yang, F.; Guo, L.; Li, Y.; Wang, G.; Wang, J.; Zhang, C.; Fang, G.-X.; Chen, X.; Liu, L.; Yan, X.; et al. Structure, function and pharmacology of human itch receptor complexes. Nature 2021, 600, 164–169. [Google Scholar] [CrossRef]
- Lu, C.; Wu, C.; Ghoreishi, D.; Chen, W.; Wang, L.; Damm, W.; Ross, G.A.; Dahlgren, M.K.; Russell, E.; Von Bargen, C.D.; et al. OPLS4: Improving Force Field Accuracy on Challenging Regimes of Chemical Space. J. Chem. Theory Comput. 2021, 17, 4291–4300. [Google Scholar] [CrossRef]
- Righino, B.; Galisson, F.; Pirolli, D.; Vitale, S.; Réty, S.; Gouet, P.; De Rosa, M.C. Structural model of the full-length Ser/Thr protein kinase StkP from S. pneumoniae and its recognition of peptidoglycan fragments. J. Biomol. Struct. Dyn. 2018, 36, 3666–3679. [Google Scholar] [CrossRef]
- De Donato, M.; Righino, B.; Filippetti, F.; Battaglia, A.; Petrillo, M.; Pirolli, D.; Scambia, G.; De Rosa, M.C.; Gallo, D. Identification and antitumor activity of a novel inhibitor of the NIMA-related kinase NEK6. Sci. Rep. 2018, 8, 16047. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring I5corporating a Model of Hydrophobic Enclosure for Protein-Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Pagani, M.; Bavbek, S.; Alvarez-Cuesta, E.; Berna Dursun, A.; Bonadonna, P.; Castells, M.; Cernadas, J.; Chiriac, A.; Sahar, H.; Madrigal-Burgaleta, R.; et al. Hypersensitivity reactions to chemotherapy: An EAACI Position Paper. Allergy 2022, 77, 388–403. [Google Scholar] [CrossRef]
- Sabato, V.; Elst, J.; Van Houdt, M.; Bridts, C.; Mertens, C.; Ebo, D.G. Surface expression of MRGPRX2 on resting basophils: An area of controversy. Allergy 2020, 75, 2421–2422. [Google Scholar] [CrossRef] [PubMed]
- Wedi, B.; Gehring, M.; Kapp, A. Reply to Sabato V et al. “Surface expression of MRGPRX2 expression on resting basophils: An area of controversy”. Allergy 2020, 75, 2424–2427. [Google Scholar] [CrossRef] [PubMed]
- MacGlashan, D., Jr.; Honigberg, L.A.; Smith, A.; Buggy, J.; Schroeder, J.T. Inhibition of IgE-mediated secretion from human basophils with a highly selective Bruton’s tyrosine kinase, Btk, inhibitor. Int. Immunopharmacol. 2011, 11, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Yamasaki, S.; Ito, Y.; Kabu, K.; Hattori, K.; Tezuka, T.; Nishizumi, H.; Kitamura, D.; Goitsuka, R.; Geha, R.S.; et al. Fc{epsilon}RI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J. Cell Biol. 2005, 170, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr. Med. Chem. Anticancer. Agents 2002, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- González-Díaz, S.N.; Canel-Paredes, A.; Macías-Weinmann, A.; Vidal-Gutiérrez, O.; Villarreal-González, R.V. Atopy, allergen sensitization and development of hypersensitivity reactions to paclitaxel. J. Oncol. Pharm. Pract. 2023, 29, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Weiszhár, Z.; Czúcz, J.; Révész, C.; Rosivall, L.; Szebeni, J.; Rozsnyay, Z. Complement activation by polyethoxylated pharmaceutical surfactants: Cremophor-EL, Tween-80 and Tween-20. Eur. J. Pharm. Sci. 2012, 45, 492–498. [Google Scholar] [CrossRef]
- Essayan, D.M.; Kagey-Sobotka, A.; Colarusso, P.J.; Lichtenstein, L.M.; Ozols, R.F.; King, E.D. Successful parenteral desensitization to paclitaxel. J. Allergy Clin. Immunol. 1996, 97 Pt 1, 42–46. [Google Scholar] [CrossRef]
- Ebo, D.G.; Van der Poorten, M.L.; Elst, J.; Van Gasse, A.L.; Mertens, C.; Bridts, C.; Garvey, L.H.; Horiuchi, T.; Sabato, V. Immunoglobulin E cross-linking or MRGPRX2 activation: Clinical insights from rocuronium hypersensitivity. Br. J. Anaesth. 2021, 126, e27–e29. [Google Scholar] [CrossRef]
- Tatemoto, K.; Nozaki, Y.; Tsuda, R.; Konno, S.; Tomura, K.; Furuno, M.; Ogasawara, H.; Edamura, K.; Takagi, H.; Iwamura, H.; et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem. Biophys. Res. Commun. 2006, 349, 1322–1328. [Google Scholar] [CrossRef]
- Kumar, M.; Duraisamy, K.; Annapureddy, R.R.; Chan, C.B.; Chow, B.K.C. Novel small molecule MRGPRX2 antagonists inhibit a murine model of allergic reaction. J. Allergy Clin. Immunol. 2023, 151, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.N.; Kammala, A.K.; Syed, M.; Yang, C.; Occhiuto, C.J.; Nellutla, R.; Chumanevich, A.P.; Oskeritzian, C.A.; Das, R.; Subramanian, H. Osthole, a Natural Plant Derivative Inhibits MRGPRX2 Induced Mast Cell Responses. Front. Immunol. 2020, 11, 703. [Google Scholar] [CrossRef] [PubMed]
- Bawazir, M.; Amponnawarat, A.; Hui, Y.; Oskeritzian, C.A.; Ali, H. Inhibition of MRGPRX2 but not FcεRI or MrgprB2-mediated mast cell degranulation by a small molecule inverse receptor agonist. Front. Immunol. 2022, 13, 1033794. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.A.; Cao, Y.; Dispenza, M.C.; Ma, S.; Gordon, L.I.; Petrich, A.M.; Bochner, B.S. Ibrutinib, a Bruton’s tyrosine kinase inhibitor used for treatment of lymphoproliferative disorders, eliminates both aeroallergen skin test and basophil activation test reactivity. J. Allergy Clin. Immunol. 2017, 140, 875–879.e1. [Google Scholar] [CrossRef]
- Estupiñán, H.Y.; Berglöf, A.; Zain, R.; Smith, C.I.E. Comparative Analysis of BTK Inhibitors and Mechanisms Underlying Adverse Effects. Front. Cell Dev. Biol. 2021, 9, 630942. [Google Scholar] [CrossRef]

| Culprit Drug | SI Calculation Method | Cutoff Value | SE | SP | iHSRpos Patients above Cutoff (Out of Total) | iHSRneg Patients above Cutoff (Out of Total) |
|---|---|---|---|---|---|---|
| Paclitaxel | SI-CD63% | >0.22 | 90.91% | 90.91% | 10/11 | 1/11 |
| Paclitaxel | SI-CD203cMFI | >0.024 | 81.82% | 90.91% | 9/11 | 1/11 |
| Paclitaxel | SI-CD63% + SI-CD203cMFI | >0.1809 | 90.91% | 90.91% | 10/11 | 1/11 |
| Carboplatin | SI-CD63% | >0.665 | 78.57% | 91.67% | 11/14 | 1/12 |
| Carboplatin | SI-CD203cMFI | >0.115 | 64.29% | 91.67% | 9/14 | 1/12 |
| Carboplatin | SI-CD63% + SI-CD203cMFI | >0.78 | 78.57% | 91.67% | 11/14 | 1/12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Sorda, M.; Fossati, M.; Graffeo, R.; Ferraironi, M.; De Rosa, M.C.; Buzzonetti, A.; Righino, B.; Zampetti, N.; Fattorossi, A.; Nucera, E.; et al. A Modified Basophil Activation Test for the Clinical Management of Immediate Hypersensitivity Reactions to Paclitaxel: A Proof-of-Concept Study. Cancers 2023, 15, 5818. https://doi.org/10.3390/cancers15245818
La Sorda M, Fossati M, Graffeo R, Ferraironi M, De Rosa MC, Buzzonetti A, Righino B, Zampetti N, Fattorossi A, Nucera E, et al. A Modified Basophil Activation Test for the Clinical Management of Immediate Hypersensitivity Reactions to Paclitaxel: A Proof-of-Concept Study. Cancers. 2023; 15(24):5818. https://doi.org/10.3390/cancers15245818
Chicago/Turabian StyleLa Sorda, Marilena, Marco Fossati, Rosalia Graffeo, Manuela Ferraironi, Maria Cristina De Rosa, Alexia Buzzonetti, Benedetta Righino, Nicole Zampetti, Andrea Fattorossi, Eleonora Nucera, and et al. 2023. "A Modified Basophil Activation Test for the Clinical Management of Immediate Hypersensitivity Reactions to Paclitaxel: A Proof-of-Concept Study" Cancers 15, no. 24: 5818. https://doi.org/10.3390/cancers15245818
APA StyleLa Sorda, M., Fossati, M., Graffeo, R., Ferraironi, M., De Rosa, M. C., Buzzonetti, A., Righino, B., Zampetti, N., Fattorossi, A., Nucera, E., Aruanno, A., Ferrandina, G., Apostol, A. I., Buonomo, A., Scambia, G., Sanguinetti, M., & Battaglia, A. (2023). A Modified Basophil Activation Test for the Clinical Management of Immediate Hypersensitivity Reactions to Paclitaxel: A Proof-of-Concept Study. Cancers, 15(24), 5818. https://doi.org/10.3390/cancers15245818








