Experimental Modeling of Host–Bacterial Interactions in Head and Neck Squamous Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Microbiome Plays a Role in Head and Neck Homeostasis
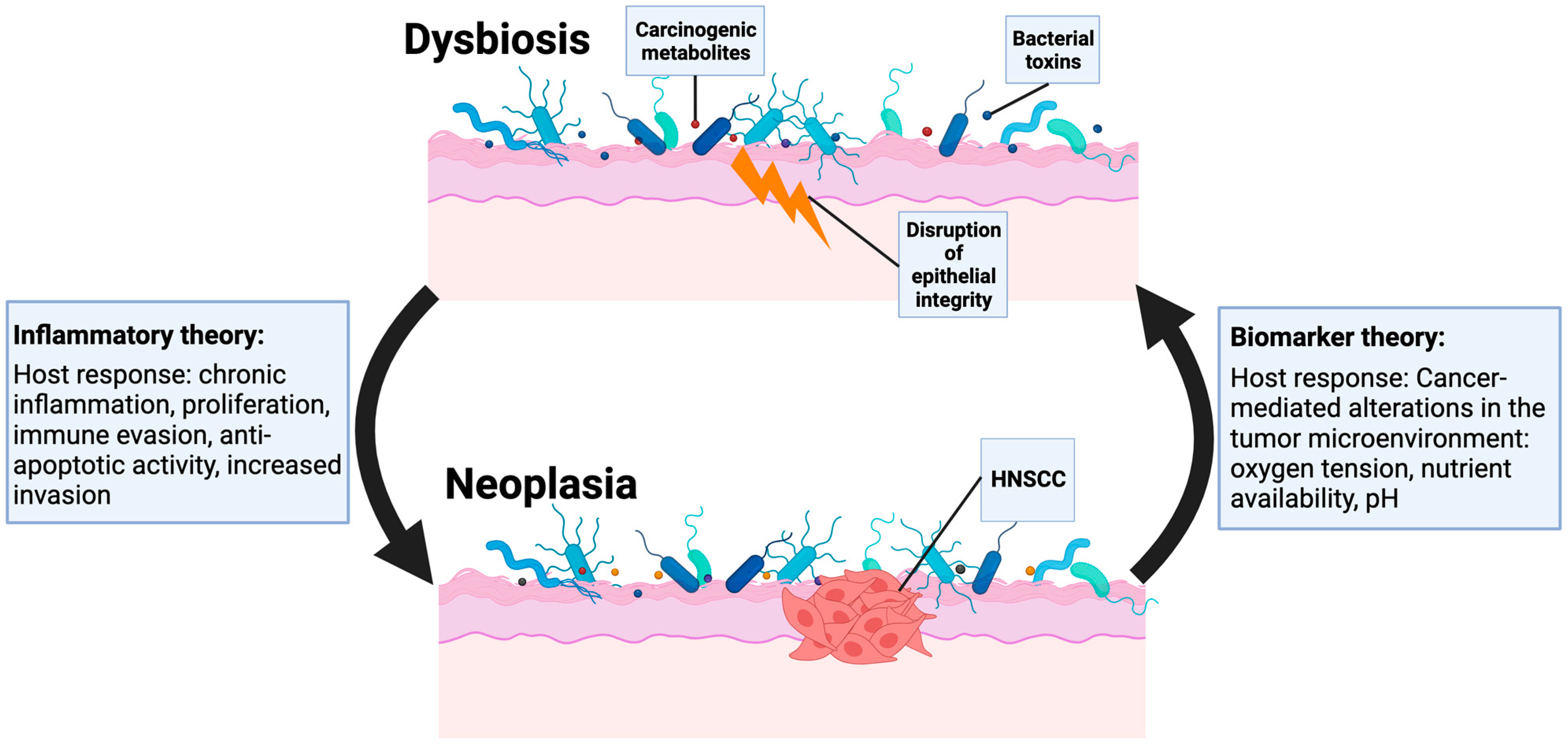
3. The Microbiome May Be Altered in HNSCC
| Sample | Citation Number | Technique | Bacterial Diversity | Notable Specific Bacterial Populations Enriched or Depleted in Cancer | ||
|---|---|---|---|---|---|---|
| Alpha | Beta | Enriched | Depleted | |||
| Tissue | [20] | 16S rRNAseq | NR | NR | Entereobacteriaceae | |
| [25] | 16S rRNAseq | Increased | NR | Fusobacterium, Capnocytophaga, Alloprevotella | Streptococcus, Veillonella, Lautropia | |
| [5] | 16S rRNAseq | Increased | + | Fusobacterium, Prevotella, Capnocytophaga | Streptococcus, Veillonella, Parvimonas | |
| [17] | 16s rDNAseq | No difference | − | Parvimonas | Actinomyces | |
| [27] | 16S rRNAseq | Increased | + | Fusobacterium, Prevotella, Porphyromonas | Streptococcus, Veillonella, Rothia | |
| [29] | 16S rRNAseq | Decreased | + | Stenotrophomonas, Ruminococcus, Comamonadaceae | Tannerella, Veillonella, Kingella | |
| [40] | PathoChip | NR | Lactobacillus, Lactococcus, Proteus | NR | ||
| [35] | 16S rRNAseq | No difference | + | Firmicutes, Actinobacteria | Spirochetes, Synergistetes, Fusobacteria | |
| [47] | 16s rRNAseq | NR | + | Fusobacteria and Spirochaetes | Firmicutes and Actinobacteria | |
| Saliva | [39] | 16S rRNAseq | Increased | + | Lactobacillus, Streptococcus, Staphylococcus | Aggregatibacter, Haemophillus, Neisseria |
| [21] | 16S rRNAseq | Increased | + | Streptococcus, Lactobacillus, parvmonas | Leptotrichia, Fusobacterium | |
| [33] | 16S rRNAseq | No difference | - | Actinobacteria | NR | |
| [42] | RNASeq | NR | - | Fusobacteria, Selenomonas, Capnocytophaga | NR | |
| [26] | 16S rRNAseq | Increased | + | Fusobacterium | Streptococcus, Haemophilus, Porphyromonas | |
| [6] | 16S rRNAseq | NR | Prevotella, Fusobacterium | Streptococcus | ||
| [34] | 16S rRNAseq | No difference | + | None | None | |
| [44] | 16S rRNAseq | No difference | + | Fusobacterium, Prevotella, Alloprevotella | Streptococcus | |
| [31] | 16S rRNAseq | Decreased | + | Streptococcus, Gemella, Veillonella | Haemophilus, Veillonella, Fusobacterium | |
| [48] | 16S rRNAseq | No difference | + | Fusobacteria, Prevotella, Veillonella | Neisseria, Rothia, Rhodotorula | |
| [49] | 16S rDNAseq | NR | Granulicatella, Alloscardovia, Stenotrophomonas | Moryella, Kingella, Centipeda | ||
| [30] | 16S rRNAseq | Decreased | + | Lactobacillus, Ochrobactrum, Parvimonas | Neisseria and Phyllobacterium | |
| [50] | 16S rRNAseq | No difference | + | Lachnospiraceae, Eikenella | Lactobacillus, Bacillus, Bifidobacteriaceae | |
| Tissue/ Saliva | [32] | 16S rRNAseq | Decreased | + | Tissue: Acinetobacter, Fusobacterium, Campylobacter Saliva: Streptococcus, Prevotella | NR |
| [19] | 16S rRNAseq | Decreased | + | Tissue: Fusobacterium, Peptostreptococcus, Johnsonsella Saliva: Fusobacterium, Alloprevotella, Prevotella | Tissue: Streptoccocus, Neisseria, Veillonella Saliva: Streptoccocus, Neisseria, Rothia | |
| [43] | 16S rDNAseq | Decreased | + | Fusobacterium, Peptostreptococcus, Prevotella | Streptococcus, Neisseria, Haemophilus | |
| [51] | 16S rDNAseq | Increased * | + | Fusobacterium, Prevotella, Actinomyces | Streptococcus, Veillonella, Rothia | |
| [52] | 16S rDNAseq | Increased | +/− ** | Veillonella, Fusobacterium | Streptococcus, Neisseria, Prevotella | |
| Swab | [28] | 16S rRNAseq | Increased | + | Fusobacterium, Peptostreptococcus, Prevotella | Streptococcus |
| Sample | Citation Number | Technique | Stratification | Bacterial Diversity | Notable Specific Bacterial Populations Enriched or Depleted | Key Findings | |
|---|---|---|---|---|---|---|---|
| Alpha | Beta | ||||||
| Tissue | [53] | 16S rRNAseq | Chemotherapy induction | Decrease | + | Enriched in induced chemotherapy group:
Mycoplasma and unidentified Veilloneliaceae. Depleted in induced chemotherapy group: Veillonella, Rhodococcus and Acinetobacter. | In a non-induced chemotherapy group, Fusobacterium and Actinomyces were associated with more advanced stage of disease. |
| [54] | 16S rRNAseq | Length of survival | Decreased diversity in patients who survived less than 3 years compared with those who survived greater than 3 years | Significant difference in bacterial communities between those who survived less than 3 years and those who survived greater than 3 years. | Enriched in cases with survival less than 3 years:
Methyloversatilis and Schlegelella. Enriched in cases with survival greater than 3 years: Bacillus, Lactobacillus and Sphingomonas. | Patients with tumors with increased dysbiosis exhibited shorter overall survival than those with less dysbiosis. | |
| [55] | RNAseq | Subsite Location | NR | Enriched Oral HNSCC:
Fusobacterium, Leptotrichia, Selenomonas and
Treponema. Enriched Nonoral HNSCC: Clostridium and Pseudoalteromonas. | Microbial signatures were correlated with the Kyoto Encyclopedia of Genes and Genomes pathways for both oral and non-oral cancers. Oral cancers showed signatures involved in neurodegenerative diseases and non-oral cancers showed signatures involved in HSV-1 infection. | ||
| [56] | RNAseq | NR | Enriched by Subsite Oral Cavity: Pseudomonas Oropharynx: Actinomyces and Sulfurimonas Larynx: Filifactor, Pseudomonas, and Actinomyces | Microbial diversity was dependent on tumor location (oral cavity, oropharynx, larynx). | |||
| Saliva | [36] | 16S rRNAseq | Tumor Mutational Characterization | Significant difference between mutational signal cluster cluster 2 and 3 | Slight difference in bacterial communities between mutational signal cluster 1, 2 and 3 | Enriched in MSC1 and MSC2: Rothia Enriched in MSC2: Firmicutes Enriched in MSC2 and MSC3: Selenomonas Enriched in MSC3: Capnocytophaga | Inferred functional assessment for microbial communities across mutational states showed differential enrichment in pathways linked to cell-mobility |
| [57] | 16S rRNAseq | Development of Oral Mucositis | No difference * | − * | See key findings | Patients with enrichment in Cardiobacterium, Granulicatella, Prevotella, and Fusobacterium had increased risk of developing early onset severe oral mucositis after chemoradiation. Patients with enrichment of Streptococcus had a decreased risk of developing early onset severe oral mucositis after chemoradiation. | |
| [45] | 16S rRNAseq | Metastasis | No difference between metastatic cancer and non-metastatic cancer | Significant difference between metastatic cancer and non-metastatic cancer | Enriched in metastatic group: Prevotella, Stomatobaculum, Bifidobacterium, Peptostreptococcaceae, Shuttleworthia and Finegoldia Enriched in non-metastatic group: Neisseria, Haemophilus. | A machine learning program used the oral microbiome to predict lymph node metastases with 86.3% accuracy. | |
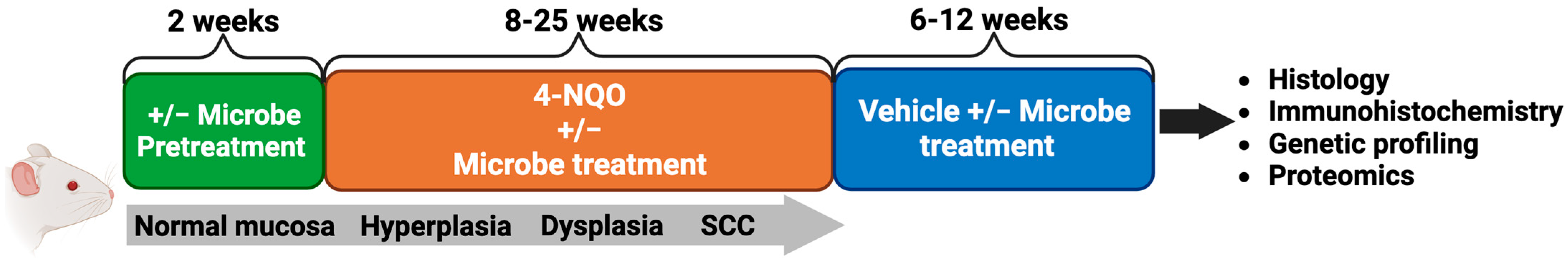
4. Animal Models Suggest the Microbiome May Contribute to the Development and Progression of HNSCC
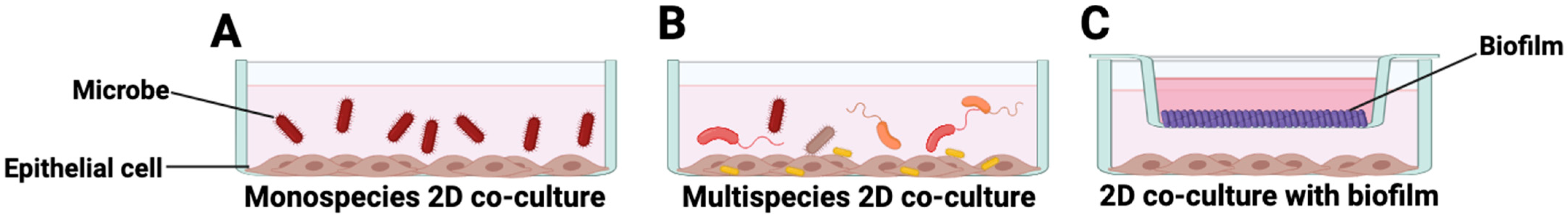
5. In Vitro Modeling of Host–Microbe Interactions in HNSCC
6. Organotypic 3D Culture Systems to Study Host–Microbe Interactions
7. Three-Dimensional Organoids to Model Host–Microbe Interactions
7.1. Co-Culture via Microinjection
7.2. Microbial Infection of Dissociated Epithelial Cells
7.3. Epithelial-Microbial Co-Culture Using Organoid-Derived Monolayers
7.4. Three-Dimensional Organoids to Examine Microbial Contributions to Carcinogenesis in the Aerodigestive Tract
8. Future Directions: 3D Microbiome Co-Culture Models to Investigate HNSCC Carcinogenesis
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef]
- Koyfman, S.A.; Ismaila, N.; Crook, D.; D’Cruz, A.; Rodriguez, C.P.; Sher, D.J.; Silbermins, D.; Sturgis, E.M.; Tsue, T.T.; Weiss, J.; et al. Management of the Neck in Squamous Cell Carcinoma of the Oral Cavity and Oropharynx: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1753–1774. [Google Scholar] [CrossRef]
- D’Cruz, A.K.; Vaish, R.; Kapre, N.; Dandekar, M.; Gupta, S.; Hawaldar, R.; Agarwal, J.P.; Pantvaidya, G.; Chaukar, D.; Deshmukh, A.; et al. Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. N. Engl. J. Med. 2015, 373, 521–529. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Shin, J.M.; Luo, T.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Microbial Communities Associated with Primary and Metastatic Head and Neck Squamous Cell Carcinoma—A High Fusobacterial and Low Streptococcal Signature. Sci. Rep. 2017, 7, 9934. [Google Scholar] [CrossRef]
- Hsiao, J.-R.; Chang, C.-C.; Lee, W.-T.; Huang, C.-C.; Ou, C.-Y.; Tsai, S.-T.; Chen, K.-C.; Huang, J.-S.; Wong, T.-Y.; Lai, Y.-H.; et al. The Interplay between Oral Microbiome, Lifestyle Factors and Genetic Polymorphisms in the Risk of Oral Squamous Cell Carcinoma. Carcinogenesis 2018, 39, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Luo, W. Nasopharyngeal Carcinoma Ecology Theory: Cancer as Multidimensional Spatiotemporal “Unity of Ecology and Evolution” Pathological Ecosystem. Theranostics 2023, 13, 1607–1631. [Google Scholar] [CrossRef]
- Binder Gallimidi, A.; Fischman, S.; Revach, B.; Bulvik, R.; Maliutina, A.; Rubinstein, A.M.; Nussbaum, G.; Elkin, M. Periodontal Pathogens Porphyromonas gingivalis and Fusobacterium nucleatum Promote Tumor Progression in an Oral-Specific Chemical Carcinogenesis Model. Oncotarget 2015, 6, 22613–22623. [Google Scholar] [CrossRef]
- Hoppe, T.; Kraus, D.; Novak, N.; Probstmeier, R.; Frentzen, M.; Wenghoefer, M.; Jepsen, S.; Winter, J. Oral Pathogens Change Proliferation Properties of Oral Tumor Cells by Affecting Gene Expression of Human Defensins. Tumour Biol. 2016, 37, 13789–13798. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, C.; Roselli, R.; Biagioli, M.; Marchianò, S.; Distrutti, E.; Bordoni, M.; Donini, A.; Fiorucci, S. Organoids as Ex Vivo Culture System to Investigate Infection-Host Interaction in Gastric Pre-Carcinogenesis. Recent Adv. Inflamm. Allergy Drug Discov. 2022, 15, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Samarrai, R.; Frank, S.; Lum, A.; Woodis, K.; Weinstock, G.; Roberts, D. Defining the Microbiome of the Head and Neck: A Contemporary Review. Am. J. Otolaryngol. 2022, 43, 103224. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef]
- Annavajhala, M.K.; May, M.; Compres, G.; Freedberg, D.E.; Graham, R.; Stump, S.; Que, J.; Korem, T.; Uhlemann, A.-C.; Abrams, J.A. Relationship of the Esophageal Microbiome and Tissue Gene Expression and Links to the Oral Microbiome: A Randomized Clinical Trial. Clin. Transl. Gastroenterol. 2020, 11, e00235. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Zhang, L. Role of the Microbiome in Oral Cancer Occurrence, Progression and Therapy. Microb. Pathog. 2022, 169, 105638. [Google Scholar] [CrossRef]
- van Vliet, M.J.; Harmsen, H.J.M.; de Bont, E.S.J.M.; Tissing, W.J.E. The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced Mucositis. PLoS Pathog. 2010, 6, e1000879. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Ando, M.; Kamada, N.; Nagano, Y.; Narushima, S.; Suda, W.; Imaoka, A.; Setoyama, H.; Nagamori, T.; et al. Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell 2015, 163, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Funchain, P.; Bebek, G.; Altemus, J.; Zhang, H.; Niazi, F.; Peterson, C.; Lee, W.T.; Burkey, B.B.; Eng, C. Microbiomic Differences in Tumor and Paired-Normal Tissue in Head and Neck Squamous Cell Carcinomas. Genome Med. 2017, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Stratiki, Z.; Costalos, C.; Sevastiadou, S.; Kastanidou, O.; Skouroliakou, M.; Giakoumatou, A.; Petrohilou, V. The Effect of a Bifidobacter Supplemented Bovine Milk on Intestinal Permeability of Preterm Infants. Early Hum. Dev. 2007, 83, 575–579. [Google Scholar] [CrossRef]
- Chen, Z.; Wong, P.Y.; Ng, C.W.K.; Lan, L.; Fung, S.; Li, J.W.; Cai, L.; Lei, P.; Mou, Q.; Wong, S.H.; et al. The Intersection between Oral Microbiota, Host Gene Methylation and Patient Outcomes in Head and Neck Squamous Cell Carcinoma. Cancers 2020, 12, 3425. [Google Scholar] [CrossRef]
- Bebek, G.; Bennett, K.L.; Funchain, P.; Campbell, R.; Seth, R.; Scharpf, J.; Burkey, B.; Eng, C. Microbiomic Subprofiles and MDR1 Promoter Methylation in Head and Neck Squamous Cell Carcinoma. Hum. Mol. Genet. 2012, 21, 1557–1565. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; White, J.R.; Godoy-Vitorino, F.; Rodríguez-Hilario, A.; Navarro, K.; González, H.; Michailidi, C.; Jedlicka, A.; Canapp, S.; Bondy, J.; et al. High-Resolution Microbiome Profiling Uncovers Fusobacterium Nucleatum, Lactobacillus Gasseri/Johnsonii, and Lactobacillus Vaginalis Associated to Oral and Oropharyngeal Cancer in Saliva from HPV Positive and HPV Negative Patients Treated with Surgery and Chemo-Radiation. Oncotarget 2017, 8, 110931–110948. [Google Scholar] [CrossRef] [PubMed]
- Groeger, S.; Denter, F.; Lochnit, G.; Schmitz, M.L.; Meyle, J. Porphyromonas gingivalis Cell Wall Components Induce Programmed Death Ligand 1 (PD-L1) Expression on Human Oral Carcinoma Cells by a Receptor-Interacting Protein Kinase 2 (RIP2)-Dependent Mechanism. Infect. Immun. 2020, 88, e00051-20. [Google Scholar] [CrossRef] [PubMed]
- Groeger, S.; Howaldt, H.P.; Raifer, H.; Gattenloehner, S.; Chakraborty, T.; Meyle, J. Oral Squamous Carcinoma Cells Express B7-H1 and B7-DC Receptors in Vivo. Pathol. Oncol. Res. 2017, 23, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Chen, H.-M.; Yang, S.-F.; Liang, C.; Peng, C.-Y.; Lin, F.-M.; Tsai, L.-L.; Wu, B.-C.; Hsin, C.-H.; Chuang, C.-Y.; et al. Bacterial Alterations in Salivary Microbiota and Their Association in Oral Cancer. Sci. Rep. 2017, 7, 16540. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chu, M.; Huang, Z.; Yang, X.; Ran, S.; Hu, B.; Zhang, C.; Liang, J. Variations in Oral Microbiota Associated with Oral Cancer. Sci. Rep. 2017, 7, 11773. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Yeh, Y.-M.; Yu, H.-Y.; Chin, C.-Y.; Hsu, C.-W.; Liu, H.; Huang, P.-J.; Hu, S.-N.; Liao, C.-T.; Chang, K.-P.; et al. Oral Microbiota Community Dynamics Associated with Oral Squamous Cell Carcinoma Staging. Front. Microbiol. 2018, 9, 862. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The Oral Microbiota May Have Influence on Oral Cancer. Front. Cell Infect. Microbiol. 2019, 9, 476. [Google Scholar] [CrossRef]
- Su, S.-C.; Chang, L.-C.; Huang, H.-D.; Peng, C.-Y.; Chuang, C.-Y.; Chen, Y.-T.; Lu, M.-Y.; Chiu, Y.-W.; Chen, P.-Y.; Yang, S.-F. Oral Microbial Dysbiosis and Its Performance in Predicting Oral Cancer. Carcinogenesis 2021, 42, 127–135. [Google Scholar] [CrossRef]
- Sharma, A.K.; DeBusk, W.T.; Stepanov, I.; Gomez, A.; Khariwala, S.S. Oral Microbiome Profiling in Smokers with and without Head and Neck Cancer Reveals Variations Between Health and Disease. Cancer Prev. Res. 2020, 13, 463–474. [Google Scholar] [CrossRef]
- Frank, D.N.; Qiu, Y.; Cao, Y.; Zhang, S.; Lu, L.; Kofonow, J.M.; Robertson, C.E.; Liu, Y.; Wang, H.; Levens, C.L.; et al. A Dysbiotic Microbiome Promotes Head and Neck Squamous Cell Carcinoma. Oncogene 2022, 41, 1269. [Google Scholar] [CrossRef]
- Shay, E.; Sangwan, N.; Padmanabhan, R.; Lundy, S.; Burkey, B.; Eng, C. Bacteriome and Mycobiome and Bacteriome-Mycobiome Interactions in Head and Neck Squamous Cell Carcinoma. Oncotarget 2020, 11, 2375–2386. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Feng, Q.; Chen, B.; Li, M.; Liang, C.; Li, M.; Li, Z.; Xu, Q.; Zhang, L.; et al. Compositional and Functional Analysis of the Microbiome in Tissue and Saliva of Oral Squamous Cell Carcinoma. Front. Microbiol. 2019, 10, 1439. [Google Scholar] [CrossRef]
- Hayes, R.B.; Ahn, J.; Fan, X.; Peters, B.A.; Ma, Y.; Yang, L.; Agalliu, I.; Burk, R.D.; Ganly, I.; Purdue, M.P.; et al. Association of Oral Microbiome with Risk for Incident Head and Neck Squamous Cell Cancer. JAMA Oncol. 2018, 4, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Vesty, A.; Gear, K.; Biswas, K.; Radcliff, F.J.; Taylor, M.W.; Douglas, R.G. Microbial and Inflammatory-Based Salivary Biomarkers of Head and Neck Squamous Cell Carcinoma. Clin. Exp. Dent. Res. 2018, 4, 255–262. [Google Scholar] [CrossRef] [PubMed]
- De Martin, A.; Lütge, M.; Stanossek, Y.; Engetschwiler, C.; Cupovic, J.; Brown, K.; Demmer, I.; Broglie, M.A.; Geuking, M.B.; Jochum, W.; et al. Distinct Microbial Communities Colonize Tonsillar Squamous Cell Carcinoma. Oncoimmunology 2021, 10, 1945202. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-F.; Huang, H.-D.; Fan, W.-L.; Jong, Y.-J.; Chen, M.-K.; Huang, C.-N.; Chuang, C.-Y.; Kuo, Y.-L.; Chung, W.-H.; Su, S.-C. Compositional and Functional Variations of Oral Microbiota Associated with the Mutational Changes in Oral Cancer. Oral Oncol. 2018, 77, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y. Bioinformatic and Statistical Analysis of Microbiome Data. Methods Mol. Biol. 2023, 2629, 183–229. [Google Scholar] [CrossRef]
- Coletta, R.D.; Yeudall, W.A.; Salo, T. Grand Challenges in Oral Cancers. Front. Oral Health 2020, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Preston, R.; Godoy-Vitorino, F.; Jedlicka, A.; Rodríguez-Hilario, A.; González, H.; Bondy, J.; Lawson, F.; Folawiyo, O.; Michailidi, C.; Dziedzic, A.; et al. 16S rRNA Amplicon Sequencing Identifies Microbiota Associated with Oral Cancer, Human Papilloma Virus Infection and Surgical Treatment. Oncotarget 2016, 7, 51320–51334. [Google Scholar] [CrossRef]
- Rajasekaran, K.; Carey, R.M.; Lin, X.; Seckar, T.D.; Wei, Z.; Chorath, K.; Newman, J.G.; O’Malley, B.W.; Weinstein, G.S.; Feldman, M.D.; et al. The Microbiome of HPV-Positive Tonsil Squamous Cell Carcinoma and Neck Metastasis. Oral Oncol. 2021, 117, 105305. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lin, W.-C.; Kong, M.-S.; Shi, H.N.; Walker, W.A.; Lin, C.-Y.; Huang, C.-T.; Lin, Y.-C.; Jung, S.-M.; Lin, T.-Y. Oral Inoculation of Probiotics Lactobacillus acidophilus NCFM Suppresses Tumour Growth Both in Segmental Orthotopic Colon Cancer and Extra-Intestinal Tissue. Br. J. Nutr. 2012, 107, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Yost, S.; Stashenko, P.; Choi, Y.; Kukuruzinska, M.; Genco, C.A.; Salama, A.; Weinberg, E.O.; Kramer, C.D.; Frias-Lopez, J. Increased Virulence of the Oral Microbiome in Oral Squamous Cell Carcinoma Revealed by Metatranscriptome Analyses. Int. J. Oral Sci. 2018, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, X.; Zhao, X.; Xu, Z.; Dai, W.; Duan, W.; Huang, S.; Zhang, E.; Liu, J.; Zhang, S.; et al. Composition and Function of Oral Microbiota between Gingival Squamous Cell Carcinoma and Periodontitis. Oral Oncol. 2020, 107, 104710. [Google Scholar] [CrossRef] [PubMed]
- Ganly, I.; Yang, L.; Giese, R.A.; Hao, Y.; Nossa, C.W.; Morris, L.G.T.; Rosenthal, M.; Migliacci, J.; Kelly, D.; Tseng, W.; et al. Periodontal Pathogens Are a Risk Factor of Oral Cavity Squamous Cell Carcinoma, Independent of Tobacco and Alcohol and Human Papillomavirus. Int. J. Cancer 2019, 145, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Eun, Y.-G.; Lee, J.-W.; Kim, S.W.; Hyun, D.-W.; Bae, J.-W.; Lee, Y.C. Oral Microbiome Associated with Lymph Node Metastasis in Oral Squamous Cell Carcinoma. Sci. Rep. 2021, 11, 23176. [Google Scholar] [CrossRef] [PubMed]
- Debelius, J.W.; Huang, T.; Cai, Y.; Ploner, A.; Barrett, D.; Zhou, X.; Xiao, X.; Li, Y.; Liao, J.; Zheng, Y.; et al. Subspecies Niche Specialization in the Oral Microbiome Is Associated with Nasopharyngeal Carcinoma Risk. mSystems 2020, 5, e00065-20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Wang, J. A Comprehensive Analysis of Intratumor Microbiome in Head and Neck Squamous Cell Carcinoma. Eur. Arch. Otorhinolaryngol. 2022, 279, 4127–4136. [Google Scholar] [CrossRef]
- Rai, A.K.; Panda, M.; Das, A.K.; Rahman, T.; Das, R.; Das, K.; Sarma, A.; Kataki, A.C.; Chattopadhyay, I. Dysbiosis of Salivary Microbiome and Cytokines Influence Oral Squamous Cell Carcinoma through Inflammation. Arch. Microbiol. 2021, 203, 137–152. [Google Scholar] [CrossRef]
- Granato, D.C.; Neves, L.X.; Trino, L.D.; Carnielli, C.M.; Lopes, A.F.B.; Yokoo, S.; Pauletti, B.A.; Domingues, R.R.; Sá, J.O.; Persinoti, G.; et al. Meta-Omics Analysis Indicates the Saliva Microbiome and Its Proteins Associated with the Prognosis of Oral Cancer Patients. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140659. [Google Scholar] [CrossRef]
- Benjamin, W.J.; Wang, K.; Zarins, K.; Bellile, E.; Blostein, F.; Argirion, I.; Taylor, J.M.G.; D’Silva, N.J.; Chinn, S.B.; Rifkin, S.; et al. Oral Microbiome Community Composition in Head and Neck Squamous Cell Carcinoma. Cancers 2023, 15, 2549. [Google Scholar] [CrossRef]
- Torralba, M.G.; Aleti, G.; Li, W.; Moncera, K.J.; Lin, Y.-H.; Yu, Y.; Masternak, M.M.; Golusinski, W.; Golusinski, P.; Lamperska, K.; et al. Oral Microbial Species and Virulence Factors Associated with Oral Squamous Cell Carcinoma. Microb. Ecol. 2021, 82, 1030–1046. [Google Scholar] [CrossRef]
- Zhou, X.; Hao, Y.; Peng, X.; Li, B.; Han, Q.; Ren, B.; Li, M.; Li, L.; Li, Y.; Cheng, G.; et al. The Clinical Potential of Oral Microbiota as a Screening Tool for Oral Squamous Cell Carcinomas. Front. Cell Infect. Microbiol. 2021, 11, 728933. [Google Scholar] [CrossRef]
- Lau, H.-C.; Hsueh, C.-Y.; Gong, H.; Sun, J.; Huang, H.-Y.; Zhang, M.; Zhou, L. Oropharynx Microbiota Transitions in Hypopharyngeal Carcinoma Treatment of Induced Chemotherapy Followed by Surgery. BMC Microbiol. 2021, 21, 310. [Google Scholar] [CrossRef]
- Dou, Y.; Ma, C.; Wang, K.; Liu, S.; Sun, J.; Tan, W.; Neckenig, M.; Wang, Q.; Dong, Z.; Gao, W.; et al. Dysbiotic Tumor Microbiota Associates with Head and Neck Squamous Cell Carcinoma Outcomes. Oral Oncol. 2022, 124, 105657. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kwon, E.J.; Yu, Y.; Kim, J.; Woo, S.-Y.; Choi, H.-S.; Kwon, M.; Jung, K.; Kim, H.-S.; Park, H.R.; et al. Microbial and Molecular Differences According to the Location of Head and Neck Cancers. Cancer Cell Int. 2022, 22, 135. [Google Scholar] [CrossRef]
- Dhakal, A.; Upadhyay, R.; Wheeler, C.; Hoyd, R.; Karivedu, V.; Gamez, M.E.; Valentin, S.; Vanputten, M.; Bhateja, P.; Bonomi, M.; et al. Association between Tumor Microbiome and Hypoxia across Anatomic Subsites of Head and Neck Cancers. Int. J. Mol. Sci. 2022, 23, 15531. [Google Scholar] [CrossRef]
- Reyes-Gibby, C.C.; Wang, J.; Zhang, L.; Peterson, C.B.; Do, K.-A.; Jenq, R.R.; Shelburne, S.; Shah, D.P.; Chambers, M.S.; Hanna, E.Y.; et al. Oral Microbiome and Onset of Oral Mucositis in Patients with Squamous Cell Carcinoma of the Head and Neck. Cancer 2020, 126, 5124–5136. [Google Scholar] [CrossRef] [PubMed]
- Steidler, N.E.; Reade, P.C. Experimental Induction of Oral Squamous Cell Carcinomas in Mice with 4-Nitroquinolone-1-Oxide. Oral Surg. Oral Med. Oral Pathol. 1984, 57, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.L.; Heniford, B.W.; Ackermann, D.M.; Leonberger, M.; Martinez, S.A.; Hendler, F.J. 4NQO Carcinogenesis: A Mouse Model of Oral Cavity Squamous Cell Carcinoma. Head Neck 1994, 16, 424–432. [Google Scholar] [CrossRef]
- Downes, D.J.; Chonofsky, M.; Tan, K.; Pfannenstiel, B.T.; Reck-Peterson, S.L.; Todd, R.B. Characterization of the Mutagenic Spectrum of 4-Nitroquinoline 1-Oxide (4-NQO) in Aspergillus Nidulans by Whole Genome Sequencing. G3 2014, 4, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Sagheer, S.H.; Whitaker-Menezes, D.; Han, J.Y.S.; Curry, J.M.; Martinez-Outschoorn, U.; Philp, N.J. 4NQO Induced Carcinogenesis: A Mouse Model for Oral Squamous Cell Carcinoma. Methods Cell Biol. 2021, 163, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, P.; Yost, S.; Choi, Y.; Danciu, T.; Chen, T.; Yoganathan, S.; Kressirer, C.; Ruiz-Tourrella, M.; Das, B.; Kokaras, A.; et al. The Oral Mouse Microbiome Promotes Tumorigenesis in Oral Squamous Cell Carcinoma. mSystems 2019, 4, e00323-19. [Google Scholar] [CrossRef] [PubMed]
- Abusleme, L.; Hong, B.-Y.; Hoare, A.; Konkel, J.E.; Diaz, P.I.; Moutsopoulos, N.M. Oral Microbiome Characterization in Murine Models. Bio Protoc. 2017, 7, e2655. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Hughes, C.C.W. Of Mice and Not Men: Differences between Mouse and Human Immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef]
- Mountcastle, S.E.; Cox, S.C.; Sammons, R.L.; Jabbari, S.; Shelton, R.M.; Kuehne, S.A. A Review of Co-Culture Models to Study the Oral Microenvironment and Disease. J. Oral Microbiol. 2020, 12, 1773122. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Moffatt, C.E.; Hagerty, D.; Whitmore, S.E.; Brown, T.A.; Graves, D.T.; Lamont, R.J. Interaction of Oral Bacteria with Gingival Epithelial Cell Multilayers. Mol. Oral Microbiol. 2011, 26, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Millhouse, E.; Jose, A.; Sherry, L.; Lappin, D.F.; Patel, N.; Middleton, A.M.; Pratten, J.; Culshaw, S.; Ramage, G. Development of an in Vitro Periodontal Biofilm Model for Assessing Antimicrobial and Host Modulatory Effects of Bioactive Molecules. BMC Oral Health 2014, 14, 80. [Google Scholar] [CrossRef]
- Geng, F.; Liu, J.; Guo, Y.; Li, C.; Wang, H.; Wang, H.; Zhao, H.; Pan, Y. Persistent Exposure to Porphyromonas gingivalis Promotes Proliferative and Invasion Capabilities, and Tumorigenic Properties of Human Immortalized Oral Epithelial Cells. Front. Cell Infect. Microbiol. 2017, 7, 57. [Google Scholar] [CrossRef]
- Ha, N.H.; Woo, B.H.; Kim, D.J.; Ha, E.S.; Choi, J.I.; Kim, S.J.; Park, B.S.; Lee, J.H.; Park, H.R. Prolonged and Repetitive Exposure to Porphyromonas gingivalis Increases Aggressiveness of Oral Cancer Cells by Promoting Acquisition of Cancer Stem Cell Properties. Tumour Biol. 2015, 36, 9947–9960. [Google Scholar] [CrossRef]
- Yilmaz, Ö.; Jungas, T.; Verbeke, P.; Ojcius, D.M. Activation of the Phosphatidylinositol 3-Kinase/Akt Pathway Contributes to Survival of Primary Epithelial Cells Infected with the Periodontal Pathogen Porphyromonas gingivalis. Infect. Immun. 2004, 72, 3743–3751. [Google Scholar] [CrossRef]
- Moffatt, C.E.; Lamont, R.J. Porphyromonas gingivalis Induction of MicroRNA-203 Expression Controls Suppressor of Cytokine Signaling 3 in Gingival Epithelial Cells. Infect. Immun. 2011, 79, 2632–2637. [Google Scholar] [CrossRef] [PubMed]
- Harrandah, A.M.; Chukkapalli, S.S.; Bhattacharyya, I.; Progulske-Fox, A.; Chan, E.K.L. Fusobacteria Modulate Oral Carcinogenesis and Promote Cancer Progression. J. Oral Microbiol. 2021, 13, 1849493. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.H.; Park, D.G.; Woo, B.H.; Kim, D.J.; Choi, J.I.; Park, B.S.; Kim, Y.D.; Lee, J.H.; Park, H.R. Porphyromonas gingivalis Increases the Invasiveness of Oral Cancer Cells by Upregulating IL-8 and MMPs. Cytokine 2016, 86, 64–72. [Google Scholar] [CrossRef]
- Sztukowska, M.N.; Ojo, A.; Ahmed, S.; Carenbauer, A.L.; Wang, Q.; Shumway, B.; Jenkinson, H.F.; Wang, H.; Darling, D.S.; Lamont, R.J. Porphyromonas gingivalis Initiates a Mesenchymal-like Transition through ZEB1 in Gingival Epithelial Cells. Cell Microbiol. 2016, 18, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Groeger, S.; Domann, E.; Gonzales, J.R.; Chakraborty, T.; Meyle, J. B7-H1 and B7-DC Receptors of Oral Squamous Carcinoma Cells Are Upregulated by Porphyromonas gingivalis. Immunobiology 2011, 216, 1302–1310. [Google Scholar] [CrossRef]
- Law, A.M.K.; Rodriguez de la Fuente, L.; Grundy, T.J.; Fang, G.; Valdes-Mora, F.; Gallego-Ortega, D. Advancements in 3D Cell Culture Systems for Personalizing Anti-Cancer Therapies. Front. Oncol. 2021, 11, 782766. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D Cell Cultures—A Comparison of Different Types of Cancer Cell Cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the Third Dimension: How 3D Culture Microenvironments Alter Cellular Cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Hickman, J.A.; Graeser, R.; de Hoogt, R.; Vidic, S.; Brito, C.; Gutekunst, M.; van der Kuip, H.; IMI PREDECT Consortium. Three-Dimensional Models of Cancer for Pharmacology and Cancer Cell Biology: Capturing Tumor Complexity in Vitro/Ex Vivo. Biotechnol. J. 2014, 9, 1115–1128. [Google Scholar] [CrossRef]
- Bissell, M.J.; Rizki, A.; Mian, I.S. Tissue Architecture: The Ultimate Regulator of Breast Epithelial Function. Curr. Opin. Cell Biol. 2003, 15, 753–762. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The Third Dimension Bridges the Gap between Cell Culture and Live Tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Andrian, E.; Mostefaoui, Y.; Rouabhia, M.; Grenier, D. Regulation of Matrix Metalloproteinases and Tissue Inhibitors of Matrix Metalloproteinases by Porphyromonas gingivalis in an Engineered Human Oral Mucosa Model. J. Cell Physiol. 2007, 211, 56–62. [Google Scholar] [CrossRef]
- Andrian, E.; Grenier, D.; Rouabhia, M. In Vitro Models of Tissue Penetration and Destruction by Porphyromonas gingivalis. Infect. Immun. 2004, 72, 4689–4698. [Google Scholar] [CrossRef]
- Gursoy, U.K.; Pöllänen, M.; Könönen, E.; Uitto, V.-J. A Novel Organotypic Dento-Epithelial Culture Model: Effect of Fusobacterium Nucleatum Biofilm on B-Defensin-2, -3, and LL-37 Expression. J. Periodontol. 2012, 83, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Johnston, W.; Delaney, C.; Rajendran, R.; Butcher, J.; Khan, S.; Bradshaw, D.; Ramage, G.; Culshaw, S. Biofilm-Stimulated Epithelium Modulates the Inflammatory Responses in Co-Cultured Immune Cells. Sci. Rep. 2019, 9, 15779. [Google Scholar] [CrossRef] [PubMed]
- Karakasheva, T.A.; Kijima, T.; Shimonosono, M.; Maekawa, H.; Sahu, V.; Gabre, J.T.; Cruz-Acuña, R.; Giroux, V.; Sangwan, V.; Whelan, K.A.; et al. Generation and Characterization of Patient-Derived Head and Neck, Oral, and Esophageal Cancer Organoids. Curr. Protoc. Stem Cell Biol. 2020, 53, e109. [Google Scholar] [CrossRef]
- Driehuis, E.; Kretzschmar, K.; Clevers, H. Establishment of Patient-Derived Cancer Organoids for Drug-Screening Applications. Nat. Protoc. 2020, 15, 3380–3409. [Google Scholar] [CrossRef]
- Driehuis, E.; Kolders, S.; Spelier, S.; Lõhmussaar, K.; Willems, S.M.; Devriese, L.A.; de Bree, R.; de Ruiter, E.J.; Korving, J.; Begthel, H.; et al. Oral Mucosal Organoids as a Potential Platform for Personalized Cancer Therapy. Cancer Discov. 2019, 9, 852–871. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.S.; Tocchi, A.; Holly, M.K.; Parks, W.C.; Smith, J.G. A Small Intestinal Organoid Model of Non-Invasive Enteric Pathogen-Epithelial Cell Interactions. Mucosal Immunol. 2015, 8, 352–361. [Google Scholar] [CrossRef]
- Sittipo, P.; Lee, Y.K. The Application of Intestinal Organoids and Their Co-Culture Systems in the Study of Gastrointestinal Diseases. Organoid 2022, 2, e3. [Google Scholar] [CrossRef]
- Tiefenboeck, P.; Kim, J.A.; Leroux, J.-C. Intracellular Delivery of Colloids: Past and Future Contributions from Microinjection. Adv. Drug Deliv. Rev. 2018, 132, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, L.-C. Microinjection as a Tool of Mechanical Delivery. Curr. Opin. Biotechnol. 2008, 19, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Williamson, I.A.; Arnold, J.W.; Samsa, L.A.; Gaynor, L.; DiSalvo, M.; Cocchiaro, J.L.; Carroll, I.; Azcarate-Peril, M.A.; Rawls, J.F.; Allbritton, N.L.; et al. A High-Throughput Organoid Microinjection Platform to Study Gastrointestinal Microbiota and Luminal Physiology. Cell Mol. Gastroenterol. Hepatol. 2018, 6, 301–319. [Google Scholar] [CrossRef]
- Puschhof, J.; Pleguezuelos-Manzano, C.; Clevers, H. Organoids and Organs-on-Chips: Insights into Human Gut-Microbe Interactions. Cell Host Microbe 2021, 29, 867–878. [Google Scholar] [CrossRef]
- Dang, J.; Tiwari, S.K.; Lichinchi, G.; Qin, Y.; Patil, V.S.; Eroshkin, A.M.; Rana, T.M. Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell Stem Cell 2016, 19, 258–265. [Google Scholar] [CrossRef]
- Forbester, J.L.; Goulding, D.; Vallier, L.; Hannan, N.; Hale, C.; Pickard, D.; Mukhopadhyay, S.; Dougan, G. Interaction of Salmonella Enterica Serovar Typhimurium with Intestinal Organoids Derived from Human Induced Pluripotent Stem Cells. Infect. Immun. 2015, 83, 2926–2934. [Google Scholar] [CrossRef]
- Levy, A.; Stedman, A.; Deutsch, E.; Donnadieu, F.; Virgin, H.W.; Sansonetti, P.J.; Nigro, G. Innate Immune Receptor NOD2 Mediates LGR5+ Intestinal Stem Cell Protection against ROS Cytotoxicity via Mitophagy Stimulation. Proc. Natl. Acad. Sci. USA 2020, 117, 1994–2003. [Google Scholar] [CrossRef]
- Zhang, Y.-G.; Wu, S.; Xia, Y.; Sun, J. Salmonella-Infected Crypt-Derived Intestinal Organoid Culture System for Host–Bacterial Interactions. Physiol. Rep. 2014, 2, e12147. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Navarro-Serer, B.; Jeong, Y.J.; Chianchiano, P.; Xia, L.; Luchini, C.; Veronese, N.; Dowiak, C.; Ng, T.; Trujillo, M.A.; et al. Pattern of Invasion in Human Pancreatic Cancer Organoids is Associated with Loss of SMAD4 and Clinical Outcome. Cancer Res. 2020, 80, 2804–2817. [Google Scholar] [CrossRef]
- Dutta, D.; Heo, I.; Clevers, H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol. Med. 2017, 23, 393–410. [Google Scholar] [CrossRef]
- Thorne, C.A.; Chen, I.W.; Sanman, L.E.; Cobb, M.H.; Wu, L.F.; Altschuler, S.J. Enteroid Monolayers Reveal an Autonomous WNT and BMP Circuit Controlling Intestinal Epithelial Growth and Organization. Dev. Cell 2018, 44, 624–633.e4. [Google Scholar] [CrossRef]
- Nickerson, K.P.; Llanos-Chea, A.; Ingano, L.; Serena, G.; Miranda-Ribera, A.; Perlman, M.; Lima, R.; Sztein, M.B.; Fasano, A.; Senger, S.; et al. A Versatile Human Intestinal Organoid-Derived Epithelial Monolayer Model for the Study of Enteric Pathogens. Microbiol. Spectr. 2021, 9, e0000321. [Google Scholar] [CrossRef]
- Ranganathan, S.; Doucet, M.; Grassel, C.L.; Delaine-Elias, B.; Zachos, N.C.; Barry, E.M. Evaluating Shigella Flexneri Pathogenesis in the Human Enteroid Model. Infect. Immun. 2019, 87, e00740-18. [Google Scholar] [CrossRef]
- Rees, W.D.; Stahl, M.; Jacobson, K.; Bressler, B.; Sly, L.M.; Vallance, B.A.; Steiner, T.S. Enteroids Derived from Inflammatory Bowel Disease Patients Display Dysregulated Endoplasmic Reticulum Stress Pathways, Leading to Differential Inflammatory Responses and Dendritic Cell Maturation. J. Crohn’s Colitis 2020, 14, 948–961. [Google Scholar] [CrossRef]
- Ruan, W.; Engevik, M.A.; Chang-Graham, A.L.; Danhof, H.A.; Goodwin, A.; Engevik, K.A.; Shi, Z.; Hall, A.; Rienzi, S.C.D.; Venable, S.; et al. Enhancing Responsiveness of Human Jejunal Enteroids to Host and Microbial Stimuli. J. Physiol. 2020, 598, 3085–3105. [Google Scholar] [CrossRef]
- Zhang, J.; Hernandez-Gordillo, V.; Trapecar, M.; Wright, C.; Taketani, M.; Schneider, K.; Chen, W.L.K.; Stas, E.; Breault, D.T.; Carrier, R.L.; et al. Coculture of Primary Human Colon Monolayer with Human Gut Bacteria. Nat. Protoc. 2021, 16, 3874–3900. [Google Scholar] [CrossRef]
- Poletti, M.; Arnauts, K.; Ferrante, M.; Korcsmaros, T. Organoid-Based Models to Study the Role of Host-Microbiota Interactions in IBD. J. Crohn’s Colitis 2021, 15, 1222–1235. [Google Scholar] [CrossRef]
- Kim, M.B.; Hwangbo, S.; Jang, S.; Jo, Y.K. Bioengineered Co-Culture of Organoids to Recapitulate Host-Microbe Interactions. Mater. Today Bio 2022, 16, 100345. [Google Scholar] [CrossRef]
- Bugueno, I.M.; Batool, F.; Keller, L.; Kuchler-Bopp, S.; Benkirane-Jessel, N.; Huck, O. Porphyromonas gingivalis Bypasses Epithelial Barrier and Modulates Fibroblastic Inflammatory Response in an in Vitro 3D Spheroid Model. Sci. Rep. 2018, 8, 14914. [Google Scholar] [CrossRef]
- Bartfeld, S.; Bayram, T.; van de Wetering, M.; Huch, M.; Begthel, H.; Kujala, P.; Vries, R.; Peters, P.J.; Clevers, H. In Vitro Expansion of Human Gastric Epithelial Stem Cells and Their Responses to Bacterial Infection. Gastroenterology 2015, 148, 126–136.e6. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Piazuelo, M.B.; Chaturvedi, R.; Schumacher, M.; Aihara, E.; Feng, R.; Noto, J.M.; Delgado, A.; Israel, D.A.; Zavros, Y.; et al. Helicobacter Pylori Targets Cancer-Associated Apical-Junctional Constituents in Gastroids and Gastric Epithelial Cells. Gut 2015, 64, 720–730. [Google Scholar] [CrossRef]
- Bartfeld, S. Modeling Infectious Diseases and Host-Microbe Interactions in Gastrointestinal Organoids. Dev. Biol. 2016, 420, 262–270. [Google Scholar] [CrossRef] [PubMed]
- McCracken, K.W.; Catá, E.M.; Crawford, C.M.; Sinagoga, K.L.; Schumacher, M.; Rockich, B.E.; Tsai, Y.-H.; Mayhew, C.N.; Spence, J.R.; Zavros, Y.; et al. Modelling Human Development and Disease in Pluripotent Stem-Cell-Derived Gastric Organoids. Nature 2014, 516, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Bertaux-Skeirik, N.; Feng, R.; Schumacher, M.A.; Li, J.; Mahe, M.M.; Engevik, A.C.; Javier, J.E.; Peek, R.M., Jr.; Ottemann, K.; Orian-Rousseau, V.; et al. CD44 Plays a Functional Role in Helicobacter Pylori-Induced Epithelial Cell Proliferation. PLoS Pathog. 2015, 11, e1004663. [Google Scholar] [CrossRef] [PubMed]
- Li, V.S.W. Modelling Intestinal Inflammation and Infection Using “mini-Gut” Organoids. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 89–90. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New Insights into the Mechanisms of Epithelial-Mesenchymal Transition and Implications for Cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, C.; Liu, J.; Geng, F.; Shi, X.; Li, Q.; Lu, Z.; Pan, Y. Fusobacterium Nucleatum Promotes Epithelial-Mesenchymal Transiton through Regulation of the lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 Signaling Pathway. FEBS J. 2020, 287, 4032–4047. [Google Scholar] [CrossRef] [PubMed]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 2017, 171, 1611–1624.e24. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Lebeau, A.; Bruyere, D.; Roncarati, P.; Peixoto, P.; Hervouet, E.; Cobraiville, G.; Taminiau, B.; Masson, M.; Gallego, C.; Mazzucchelli, G.; et al. HPV Infection Alters Vaginal Microbiome through Down-Regulating Host Mucosal Innate Peptides Used by Lactobacilli as Amino Acid Sources. Nat. Commun. 2022, 13, 1076. [Google Scholar] [CrossRef]
- Sachdeva, U.M.; Shimonosono, M.; Flashner, S.; Cruz-Acuña, R.; Gabre, J.T.; Nakagawa, H. Understanding the Cellular Origin and Progression of Esophageal Cancer Using Esophageal Organoids. Cancer Lett. 2021, 509, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Abhyankar, V.P.; Bates, A.M.; Fischer, C.L.; Johnson, G.K.; Guthmiller, J.M.; Progulske-Fox, A.; Brogden, K.A. Dataset on the Chemokine and Cytokine Responses of Multi-Cell Cultures Treated with Porphyromonas gingivalis Hemagglutinin B. Data Brief. 2019, 22, 964–970. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okolo, O.; Honzel, E.; Britton, W.R.; Yu, V.X.; Flashner, S.; Martin, C.; Nakagawa, H.; Parikh, A.S. Experimental Modeling of Host–Bacterial Interactions in Head and Neck Squamous Cell Carcinoma. Cancers 2023, 15, 5810. https://doi.org/10.3390/cancers15245810
Okolo O, Honzel E, Britton WR, Yu VX, Flashner S, Martin C, Nakagawa H, Parikh AS. Experimental Modeling of Host–Bacterial Interactions in Head and Neck Squamous Cell Carcinoma. Cancers. 2023; 15(24):5810. https://doi.org/10.3390/cancers15245810
Chicago/Turabian StyleOkolo, Ogoegbunam, Emily Honzel, William R. Britton, Victoria X. Yu, Samuel Flashner, Cecilia Martin, Hiroshi Nakagawa, and Anuraag S. Parikh. 2023. "Experimental Modeling of Host–Bacterial Interactions in Head and Neck Squamous Cell Carcinoma" Cancers 15, no. 24: 5810. https://doi.org/10.3390/cancers15245810
APA StyleOkolo, O., Honzel, E., Britton, W. R., Yu, V. X., Flashner, S., Martin, C., Nakagawa, H., & Parikh, A. S. (2023). Experimental Modeling of Host–Bacterial Interactions in Head and Neck Squamous Cell Carcinoma. Cancers, 15(24), 5810. https://doi.org/10.3390/cancers15245810






