Enhanced Precision and Safety in Thermal Ablation: O-Arm Cone Beam Computed Tomography with Magnetic Resonance Imaging Fusion for Spinal Column Tumor Targeting
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Follow-Up
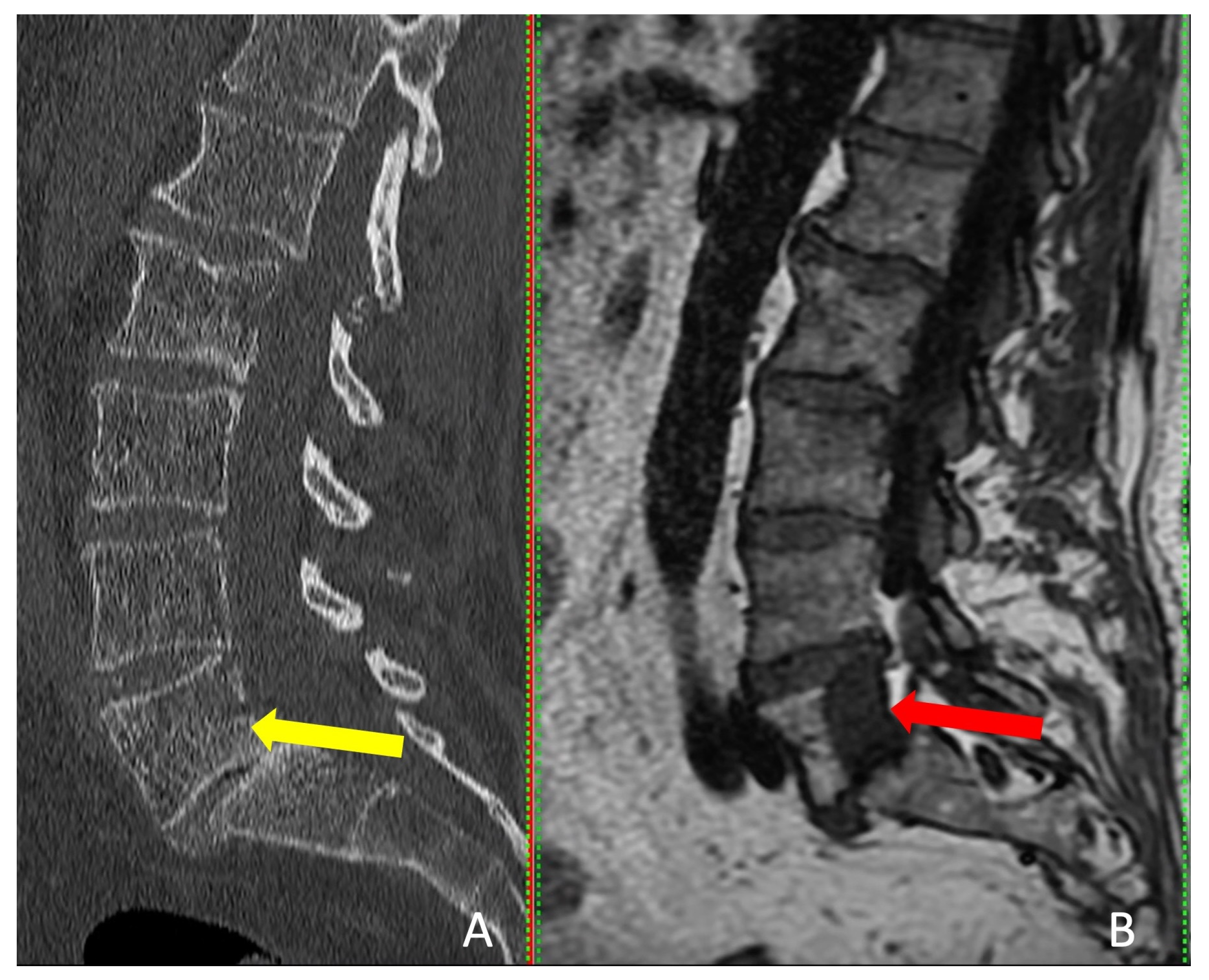
2.2. Image-Guided Navigation and O-Arm System Setup
2.3. Imaging Processing and Analysis
2.4. Biopsy and Coagulation Procedure Workflow
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Fang, Z.; Lang, N.; Yuan, H.; Su, M.-Y.; Baldi, P. A Multi-Resolution Approach for Spinal Metastasis Detection Using Deep Siamese Neural Networks. Comput. Biol. Med. 2017, 84, 137–146. [Google Scholar] [CrossRef]
- Murali, N.; Turmezei, T.; Bhatti, S.; Patel, P.; Marshall, T.; Smith, T. What Is the Effectiveness of Radiofrequency Ablation in the Management of Patients with Spinal Metastases? A Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2021, 16, 659. [Google Scholar] [CrossRef]
- Colonna, S.; Bianconi, A.; Cofano, F.; Prior, A.; Di Perna, G.; Palmieri, G.; Zona, G.; Garbossa, D.; Fiaschi, P. Radiofrequency Ablation in Vertebral Body Metastasis with and without Percutaneous Cement Augmentation: A Systematic Review Addressing the Need for SPINE Stability Evaluation. Diagnostics 2023, 13, 1164. [Google Scholar] [CrossRef] [PubMed]
- Roser, S.; Maharaj, M.M.; Taylor, M.A.; Kuru, R.; Hansen, M.A.; Ferch, R. Vertebrectomy in Metastatic Spinal Tumours: A 10 Year, Single-Centre Review of Outcomes and Survival. J. Clin. Neurosci. 2019, 68, 218–223. [Google Scholar] [CrossRef]
- Urch, C. The Pathophysiology of Cancer-Induced Bone Pain: Current Understanding. Palliat. Med. 2004, 18, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Munk, P.L.; Rashid, F.; Heran, M.K.; Papirny, M.; Liu, D.M.; Malfair, D.; Badii, M.; Clarkson, P.W. Combined Cementoplasty and Radiofrequency Ablation in the Treatment of Painful Neoplastic Lesions of Bone. J. Vasc. Interv. Radiol. 2009, 20, 903–911. [Google Scholar] [CrossRef]
- Giammalva, G.R.; Costanzo, R.; Paolini, F.; Benigno, U.E.; Porzio, M.; Brunasso, L.; Basile, L.; Gulì, C.; Pino, M.A.; Gerardi, R.M.; et al. Management of Spinal Bone Metastases with Radiofrequency Ablation, Vertebral Reinforcement and Transpedicular Fixation: A Retrospective Single-Center Case Series. Front. Oncol. 2021, 11, 818760. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Losina, E.; Bono, C.M.; Schoenfeld, A.J.; Collins, J.E.; Katz, J.N.; Harris, M.B. Clinical Outcome of Metastatic Spinal Cord Compression Treated with Surgical Excision ± Radiation versus Radiation Therapy Alone: A Systematic Review of Literature. Spine 2012, 37, 78–84. [Google Scholar] [CrossRef]
- Mankin, H.J.; Mankin, C.J.; Simon, M.A. The Hazards of the Biopsy, Revisited. Members of the Musculoskeletal Tumor Society. J. Bone Jt. Surg. Am. 1996, 78, 656–663. [Google Scholar] [CrossRef]
- Errani, C.; Traina, F.; Perna, F.; Calamelli, C.; Faldini, C. Current Concepts in the Biopsy of Musculoskeletal Tumors. Sci. World J. 2013, 2013, 538152. [Google Scholar] [CrossRef]
- Rougraff, B.T.; Aboulafia, A.; Biermann, J.S.; Healey, J. Biopsy of Soft Tissue Masses: Evidence-Based Medicine for the Musculoskeletal Tumor Society. Clin. Orthop. Relat. Res. 2009, 467, 2783–2791. [Google Scholar] [CrossRef]
- Liu, J.-C.; Chiou, H.-J.; Chen, W.-M.; Chou, Y.-H.; Chen, T.-H.; Chen, W.; Yen, C.-C.; Chiu, S.-Y.; Chang, C.-Y. Sonographically Guided Core Needle Biopsy of Soft Tissue Neoplasms. J. Clin. Ultrasound 2004, 32, 294–298. [Google Scholar] [CrossRef]
- Stringham, D.R.; Hadjipavlou, A.; Dzioba, R.B.; Lander, P. Percutaneous Transpedicular Biopsy of the Spine. Spine 1994, 19, 1985–1991. [Google Scholar] [CrossRef]
- Carrino, J.A.; Khurana, B.; Ready, J.E.; Silverman, S.G.; Winalski, C.S. Magnetic Resonance Imaging-Guided Percutaneous Biopsy of Musculoskeletal Lesions. J. Bone Jt. Surg. Am. 2007, 89, 2179–2187. [Google Scholar] [CrossRef]
- López, J.I.; Del Cura, J.L.; Zabala, R.; Bilbao, F.J. Usefulness and Limitations of Ultrasound-Guided Core Biopsy in the Diagnosis of Musculoskeletal Tumours. APMIS 2005, 113, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Möller, S.; Kothe, R.; Wiesner, L.; Werner, M.; Rüther, W.; Delling, G. Fluoroscopy-Guided Transpedicular Trocar Biopsy of the Spine--Results, Review, and Technical Notes. Acta Orthop. Belg. 2001, 67, 488–499. [Google Scholar] [PubMed]
- Rimondi, E.; Staals, E.L.; Errani, C.; Bianchi, G.; Casadei, R.; Alberghini, M.; Malaguti, M.C.; Rossi, G.; Durante, S.; Mercuri, M. Percutaneous CT-Guided Biopsy of the Spine: Results of 430 Biopsies. Eur. Spine J. 2008, 17, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, A.; Grady, J.J.; Garges, K.J. Percutaneous Spine Biopsy: A Meta-Analysis. J. Bone Jt. Surg. Am. 2008, 90, 1722–1725. [Google Scholar] [CrossRef]
- Al-Smadi, M.W.; Kozma, I.; Aslan, S.; Bölöni, B.; Viola, Á. Percutaneous Superimposed O-Arm-MRI-Navigated Biopsy for Spinal Column Pathologies. Diagnostics 2023, 13, 2252. [Google Scholar] [CrossRef]
- Gangi, A. Percutaneous Spinal Tumor Management. Neuroradiol. J. 2009, 22 (Suppl. S1), 131–139. [Google Scholar] [CrossRef]
- Tomasian, A.; Hillen, T.J.; Chang, R.O.; Jennings, J.W. Simultaneous Bipedicular Radiofrequency Ablation Combined with Vertebral Augmentation for Local Tumor Control of Spinal Metastases. AJNR Am. J. Neuroradiol. 2018, 39, 1768–1773. [Google Scholar] [CrossRef] [PubMed]
- Tomasian, A.; Jennings, J.W. Percutaneous Interventional Techniques for Treatment of Spinal Metastases. Semin. Intervent. Radiol. 2020, 37, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Urbisci, T.; Thomas, B.; Tran, N. SURG-28. Radiofrequency ablation as an adjunct for pain and tumor control in spinal metastatic diseasE. Neuro. Oncol. 2019, 21 (Suppl. S6). [Google Scholar] [CrossRef]
- Tsoumakidou, G.; Koch, G.; Caudrelier, J.; Garnon, J.; Cazzato, R.L.; Edalat, F.; Gangi, A. Image-Guided Spinal Ablation: A Review. Cardiovasc. Intervent. Radiol. 2016, 39, 1229–1238. [Google Scholar] [CrossRef]
- Grönemeyer, D.H.W.; Schirp, S.; Gevargez, A. Image-Guided Radiofrequency Ablation of Spinal Tumors: Preliminary Experience with an Expandable Array Electrode. Cancer J. 2002, 8, 33–39. [Google Scholar] [CrossRef]
- Racadio, J.M.; Babic, D.; Homan, R.; Rampton, J.W.; Patel, M.N.; Racadio, J.M.; Johnson, N.D. Live 3D Guidance in the Interventional Radiology Suite. AJR Am. J. Roentgenol. 2007, 189, W357–W364. [Google Scholar] [CrossRef]
- Carriero, S.; Della Pepa, G.; Monfardini, L.; Vitale, R.; Rossi, D.; Masperi, A.; Mauri, G. Role of Fusion Imaging in Image-Guided Thermal Ablations. Diagnostics 2021, 11, 549. [Google Scholar] [CrossRef]
- Biondetti, P.; Ascenti, V.; Shehab, A.; Ierardi, A.M.; Carriero, S.; Lanza, C.; Angileri, S.A.; Guzzardi, G.; Carrafiello, G. Percutaneous Microwave Ablation of Hepatocellular Carcinoma with “Double Fusion” Technique: Technical Note and Single-Center Preliminary Experience. Diagnostics 2023, 13, 2349. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Carnevale, A.; Stellato, E.; De Lorenzis, E.; Uccelli, L.; Dionigi, G.; Giganti, M.; Montanari, E.; Carrafiello, G. Cone Beam Computed Tomography Image Fusion with Cross Sectional Images for Percutaneous Renal Tumor Ablation: Preliminary Data. Technol. Cancer Res. Treat. 2023, 22, 15330338231154994. [Google Scholar] [CrossRef]
- Akgun, B.; Ergun, A.C.; Ozercan, I.H.; Kok, S. Intradural Extramedullary Epidermoid Cyst at the Conus Medullaris Level with Thoracic Syringomyelia: A Case Report. Acta Medica 2019, 62, 39–42. [Google Scholar] [CrossRef]
- Arseni, C.N.; Simionescu, M.D.; Horwath, L. Tumors of the Spine. A Follow-up Study of 350 Patients with Neurosurgical Considerations. Acta Psychiatr. Scand. 1959, 34, 398–410. [Google Scholar] [CrossRef]
- Black, P. Spinal Metastasis: Current Status and Recommended Guidelines for Management. Neurosurgery 1979, 5, 726–746. [Google Scholar] [CrossRef]
- Larson, S.; Wetzel, N.; Brochner, R.; Ruge, D. The Surgical Treatment of Metastatic Epidural Tumors. Q. Bull. Northwestern Univ. Med. Sch. 1961, 35, 42–44. [Google Scholar]
- Perese, D.M. Treatment of Metastatic Extradural Spinal Cord Tumors; a Series of 30 Cases. Cancer 1958, 11, 214–221. [Google Scholar] [CrossRef]
- Klimo, P.; Schmidt, M.H. Surgical Management of Spinal Metastases. Oncologist 2004, 9, 188–196. [Google Scholar] [CrossRef]
- Barr, J.D.; Barr, M.S.; Lemley, T.J.; McCann, R.M. Percutaneous Vertebroplasty for Pain Relief and Spinal Stabilization. Spine 2000, 25, 923–928. [Google Scholar] [CrossRef]
- Renfrew, D.L.; Whitten, C.G.; Wiese, J.A.; el-Khoury, G.Y.; Harris, K.G. CT-Guided Percutaneous Transpedicular Biopsy of the Spine. Radiology 1991, 180, 574–576. [Google Scholar] [CrossRef]
- Liu, M.; Sequeiros, R.B.; Xu, Y.; He, X.; Zhu, T.; Li, L.; Lü, Y.; Huang, J.; Li, C. MRI-Guided Percutaneous Transpedicular Biopsy of Thoracic and Lumbar Spine Using a 0.23t Scanner with Optical Instrument Tracking. J. Magn. Reson. Imaging 2015, 42, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Kerimaa, P.; Marttila, A.; Hyvönen, P.; Ojala, R.; Lappi-Blanco, E.; Tervonen, O.; Blanco Sequeiros, R. MRI-Guided Biopsy and Fine Needle Aspiration Biopsy (FNAB) in the Diagnosis of Musculoskeletal Lesions. Eur. J. Radiol. 2013, 82, 2328–2333. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.R.; Thomas, S. Complications of Image-Guided Thermal Ablation of Liver and Kidney Neoplasms. Semin. Intervent. Radiol. 2014, 31, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Solbiati, L.; Meloni, M.F.; Gazelle, G.S.; Halpern, E.F.; Goldberg, S.N. Treatment of Focal Liver Tumors with Percutaneous Radio-Frequency Ablation: Complications Encountered in a Multicenter Study. Radiology 2003, 226, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Straub, R.; Eichler, K.; Woitaschek, D.; Mack, M.G. Malignant Liver Tumors Treated with MR Imaging-Guided Laser-Induced Thermotherapy: Experience with Complications in 899 Patients (2520 Lesions). Radiology 2002, 225, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Callstrom, M.R.; Charboneau, J.W.; Goetz, M.P.; Rubin, J.; Wong, G.Y.; Sloan, J.A.; Novotny, P.J.; Lewis, B.D.; Welch, T.J.; Farrell, M.A.; et al. Painful Metastases Involving Bone: Feasibility of Percutaneous CT- and US-Guided Radio-Frequency Ablation. Radiology 2002, 224, 87–97. [Google Scholar] [CrossRef]
- Citone, M.; Fanelli, F.; Falcone, G.; Mondaini, F.; Cozzi, D.; Miele, V. A Closer Look to the New Frontier of Artificial Intelligence in the Percutaneous Treatment of Primary Lesions of the Liver. Med. Oncol. 2020, 37, 55. [Google Scholar] [CrossRef]
- Huang, Q.; Zeng, Q.; Long, Y.; Tan, L.; Zheng, R.; Xu, E.; Li, K. Fusion Imaging Techniques and Contrast-Enhanced Ultrasound for Thermal Ablation of Hepatocellular Carcinoma—A Prospective Randomized Controlled Trial. Int. J. Hyperth. 2019, 36, 1207–1215. [Google Scholar] [CrossRef] [PubMed]




| Patients # (Tumor Level) | Primary Tumor | Previous Treatment | First Radiation of the Primary Tumors to Ablation (Months) | Diagnosis of Metastatic Spinal Column Tumor to Ablation (Days) | Interventions |
|---|---|---|---|---|---|
| 1 (Th IX) | DLBCL | 6 cycles of R-MBACOD chemotherapy | 33 | 16 | Thermocoagulation + PVP |
| 2 (Th XII) | DLBCL | 4 cycles of R-CHOP chemotherapy | 18 | 21 | Thermocoagulation + PVP |
| 3 (L IV) | PCa | 44/2 Gy radiation therapy for the small pelvic field; 54/2 Gy for prostate and seminal vesicles and 78/2 Gy and hormone therapy for prostate | 12 | 22 | Thermocoagulation |
| 4 (L V) | FL | 5 cycles of R-CHOP chemotherapy | 13 | 12 | Thermocoagulation |
| Patient (Tumor Level) | Tumor Dimension (mm) | |||||
|---|---|---|---|---|---|---|
| CT | MRI | |||||
| A | S | C | A | S | C | |
| 1 (Th IX) | 17.5 | 18.9 | 9.8 | 35.4 | 21.5 | 19.3 |
| 2 (Th XII) | NV | 21.6 | 26.3 | 10.8 | ||
| 3 (L IV) | NV | 34 | 39.2 | 21.2 | ||
| 4 (L V) | NV | 28.5 | 29.8 | 23.2 | ||
| Patient # (Tumor Level) | Pre-Surgery VAS | 3 Months VAS | 9 Months VAS | 12 Months VAS | 18 Months VAS |
|---|---|---|---|---|---|
| 1 (Th IX) | 8/10 | 3/10 | 4/10 | 3/10 | 3/10 |
| 2 (Th XII) | 7/10 | 1/10 | 0/10 | 0/10 | 0/10 |
| 3 (L IV) | 6/10 | 0/10 | 3/10 | 3/10 | NA |
| 4 (L V) | 7/10 | 0/10 | 0/10 | 0/10 | 0/10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslan, S.; Al-Smadi, M.W.; Kozma, I.; Viola, Á. Enhanced Precision and Safety in Thermal Ablation: O-Arm Cone Beam Computed Tomography with Magnetic Resonance Imaging Fusion for Spinal Column Tumor Targeting. Cancers 2023, 15, 5744. https://doi.org/10.3390/cancers15245744
Aslan S, Al-Smadi MW, Kozma I, Viola Á. Enhanced Precision and Safety in Thermal Ablation: O-Arm Cone Beam Computed Tomography with Magnetic Resonance Imaging Fusion for Spinal Column Tumor Targeting. Cancers. 2023; 15(24):5744. https://doi.org/10.3390/cancers15245744
Chicago/Turabian StyleAslan, Siran, Mohammad Walid Al-Smadi, István Kozma, and Árpad Viola. 2023. "Enhanced Precision and Safety in Thermal Ablation: O-Arm Cone Beam Computed Tomography with Magnetic Resonance Imaging Fusion for Spinal Column Tumor Targeting" Cancers 15, no. 24: 5744. https://doi.org/10.3390/cancers15245744
APA StyleAslan, S., Al-Smadi, M. W., Kozma, I., & Viola, Á. (2023). Enhanced Precision and Safety in Thermal Ablation: O-Arm Cone Beam Computed Tomography with Magnetic Resonance Imaging Fusion for Spinal Column Tumor Targeting. Cancers, 15(24), 5744. https://doi.org/10.3390/cancers15245744







