The Impact of Outpatient versus Inpatient Administration of CAR-T Therapies on Clinical, Economic, and Humanistic Outcomes in Patients with Hematological Cancer: A Systematic Literature Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Search Process
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment and Risk of Bias
2.5. Data Analysis
3. Results
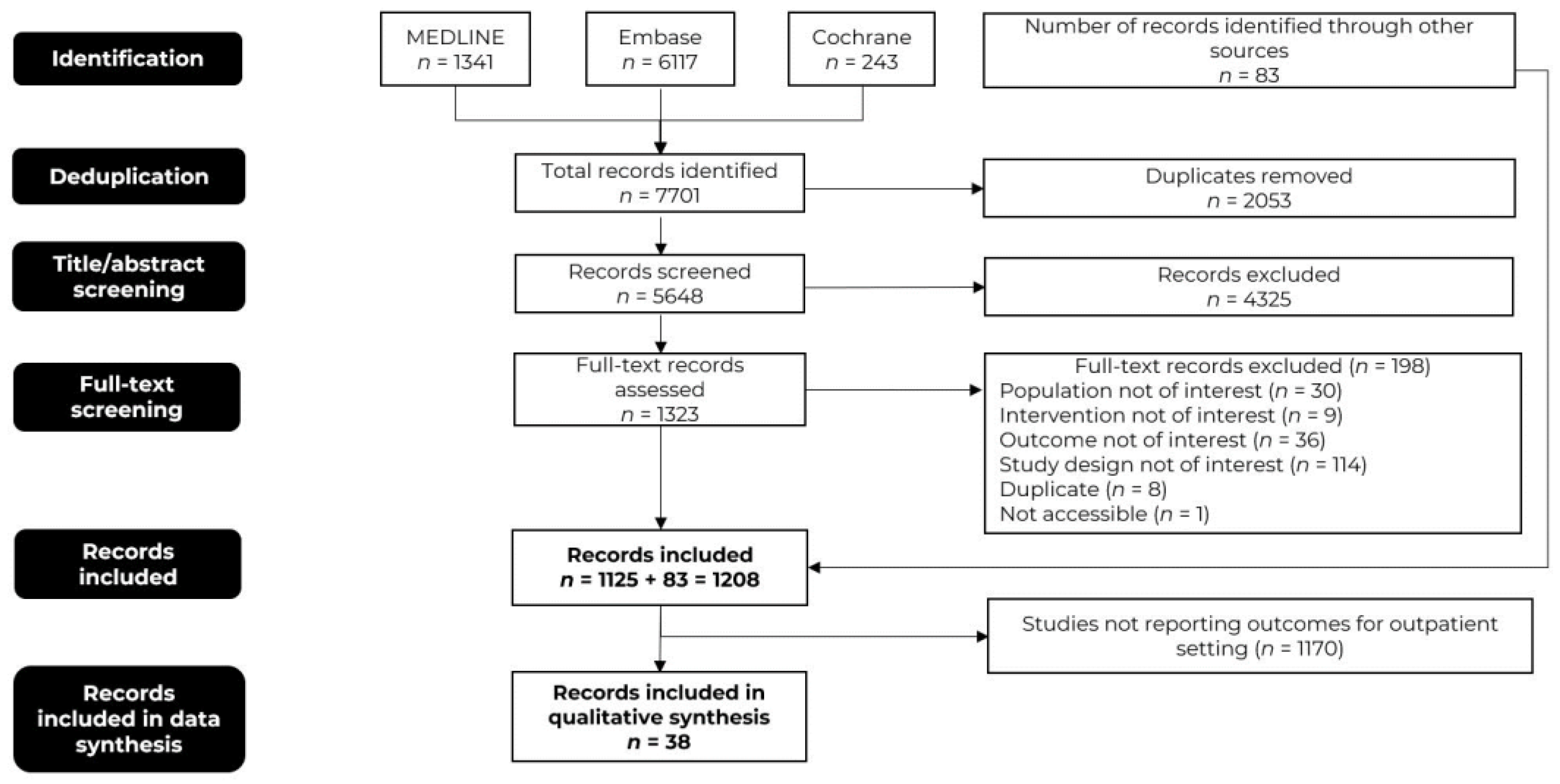
3.1. Literature Search Results
3.2. Study Characteristics
3.3. Quality Assessment
3.4. Clinical Outcomes
3.4.1. Safety
3.4.2. Efficacy: Response and Survival Outcomes
3.4.3. Quality of Life
3.5. Economic Outcomes
3.5.1. Direct Costs
3.5.2. Healthcare Resource Utilization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, M.; Chen, W.; Liu, K.; Wang, L.; Hu, Y.; Mao, Y.; Sun, X.; Luo, Y.; Shi, J.; Shao, K.; et al. The Global Burden of Leukemia and Its Attributable Factors in 204 Countries and Territories: Findings from the Global Burden of Disease 2019 Study and Projections to 2030. J. Oncol. 2022, 2022, 1612702. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Allart-Vorelli, P.; Porro, B.; Baguet, F.; Michel, A.; Cousson-Gélie, F. Haematological cancer and quality of life: A systematic literature review. Blood Cancer J. 2015, 5, e305. [Google Scholar] [CrossRef]
- La Nasa, G.; Caocci, G.; Morelli, E.; Massa, E.; Farci, A.; Deiana, L.; Pintus, E.; Scartozzi, M.; Sancassiani, F. Health Related Quality of Life in Patients with Onco-hematological Diseases. Clin. Pract. Epidemiol. Ment. Health 2020, 16, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Burns, R.; Leal, J.; Sullivan, R.; Luengo-Fernandez, R. Economic burden of malignant blood disorders across Europe: A population-based cost analysis. Lancet Haematol. 2016, 3, e362–e370. [Google Scholar] [CrossRef] [PubMed]
- Yucel, E.; Zhang, S.; Panjabi, S. Health-Related and Economic Burden Among Family Caregivers of Patients with Acute Myeloid Leukemia or Hematological Malignancies. Adv. Ther. 2021, 38, 5002–5024. [Google Scholar] [CrossRef]
- Sochacka-Ćwikła, A.; Mączyński, M.; Regiec, A. FDA-Approved Drugs for Hematological Malignancies-The Last Decade Review. Cancers 2021, 14, 87. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Lei, W.; Xie, M.; Jiang, Q.; Xu, N.; Li, P.; Liang, A.; Young, K.H.; Qian, W. Treatment-Related Adverse Events of Chimeric Antigen Receptor T-Cell (CAR T) in Clinical Trials: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 3912. [Google Scholar] [CrossRef]
- Parikh, R.H.; Lonial, S. Chimeric antigen receptor T-cell therapy in multiple myeloma: A comprehensive review of current data and implications for clinical practice. CA Cancer J. Clin. 2023, 73, 275–285. [Google Scholar] [CrossRef]
- Chimeric Antigen Receptor CAR T Cell Therapy Cancer Treatment Market Size Report. Available online: https://www.globenewswire.com/news-release/2022/06/25/2469076/0/en/Chimeric-Antigen-Receptor-CAR-T-Cell-Therapy-Cancer-Treatment-Market-Size-Report.html (accessed on 18 July 2023).
- Facts about Chimeric Antigen Receptor (CAR) T-Cell Therapy. Available online: https://www.lls.org/sites/default/files/2021-05/FSHP1_CART_Factsheet_Sept2020_Rev.pdf (accessed on 18 July 2023).
- Barros, L.R.C.; Couto, S.C.F.; da Silva Santurio, D.; Paixão, E.A.; Cardoso, F.; da Silva, V.J.; Klinger, P.; Ribeiro, P.; Rós, F.A.; Oliveira, T.G.M.; et al. Systematic Review of Available CAR-T Cell Trials around the World. Cancers 2022, 14, 2667. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.; Barrett, D.M. Current status of chimeric antigen receptor therapy for haematological malignancies. Br. J. Haematol. 2016, 172, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Davila, M.L.; Riviere, I.; Wang, X.; Bartido, S.; Park, J.; Curran, K.; Chung, S.S.; Stefanski, J.; Borquez-Ojeda, O.; Olszewska, M.; et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014, 6, 224ra225. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 2015, 385, 517–528. [Google Scholar] [CrossRef]
- Quintás-Cardama, A. CD19 directed CAR T cell therapy in diffuse large B-cell lymphoma. Oncotarget 2018, 9, 29843–29844. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; An, G.; Sui, W.; Wang, T.; Zhang, X.; Yang, J.; Zhang, Y.; Zhang, L.; Zhu, D.; Huang, J.; et al. Phase 1 study of C-CAR088, a novel humanized anti-BCMA CAR T-cell therapy in relapsed/refractory multiple myeloma. J. Immunother. Cancer 2022, 10, e005145. [Google Scholar] [CrossRef]
- Gagelmann, N.; Riecken, K.; Wolschke, C.; Berger, C.; Ayuk, F.A.; Fehse, B.; Kröger, N. Development of CAR-T cell therapies for multiple myeloma. Leukemia 2020, 34, 2317–2332. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- San-Miguel, J.; Dhakal, B.; Yong, K.; Spencer, A.; Anguille, S.; Mateos, M.V.; Fernández de Larrea, C.; Martínez-López, J.; Moreau, P.; Touzeau, C.; et al. Cilta-cel or Standard Care in Lenalidomide-Refractory Multiple Myeloma. N. Engl. J. Med. 2023, 389, 335–347. [Google Scholar] [CrossRef]
- Myers, G.D.; Verneris, M.R.; Goy, A.; Maziarz, R.T. Perspectives on outpatient administration of CAR-T cell therapy in aggressive B-cell lymphoma and acute lymphoblastic leukemia. J. Immunother. Cancer 2021, 9, e002056. [Google Scholar] [CrossRef]
- Bachier, C.; Palomba, M.; Abramson, J.; Andreadis, C.; Sehgal, A.; Godwin, J.; Hildebrandt, G.; Siddiqi, T.; Stevens, D.; Farazi, T.; et al. Outpatient Treatment with Lisocabtagene Maraleucel (liso-cel) in 3 Ongoing Clinical Studies in Relapsed/Refractory (R/R) Large B Cell Non-Hodgkin Lymphoma (NHL), Including Second-Line Transplant Noneligible (TNE) Patients: Transcend NHL 001, Outreach, and PILOT. Biol. Blood Marrow Transplant. 2020, 26, S25–S26. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Schuster, S.; Tam, C.; Borchmann, P.; Jaeger, U.; Waller, E.; Holte, H.; McGuirk, J.; Jaglowski, S.; Tobinai, K.; Andreadis, C.; et al. Sustained Disease Control for Adult Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma: An Updated Analysis of Juliet, a Global Pivotal Phase 2 Trial of Tisagenlecleucel. Blood 2018, 132, 1684. [Google Scholar] [CrossRef]
- Yang, H.; Bollu, V.; Lim, S.; Tesfaye, M.; Dalal, A.A.; Lax, A.; Sethi, S.; Zhao, J. Healthcare resource use and reimbursement amount by site of care in patients with diffuse large B-cell lymphoma receiving chimeric antigen receptor T-cell (CAR-T) therapy–a retrospective cohort study using CMS 100% Medicare claims database. Leuk. Lymphoma 2022, 64, 339–348. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022); Cochrane: London, UK, 2022; Available online: www.training.cochrane.org/handbook (accessed on 18 July 2023).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Newcastle-Ottawa Quality Assessment form for Cohort and Case-Control Studies. Available online: https://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf (accessed on 18 July 2023).
- Godwin, J.; Freytes, C.O.; Maris, M.; Stevens, D.A.; Hoda, D.; Mattar, B.; Varela, J.C.; Cherry, M.; Essell, J.; Courtright, J.; et al. Outcomes of Treatment with the Chimeric Antigen Receptor (CAR) T Cell Therapy Lisocabtagene Maraleucel (liso-cel) at Nonuniversity Medical Centers (NMCs): Initial Results from the Outreach Study in Patients with Relapsed/Refractory (R/R) Large B-Cell Lymphoma (LBCL). Transplant. Cell Ther. 2021, 27, S203–S204. [Google Scholar]
- Godwin, J.E.; Freytes, C.O.; Maris, M.; Stevens, D.A.; Hoda, D.; Mattar, B.; Varela, J.C.; Cherry, M.; Essell, J.; Courtright, J.; et al. Outcomes of Treatment with the Chimeric Antigen Receptor (CAR) T Cell Therapy Lisocabtagene Maraleucel (liso-cel) in the Nonuniversity Setting: Initial Results from the Outreach Study. Blood 2020, 136, 50–52. [Google Scholar] [CrossRef]
- Godwin, J.E.; Mattar, B.; Maris, M.; Bachier, C.; Stevens, D.A.; Hoda, D.; Varela, J.C.; Cherry, M.; Fanning, S.; Essell, J.; et al. Outreach: Preliminary safety & efficacy results from a phase 2 study of lisocabtagene maraleucel (LISO-CEL) in the nonuniversity setting. Hematol. Oncol. 2021, 39, 368–370. [Google Scholar] [CrossRef]
- Godwin, J.E.; Mattar, B.I.; Maris, M.B.; Bachier, C.R.; Stevens, D.A.; Hoda, D.; Varela, J.C.; Cherry, M.; Fanning, S.R.; Essell, J.H.; et al. Outreach: Preliminary safety and efficacy results from a phase 2 study of lisocabtagene maraleucel (liso-cel) in the non university setting. J. Clin. Oncol. 2021, 39, e19513. [Google Scholar] [CrossRef]
- Godwin, J.E.; Mattar, B.; Maris, M.; Bachier, C.; Stevens, D.; Hoda, D.; Varela, J.C.; Cherry, M.; Fanning, S.; Essell, J.; et al. Outreach: Results from a Phase 2 Study of Lisocabtagene Maraleucel (liso-cel) Administered As Inpatient (Inpt) or Outpatient (Outpt) Treatment in the Nonuniversity Setting in Patients (Pts) with R/R Large B-Cell Lymphoma (LBCL). Blood 2021, 138, 1762. [Google Scholar] [CrossRef]
- Linhares, Y.; Freytes, C.; Cherry, M.; Bachier, C.; Maris, M.; Hoda, D.; Varela, J.C.; Bellomo, C.; Cross, S.; Essell, J. Results from Outreach: A Phase 2 Study of Lisocabtagene Maraleucel (Liso-cel) Administered As Outpatient (Outpt) or Inpatient (Inpt) Treatment in the Community/Nonuniversity Setting in Patients (Pts) with Relapsed or Refractory (R/R) Large B-Cell Lymphoma (LBCL). Blood 2022, 140, 10416–10418. [Google Scholar] [CrossRef]
- Linhares, Y.; Liu, F.F.; Freytes, C.; Cherry, M.; Shi, L.; Liao, W.; Braverman, J.; Espinola, R.; Vedal, M.; Mattar, B. Lisocabtagene Maraleucel (Liso-cel) in Patients with Relapsed or Refractory (R/R) Large B-Cell Lymphoma (LBCL) Treated As Outpatients or Inpatients in the Community/Nonuniversity Setting: Patient-Reported Outcomes/Health-Related Quality of Life from the Outreach Study. Blood 2022, 140, 10930–10931. [Google Scholar] [CrossRef]
- Sehgal, A.; Hoda, D.; Riedell, P.A.; Ghosh, N.; Hamadani, M.; Hildebrandt, G.C.; Godwin, J.E.; Reagan, P.M.; Wagner-Johnston, N.; Essell, J. Lisocabtagene maraleucel as second-line therapy in adults with relapsed or refractory large B-cell lymphoma who were not intended for haematopoietic stem cell transplantation (PILOT): An open-label, phase 2 study. Lancet Oncol. 2022, 23, 1066–1077. [Google Scholar] [CrossRef]
- Sehgal, A.R.; Godwin, J.; Pribble, J.; Wang, L.; Thorpe, J.; Hildebrandt, G.C. Lisocabtagene maraleucel (liso-cel) for treatment of second-line transplant noneligible (TNE) relapsed/refractory (R/R) aggressive non-hodgkin lymphoma (NHL): Initial results from the PILOT Study. Blood 2019, 134, 2882. [Google Scholar] [CrossRef]
- McGarvey, N.; Gitlin, M.; Lee, A.; Keating, S. Post-infusion monitoring costs by site of care among patients with relapsed or refractory large B-cell lymphoma who received second-line treatment with lisocabtagene maraleucel in the PILOT study. J. Manag. Care Spec. Pharm. 2022, 28, S32–S33. [Google Scholar]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Maloney, D.G.; Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Arnason, J.E.; Wang, M.; Forero, A.; Albertson, T.; Garcia, J.; et al. Preliminary Safety Profile of the CD19-Directed Defined Composition CAR T Cell Product JCAR017 in Relapsed/Refractory Aggressive B-NHL Patients: Potential for Outpatient Administration. Blood 2017, 130, 1552. [Google Scholar] [CrossRef]
- Palomba, M.L.; Garcia, J.; Wang, L.; Dehner, C.; Chung, K.C.; Maloney, D.G. TRANSCEND: Lisocabtagene Maraleucel (liso-cel; JCAR017) Healthcare Resource Utilization in Patients with Relapsed/Refractory Diffuse Large B-Cell Lymphoma (DLBCL). Blood 2018, 132, 3545. [Google Scholar] [CrossRef]
- Gofshteyn, J.S.; Shaw, P.A.; Teachey, D.T.; Grupp, S.A.; Maude, S.; Banwell, B.; Chen, F.; Lacey, S.F.; Melenhorst, J.J.; Edmonson, M.J. Neurotoxicity after CTL019 in a pediatric and young adult cohort. Ann. Neurol. 2018, 84, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.M.; Fitzgerald, J.C.; DiNofia, A.; Wray, L.; Leahy, A.B.; Li, Y.; Smith, L.T.; Burrows, E.K.; Ramos, M.; Motley, L.S. Inpatient and intensive care unit resource utilization after CD19-targeted chimeric antigen receptor T-cell therapy (CART19) for pediatric acute lymphoblastic leukemia (ALL). Biol. Blood Marrow Transplant. 2020, 26, S202–S203. [Google Scholar] [CrossRef]
- Shadman, M.; Yeung, C.; Redman, M.; Lee, S.Y.; Lee, D.H.; Ra, S.; Ujjani, C.S.; Dezube, B.J.; Poh, C.; Warren, E.H.; et al. Safety and Efficacy of Third Generation CD20 Targeted CAR-T (MB-106) for Treatment of Relapsed/Refractory B-NHL and CLL. Blood 2021, 138, 3872. [Google Scholar] [CrossRef]
- Shadman, M.; Yeung, C.; Redman, M.W.; Lee, S.Y.; Lee, D.H.; Ramachandran, A.; Ra, S.; Marzbani, E.A.; Graf, S.A.; Warren, E.H.; et al. Third Generation CD20 Targeted CAR T-Cell Therapy (MB-106) for Treatment of Patients with Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma. Blood 2020, 136, 38–39. [Google Scholar] [CrossRef]
- Shadman, M.; Yeung, C.; Redman, M.; Lee, S.; Lee, D.; Ra, S.; Ramachandran, A.; Lynch, R.; Smith, S.; Poh, C.; et al. Immunotherapy Using a 3rd Generation CD20 Targeted CAR T-Cell (MB-106) for Treatment of B-Cell Non-Hodgkin Lymphoma (B-NHL) and Chronic Lymphocytic Leukemia (CLL). HemaSphere 2021, 5, 335. [Google Scholar]
- Shadman, M.; Yeung, C.; Redman, M.; Lee, S.Y.; Lee, D.H.; Ra, S.; Qian, D.; Ujjani, C.; Dezube, B.; Poh, C.; et al. Efficacy and safety of a third generation CD20 CART (MB-106) for treatment of Relapsed/Refractory Follicular Lymphoma (FL). HemaSphere 2022, 6, 108–109. [Google Scholar] [CrossRef]
- Turtle, C.J.; Hay, K.A.; Gust, J.; Hanaff, L.A.; Li, D.; Liles, W.C.; Wurfel, M.; Harju-Baker, S.; Myerson, D.; Gonzalez-Cuyar, L.; et al. Cytokine release syndrome (CRS) and neurotoxicity (NT) after CD19-specific chimeric antigen receptor-(CAR-) modified T cells. J. Clin. Oncol. 2017, 35, 3020. [Google Scholar] [CrossRef]
- Palomba, M.L.; Jun, M.P.; Garcia, J.; Lymp, J.; McGarvey, N.; Gitlin, M.; Pelletier, C.; Nguyen, A. Costs of Postinfusion Monitoring By Site of Care for Patients with Relapsed/Refractory (R/R) Large B-Cell Lymphoma (LBCL) Who Received Third-Line or Later Treatment with Lisocabtagene Maraleucel (liso-cel) in the Transcend NHL 001 and Outreach Trials. Blood 2020, 136, 16–17. [Google Scholar] [CrossRef]
- Denlinger, N.; Huang, Y.; Braunstein, Z.; Sigmund, A.; Bajwa, A.; Kapoor, N.; Agyeman, A.; Fisher, S.; Purdin, Z.; Neal, A.; et al. ABCL-509 Healthcare Utilization and Costs in Chimeric Antigen Receptor T-Cell Therapy for B-Cell Lymphoma. Clin. Lymphoma Myeloma Leuk. 2022, 22, S381–S382. [Google Scholar] [CrossRef]
- Chihara, D.; Liao, L.; Tkacz, J.; Lewing, B.; Franco, A.; Kilgore, K.M.; Nastoupil, L.J.; Chen, L. Real-World Effectiveness and Economic Impact Associated with Chimeric Antigen Receptor T-Cell Therapy Among Older Patients with Relapsed/Refractory Diffuse Large B-Cell Lymphoma in US. Blood 2022, 140, 2421–2423. [Google Scholar] [CrossRef]
- Borogovac, A.; Keruakous, A.; Bycko, M.; Chakrabarty, J.H.; Ibrahimi, S.; Khawandanah, M.; Selby, G.B.; Yuen, C.; Schmidt, S.; Autry, M.T.; et al. Safety and feasibility of outpatient chimeric antigen receptor (CAR) T-cell therapy: Experience from a tertiary care center. Bone Marrow Transplant. 2022, 57, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Borogovac, A.; Keruakous, A.R.; Bycko, M.; Chakrabarty, J.H.; Ibrahimi, S.; Khawandanah, M.O.; Selby, G.B.; Yuen, C.; Schmidt, S.A.; Al-Juhaishi, T.; et al. Successful Development of an Outpatient Chimeric Antigen Receptor (CAR) T Cell Therapy Program. Blood 2021, 138, 4821. [Google Scholar] [CrossRef]
- Shao, Y.F.; Modi, D.; Kin, A.; Alavi, A.; Ayash, L.; Ratanatharathorn, V.; Uberti, J.P.; Deol, A. Feasibility of Outpatient CAR T Cell Therapy: Experience of a Single Institution. Blood 2021, 138, 4828. [Google Scholar] [CrossRef]
- Zhao, J.; Bollu, V.; Yang, H.; Dalal, A.; Tesfaye, M.; Ma, Q.; Lax, A.; Lim, S. Healthcare resource use (HRU) by infusion setting of chimeric antigen receptor T-cell (CAR-T) in patients with relapsed and refractory (r/r) diffuse large B-cell lymphoma (DLBCL): A retrospective cohort study using CMS 100% Medicare database. J. Clin. Oncol. 2021, 39, e19550. [Google Scholar] [CrossRef]
- Farooqui, N.; Sy-Go, J.P.T.; Miao, J.; Mehta, R.; Vaughan, L.E.; Bennani, N.N.; Wang, Y.; Bansal, R.; Hathcock, M.A.; Hayman, S.R. Incidence and Risk Factors for Acute Kidney Injury After Chimeric Antigen Receptor T-Cell Therapy. Mayo Clin. Proc. 2022, 97, 1294–1304. [Google Scholar] [CrossRef]
- Nasta, S.D.; Hughes, M.E.; Namoglu, E.C.; Garfall, A.; DiFilippo, H.; Ballard, H.J.; Barta, S.K.; Chong, E.A.; Frey, N.V.; Gerson, J.N. Outcomes of Tisagenlecleucel in Lymphoma Patients With Predominant Management in an Ambulatory Setting. Clin. Lymphoma Myeloma Leuk. 2022, 22, e730–e737. [Google Scholar] [CrossRef] [PubMed]
- Maziarz, R.; Yang, H.; Liu, Q.; Zhao, J.; Lee, S.; Dalal, A.; Lim, S.; Bollu, V. Real-world healthcare resource utilization and costs associated with tisagenlecleucel and axicabtagene ciloleucel among patients with diffuse large B-cell lymphoma: An analysis of hospital data. J. Manag. Care Spec. Pharm. 2021, 27, S37–S38. [Google Scholar] [CrossRef]
- Maziarz, R.T.; Yang, H.; Liu, Q.; Wang, T.; Zhao, J.; Lim, S.; Lee, S.; Dalal, A.; Bollu, V. Real-world healthcare resource utilization and costs associated with tisagenlecleucel and axicabtagene ciloleucel among patients with diffuse large B-cell lymphoma: An analysis of hospital data in the United States. Leuk. Lymphoma 2022, 63, 2052–2062. [Google Scholar] [CrossRef]
- Kirby, S.; Hoda, D.; Hunter, B. Successful Outpatient Treatment and Monitoring Following Administration of Various Anti-CD19 Chimeric Antigen Receptor Therapies in B-Cell Lymphomas. Blood 2022, 140, 10812–10813. [Google Scholar] [CrossRef]
- Kamdar, M.; Solomon, S.R.; Arnason, J.; Johnston, P.B.; Glass, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.; Hernandez-Ilizaliturri, F. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): Results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet 2022, 399, 2294–2308. [Google Scholar] [CrossRef]
- McGarvey, N.; Gitlin, M.; Lee, A.; Keating, S. Post-infusion monitoring costs by site of care among patients with relapsed or refractory large B-cell lymphoma who received second-line treatment with lisocabtagene maraleucel in the TRANSFORM study. J. Manag. Care Spec. Pharm. 2022, 28, S33. [Google Scholar]
- Fowler, N.H.; Dickinson, M.; Ghosh, M.; Chen, A.; Andreadis, C.; Tiwari, R.; Masood, A.; Ramos, R.; Bollu, V.; Jousseaume, E.; et al. Assessment of Healthcare Resource Utilization and Costs in Patients with Relapsed or Refractory Follicular Lymphoma Undergoing CAR-T Cell Therapy with Tisagenlecleucel: Results from the Elara Study. Blood 2021, 138, 3533. [Google Scholar] [CrossRef]
- Fowler, N.H.; Dickinson, M.; Ghosh, M.; Chen, A.I.; Andreadis, C.; Tiwari, R.; Masood, A.; Ramos, R.; Jousseaume, E.; Thieblemont, C. Assessment of Healthcare Resource Utilization and Hospitalization Costs in Patients with Relapsed or Refractory Follicular Lymphoma Undergoing CAR-T Cell Therapy With Tisagenlecleucel: Results From the ELARA Study. Transplant. Cell Ther. 2023, 29, 60.e61–60.e64. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Anstadt, E.; Baron, J.; LaRiviere, M.; LaRose, M.; Landsburg, D.; Svoboda, J.; Nasta, S.; Gerson, J.; Barta, S.; et al. Impact of Radiotherapy on Hospitalization Burden Surrounding Chimeric Antigen Receptor T-Cell Therapy in Patients with Relapsed/Refractory Non-Hodgkin Lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, E51–E52. [Google Scholar] [CrossRef]
- Delgado, A.; Guddati, A.K. Clinical endpoints in oncology—A primer. Am. J. Cancer Res. 2021, 11, 1121–1131. [Google Scholar]
- Senf, B.; Grabowski, K.; Spielmann, N.; Fettel, J. Quality of life and distress assessed with self and external assessment screening tools in patients with hematologic malignancies attending treatment in an acute hospital. Qual. Life Res. 2020, 29, 3375–3385. [Google Scholar] [CrossRef]
- Hubbard, G.; Kidd, L.; Donaghy, E. Preferences for involvement in treatment decision making of patients with cancer: A review of the literature. Eur. J. Oncol. Nurs. 2008, 12, 299–318. [Google Scholar] [CrossRef]
- Lyman, G.H.; Nguyen, A.; Snyder, S.; Gitlin, M.; Chung, K.C. Economic Evaluation of Chimeric Antigen Receptor T-Cell Therapy by Site of Care Among Patients with Relapsed or Refractory Large B-Cell Lymphoma. JAMA Netw. Open 2020, 3, e202072. [Google Scholar] [CrossRef]
| Study | Country | Trial Name/ID | Study Design | Treatment | Patient Population | Setting (IP/OP/Both) | Sample Size, n | Reason for Different Sample Sizes | List of Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Kamdar (2022) [64] | US, Europe, and Japan | TRANSFORM/NCT03575351 | Ph 3 trial | Liso-cel | R/R LBCL (2L+) | Both | 91 | Response, LOS | |
| McGarvey (2022) [65] | 90 | Total post-infusion monitoring costs, hospitalizations cost | |||||||
| Godwin (2021) [32] | US | OUTREACH/NCT03744676 | Ph 2 trial | Liso-cel | R/R LBCL (3L+) | Both | 34 | Preliminary results 1 | Response, AEs, hospitalizations, LOS |
| Godwin (2020) [33] | 34 | Response, AEs, hospitalizations, time to hospitalization, LOS | |||||||
| Godwin (2021) [34] | 46 | Preliminary results 2 | Response, AEs | ||||||
| Godwin (2021) [35] | 46 | Response, AEs, hospitalizations, time to hospitalization, LOS | |||||||
| Godwin (2021) [36] | 71 | Preliminary results 3 | Response, AEs, time to hospitalization, LOS | ||||||
| Linhares (2022) [37] | 82 | Updated results | Response, AEs, hospitalizations, time to hospitalization | ||||||
| Linhares (2022) [38] | 82 | Response, AEs, hospitalizations, time to hospitalization, QoL | |||||||
| Sehgal (2022) [39] | US | PILOT/NCT03483103 | Ph 2 trial | Liso-cel | R/R LBCL (2L+ not intended for HSCT) | Both | 61 | Response, time to response, DOR, PFS, OS, EFS, AEs, hospitalization, ICU admission | |
| Sehgal (2019) [40] | R/R aggressive NHL (2L+) | 10 | Subgroup | Response, AEs, HCRU | |||||
| McGarvey (2022) [41] | R/R LBCL (2L+ not intended for HSCT) | 61 | Hospitalizations, LOS, monitoring costs | ||||||
| Abramson (2020) [42] | US | TRANSCEND NHL 001/NCT02631044 | Ph 1 trial | Liso-cel | R/R LBCL (2L+) | Both | 25 | Preliminary results 1 | Response, PFS, AEs |
| Maloney (2017) [43] | R/R B-cell NHL (2L+) | 69 | Preliminary results 2 | AEs | |||||
| Palomba (2018) [44] | R/R DLBCL (2L+) | 94 | Updated results | Response, AEs, hospitalizations, time to hospitalization, ICU admission, LOS, OP visits | |||||
| Gofshteyn (2018) [45] | US | Pedi CART19/NCT01626495 | Ph 1/2a trial | Tisa-cel | Pediatric and young adults with R/R ALL | OP | 51 | NA | AEs |
| Fowler (2021) [66], Fowler (2023) [67] | Multinational | ELARA/NCT03568461 | Ph 2 trial | Tisa-cel | R/R FL (3L+) | Both | 97 | NA | Hospitalizations, LOS, ICU admission, hospitalization costs |
| Myers (2020) [46] | US | NCT01626495/NCT02906371/NCT02374333 | Pooled analysis (3 Ph 1/2 trials) | Tisa-cel | Pediatric ALL | 93% patients treated as OPs | 213 | NA | Hospitalizations, LOS, ICU admission, mortality rate, other HCRU |
| Shadman (2021) [47] | US | NCT03277729 | Ph 1/2 trial | MB-106 | R/R B-NHL (FL, MCL, DLBCL) and CLL | OP | 25 | Updated results | Response, AEs |
| Shadman (2020) [48] | 12 | Preliminary results | |||||||
| Shadman (2021) [49] | 12 | ||||||||
| Shadman (2022) [50] | R/R FL | 16 | Updated results for a subgroup | ||||||
| Turtle (2017) [51] | US | NCT01865617 | Ph 1/2 trial | CD19 CAR-T cells | B-ALL, NHL or CLL | OP | 161 | NA | AEs |
| Palomba (2020) [52] | US | - | Cost analysis (pooled trial data) | Liso-cel | R/R LBCL (3L+) | Both | 303 | NA | Standard IP and ICU LOS, costs (diagnostics and procedures, medications, hospitalization/ICU) |
| Denlinger (2022) [53] | US | - | Retrospective study | Tisa-cel, axi-cel | BCL | Both | 63 | NA | AEs, LOS, costs and charges, out-of-pocket charges |
| Chihara (2022) [54] | US | - | RWE | Any CAR-T | R/R DLBCL | Both | 551 | NA | PFS, OS, hospitalization, ER visits, OP visits (initial and follow-up), costs |
| Borogovac (2022) [55], Borogovac (2021) [56] | US | - | Retrospective study | Any CAR-T | DLBCL, FL and ALL, MCL | 91% patients treated as OPs | 23 | NA | Response, AEs |
| Shao (2021) [57] | US | - | Retrospective study | Tisa-cel | DLBCL (3L+) | OP | 12 | NA | Response, AEs, hospitalization, LOS |
| Yang (2022) [26], Zhao (2021) [58] | US | - | RWE (Medicare claims database) | Tisa-cel, axi-cel | R/R DLBCL | Both | 430 | NA | ICU admission, LOS, costs |
| Farooqui (2022) [59] | US | - | Retrospective study | Axi-cel | NHL | OP | 83 | NA | AEs, ICU admission |
| Nasta (2022) [60] | US | - | Retrospective study | Tisa-cel | Lymphoma (3L+) | OP | 72 | NA | Response, OS, PFS, AEs |
| Maziarz (2021) [61] Maziarz (2022) [62] | US | - | Retrospective study | Tisa-cel, axi-cel | R/R DLBCL | Both | 119 | NA | Hospitalizations, ICU admissions, LOS, OP visits, costs |
| Hospitalizations, ICU, LOS, OP visits (initial and follow-up), costs | |||||||||
| Wright (2020) [68] | NR | - | Retrospective study | Axi-cel/tisa-cel | R/R NHL | Both | 31 | NA | Hospitalizations, LOS |
| Kirby (2022) [63] | US | - | Retrospective study | Axi-cel, tisa-cel, brexu-cel, liso-cel | R/R BCL | OP | 20 | NA | PFS, OS, AEs, hospitalizations |
| Study | Study/Trial Name/Trial ID | Treatment | Setting (IP/OP) | n | Age, Median (Years) | Age ≥ 65, (%) | Male (%) | ECOG PS (%) | Number of Prior Lines | Prior Transplant Therapy, (%) | Refractory Patients, (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Godwin (2021) [36] | OUTREACH/NCT03744676 | Liso-cel | OP | 54 | 64.5 | 50.0 | 70.0 | 0: 33.0 1: 67.0 | 2.0 (2.0–4.0) * | 20.0 | 89.0 |
| IP | 23 | 68.0 | 61.0 | 61.0 | 0: 26.0 1: 74.0 | 2.0 (1.0–6.0) * | 9.0 | 96.0 | |||
| Fowler (2021) [66], Fowler (2023) [67] | ELARA/NCT03568461 | Tisa-cel | OP | 17 | NR | 23.5 | 76.5 | ≥1: 23.5 | ≥5: 41.2% | NR | 41.2 |
| IP | 80 | NR | 25.0 | 63.8 | ≥1: 47.5 | ≥5: 25.0% | NR | 26.3 | |||
| Gofshteyn (2018) [45] | Pedi CART19—NCT01626495 | Liso-cel | OP | 51 | 11.5 | NR | 49.0 | NR | NR | NR | NR |
| Shadman (2022) [50] | NCT03277729 | MB-106 (CD20 CART-T) | OP | 16 | 61.5 | NR | NR | NR | NR | NR | NR |
| Myers (2020) [46] | NCT01626495/NCT02906371/NCT02374333 | Tisa-cel | OP | 213 | 12.4 | NR | 60.0 | NR | NR | NR | NR |
| Shao (2021) [57] | NR | Tisa-cel | OP | 12 | 69.5 | 83.0 | 66.7 | NR | 2: 75.0% 3: 25.0% | 16.7 | NR |
| Zhao (2021) [58], Yang (2022) [26] | NR | Tisa-cel, axi-cel | OP | 50 | Mean: 68.4 | NR | 70.0 | NR | NR | NR | NR |
| IP | 380 | Mean: 70.8 | NR | 62.4 | NR | NR | NR | NR | |||
| Farooqui (2022) [59] | NR | Axi-cel | OP | 83 | Mean: 55.2 | NR | 65.1 | NR | NR | NR | NR |
| Nasta (2022) [60] | NR | Tisa-cel | OP | 72 | 65.7 | NR | 58.3 | 0: 31.9 1: 62.5 2: 4.2 NR: 1.4 | NR | 18.1 | NR |
| Kirby (2022) [63] | NR | Tisa-cel, axi-cel, liso-cel, brexu-cel | OP | 20 | 69.5 | NR | 60.0 | ≥2: 25.0 | NR | NR | NR |
| First Author, Year | Trial Name/ID | Study Design | Patient Population | Treatment | Site of Care | Follow-Up | n | CRS-Related Toxicity (%) | Neurologic Toxicity (%) | Other AEs (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Linhares (2022) [37] | OUTREACH/NCT03744676 | Ph 2 trial | R/R LBCL (3L+) | Liso-cel | Outpatient vs. inpatient | NA | 54 vs. 25 | Any grade: 37.0 vs. 44.0 | Any grade: 30.0 vs. 32.0 Grade 3–4: 13.0 vs. 4.0 | Infection: Any grade, 33 vs. 32; Grade 3–4, 13.0 vs. 4.0. Prolonged cytopenia at Day 29 visit: Any grade, 33.0 vs. 32.0. Hypogammaglobulinemia: Any grade, 11.0 vs. 4.0 |
| Fowler (2023) [67] | ELARA/NCT03568461 | Ph 2 trial | R/R FL (3L+) | Tisa-cel | Outpatient vs. inpatient | Median: 20 months | 17 vs. 80 | Any grade: 52.9 vs. 47.5 | Any grade: 5.9 vs. 11.3 Grade 3–4: 0.0 vs. 1.3 | At least 1 AE: Any grade, 82.4 vs. 92.5; Grade 3–4, 47.1 vs. 75. Hematological disorders, including cytopenias: Any grade, 64.7 vs. 77.5; Grade 3–4, 47.1 vs. 73.8. Infections: Any grade, 17.6 vs. 21.3; Grade 3–4, 0.0 vs. 7.5. Prolonged depletion of normal B cells or agammaglobulinemia: Any grade, 11.8 vs. 10.0. Tumor lysis syndrome: Any grade: 0.0 vs. 1.3; Grade 3–4, 0.0 vs. 1.3 |
| Sehgal (2022) [39] | PILOT/NCT03483103 | Ph 2 trial | R/R LBCL (2L+ not intended for HSCT) | Liso-cel | Outpatient vs. inpatient | Median: 12.3 months | 20 vs. 41 | Any grade: 15.0 vs. 48.0 | Any grade: 10.0 vs. 41.0 | NA |
| Abramson (2020) [42] | TRANSCEND NHL 001/NCT02631044 | Ph 3 trial | R/R LBCL (2L+) | Liso-cel | Outpatient vs. inpatient | Median: 18.8 months | 25 vs. 244 | Any grade: 40.0 vs. 42.0 Grade 3–4: 4.0 vs. 2.0 | Any grade: 44.0 vs. 28.0 Grade 3–4: 8.0 vs. 10.0 | NA |
| Denlinger (2022) [53] * | NA | Retrospective study | BCL | Tisa-cel vs. axi-cel | Outpatient vs. inpatient | NA | 18 vs. 45 | Any grade: 44.0 vs. 96.0 | Any grade: 22.0 vs. 73.0 | NA |
| First Author, Year | Trial Name/ID | Study Design | Patient Population | Treatment | Follow-Up | Sample Size, n | CRS-Related Toxicity (%) | Neurologic Toxicity (%) | Other AE (%) |
|---|---|---|---|---|---|---|---|---|---|
| Gofshteyn (2018) [45] | Pedi CART19/NCT01626495 | Ph 1/2a trial | Pediatric and young adult patients with R/R ALL | Tisa-cel | NA | 51 | Any grade: 92.0 | Any neurotoxicity: 45.0 Common neurotoxicity: 41.0 | NA |
| Turtle (2017) [51] | NCT01865617 | Ph 1/2 trial | B-ALL, NHL or CLL | Anti-CD19 CAR-T cell therapy | NA | 133 | Any grade: 71.0 Grade 1–2: 60.0 Grade 3: 4.0 Grade ≥ 4: 8.0 | Any grade: 40.0 Grade 1–2: 19.0 Grade 3: 16.0 Grade ≥ 4: 5.0 | NA |
| Shadman (2021) [49] | NCT03277729 | Ph 1/2 trial | R/R B-NHL and CLL (FL, MCL, CLL, DLBCL) Entire cohort | CD20 CAR-T cells | NA | 12 | Grade 1: 16.0 Grade 2: 8.0 | NA | NA |
| Shadman (2021) [47] | NCT03277729 | Ph 1/2 trial | FL | CD20 Targeted CAR T-cell therapy (MB-106) | Maximum 13.0 months post-infusion | 18 | Grade 1: 22.0 Grade 2: 5.5 Grade 3: 0.0 Grade 4: 0.0 | Grade 1: 0.0 Grade 2: 0.0 Grade 3: 0.0 Grade 4: 0.0 | NA |
| CLL, MCL, DLBCL, WM | 7 | Grade 1: 28.0 Grade 2: 28.0 Grade 3: 0.0 Grade 4: 0.0 | Grade 1: 14.0 Grade 2: 14.0 Grade 3: 0.0 Grade 4: 0.0 | NA | |||||
| FL, CLL, MCL, DLBCL, WM | 25 | Grade 1: 24.0 Grade 2: 12.0 Grade 3: 0.0 Grade 4: 0.0 | Grade 1: 4.0 Grade 2: 4.0 Grade 3: 0.0 Grade 4: 0.0 | NA | |||||
| Shadman (2020) [48] | NCT03277729 | Ph 1/2 trial | R/R B-cell NHL | CD20 CAR-T | NA | 11 | NA | NA | Grade ≥ 3 AEs Anemia: 36.0 Lymphopenia: 27.0 Neutropenia: 18.0 Hypertension: 9.0 Hypotension: 9.0 Thromboembolic event: 9.0 Neutropenia: 9.0 Elevated alkaline phosphatase: 9.0 Pneumonia: 9.0 Bacteremia: 9.0 Hyperglycemia: 9.0 Pleural effusion: 9.0 Generalized pain: 9.0 |
| Shao (2021) [57] | NA | Retrospective study | DLBCL | Tisa-cel | Median: 29.1 weeks, (range: 2.6–60.0) | 12 | Any grade: 50.0 Grade 3–4: 8.3 | Any grade: 8.3 Grade 3–4: 0.0 | Any grade AEs Anemia: 75.0 Thrombocytopenia: 66.7 Neutropenia: 66.7 Grade 3–4 AEs Anemia: 33.3 Thrombocytopenia: 37.5 Neutropenia: 66.7 |
| Borogovac (2022) [55] | NA | Retrospective study | DLBCL, ALL, MCL | Axi-cel | NA | 13 | Any grade: 69.0 Grade 3–4: 8.0 | Any grade: 31.0 Grade 3–4: 0.0 | NA |
| Tisa-cel | NA | 6 | Any grade: 50.0 Grade 3–4: 17.0 | Any grade: 17.0 Grade 3–4: 17.0 | NA | ||||
| Brexu-cel | NA | 1 | Any grade: 0.0 Grade 3–4: 0.0 | Any grade: 100 Grade 3–4: 0.0 | NA | ||||
| Liso-cel | NA | 1 | Any grade: 0.0 Grade 3–4: 0.0 | Any grade: 0.0 Grade 3–4: 0.0 | NA | ||||
| Farooqui (2022) [59] | NA | Retrospective study | Refractory NHL, patients without acute kidney injury | Axi-cel | NA | 69 | Incidence: 85.5 Grade None: 14.5 Grade 1: 49.3 Grade 2: 34.8 Grade 3: 0.0 Grade 4: 1.4 | Incidence: 52.2 Grade None: 47.8 Grade 1: 21.7 Grade 2: 18.8 Grade 3: 7.2 Grade 4: 4.3 | NA |
| Refractory NHL, patients with acute kidney injury | Axi-cel | NA | 14 | Incidence: 85.7 Grade None: 14.3 Grade 1: 35.7 Grade 2: 42.9 Grade 3: 0.0 Grade 4: 7.1 | Incidence: 57.1 Grade None: 42.9 Grade 1: 7.1 Grade 2: 21.4 Grade 3: 21.4 Grade 4: 7.1 | NA | |||
| Nasta (2022) [60] | NA | Retrospective study | Lymphoma (3L+) | Tisa-cel | Median: 39.5 weeks (range: 3.0–127.3) | 72 | None: 59.7 Grade 1: 22.2 Grade 2: 18.1 | None: 94.4 Grade 1: 2.8 Grade 3–4: 2.8 | NA |
| Kirby (2022) [63] | NA | Retrospective study | R/R BCL | Tisa-cel, axi-cel, liso-cel, and brexu-cel | NA | 20 | Any grade: 55.0 Grade ≥ 3: 5.0 | All grades: 45.0 Grade ≥ 3: 25.0 | Late infection events: 29.0, Hypogammaglobulinemia (IgG < 400 mg/dL or IVIg) Pre-lymphodepletion: 31.0 Late hypogammaglobulinemia: 83.0 |
| Axi-cel | NA | 3 | Grade ≥ 3: 33.0 | Grade ≥ 3: 67.0 | NA | ||||
| Liso-cel | NA | 14 | NA | Grade ≥ 3: 7.0 | NA | ||||
| Brexu-cel | NA | 1 | NA | Grade ≥ 3: 100 | NA |
| Study | Trial Name/ID | Study Design | Patient Population | Treatment | Follow-Up | Outpatients | Inpatients | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | ORR % (95% CI) | CR % (95% CI) | PR % (95% CI) | Other Details | n | ORR % (95% CI) | CR % (95% CI) | PR % (95% CI) | Other Details | ||||||
| Linhares (2022) [37], Godwin (2021) [36] | OUTREACH | Ph 2 trial | R/R LBCL (3L+) | Liso-cel | NR | 57 | 82.4 (70.0–91.0) | 58.0 | 25.0 | SD: 5.0% Median DOR: 15.1 (3.9–NR) | 25 | 76.0 (55.0–91.0) | 44.0 | 32.0 | SD: 12.0; median DOR: 14.8 (2.0–NR) |
| Sehgal (2022) [39] | PILOT | Ph 2 trial | R/R LBCL (2L+) | Liso-cel | Median: 104.3 weeks | 20 | 80.0 (56.3–94.3) | 50.0 (27.2–72.8) | 30.0 | Median DOR: 8.2 (2.1–NR) | 41 | 80.0 | 56.0 | 24.0 | IP/OP (n = 61); SD: 5.0%; median DOR: 12.1 (6.2–NR) |
| Abramson (2020) [42] | TRANSCEND NHL 001 | Ph 2 trial | R/R LBCL (2L+) | Liso-cel | NR | 25 | 80.0 (59.3–93.2) | 56.0 (34.9–75.6) | 24.0 | Median DOR: NR (2.4–NR) | 231 | 72.0 | 53.0 | 19.0 | IP/OP (n = 256): ORR: 73.0%, CR: 53.0% |
| Shadman (2021) [47] | NCT03277729 | Ph 1/2 trial | R/R FL | CAR-T cells | 13.0 months post-infusion | 18 | 94.0 | 78.0 | 17.0 | NR | NA | NA | NA | NA | NA |
| R/R MCL, CLL, DLBCL, WM/LPL | 7 | 100.0 | 57.0 | 43.0 | NR | NA | NA | NA | NA | NA | |||||
| Borogovac (2022) [57] | - | Obs. study | DLBCL and ALL | Axi-cel | 1.0 month | 13 | 77.0 | 69.0 | 8.0 | PR or SD: 8.0% | NR | NR | NR | NR | NR |
| Tisa-cel, brexu-cel, liso-cel | 8 | 87.5 | 75.0 | 12.5 | PR or SD: 13.0% | NR | NR | NR | NR | NR | |||||
| Nasta (2022) [60] | - | Obs. Study | Lymphoma | Tisa-cel | 39.5 weeks | 72 | 43.0 | 34.7 | 8.3 | SD: 5.6% | NR | NR | NR | NR | NR |
| Shao (2021) [57] | - | Obs. study | DLBCL | Tisa-cel | 29.1 weeks | 12 | 58.0 | 25.0 | 33.0 | SD: 8.3% | NR | NR | NR | NR | NR |
| Shadman (2021) [47] | NCT03277729 | Ph 1/2, CT | FL | CAR-T cells | 13.0 months post-infusion | 18 | 94.0 | 78.0 | 17.0 | NR | NR | NR | NR | NR | NR |
| DLBCL | 2 | 100 | 50.0 | 50.0 | NR | NR | NR | NR | NR | NR | |||||
| WM/LPL | 2 | 100 | 100 | 0 | NR | NR | NR | NR | NR | NR | |||||
| CLL | 1 | 100 | 100 | 0 | NR | NR | NR | NR | NR | NR | |||||
| MCL | 2 | 100 | NR | 100 | NR | NR | NR | NR | NR | NR | |||||
| Borogovac (2022) [57] | - | Obs. Study | DLBCL and ALL | Axi-cel | 1.0 month | 13 | 77.0 | 69.0 | 8.0 | PR or SD: 8.0% | NR | NR | NR | NR | NR |
| Tisa-cel | 6 | 100 | 83.0 | 17.0 | PR or SD: 17.0% | NR | NR | NR | NR | NR | |||||
| Brexu-cel | 1 | 100 | 100 | 0 | PR or SD: 0.0% | NR | NR | NR | NR | NR | |||||
| Liso-cel | 1 | 0 | 0 | 0 | PR or SD: 0.0% | NR | NR | NR | NR | NR | |||||
| Nasta (2022) [60] | - | Obs. Study | Lymphoma | Tisa-cel No Bridging | 39.5 weeks | 17 | 53.0 | 29.4 | 23.5 | SD: 5.9% | NR | NR | NR | NR | NR |
| Tisa-cel Bridging | 55 | 40.0 | 36.4 | 3.6 | SD: 5.5% | NR | NR | NR | NR | NR | |||||
| Study | Trial Name/ID | Study Design | Patient Population | Treatment | Follow-Up | Outpatient | Inpatient | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Median PFS in Months (95% CI) | PFS Rate (95% CI) | Other Data | n | Median PFS in Months (95% CI) | PFS Rate (95% CI) | Other Data | ||||||
| Linhares (2022) [37] | OUTREACH | Ph 2 trial | R/R LBCL (3L+) | Liso-cel | 57 | 6.1 (2.9-NR) | 12-mo: 41.0% | - | 25 | 4.3 (2.8-NR) | 12-mo: 39.0% | ||
| Fowler (2023) [67] | ELARA | Ph 2 trial | R/R FL (3L+) | Tisa-cel | 20.0 months | 17 | NA | 12-mo: 60% 18-mo: 57.0% | - | 80 | NA | 12-mo: 70.0% 18-mo: 62.5 (34.9–81.1) | - |
| Sehgal (2022) [39] | PILOT | Ph 2 trial | R/R LBCL (2L+) | Liso-cel | 12.3 (IQR, 6.1–18.0) | 20 | 7.2 (2.4- 13.0) | NR | Median EFS: 7.1 months (2.4–13.0) | NA | NA | NA | Overall patients (n = 61) Median PFS: 9.0 months (4.2–NR) |
| Abramson (2020) [42] | TRANSCEND NHL 001 | Ph 1 trial | R/R LBCL (2L+) | Liso-cel | 18.8 months | 25 | NR (3.0–NR) | NR | - | NA | NA | NA | Overall patients (n = 256) Median PFS: 6.8 months (3.3–14.1) 6-mo PFS: 51.4% (45.0–58.0) 12-mo PFS: 44.1% (37.3–50.7) |
| Kirby (2022) [63] | Kirby 2022 | Retr. study | R/R BCL | Liso-cel, axi-cel, tisa-cel, brexu-cel | >3.3 months | 20 | NR | 6-mo: 65.0% 12-mo: 60.0% | - | - | - | - | - |
| Study | Trial Name/ID | Study Design | Patient Population | Treatment | Follow-Up | Outpatient | Inpatient | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Median OS in Months (95% CI) | OS Rate (95% CI) | Other Data | n | Median OS in Months (95% CI) | OS Rate (95% CI) | Other Data | ||||||
| Linhares (2022) [37] | OUTREACH | Ph 2 trial | R/R LBCL (3L+) | Liso-cel | - | 57 | NR (NR-NR) | 12-mo: 60.0% | - | 25 | 22.2 (8.0-NR) | 12-mo: 60.0% | - |
| Sehgal (2022) [39] | PILOT | Ph 2 trial | R/R LBCL (2L+) | Liso-cel | 12.3 (IQR, 6.1–18.0) | 20 | NR (10.5–NR) | NA | - | NA | NA | NA | Overall patients (n = 61): 17.6 months Median (months) (95% CI): Not reached (19.3-not reached) |
| Nasta (2022) [60] | Nasta 2022 | Retr. study | Lymphoma | tisa-cel | 9.1 | 72 | 26.5 (19.0–NR) | NA | - | - | - | - | - |
| Kirby (2022) [63] | Kirby 2022 | Retr. study | R/R BCL | Liso-cel, axi-cel, tisa-cel, brexu-cel | >3.3 months | 20 | NA | 6-mo: 85.0% 12-mo: 75.0% | - | - | - | - | - |
| Shadman (2021) [47] | NCT03277729 | Ph 1/2 trial | R/R B-NHL and CLL | MB-106 (CD20 CAR-T) | 8.9 | 25 | NA | 1.0 | 1 death over FU | - | - | - | - |
| R/R FL | MB-106 (CD20 CAR-T) | 9.3 | 18 | NA | 0.9 | 1 death over FU | - | - | - | - | |||
| Study | Instruments | Domains | Outpatient (n = 54), LS Mean Change from Baseline (95% CI) | Inpatient (n = 28), LS Mean Change from Baseline (95% CI) | p Value |
|---|---|---|---|---|---|
| Linhares (2022) [38] (OUTREACH) | EORTC QLQ-C30 | GH/QoL | 7.80 (3.99–11.61) | 10.39 (5.37–15.42) | 0.415 |
| Physical functioning | 0.38 (−1.11 to 4.08) | 3.50 (−1.33 to 8.34) | 0.312 | ||
| Role functioning | 5.17 (0.27–10.01) | 4.8 (−1.63 to 11.21) | 0.927 | ||
| Cognitive functioning | 0.71 (−1.08 to 4.51) | 1.83 (−3.19 to 6.84) | 0.716 | ||
| Fatigue | −6.28 (−10.73 to −1.82) | −11.18 (−16.99 to −5.36) | 0.188 | ||
| Pain | −13.46 (−17.50 to −9.41) | −13.25 (−18.51 to −8.00) | 0.951 | ||
| EQ-5D-5L | HUI | 0.02 (−0.02 to 0.06) | 0.04 (−0.01 to 0.10) | 0.51 | |
| VAS | 7.48 (4.10–10.86) | 10.34 (5.76–14.91) | 0.31 |
| First Author, Year | Trial Name/ID | Country for Cost Analysis | Study Design | Patient Population | Treatment | Cost Components | Follow-Up | Site of Care: Outpatient | Site of care: Inpatient | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Total Costs | Hosp. Costs | Other Costs | n | Total Costs | Hosp. Costs | Other Costs | ||||||||
| Palomba (2020) [52] | TRANSCEND NHL 001 and OUTREACH | US | Pooled analysis | R/R LBCL (3L+) | Liso-cel | IP and ICU LOS, diagnostics, procedures, medications | 6.0 months | 47 | 6-month post-infusion cost: USD 36,702 First month: USD 19,837 | NA | NA | 256 | 6-month post-infusion cost: USD 89,535 First month: USD 50,369 | NA | NA |
| Denlinger (2022) [53] * | NA | US | Retrospective study | BCL | Tisa-cel; axi-cel | NA | Median: Axi-cel: 31.4 months Tisa-cel: 23.8 months | 18 | Tisa-cel (outpatient *): Median (range): USD 64,834 (USD 4007–USD 429,380) | NA | NA | 45 | Axi-cel (inpatient *): Median (range): USD 176,535 (USD 30,977–USD 1187,965) | NA | NA |
| McGarvey (2022) [41] | PILOT/NCT03483103 | US | Ph 2 trial | R/R LBCL (2L+) not intended for HSCT | Liso-cel | IP and ICU LOS, diagnostics, procedures, medications | NA | 20 | 6-month post-infusion monitoring cost: USD 16,172 First month: USD 13,261 | USD 9455 | NA | 41 | 6-month post-infusion monitoring cost: USD 61,772 First month: USD 46,947 | USD 49,495 | NA |
| McGarvey (2022) [65] | TRANSFORM/NCT03575351 | US | Ph 3 trial | R/R LBCL (2L+) | Liso-cel | IP and ICU LOS, diagnostics, procedures, medications | NA | 19 | 6-month post-infusion monitoring cost: USD 38,314 First month: USD 18,774 | USD 20,867 | NA | 71 | 6-month post-infusion monitoring cost: USD 96,297 First month: USD 49,111 | USD 69,153 | NA |
| Fowler (2023) [67] | ELARA/NCT03568461 | US | Ph 2 trial | R/R FL (3L+) | Tisa-cel | IP and ICU LOS | Median: 20.0 months | 17 | NA | USD 7477 | NA | 80 | NA | USD 40,054 | NA |
| Maziarz (2022) [62] | NA | US | Retrospective study | R/R DLBCL | Tisa-cel, axi-cel | IP and OP | Mean: 5.0 months | Tisa-cel: 8 | For FU period: Tisa-cel: USD 13,389 For infusion encounter: Tisa-cel: USD 4741 | Tisa-cel: USD 4753 | OP costs: Tisa-cel: USD 8636 | Tisa-cel: 25 Axi-cel: 86 | For FU period: Axi-cel: USD 46,575 Tisa-cel: USD 33,701 Infusion encounter: Axi-cel: USD 51,378 Tisa-cel: USD 34,908 | For FU period: Axi-cel: USD 44,561 Tisa-cel: USD 29,953 | For FU period: OP costs: Axi-cel: USD 2014 Tisa-cel: USD 3748 |
| Yang (2022) [26] ** | NA | US | RWE | R/R DLBCL | Tisa-cel, axi-cel | IP, ER, OP, other medical services, medications | CAR-T IP: 6.6 months, CAR-T OP: 6.0 months | 50 | Total Medicare reimbursement amounts for months 1, 2, 3, 4, 5, 6, and 7: USD 371,839, USD 9120, USD 4927, USD 8300, USD 8102, USD 6724, and USD 10,883, respectively | NA | NA | 380 | Total Medicare reimbursement amounts for months 1, 2, 3, 4, 5, 6, and 7: USD 348,364, USD 9756, USD 7318, USD 8259, USD 7052, USD 5748, and USD 6741, respectively | NA | NA |
| Study Details | Treatment | Outpatient Cohort | Inpatient Cohort | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | OP Visits | Hospitalization Rate | Time to Hospitalization | Reasons for Hospitalization | LOS | ICU Admissions | n | OP Visits | Hospitalization Rate | Time to Hospitalization | LOS | ICU Admissions | ||
| Chihara (2022) [54] | Any CAR-T therapy | 95 | NA | Follow-up hospitalization: 41.8% | NA | NA | NA | NA | 456 | NA | Within 90 days: rehospitalization: 28.7% | NA | 21.4 days | NA |
| Wright (2020) [68] | Tisa-cel, axi-cel | 12 | NA | Unplanned hospitalization: 33.0% | NA | NA | NA | NA | 19 | NA | Unplanned hospitalization: 26.0% | NA | NA | NA |
| Palomba (2020) [52] | Liso-cel | 47 | 100% | 62.0% | NA | NA | Total: 7.8 (SD, 13.1) days ICU: 0.6 (SD, 2.8) days | 6.0% | 256 | 93.0% | ~100% | NA | Total: 20.1 (SD, 15.1) days ICU: 1.1 (SD, 5.5) days | 7.0% |
| Sehgal (2022) [39] | Liso-cel | 20 | NA | Total: 45.0% Within 72 h: 10.0% | Median: 6.0 (IQR, 5.0–10.0) days | AEs: 78.0% Other: 22.0% | Initial hospitalization: Mean: 2.5 (SD, 3.2) days Median: 5.0 (IQR, 4.0–7.0) days ICU: Mean: 3.0 days Median: 3.0 (IQR, 3.0–3.0) days | 5.0% | 41 | NA | Total: 100% | NA | Initial hospitalization: Mean: 11.9 (SD, 5.1) days Median: 12.0 (8.0–15.0) days ICU: Mean: 2.5 (SD, 0.7) days Median: 2.5 (IQR, 2.0–3.0) days | 20.0% |
| Yang (2022) [26] | Tisa-cel, axi-cel | 50 | NA | Within the 1st month: 52.0% | NA | NA | LOS for months 1, 2, 3, 4, 5, 6, and 7: 5.2, 1.6, 1.2, 1.3, 1.4, 0.7, and 0.9 days, respectively ICU LOS: for months 1, 2, 3, 4, 5, 6, and 7: 0.6, 0.1, 0.1, 0.3, 0, 0, and 0.1 days, respectively | NA | 380 | NA | Within the 1st month: 100% (by definition) | NA | LOS for months 1, 2, 3, 4, 5, 6 and 7: 20.4, 4.6, 2.4, 2.0, 1.4, 1.1 and 1.0 days, respectively ICU LOS: for months 1, 2, 3, 4, 5, 6 and 7: 2.4, 0.1, 0.1, 0.1, 0.1, 0.1, and 0.1 days, respectively | NA |
| Fowler (2023) [67] | Tisa-cel | 17 | NA | 59.0% | 5.8 (SD, 7.1) days | CRS: 53.0% | Total: Mean: 4.3 (SD, 1.4) days Median: 4.5 days ICU: 0.0 days | 0.0% | 80 | NA | 100% | NA | Total: Mean: 13.8 (SD, 8.5) days Median: 12.5 days ICU: Median: 4.0 days | 9.0% |
| Linhares (2022) [37] | Liso-cel | 54 | NA | Overall hospitalization: 76.0% Within 4 days: 31.0% | Median: 5.0 days (2.0–310.0 days) | AEs: 83.0% Other: 17.0% | Initial hospital stay: Median: 6.0 (1.0–28.0) days ICU: Median: 3.5 (2.0–5.0) days | 4.0% | 25 | NA | 100% | NA | Initial hospital stay: Median initial stay: 13.0 days (1.0–31.0) | NA |
| McGarvey (2022) [65] | Liso-cel | 19 | NA | NA | NA | NA | Median: 9.0 (range, 4.0–33.0) days | NA | 71 | NA | NA | NA | Median: 15.0 (range, 1.0–164.0) days | NA |
| Denlinger (2022) [53] | Tisa-cel, axi-cel | Tisa-cel: 18 | NA | NA | NA | NA | Tisa-cel: 9.0 days | NA | Axi-cel: 45 | NA | NA | NA | Axi-cel: 14.0 days | NA |
| Maziarz (2022) [61] | Tisa-cel, axi-cel | Tisa-cel: 8 | 100% | 63.0% | NA | NA | Mean: 1.7 days ICU: 0.4 days | 38.0% | Axi-cel: 86 Tisa-cel: 25 | Axi-cel: 52.0% Tisa-cel: 76.0% | NA | NA | Axi-cel: 6.9 days Tisa-cel: 4.6 days | Axi-cel: 30.0% Tisa-cel: 16.0% |
| Myers (2020) [46] | Tisa-cel | 198 * | NA | Within 30 days: 70.0% | NA | NA | Median: 7.0 (IQR, 4.0–13.0) days | 23.0% | 15 * | NA | NA | NA | NA | NA |
| Kamdar (2022) [64] | Liso-cel | 19 | NA | 68.0% | Median: 9.0 (IQR, 4.0–19.0) days | CRS: 38.0% Other AEs: 38.0% PD: 8.0% Other: 15.0% | Median: 9.0 (IQR, 5.0–9.0) days | 0.0% | NA | NA | NA | NA | NA | NA |
| Kirby (2022) [63] | Tisa-cel, axi-cel, liso-cel, brexu-cel | 20 | NA | Within 1st month of therapy: Overall: 50.0% Axi-cel: 67.0% Liso-cel: 36.0% Tisa-cel: 100% Brexu-cel: 100% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Nasta (2022) [60] | Tisa-cel | 68 | NA | Within 72 h: 19.4% Within 30 days: 36.1% | NA | CRS: 85% Infection: 8% Colitis: 4% Catatonia: 4% | Median: 5.0 days | NA | 4 | NA | NA | NA | NA | NA |
| Shao (2021) [57] | Tisa-cel | 12 | NA | Within 30 days: 50.0% | Median: 4.0 days (2.0–12.0 days) | CRS: 83.0% Colitis: 17.0% | Median: 5.5 days (2.0–9.0) days | NA | NA | NA | NA | NA | NA | NA |
| Borogovac (2022) [55] | Axi-cel, tisa-cel, brexu-cel, liso-cel | 21 | NA | Post therapy: Within 72 h/within 1st month: Overall: 24.0%/71.0% Axi-cel: 23.0%/76.0% Liso-cel: 0.0%/0.0% Tisa-cel: 33.0%/67.0% Brexu-cel: 0.0%/100% | Median: 4.0 (1.0–28.0) days | Fever: 87.0% CNS toxicity: 13.0% | Median: 8.0 days (1.0–30.0) | NA | 2 | NA | NA | NA | NA | NA |
| Farooqui (2022) [59] | Axi-cel | With AKI: 14 Without AKI: 69 | NA | NA | NA | NA | NA | With AKI: 42.9% Without AKI: 29.0% | NA | NA | NA | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, D.K.; Liu, Y.-H.; Ranjan, S.; Bhandari, H.; Potluri, R.; McFarland, L.; De Braganca, K.C.; Huo, S. The Impact of Outpatient versus Inpatient Administration of CAR-T Therapies on Clinical, Economic, and Humanistic Outcomes in Patients with Hematological Cancer: A Systematic Literature Review. Cancers 2023, 15, 5746. https://doi.org/10.3390/cancers15245746
Hansen DK, Liu Y-H, Ranjan S, Bhandari H, Potluri R, McFarland L, De Braganca KC, Huo S. The Impact of Outpatient versus Inpatient Administration of CAR-T Therapies on Clinical, Economic, and Humanistic Outcomes in Patients with Hematological Cancer: A Systematic Literature Review. Cancers. 2023; 15(24):5746. https://doi.org/10.3390/cancers15245746
Chicago/Turabian StyleHansen, Doris K., Yi-Hsuan Liu, Sandip Ranjan, Hitesh Bhandari, Ravi Potluri, Lindsay McFarland, Kevin C. De Braganca, and Stephen Huo. 2023. "The Impact of Outpatient versus Inpatient Administration of CAR-T Therapies on Clinical, Economic, and Humanistic Outcomes in Patients with Hematological Cancer: A Systematic Literature Review" Cancers 15, no. 24: 5746. https://doi.org/10.3390/cancers15245746
APA StyleHansen, D. K., Liu, Y.-H., Ranjan, S., Bhandari, H., Potluri, R., McFarland, L., De Braganca, K. C., & Huo, S. (2023). The Impact of Outpatient versus Inpatient Administration of CAR-T Therapies on Clinical, Economic, and Humanistic Outcomes in Patients with Hematological Cancer: A Systematic Literature Review. Cancers, 15(24), 5746. https://doi.org/10.3390/cancers15245746





