New Insights on Liver-Directed Therapies in Hepatocellular Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Overview of Current Liver Directed Therapies for HCC
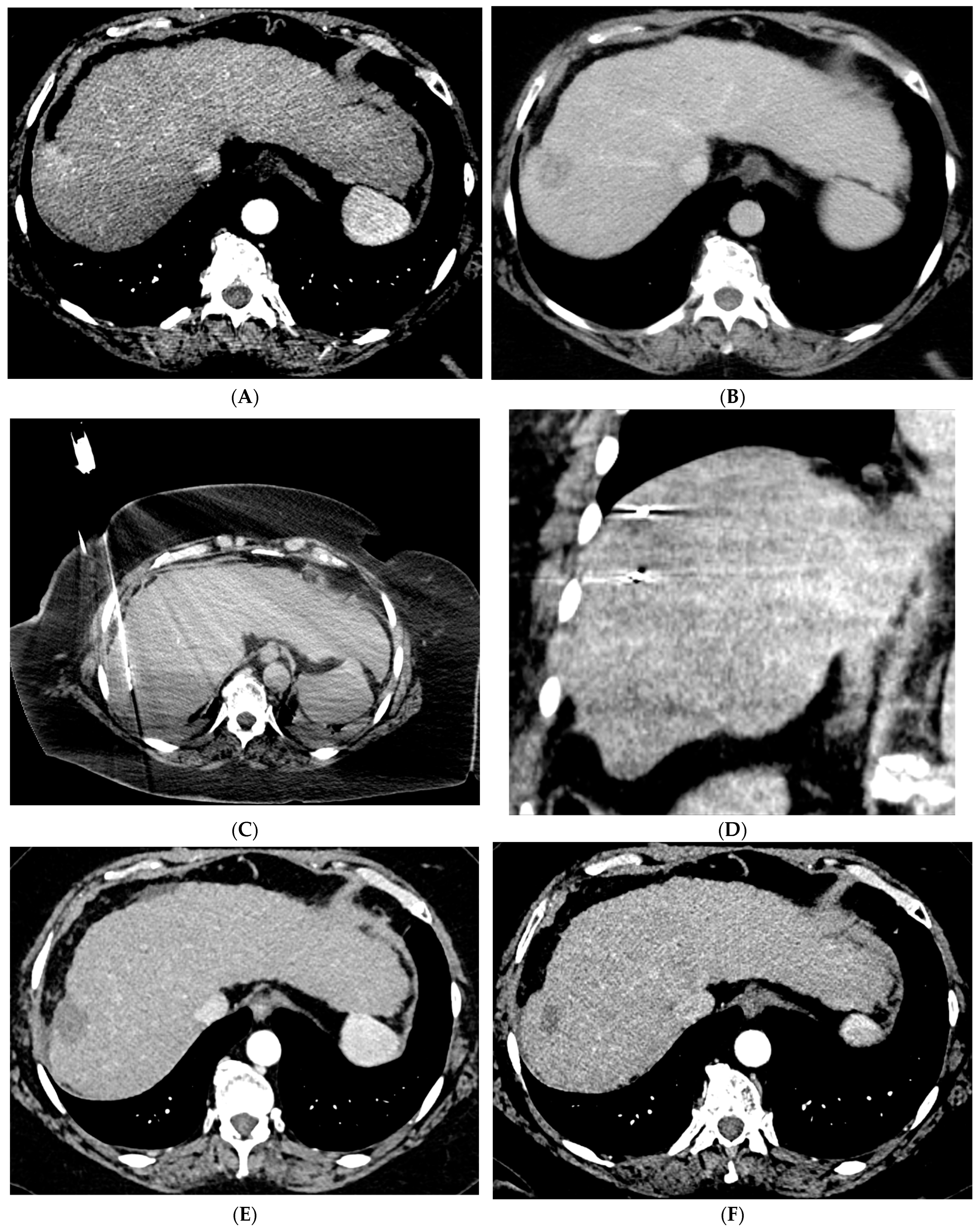
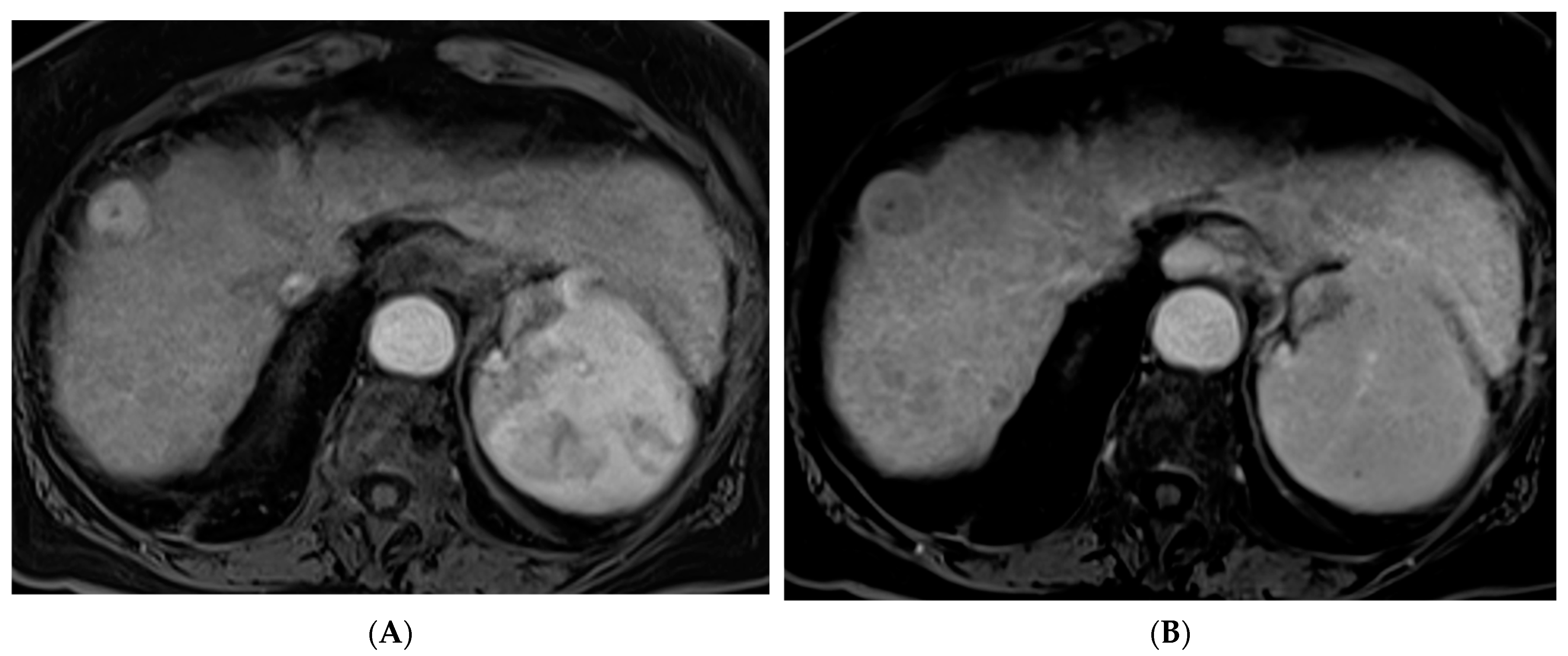
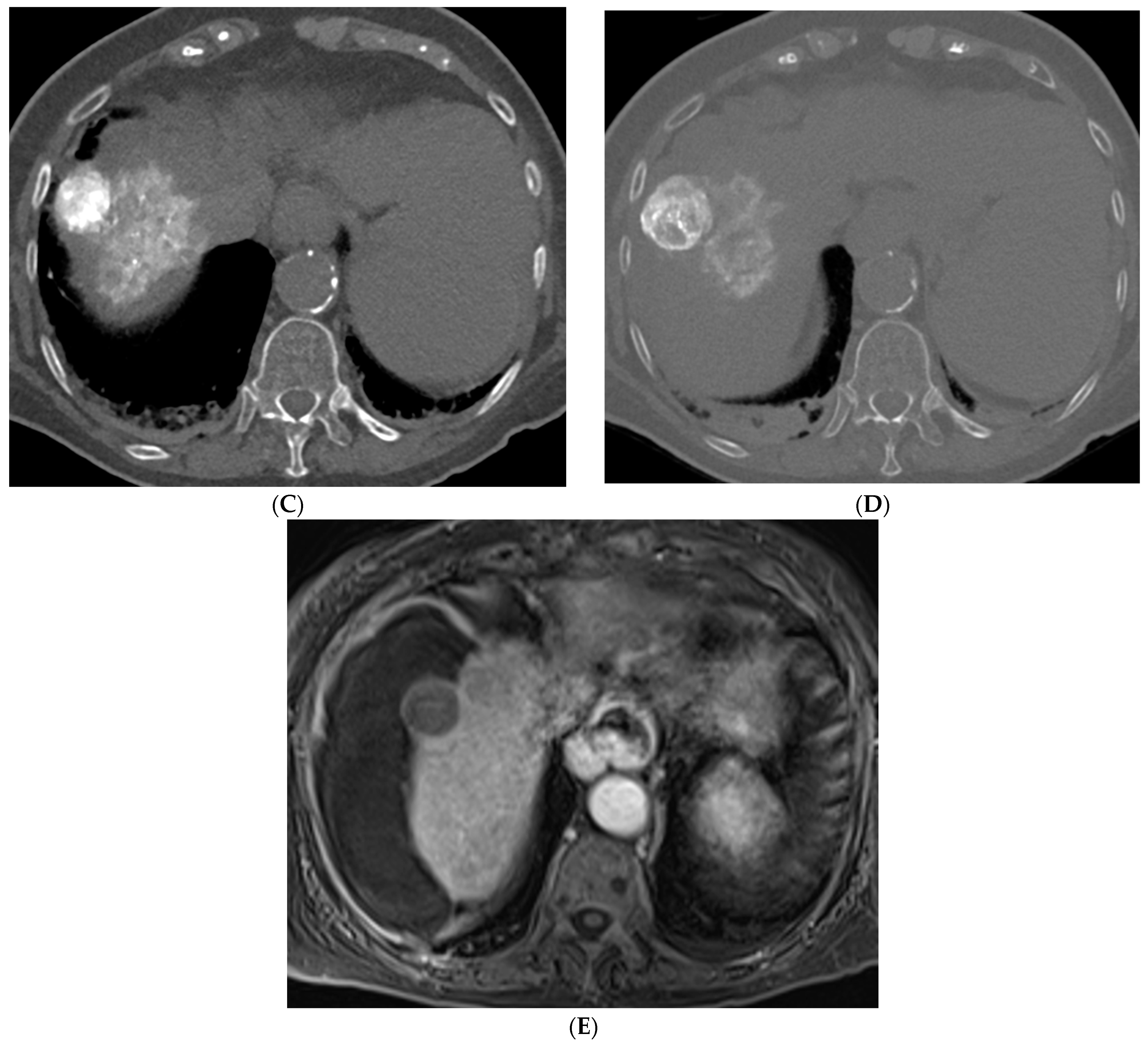
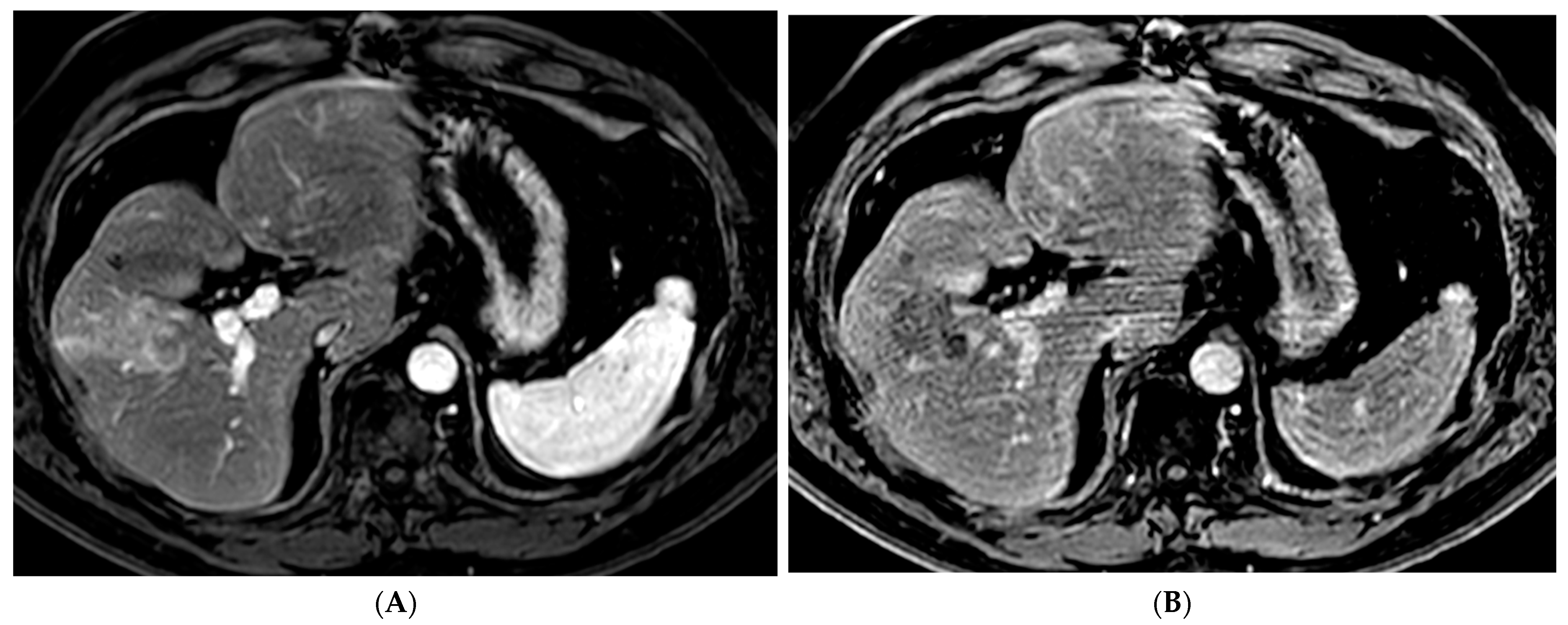
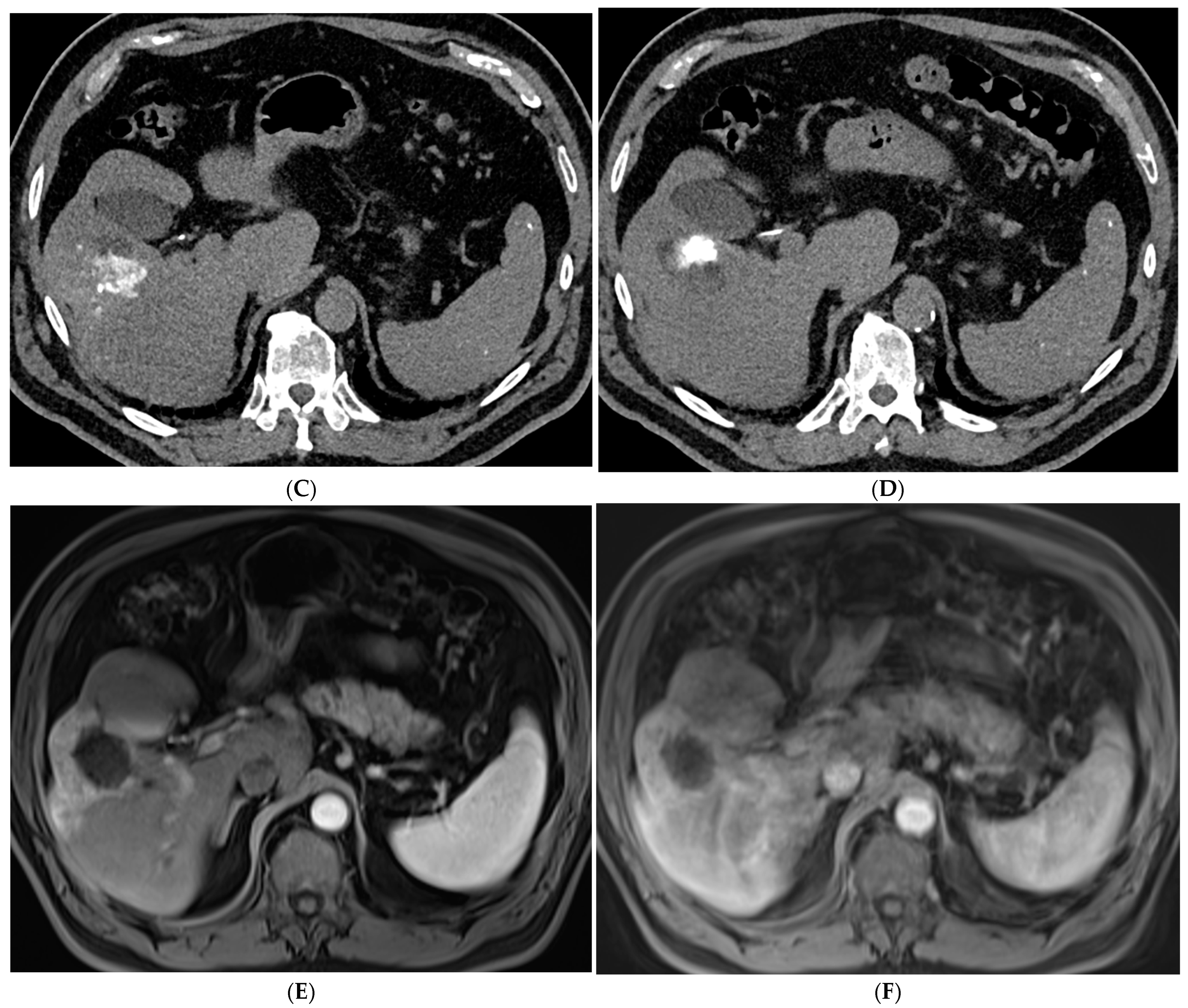
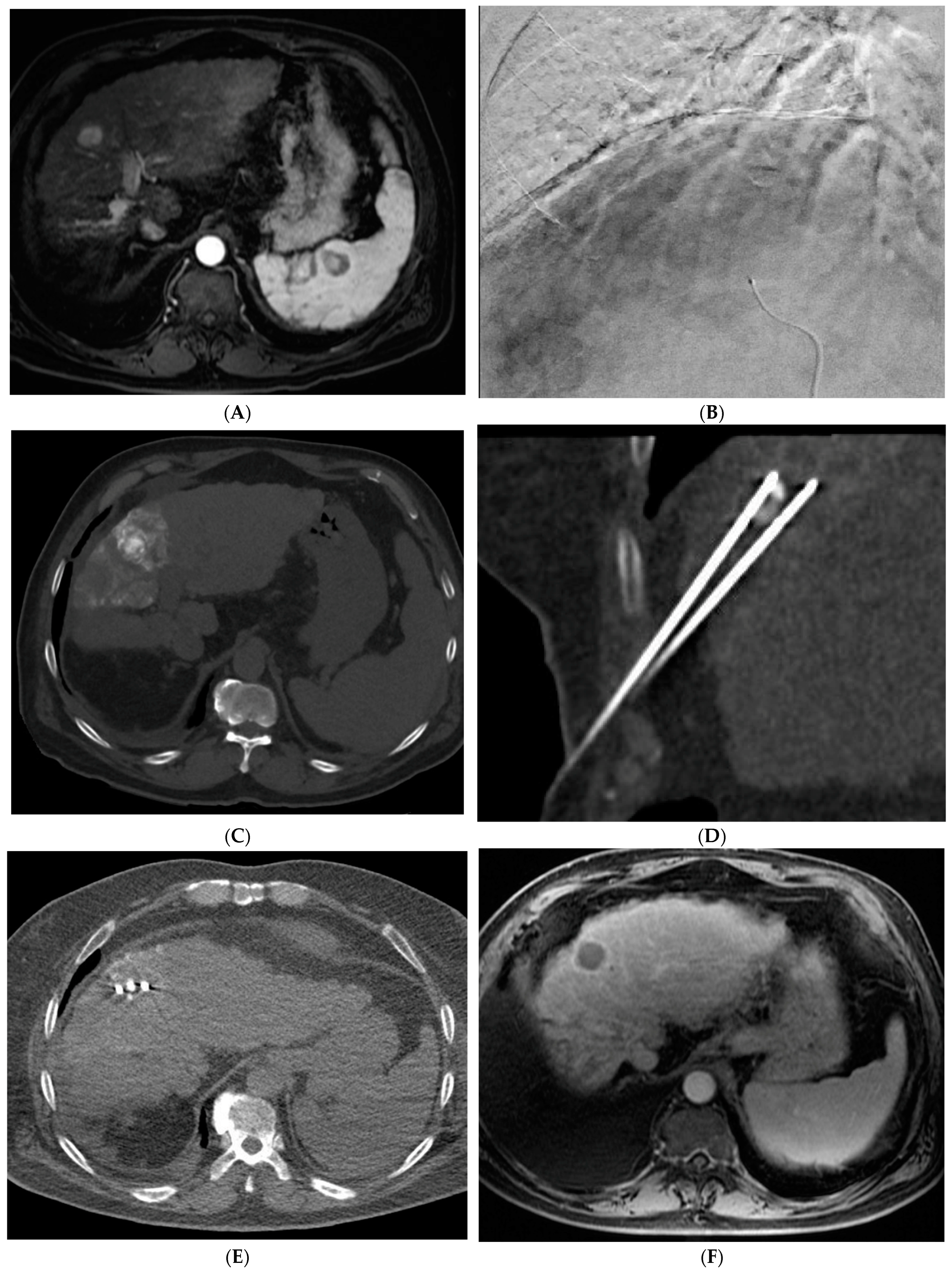
2.1. Percutaneous Ablation
2.2. Transarterial Chemoembolization (TACE)
2.3. Transarterial Radioembolization (TARE)
2.4. Other Locoregional Therapies
3. Recent Advances and Innovations in Liver Directed Therapies for HCC
3.1. Precision Medicine and Personalized Approaches
3.2. Combination Therapies
3.3. Targeted Drug Delivery
3.4. Other Emerging Techniques
4. Patient Selection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Kulik, L.M. Hepatocellular Carcinoma: New Developments. Clin. Liver Dis. 2023, 27, 85–102. [Google Scholar] [CrossRef]
- Singal, A.G.; Kudo, M.; Bruix, J. Breakthroughs in Hepatocellular Carcinoma Therapies. Clin. Gastroenterol. Hepatol. 2023, 21, 2135–2149. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Martinez, E.; Landazuri-Navas, S.; Vilchez, E.; Cantu-Hernandez, R.; Mosquera-Moscoso, J.; Encalada, S.; Al Lami, Z.; Zevallos-Delgado, C.; Cinicola, J. Prognostic Scores and Survival Rates by Etiology of Hepatocellular Carcinoma: A Review. J. Clin. Med. Res. 2023, 15, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Brú, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Saldanha, D.F.; Khiatani, V.L.; Carrillo, T.C.; Yap, F.Y.; Bui, J.T.; Knuttinen, M.G.; Owens, C.; Gaba, R.C. Current tumor ablation technologies: Basic science and device review. Semin. Interv. Radiol. 2010, 27, 247–254. [Google Scholar] [CrossRef]
- Kallini, J.R.; Gabr, A.; Salem, R.; Lewandowski, R.J. Transarterial Radioembolization with Yttrium-90 for the Treatment of Hepatocellular Carcinoma. Adv. Ther. 2016, 33, 699–714. [Google Scholar] [CrossRef]
- Saini, A.; Wallace, A.; Alzubaidi, S.; Knuttinen, M.G.; Naidu, S.; Sheth, R.; Albadawi, H.; Oklu, R. History and Evolution of Yttrium-90 Radioembolization for Hepatocellular Carcinoma. J. Clin. Med. 2019, 8, 55. [Google Scholar] [CrossRef]
- Lin, D.Y.; Liaw, Y.F.; Lee, T.Y.; Lai, C.M. Hepatic arterial embolization in patients with unresectable hepatocellular carcinoma--a randomized controlled trial. Gastroenterology 1988, 94, 453–456. [Google Scholar] [CrossRef]
- Yamada, R.; Sato, M.; Kawabata, M.; Nakatsuka, H.; Nakamura, K.; Takashima, S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology 1983, 148, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Nemoto, R.; Mori, H.; Takahashi, M.; Tamakawa, Y.; Harada, M. Arterial chemoembolization with microencapsulated anticancer drug. An approach to selective cancer chemotherapy with sustained effects. JAMA 1981, 245, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Real, M.I.; Montaña, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Solà, R.; et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet 2002, 359, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Wollner, I.S.; Knutsen, C.A.; Ullrich, K.A.; Chrisp, C.E.; Juni, J.E.; Andrews, J.C.; Tuscan, M.J.; Stetson, P.L.; Ensminger, W.D. Effects of hepatic arterial yttrium-90 microsphere administration alone and combined with regional bromodeoxyuridine infusion in dogs. Cancer Res. 1987, 47, 3285–3290. [Google Scholar] [PubMed]
- Lau, W.Y.; Leung, W.T.; Ho, S.; Leung, N.W.; Chan, M.; Lin, J.; Metreweli, C.; Johnson, P.; Li, A.K. Treatment of inoperable hepatocellular carcinoma with intrahepatic arterial yttrium-90 microspheres: A phase I and II study. Br. J. Cancer 1994, 70, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Herba, M.J.; Illescas, F.F.; Thirlwell, M.P.; Boos, G.J.; Rosenthall, L.; Atri, M.; Bret, P.M. Hepatic malignancies: Improved treatment with intraarterial Y-90. Radiology 1988, 169, 311–314. [Google Scholar] [CrossRef]
- Chen, M.-S.; Li, J.-Q.; Zheng, Y.; Guo, R.-P.; Liang, H.-H.; Zhang, Y.-Q.; Lin, X.-J.; Lau, W.Y. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann. Surg. 2006, 243, 321–328. [Google Scholar] [CrossRef]
- Feng, K.; Yan, J.; Li, X.; Xia, F.; Ma, K.; Wang, S.; Bie, P.; Dong, J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J. Hepatol. 2012, 57, 794–802. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, W.; Liang, X.; Li, D.; Lou, H.; Chen, R.; Wang, K.; Pan, H. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2014, 29, 193–200. [Google Scholar] [CrossRef]
- Lee, K.F.; Wong, J.; Hui, J.W.; Cheung, Y.S.; Chong, C.C.; Fong, A.K.; Yu, S.C.; Lai, P.B. Long-term outcomes of microwave versus radiofrequency ablation for hepatocellular carcinoma by surgical approach: A retrospective comparative study. Asian J. Surg. 2017, 40, 301–308. [Google Scholar] [CrossRef]
- Erinjeri, J.P.; Clark, T.W. Cryoablation: Mechanism of action and devices. J. Vasc. Interv. Radiol. 2010, 21 (Suppl. S8), S187–S191. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Noda, C.; Erickson, A.; Mokkarala, M.; Charalel, R.; Ramaswamy, R.; Tao, Y.; Akinwande, O. Radiofrequency Ablation vs. Cryoablation for Localized Hepatocellular Carcinoma: A Propensity-matched Population Study. Anticancer Res. 2018, 38, 6381–6386. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ren, Y.; Sun, T.; Cao, Y.; Yan, L.; Zhang, W.; Ouyang, T.; Zheng, C. The efficacy of radiofrequency ablation versus cryoablation in the treatment of single hepatocellular carcinoma: A population-based study. Cancer Med. 2021, 10, 3715–3725. [Google Scholar] [CrossRef] [PubMed]
- Knavel, E.M.; Brace, C.L. Tumor ablation: Common modalities and general practices. Technol. Vasc. Interv. Radiol. 2013, 16, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Kakimi, K.; Takeuchi, H.; Fujieda, N.; Saito, K.; Sato, E.; Sakamaki, K.; Moriyasu, F.; Itoi, T. Irreversible Electroporation versus Radiofrequency Ablation: Comparison of Systemic Immune Responses in Patients with Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2019, 30, 845–853.e6. [Google Scholar] [CrossRef]
- Lammer, J.; Malagari, K.; Vogl, T.; Pilleul, F.; Denys, A.; Watkinson, A.; Pitton, M.; Sergent, G.; Pfammatter, T.; Terraz, S.; et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepato-cellular carcinoma: Results of the PRECISION V study. Cardiovasc. Interv. Radiol. 2010, 33, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, N.; Shi, C.; Liu, Q.; Song, J.; Ye, X. Short-term efficacy and safety of callispheres drug-loaded microsphere embolization in primary hepatocellular carcinoma. J. Cancer Res. Ther. 2021, 17, 733–739. [Google Scholar]
- Ikeda, M.; Arai, Y.; Inaba, Y.; Tanaka, T.; Sugawara, S.; Kodama, Y.; Aramaki, T.; Anai, H.; Morita, S.; Tsukahara, Y.; et al. Conventional or Drug-Eluting Beads? Randomized Controlled Study of Chemoembolization for Hepatocellular Carcinoma: JIVROSG-1302. Liver Cancer 2022, 11, 440–450. [Google Scholar] [CrossRef]
- Lo, C.-M.; Ngan, H.; Tso, W.-K.; Liu, C.-L.; Lam, C.-M.; Poon, R.T.-P.; Fan, S.-T.; Wong, J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002, 35, 1164–1171. [Google Scholar] [CrossRef]
- Larijani, R.S.; Ravari, N.S.; Goodarzi, N.; Akhlaghpour, S.; Larijani, S.S.; Rouini, M.R.; Dinarvand, R. Current status of transarterial chemoembolization (TACE) agents in hepatocellular carcinoma treatment. J. Drug Deliv. Sci. Technol. 2022, 77, 103905. [Google Scholar] [CrossRef]
- Salem, R.; Lewandowski, R.; Roberts, C.; Goin, J.; Thurston, K.; Abouljoud, M.; Courtney, A. Use of Yttrium-90 Glass Microspheres (TheraSphere) for the Treatment of Unresectable Hepatocellular Carcinoma in Patients with Portal Vein Thrombosis. J. Vasc. Interv. Radiol. 2004, 15, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Johnson, G.E.; Kim, E.; Riaz, A.; Bishay, V.; Boucher, E.; Fowers, K.; Lewandowski, R.; Padia, S.A. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021, 74, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Gordon, A.C.; Mouli, S.; Hickey, R.; Kallini, J.; Gabr, A.; Mulcahy, M.F.; Baker, T.; Abecassis, M.; Miller, F.H.; et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared with Chemoembolization in Patients with Hepatocellular Carcinoma. Gastroenterology 2016, 151, 1155–1163.e2. [Google Scholar] [CrossRef] [PubMed]
- Dhondt, E.; Lambert, B.; Hermie, L.; Huyck, L.; Vanlangenhove, P.; Geerts, A.; Verhelst, X.; Aerts, M.; Vanlander, A.; Berrevoet, F.; et al. 90Y Radioembolization versus Drug-eluting Bead Chemoembolization for Unresectable Hepatocellular Carcinoma: Results from the TRACE Phase II Randomized Controlled Trial. Radiology 2022, 303, 699–710. [Google Scholar] [CrossRef] [PubMed]
- El Fouly, A.; Ertle, J.; El Dorry, A.; Shaker, M.K.; Dechêne, A.; Abdella, H.; Mueller, S.; Barakat, E.; Lauenstein, T.; Bockisch, A.; et al. In intermediate stage hepatocellular carcinoma: Radioembolization with yttrium 90 or chemoembolization? Liver Int. 2015, 35, 627–635. [Google Scholar] [CrossRef]
- Hilgard, P.; Hamami, M.; Fouly, A.E.; Scherag, A.; Müller, S.; Ertle, J.; Heusner, T.; Cicinnati, V.R.; Paul, A.; Bockisch, A.; et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 2010, 52, 1741–1749. [Google Scholar] [CrossRef]
- Kim, E.; Sher, A.; Abboud, G.; Schwartz, M.; Facciuto, M.; Tabrizian, P.; Knešaurek, K.; Fischman, A.; Patel, R.; Nowakowski, S.; et al. Radiation segmentectomy for curative intent of unresectable very early to early stage hepatocellular carcinoma (RASER): A single-centre, single-arm study. Lancet Gastroenterol. Hepatol. 2022, 7, 843–850. [Google Scholar] [CrossRef]
- Garin, E.; Tselikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; Assenat, E.; Tacher, V.; Terroir-Cassou-Mounat, M.; Mariano-Goulart, D.; Amaddeo, G.; et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): A randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 17–29. [Google Scholar] [CrossRef]
- Song, M.J. Hepatic artery infusion chemotherapy for advanced hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 3843–3849. [Google Scholar] [CrossRef]
- Lyu, N.; Wang, X.; Li, J.-B.; Lai, J.-F.; Chen, Q.-F.; Li, S.-L.; Deng, H.-J.; He, M.; Mu, L.-W.; Zhao, M. Arterial Chemotherapy of Oxaliplatin Plus Fluorouracil Versus Sorafenib in Advanced Hepatocellular Carcinoma: A Biomolecular Exploratory, Randomized, Phase III Trial (FOHAIC-1). J. Clin. Oncol. 2022, 40, 468–480. [Google Scholar] [CrossRef]
- Chen, C.P. Role of External Beam Radiotherapy in Hepatocellular Carcinoma. Clin. Liver Dis. 2020, 24, 701–717. [Google Scholar] [CrossRef]
- Sapisochin, G.; Barry, A.; Doherty, M.; Fischer, S.; Goldaracena, N.; Rosales, R.; Russo, M.; Beecroft, R.; Ghanekar, A.; Bhat, M.; et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J. Hepatol. 2017, 67, 92–99. [Google Scholar] [CrossRef]
- Galun, D.; Mijac, D.; Filipovic, A.; Bogdanovic, A.; Zivanovic, M.; Masulovic, D. Precision Medicine for Hepatocellular Carcinoma: Clinical Perspective. J. Pers. Med. 2022, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sun, H.; Wang, Z.; Cong, W.; Wang, J.; Zeng, M.; Zhou, W.; Bie, P.; Liu, L.; Wen, T.; et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer 2020, 9, 682–720. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.L.; Yang, J.; Bie, P.; Zhang, S.; Chen, X.; Liu, F.; Liu, L.; Zhou, J.; Dou, K.; Hao, C.; et al. Safety assessment of sorafenib in Chinese patients with unresectable hepatocellular carcinoma: Subgroup analysis of the GIDEON study. BMC Cancer 2018, 18, 247. [Google Scholar] [CrossRef] [PubMed]
- Ziv, E.; Zhang, Y.; Kelly, L.; Nikolovski, I.; Boas, F.E.; Erinjeri, J.P.; Cai, L.; Petre, E.N.; Brody, L.A.; Covey, A.M.; et al. NRF2 dysregulation in hepatocellular carcinoma and ischemia: A cohort study and laboratory investigation. Radiology 2020, 297, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Viveiros, P.; Riaz, A.; Lewandowski, R.J.; Mahalingam, D. Current State of Liver-Directed Therapies and Combinatory Approaches with Systemic Therapy in Hepatocellular Carcinoma (HCC). Cancers 2019, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Hack, S.P.; Spahn, J.; Chen, M.; Cheng, A.L.; Kaseb, A.; Kudo, M.; Lee, H.C.; Yopp, A.; Chow, P.; Qin, S. IMbrave 050: A Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020, 16, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.; Chen, M.; Cheng, A.-L.; Kaseb, A.; Kudo, M.; Lee, H.C.; Yopp, A.; Zhou, J.; Wang, L.; Wen, X.; et al. IMbrave050: Phase 3 study of adjuvant atezolizumab+ bevacizumab versus active surveillance in patients with hepatocellular carcinoma (HCC) at high risk of disease recurrence following resection or ablation. In Proceedings of the American Association for Cancer Research (AACR) Annual Conference, Orlando, FL, USA, 14–19 April 2023; Volume 16. [Google Scholar]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef]
- Qin, S.; Chan, S.L.; Gu, S.; Bai, Y.; Ren, Z.; Lin, X.; Chen, Z.; Jia, W.; Jin, Y.; Guo, Y.; et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): A randomised, open-label, international phase 3 study. Lancet 2023, 402, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M.; Han, G.; Tak, W.Y.; Yang, J.; Guglielmi, A.; Paik, S.W.; Reig, M.; Kim, D.Y.; Chau, G.Y.; et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J. Hepatol. 2016, 64, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Fox, R.; Ma, Y.T.; Ross, P.J.; James, M.W.; Sturgess, R.; Stubbs, C.; Stocken, D.D.; Wall, L.; Watkinson, A.; et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): A randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol. Hepatol. 2017, 2, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Proposal of Primary Endpoints for TACE Combination Trials with Systemic Therapy: Lessons Learned from 5 Negative Trials and the Positive TACTICS Trial. Liver Cancer 2018, 7, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Final Results of TACTICS: A Randomized, Prospective Trial Comparing Transarterial Chemoembolization Plus Sorafenib to Transarterial Chemoembolization Alone in Patients with Unresectable Hepatocellular Carcinoma. Liver Cancer 2022, 11, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Bai, W.; Wang, E.; Li, J.; Chen, X.; Wang, Z.; Huang, M.; Huang, M.; Sun, J.; Yang, W.; et al. Lenvatinib with or without Concurrent Drug-Eluting Beads Transarterial Chemoembolization in Patients with Unresectable, Advanced Hepatocellular Carcinoma: A Real-World, Multicenter, Retrospective Study. Liver Cancer 2022, 11, 368–382. [Google Scholar] [CrossRef]
- Peng, Z.; Fan, W.; Zhu, B.; Wang, G.; Sun, J.; Xiao, C.; Huang, F.; Tang, R.; Cheng, Y.; Huang, Z.; et al. Lenvatinib Combined with Transarterial Chemoembolization as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Phase III, Randomized Clinical Trial (LAUNCH). J. Clin. Oncol. 2023, 41, 117–127. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yang, X.; Wang, Y.C.; Long, J.Y.; Sun, H.S.; Li, Y.R.; Xun, Z.Y.; Zhang, N.; Xue, J.N.; Ning, C.; et al. Clinical outcomes of lenvatinib plus transarterial chemoembolization with or without programmed death receptor-1 inhibitors in unresectable hepatocellular carcinoma. World J. Gastroenterol. 2023, 29, 1614–1626. [Google Scholar] [CrossRef]
- Han, Z.; Yang, F.; Zhang, Y.; Wang, J.; Ni, Q.; Zhu, H.; Zhou, X.; Gao, H.; Lu, J. Prognostic efficacy and prognostic factors of TACE plus TKI with ICIs for the treatment of unresectable hepatocellular carcinoma: A retrospective study. Front. Oncol. 2022, 12, 1029951. [Google Scholar] [CrossRef]
- Meng, M.; Li, W.; Yang, X.; Huang, G.; Wei, Z.; Ni, Y.; Han, X.; Wang, J.; Ye, X. Transarterial chemoembolization, ablation, tyrosine kinase inhibitors, and immunotherapy (TATI): A novel treatment for patients with advanced hepatocellular carcinoma. J. Cancer Res. Ther. 2020, 16, 327–334. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, H.; Yu, H.; Cheng, Y.; Xiang, Y.; Cheng, Z.; Li, Y.; Li, T.; Wang, D.; Zhu, Z.; et al. Early Experience of TACE Combined with Atezolizumab plus Bevacizumab for Patients with Intermediate-Stage Hepatocellular Carcinoma beyond Up-to-Seven Criteria: A Multicenter, Single-Arm Study. J. Oncol. 2023, 2023, 6353047. [Google Scholar] [CrossRef]
- Britten, C.D.; Gomes, A.S.; Wainberg, Z.A.; Elashoff, D.; Amado, R.; Xin, Y.; Busuttil, R.W.; Slamon, D.J.; Finn, R.S. Transarterial chemoembolization plus or minus intravenous bevacizumab in the treatment of hepatocellular cancer: A pilot study. BMC Cancer 2012, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Wang, H.; Liu, Y.; Shen, K.; Dong, Y.; Sun, J.; Shu, Y.; Wan, X.; Ren, X.; Wei, X. A comparison between radiofrequency ablation combined with transarterial chemoembolization and surgical resection in hepatic carcinoma: A meta-analysis. J. Cancer Res. Ther. 2019, 15, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.C.; Chiang, C.-L.; Lee, A.-S.; Lee, V.H.; Yeung, C.S.; Ho, C.H.; Cheung, T.-T.; Ng, K.K.; Chok, S.-H.; Chan, A.C.; et al. Better survival after stereotactic body radiation therapy following transarterial chemoembolization in nonresectable hepatocellular carcinoma: A propensity score matched analysis. Surg. Oncol. 2019, 28, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Comito, T.; Loi, M.; Franzese, C.; Clerici, E.; Franceschini, D.; Badalamenti, M.; Teriaca, M.A.; Rimassa, L.; Pedicini, V.; Poretti, D.; et al. Stereotactic Radiotherapy after Incomplete Transarterial (Chemo-) Embolization (TAE\TACE) versus Exclusive TAE or TACE for Treatment of Inoperable HCC: A Phase III Trial (NCT02323360). Curr. Oncol. 2022, 29, 8802–8813. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cai, C.; Wang, H.; Ma, X.; Shao, A.; Sheng, J.; Yu, C. Drug delivery strategy in hepatocellular carcinoma therapy. Cell Commun. Signal. 2022, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Liu, Z.; Wang, W.; Liu, M.; Xu, Y.; Hu, W.; Fan, Y.; Zhang, X.; Liu, Y.; Si, G. Multifunctional nanoplatforms application in the transcatheter chemoembolization against hepatocellular carcinoma. J. Nanobiotechnol. 2023, 21, 68. [Google Scholar] [CrossRef]
- Bakrania, A.; Zheng, G.; Bhat, M. Nanomedicine in Hepatocellular Carcinoma: A New Frontier in Targeted Cancer Treatment. Pharmaceutics 2021, 14, 41. [Google Scholar] [CrossRef]
- Chen, M.-H.; Yang, W.; Lee, J.-C.; Zhang, Z.-Y.; Bai, X.-M.; Yin, S.-S.; Cao, K.; Wang, S.; Wu, W.; Yan, K. Thermosensitive liposomal doxorubicin plus radiofrequency ablation increased tumor destruction and improved survival in patients with medium and large hepatocellular carcinoma: A randomized, double-blinded, dummy-controlled clinical trial in a single center. J. Cancer Res. Ther. 2019, 15, 773–783. [Google Scholar]
- Regenold, M.; Bannigan, P.; Evans, J.C.; Waspe, A.; Temple, M.J.; Allen, C. Turning down the heat: The case for mild hyperthermia and thermosensitive liposomes. Nanomedicine 2022, 40, 102484. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, E.; Sarkar, D. Emerging Therapies for Hepatocellular Carcinoma (HCC). Cancers 2022, 14, 2798. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hall, T.L.; Vlaisavljevich, E.; Lee, F.T., Jr. Histotripsy: The first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound. Int. J. Hyperth. 2021, 38, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Jove, J.; Serres, X.; Vlaisavljevich, E.; Cannata, J.; Duryea, A.; Miller, R.; Merino, X.; Velat, M.; Kam, Y.; Bolduan, R.; et al. First-in-man histotripsy of hepatic tumors: The THERESA trial, a feasibility study. Int. J. Hyperth. 2022, 39, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Wah, T.M.; Pech, M.; Thormann, M.; Serres, X.; Littler, P.; Stenberg, B.; Lenton, J.; Smith, J.; Wiggermann, P.; Planert, M.; et al. A Multi-centre, Single Arm, Non-randomized, Prospective European Trial to Evaluate the Safety and Efficacy of the HistoSonics System in the Treatment of Primary and Metastatic Liver Cancers (#HOPE4LIVER). Cardiovasc. Interv. Radiol. 2023, 46, 259–267. [Google Scholar] [CrossRef]
- Chui, A.M.N.; Yau, T.C.C.; Cheung, T.T. An overview in management of hepatocellular carcinoma in Hong Kong using the Hong Kong Liver Cancer (HKLC) staging system. Glob. Health Med. 2020, 2, 312–318. [Google Scholar] [CrossRef]
- Cheung, T.T.-T.; Kwok, P.C.-H.; Chan, S.; Cheung, C.-C.; Lee, A.-S.; Lee, V.; Cheng, H.-C.; Chia, N.-H.; Chong, C.C.; Lai, T.-W.; et al. Hong Kong Consensus Statements for the Management of Unresectable Hepatocellular Carcinoma. Liver Cancer 2018, 7, 40–54. [Google Scholar] [CrossRef]
- Giannini, E.G.; Farinati, F.; Ciccarese, F.; Pecorelli, A.; Rapaccini, G.L.; Di Marco, M.; Benvegnù, L.; Caturelli, E.; Zoli, M.; Borzio, F.; et al. Prognosis of untreated hepatocellular carcinoma. Hepatology 2015, 61, 184–190. [Google Scholar] [CrossRef]
- A Phase II Study of Combined Treatment of Durvalumab, Bevacizumab, Tremelimumab, and Transarterial Chemoembolization (TACE) in Subjects with Hepatocellular Carcinoma (HCC) or Biliary Tract Carcinoma (BTC). NCT03937830. NIH Clinical Center. 2023. Available online: https://clinicalstudies.info.nih.gov/ProtocolDetails.aspx?id=19-C-0094 (accessed on 4 December 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalzell, C.G.; Taylor, A.C.; White, S.B. New Insights on Liver-Directed Therapies in Hepatocellular Carcinoma. Cancers 2023, 15, 5749. https://doi.org/10.3390/cancers15245749
Dalzell CG, Taylor AC, White SB. New Insights on Liver-Directed Therapies in Hepatocellular Carcinoma. Cancers. 2023; 15(24):5749. https://doi.org/10.3390/cancers15245749
Chicago/Turabian StyleDalzell, Christina G., Amy C. Taylor, and Sarah B. White. 2023. "New Insights on Liver-Directed Therapies in Hepatocellular Carcinoma" Cancers 15, no. 24: 5749. https://doi.org/10.3390/cancers15245749
APA StyleDalzell, C. G., Taylor, A. C., & White, S. B. (2023). New Insights on Liver-Directed Therapies in Hepatocellular Carcinoma. Cancers, 15(24), 5749. https://doi.org/10.3390/cancers15245749






