Simple Summary
Carcinoembryonic antigen (CEA) in serum is widely used as a tumor marker in colorectal cancer (CRC). The levels of fecal CEA (FCEA) are higher than serum CEA (SCEA), especially in the early stages of CRC. In this systematic review, we aimed to provide a comprehensive overview of studies that evaluated FCEA as a biomarker for the noninvasive diagnosis and diagnosis of CRC. All of the few identified studies found statistically significant differences in FCEA levels between the CRC and control groups. Moreover, the diagnostic performance of FCEA surpassed that of SCEA, suggesting a potential role as a novel, easily measurable biomarker for the diagnosis of CRC. However, evidence is still limited to a few, mostly small, studies from clinical settings, and comprehensive evaluation in screening settings is warranted.
Abstract
Carcinoembryonic antigen (CEA) is more abundant in feces than in serum; however, evidence for the role of fecal CEA (FCEA) in the detection of colorectal cancer (CRC) is limited. We conducted a systematic review of studies that evaluated FCEA for the noninvasive detection and diagnosis of CRC. PubMed and Web of Science were searched for relevant studies published until 18 January 2023. Information on publication year, study design, country, study population characteristics, FCEA and serum CEA (SCEA) concentrations, and diagnostic performance was summarized. Two authors independently extracted data and assessed the risk of bias and applicability of each included study. Seven studies published between 1979 and 2021, all conducted in clinical settings and together involving 399 CRC patients and 889 controls, were identified. Significant differences in FCEA concentrations were observed between CRC and control groups in all studies. Methods for detecting FCEA varied, with the electronic chemiluminescence immunoassay (ECLIA) being used in the most recent studies. Reported sensitivities, specificities, and area under the curves of FCEA ranged from 50.0% to 85.7%, 73.0% to 100.0%, and 0.704 to 0.831, respectively. In direct comparisons, the diagnostic performance of FCEA was better than that of SCEA. The potential role of FCEA as a novel, noninvasive, easily measurable biomarker for the diagnosis of CRC requires further evaluation in screening settings.
1. Introduction
Colorectal cancer (CRC) is the third most common malignancy and the second most common cause of cancer death, accounting for over 1.9 million new cases and over 0.9 million deaths worldwide in 2020 [1]. Despite significant improvements in treatment, the prognosis of patients with advanced TNM-stage CRC remains poor [2,3]. The 5-year relative survival rate of patients with stage IV CRC in the US was estimated to be at 12%, in contrast to 91% in patients with stage I CRC [4]. Therefore, screening for early-stage CRC is a critical strategy for reducing CRC-associated mortality.
Currently, there are various approaches to CRC screening, but colonoscopy, flexible sigmoidoscopy, and fecal occult blood testing (FOBT, i.e., fecal immunochemical test (FIT)) are the most widely used [5,6]. Although colonoscopy is the gold standard for the diagnosis of CRC and its precursors, the compliance rate for colonoscopy is low due to its invasiveness, high cost, and requirement for extensive bowel preparation [7,8,9,10]. Compared with colonoscopy, flexible sigmoidoscopy is less invasive, less costly, and does not require complete bowel preparation and sedation; however, its major drawback is the inability to visualize neoplasms in the proximal colon [11,12]. FITs are less costly and more convenient than colonoscopy but have relatively low sensitivity for detecting advanced adenoma and early-stage CRC [13,14]. The sensitivity of FIT for CRC screening ranges from 68.8% to 81.3%, whereas its sensitivity for detecting advanced adenomas is much lower (18.0–43.5%) [15]. A large screening study with more than 20,000 participants showed poor performance of FIT for detecting advanced adenoma, with a sensitivity below 22% [16]. Therefore, a novel, cost-effective, noninvasive, and easily measurable CRC screening test, with high sensitivity and specificity, would be highly desirable.
Carcinoembryonic antigen (CEA) is widely used as a tumor marker in most gastrointestinal cancers. It is formed in the cytoplasm and can be detected in serum, cerebrospinal fluid, urine, and feces [17]. Serum CEA (SCEA) levels are low in healthy individuals and may rise 4 to 8 months before cancer-related symptoms develop [18]. However, SCEA is not widely used in CRC screening because several studies have found that it does not have sufficient sensitivity and specificity to diagnose CRC, and it is more commonly used to monitor tumor recurrence [17,19].
Feces composed of undigested food, endogenous secretions, microbiota, and exfoliated host cellular components can be used to assess the entire intestinal environment, including the occurrence of CRC and its biological effects on epithelial cells [20]. As stool is a rich source of cells derived from the gastrointestinal tract, several oncoproteins derived from intact tumor cells or tumor cell debris are present in the stool of patients with CRC. The concentrations of fecal CEA (FCEA) are higher than those of SCEA, especially in the early stages of CRC, prompting researchers to advocate using FCEA for the diagnosis of CRC.
Several studies suggested FCEA to be more sensitive than SCEA for early CRC detection [21,22,23]. However, no systematic review has evaluated the potential of FCEA in the detection of CRC. In this systematic review, we aimed to provide a comprehensive overview of studies that evaluated FCEA as a biomarker for the noninvasive detection of CRC.
2. Materials and Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocols (PRISMA-P) [24]. The protocol has not been registered.
2.1. Data Sources and Search Strategy
The PubMed and Web of Science databases were searched for relevant articles published up to 18 January 2023. The search terms included (CEA OR carcinoembryonic antigen OR carcinoembryonic AND antigen) AND (faeces OR feces OR stool OR stooling OR stools) AND (CRC OR colorectal cancer OR colorectal AND cancer OR colorectal neoplasms OR colorectal AND neoplasms). Duplicate hits were removed.
2.2. Study Selection
Studies were eligible for inclusion in this systematic review if they met the following inclusion criteria: examination of CEA in stool samples from patients with CRC at various stages compared with control groups of individuals without CRC. The search was restricted to human studies published in English. The first step in the selection of eligible studies was based on reading the title and abstract. Articles were excluded if they were (1) not based on stool samples, (2) not full papers, (3) not related to the topic, (4) not original articles (e.g., case reports) or reviews, or (5) not in English. Full texts of the remaining articles were reviewed and included if deemed relevant. Finally, studies that did not report the key study characteristics, stratified results, or sufficient data for calculation were excluded.
2.3. Data Extraction
Two authors (X.L. and L.S.) independently extracted data from eligible studies. Descriptive key characteristics of eligible studies, including publication year, study design, country, study population characteristics, FCEA, and SCEA concentrations, were extracted. In addition, we extracted descriptions of the stool sampling and quantitative FCEA detection methods. Furthermore, sensitivity, specificity, and area under the curve (AUC) for evaluating the diagnostic performance of FCEA and SCEA were extracted.
2.4. Risk of Bias and Applicability Assessment of Each Study
The Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) [25] tool was used to assess the quality of each included study in terms of patient selection, index test, reference standard, flow, and timing. In QUADAS-2, each domain is evaluated for risk of bias, and the first three domains are evaluated for applicability. The risk of bias and applicability assessment for each study was rated as “high risk”, “low risk”, or “unclear risk”. Two authors (X.L. and L.S.) independently performed the assessments using Review Manager 5.4.1 (The Cochrane Collaboration, London, UK, 2020).
3. Results
3.1. Literature Search Results
The literature search and selection process are shown in Figure 1, including four stages of “identification”, “screening”, “eligibility”, and “included”. After removing duplicates, 155 articles were identified. On inspection of titles and abstracts, 136 articles were excluded because they either did not evaluate fecal samples, were not full papers, were not related to the topic, were non-original, or were not in English. We selected 19 articles for full-text assessment. Of these, 12 articles were excluded because they did not provide relevant data on key study characteristics such as FCEA values. Finally, seven studies on CEA levels in feces published up to 18 January 2023 were included in this systematic review.

Figure 1.
PRISMA flow diagram for the literature search process for records identified using PubMed and Web of Science databases.
3.2. Study Characteristics
Table 1 summarizes the key characteristics of the studies included in this review. The studies consisted of seven case–control studies [21,22,23,26,27,28,29], involving a total of 399 CRC cases and 889 controls, including patients with gastric cancer (GC), non-gastrointestinal cancer (NGIC), adenomatous polyposis coli (APC), benign gastrointestinal disorders (BGID), and healthy controls (HCs). The NGIC group included patients with head and neck, hepatobiliary, pancreatic, breast, lung, reproductive system, esophageal, and kidney cancers. The year of publication ranged from 1979 to 2021, with four studies reported between 1979 and 1989, one study in 2003, and two studies in 2021. Six studies were conducted in Asian countries (China, Japan, and South Korea) and one in a European country (the United Kingdom). Sample sizes were rather small in the earlier studies, and the largest two were in the most recent studies from China, which included 436 [21] and 298 [22] participants, with potentially overlapping study populations. Only three studies provided data on age and sex [21,22,29].

Table 1.
Key characteristics of all included studies.
3.3. FCEA and SCEA Concentrations in Different Groups
As shown in Table 1, statistically significant differences in FCEA concentrations were observed between the HC and CRC groups in all studies (p < 0.05). Two studies found that compared with the CRC group, the FCEA concentration of the NGIC group was significantly different (p < 0.001), but there was no statistically significant difference between the CRC and APC groups [21,22]. Three studies found that compared with the CRC group, the BGID group also had statistically significant differences in FCEA (p < 0.01) [23,26,27]. Kim et al. [23] found that, compared with the CRC group, there was no statistically significant difference in FCEA in either the advanced GC (p = 0.879) or the early GC group (p = 0.909). In addition, only three studies reported on SCEA concentrations. Two studies found that compared with the CRC group, there were statistically significant differences in SCEA concentrations in the NGIC, APC, and HC groups [21,22]. Another study found that compared with the CRC group, except for advanced GC, mean SCEA concentrations in the early GC, BGID, and HC groups were significantly different [23]. Although the units of measurement differed, the CEA concentrations in feces were much higher than those in blood.
3.4. Different Methods for Stool Sampling and Fecal CEA Quantitative Detection
All the included studies provided descriptions of stool sampling and FCEA quantitative detection methods, as summarized in Table 2. The two studies reported by Li et al. [22] and Li et al. [21] in 2021 were from the same research group in China; they used similar approaches in terms of stool sampling and FCEA quantitative detection. The studies conducted by Kitsukawa et al. [28] and Fujimoto et al. [29] also used the same methods. However, in general, there were notable variations in the detailed methods of stool sampling and FCEA detection across the studies. Furthermore, it is noteworthy that over time, the quantitative detection method for FCEA has evolved from radioimmunoassay [28,29] to enzyme-linked immunoassay (ELISA) [27] and, subsequently, to electronic chemiluminescence immunoassay (ECLIA) [21,22], which is currently one of the most widely used methods for protein biomarker detection.

Table 2.
Summary of different methods for stool sampling and fecal CEA quantitative detection.
3.5. Diagnostic Performance of Fecal CEA Compared with Serum CEA
Among all the included studies, five assessed or calculated the diagnostic performance (sensitivity, specificity, and AUC) of FCEA or SCEA [21,22,23,26,28]. The results are summarized in Table 3. The sensitivity of FCEA ranged from 50% to 85.7% in four studies, whereas the sensitivity of SCEA ranged from 29.8% to 39.3% in three studies [21,23,28]. The specificity of FCEA ranged from 73.0% to 100% in three studies [21,22,23,26,28], whereas the specificity of SCEA ranged from 90.0% to 98.0% in two studies [21,23]. Additionally, AUC values were reported in two studies [21,22], ranging from 0.704 to 0.831 for FCEA, and from 0.525 [23] to 0.861 for SCEA.

Table 3.
Summary of diagnostic performance of fecal CEA compared with serum CEA.
3.6. Assessment of Risk of Bias and Applicability across Studies
The results of the risk of bias and applicability assessment, based on the QUADAS-2 criteria, are summarized in Figure 2. The primary source of potential bias and applicability concerns in the studies included in the review was the selection of study participants. All studies were case–control studies, and recruited participants had already been diagnosed in clinical settings rather than in prospective true screening settings, so the “Patient Selection” for all included studies was considered high risk in risk of bias and applicability. However, the “Reference Standard” was low risk for both bias and applicability for all included studies, except one reported by Sugano et al. [26]. In four of the seven studies, the “Flow and Timing” had an unclear risk because it was unclear whether there was an appropriate interval between the measurement of FCEA and colonoscopy/pathology.
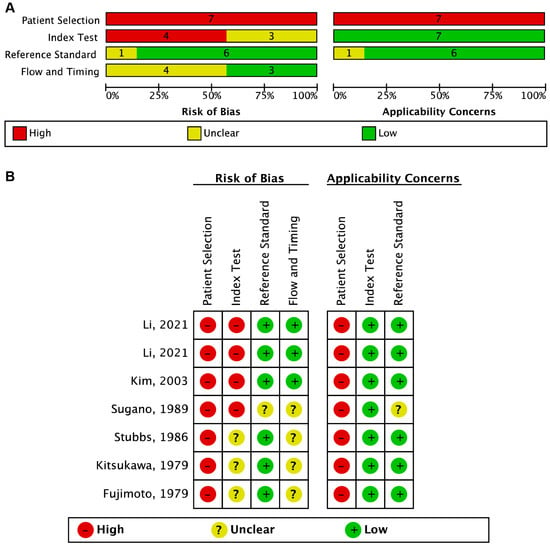
Figure 2.
The risk of bias and applicability assessment based on the QUADAS-2 criteria. (A) Risk of bias and applicability concerns graph: review authorsʹ judgments about each domain presented as percentages of the included studies. (B) Risk of bias and applicability concerns summary: review authors’ judgments about each domain for each included study [21,22,23,26,27,28,29].
4. Discussion
CRC often grows slowly through a multistep process, involving a series of histological, morphological, and genetic changes that accumulate over time. This allows for the screening and detection of early-stage precancerous lesions before they become cancerous in individuals at average risk of CRC [30]. Serum CEA has long been one of the most widely used and evaluated biomarkers for detecting CRC. Although it is often elevated, especially in the advanced stages of the disease, low sensitivity for detecting early stages of the disease limited its use for CRC diagnosis and screening [17,19]. However, CEA can be detected not only in serum but also in feces [31]. Feces are a rich source of cells derived from the gastrointestinal tract, which can be used to measure proteins such as CEA originating in the intestinal mucosa and assess the occurrence of CRC [32]. Kim et al. [23] speculated that if adequate numbers of cancer cells or their products are mixed in the feces, the amount of fecal CEA should be higher in patients with CRC than in normal controls.
However, surprisingly few studies have reported on the potential use of FCEA for CRC detection. The few studies, all of which were conducted in clinical settings, consistently found statistically significant differences in FCEA levels between the CRC and HC groups. In addition, FCEA levels in the CRC group were higher than those in the other disease groups, including APC, NGIC, and BGID. Studies reporting both FCEA and SCEA also found that the levels of CEA in feces were higher than those in the serum. The reason may be that CRC tumor cell-derived CEA is transported from the portal vein to the liver and then decomposed, decreasing CEA concentration in the blood, but it is more likely to be enriched in feces without significant degradation [22]. Li et al. [22] further found that the concentration of FCEA was correlated with tumor size: the concentration of FCEA in patients with CRC with a tumor diameter ≥5 cm was significantly higher than that in those with a tumor diameter <5 cm. A possible reason for this is that the larger the tumor size, the more CRC tumor cells secrete CEA, which is then released into the gut and enriched in feces.
Among the included studies, five evaluated the diagnostic performance of both FCEA and SCEA, and the diagnostic performance (sensitivity, specificity, and AUC) of FCEA was better than that of SCEA, suggesting that FCEA might be a better biomarker than SCEA for the diagnosis of CRC. The FIT for hemoglobin is currently the best-established test for the noninvasive detection of CRC. However, only one study compared the diagnostic performance of FCEA with that of an FIT. Li et al. [21] showed that the AUC value of FCEA for CRC diagnosis was 0.802, which was lower than 0.903 using the FIT. In an older study from 2003, Kim et al. [23] found that the sensitivity for CRC diagnosis of FCEA was 60.0%, and the specificity was 98.2%, both of which were higher than 37.5% and 91.3% using a chemical fecal occult blood test available at that time. The different patterns may primarily be explained by the different types of fecal occult blood tests. Further research should explore if FCEA, by itself or in combination with FIT or other promising fecal tests, might have a potential role in stool-based CRC screening. Compared with colonoscopy, stool-based CRC screening tests may have the advantages of being noninvasive and easily incorporated into routine clinical practice [33]. They do not require bowel preparation or sedation, making them a convenient option for patients.
In this review, we also summarized the methods used for stool sampling and quantitative detection of FCEA in various studies. Except for the studies from the same research group, differences were observed in the specific methodologies used for stool sampling and FCEA detection among the studies. In addition, it is important to highlight the evolution of quantitative methods for FCEA. Initially, a radioimmunoassay was mainly used [28,29], which was later replaced by ELISA [27]. Then, the two most recent studies from the same group in China both used ECLIA [21,22]. Compared to radioimmunoassay and ELISA, ECLIA may offer higher sensitivity and accuracy in detection, as well as simpler operational procedures. With the development of technology, quantitative detection methods for fecal CEA will continue to evolve. Therefore, more accurate and convenient detection methods may be developed.
Generally, when identifying diagnostic biomarkers for CRC screening, study participants need to be representative of the target population and adhere to the recruitment criteria [34]. Differences in the characteristics of the study populations may result in discrepancies in the diagnostic performances of the reported biomarkers. Additionally, the most suitable setting for identifying diagnostic biomarkers is real screening settings. However, all studies included in this review were case–control studies, and the participants had already been diagnosed in clinical settings, not in prospective true screening settings, which may introduce potential spectrum bias [35]. The lack of further external validation of the results of these studies in different settings may also have contributed to potential overestimation and overoptimism [36]. Among the included studies, six originated from Asian countries (China, Japan, and South Korea) and one was from a European country (the United Kingdom), which could potentially restrict the generalizability of the findings to other populations. None of the included studies provided the number of patients who failed to submit stool samples or the number of patients who submitted unusable samples. Therefore, we were unable to assess the failure rates of the FCEA or SCEA test. Moreover, only two studies [27,29] were not typical diagnostic studies because the diagnostic performance results (sensitivity, specificity, and AUC) were not provided. However, to ensure the consistency of the evaluation, we still chose the QUADAS-2 criteria to evaluate the two studies. In addition, the value of fecal CEA may be questionable for the diagnosis of CRC precancerous lesions in clinical practice due to the lack of sufficient evidence of its effectiveness. Further large-scale screening cohorts are needed to settle this issue.
Although this systematic review provided a comprehensive overview of studies investigating and assessing FCEA as a potential biomarker for CRC screening, it has some limitations. First, despite the exhaustive search conducted by two independent reviewers in two reputable databases and meticulous cross-referencing, it remains possible that relevant studies, particularly those in languages other than English, might have been inadvertently overlooked, thus introducing language bias. Second, due to the significant heterogeneity observed among the studies, it was not feasible to conduct meta-analyses to combine the study results. Finally, there was no single original article published about the detection or diagnosis of CRC via FCEA between 2004 and 2020. There might be some publication bias here (i.e., studies might have been conducted but they were simply unpublished due to the absence of significant results).
5. Conclusions
To the best of our knowledge, this systematic review is the first to provide a comprehensive overview of studies that assessed FCEA as a potential biomarker for the noninvasive detection of CRC. All of the few studies reported so far found statistically significant differences in FCEA levels between the CRC and control groups. Moreover, the diagnostic performance of FCEA surpassed that of SCEA, suggesting that it could be a potentially useful, noninvasive, and easily measurable biomarker for the diagnosis of CRC. Nevertheless, evidence is still very limited and essentially restricted to a few, mostly small, case–control studies from clinical settings, with only three studies (including two recent studies from the same group) published since 1990. Further research, in particular large-scale prospective studies with comprehensive analyses of diagnostic performance of FCEA compared to or in combination with other tests, such as FIT, is needed to explore a potential role for CRC diagnosis and screening. Such studies should not only address the detection of CRC but also precursors of CRC, whose detection is essential for CRC prevention. Further research should also address the potential impact of using FCEA or SCEA, alone or in combination with FIT, on the long-term outcomes of screening, such as CRC incidence and mortality.
Author Contributions
H.B. designed and supervised the study. X.L. and L.S. carried out the literature search, extracted the data, and performed the QUADAS-2 assessment. X.L. drafted the manuscript. X.L., P.S.-K., Z.Z., R.C., M.B., J.R.R. and H.B. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Scholarship of China Scholarship Council for X.L.
Data Availability Statement
The data presented in this study are available in article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
APC: adenomatous polyposis coli; AUC, area under the curve; BGID, benign gastrointestinal disorders; CEA, Carcinoembryonic antigen; CRC, Colorectal cancer; ECLIA, Electronic Chemiluminescence Immunoassay; ELISA, Enzyme Linked Immunoassay; FCEA, fecal CEA; FIT, fecal immunochemical tests; FOBT, fecal occult blood testing; GC, gastric cancer; HC, healthy controls; NGIC, non-gastrointestinal cancer; PBS, phosphate buffer saline; PRISMA-P, Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocols; SCEA, Serum CEA.
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from globocan. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef]
- Brenner, H.; Chen, C. The colorectal cancer epidemic: Challenges and opportunities for primary, secondary and tertiary prevention. Br. J. Cancer 2018, 119, 785–792. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.; Guo, F.; Heisser, T.; Hackl, M.; Ihle, P.; De Schutter, H.; Van Damme, N.; Valerianova, Z.; Atanasov, T.; Májek, O.; et al. Colorectal cancer incidence, mortality, and stage distribution in european countries in the colorectal cancer screening era: An international population-based study. Lancet Oncol. 2021, 22, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- González-Suárez, B.; Pagés, M.; Araujo, I.K.; Romero, C.; Rodríguez de Miguel, C.; Ayuso, J.R.; Pozo, À.; Vila-Casadesús, M.; Serradesanferm, A.; Ginès, À.; et al. Colon capsule endoscopy versus CT colonography in FIT-positive colorectal cancer screening subjects: A prospective randomised trial-the VICOCA study. BMC Med. 2020, 18, 255. [Google Scholar] [CrossRef]
- Rabeneck, L.; Saskin, R.; Paszat, L.F. Onset and clinical course of bleeding and perforation after outpatient colonoscopy: A population-based study. Gastrointest. Endosc. 2011, 73, 520–523. [Google Scholar] [CrossRef]
- Rabeneck, L.; Paszat, L.F.; Hilsden, R.J.; Saskin, R.; Leddin, D.; Grunfeld, E.; Wai, E.; Goldwasser, M.; Sutradhar, R.; Stukel, T.A. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology 2008, 135, 1899–1906. [Google Scholar] [CrossRef]
- Frazier, A.L.; Colditz, G.A.; Fuchs, C.S.; Kuntz, K.M. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA 2000, 284, 1954–1961. [Google Scholar] [CrossRef]
- Sonnenberg, A.; Delcò, F.; Inadomi, J.M. Cost-effectiveness of colonoscopy in screening for colorectal cancer. Ann. Intern. Med. 2000, 133, 573–584. [Google Scholar] [CrossRef]
- Helsingen, L.M.; Vandvik, P.O.; Jodal, H.C.; Agoritsas, T.; Lytvyn, L.; Anderson, J.C.; Auer, R.; Murphy, S.B.; Almadi, M.A.; Corley, D.A.; et al. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: A clinical practice guideline. BMJ 2019, 367, l5515. [Google Scholar] [CrossRef] [PubMed]
- Grobbee, E.J.; van der Vlugt, M.; van Vuuren, A.J.; Stroobants, A.K.; Mallant-Hent, R.C.; Lansdorp-Vogelaar, I.; Bossuyt, P.M.M.; Kuipers, E.J.; Dekker, E.; Spaander, M.C.W. Diagnostic yield of one-time colonoscopy vs. one-time flexible sigmoidoscopy vs. multiple rounds of mailed fecal immunohistochemical tests in colorectal cancer screening. Clin. Gastroenterol. Hepatol. 2020, 18, 667–675.e1. [Google Scholar] [CrossRef] [PubMed]
- Young, G.P.; Symonds, E.L.; Allison, J.E.; Cole, S.R.; Fraser, C.G.; Halloran, S.P.; Kuipers, E.J.; Seaman, H.E. Advances in fecal occult blood tests: The FIT revolution. Dig. Dis. Sci. 2015, 60, 609–622. [Google Scholar] [CrossRef]
- Lee, J.K.; Liles, E.G.; Bent, S.; Levin, T.R.; Corley, D.A. Accuracy of fecal immunochemical tests for colorectal cancer: Systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 171. [Google Scholar] [CrossRef] [PubMed]
- Gies, A.; Cuk, K.; Schrotz-King, P.; Brenner, H. Direct comparison of diagnostic performance of 9 quantitative fecal immunochemical tests for colorectal cancer screening. Gastroenterology 2018, 154, 93–104. [Google Scholar] [CrossRef]
- Morikawa, T.; Kato, J.; Yamaji, Y.; Wada, R.; Mitsushima, T.; Shiratori, Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology 2005, 129, 422–428. [Google Scholar] [CrossRef]
- Konishi, T.; Shimada, Y.; Hsu, M.; Tufts, L.; Jimenez-Rodriguez, R.; Cercek, A.; Yaeger, R.; Saltz, L.; Smith, J.J.; Nash, G.M.; et al. Association of preoperative and postoperative serum carcinoembryonic antigen and colon cancer outcome. JAMA Oncol. 2018, 4, 309–315. [Google Scholar] [CrossRef]
- Goldstein, M.J.; Mitchell, E.P. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Investig. 2005, 23, 338–351. [Google Scholar] [CrossRef]
- Wang, Y.-R.; Yan, J.-X.; Wang, L.-N. The diagnostic value of serum carcino-embryonic antigen, alpha fetoprotein and carbohydrate antigen 19-9 for colorectal cancer. J. Cancer Res. Ther. 2014, 10 (Suppl. S4), 307–309. [Google Scholar] [CrossRef]
- Cruz, A.; Carvalho, C.M.; Cunha, A.; Crespo, A.; Iglesias, Á.; García-Nimo, L.; Freitas, P.P.; Cubiella, J. Faecal diagnostic biomarkers for colorectal cancer. Cancers 2021, 13, 5568. [Google Scholar] [CrossRef]
- Li, L.; Gu, W.; Wu, X.; Ao, Y.; Song, Y.; Li, X.; Zeng, Q. Superiority of fecal carcinoembryonic antigen as diagnosis marker for adenomatous polyposis coli and asymptomatic colorectal cancer. Ther. Adv. Gastroenterol. 2021, 14, 17562848211062792. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xing, S.; Wu, M.; Ao, Y.; Zheng, X.; Cai, R.; Han, R.; Li, J.; Li, X.; Zeng, Q. Fecal CEA has an advantage in the diagnosis of colorectal cancer at early stage. Cancer Control J. Moffitt Cancer Cent. 2021, 28, 10732748211048292. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, S.; Park, S.; Jeon, H.; Lee, W.; Kim, J.K.; Cho, M.; Kim, M.; Lim, J.; Kang, C.S.; et al. Gastrointestinal tract cancer screening using fecal carcinoembryonic antigen. Ann. Clin. Lab. Sci. 2003, 33, 32–38. [Google Scholar] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Sugano, K.; Ohkura, H.; Hirohashi, S.; Shimosato, Y.; Sakurai, Y.; Kodaira, S.; Abe, O. Detection of increased fecal carcinoembryonic antigen and its characterization as a membrane-bound form in colorectal carcinoma and other gastrointestinal disorders. Jpn. J. Cancer Res. Gann 1989, 80, 1156–1160. [Google Scholar] [CrossRef]
- Stubbs, R.S.; Nadkarni, D.M.; Monsey, H.A. Faecal carcinoembryonic antigen in colorectal cancer patients. Gut 1986, 27, 901–905. [Google Scholar] [CrossRef]
- Kitsukawa, Y. Immunoreactive carcinoembryonic antigen [CEA] levels in feces from colorectal cancer patients. Jpn. J. Surg. 1979, 9, 102–109. [Google Scholar] [CrossRef]
- Fujimoto, S.; Kitsukawa, U.; Itoh, K. Carcinoembryonic antigen (CEA) in gastric juice or feces as an aid in the diagnosis of gastrointestinal cancer. Ann. Surg. 1979, 189, 34–38. [Google Scholar] [CrossRef]
- Lin, J.S.; Perdue, L.A.; Henrikson, N.B.; Bean, S.I.; Blasi, P.R. Screening for colorectal cancer: Updated evidence report and systematic review for the US preventive services task force. JAMA 2021, 325, 1978–1998. [Google Scholar] [CrossRef]
- Mo, S.; Dai, W.; Wang, H.; Lan, X.; Ma, C.; Su, Z.; Xiang, W.; Han, L.; Luo, W.; Zhang, L.; et al. Early detection and prognosis prediction for colorectal cancer by circulating tumour DNA methylation haplotypes: A multicentre cohort study. EClinicalMedicine 2023, 55, 101717. [Google Scholar] [CrossRef] [PubMed]
- Ewald, N.; Toepler, M.; Akinci, A.; Kloer, H.U.; Bretzel, R.G.; Hardt, P.D. Pyruvate kinase M2 (tumor M2-PK) as a screening tool for colorectal cancer (CRC). A review of current published data. Z. Gastroenterol. 2005, 43, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Jelski, W.; Mroczko, B. Biochemical markers of colorectal cancer—Present and future. Cancer Manag. Res. 2020, 12, 4789–4797. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhu, A.; Bhardwaj, M.; Schrotz-King, P.; Brenner, H. Fecal microRNAs, fecal microRNA panels, or combinations of fecal microRNAs with fecal hemoglobin for early detection of colorectal cancer and its precursors: A systematic review. Cancers 2021, 14, 65. [Google Scholar] [CrossRef]
- Hegedus, E.J.; Moody, J. Clinimetrics corner: The many faces of selection bias. J. Man. Manip. Ther. 2010, 18, 69–73. [Google Scholar] [CrossRef]
- Bell, K.J.; Macaskill, P.; Loy, C. Test accuracy and potential sources of bias in diagnostic test evaluation. Med. J. Aust. 2020, 212, 10–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).