Peritoneal Metastasis: A Dilemma and Challenge in the Treatment of Metastatic Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Background
1.1. Epidemiological
1.2. Occurrence of PM
1.3. Diagnosis and Treatment Development
2. Pathophysiological Process and Molecular Biology Characterization of PM
2.1. Pathophysiological Process
2.2. Molecular Biology Characterization
2.2.1. High-Frequency Mutations in PM
2.2.2. Consensus Molecular Subtypes (CMS)
3. Characterization of Tumor Immune Microenvironment
3.1. Differences between PM and Primary Foci
3.2. Phenotypic Abnormality of NK Cells
4. Diagnosis and Evaluation
4.1. Diagnosis
4.1.1. Radiological Tests
CT
MRI
PET-CT
PET-MRI
4.1.2. Serum Marker Tests
CEA
CA19-9
CA125
4.1.3. Cytological Texts
4.2. Evaluation and Staging
4.2.1. PCI
4.2.2. Peritoneal Surface Disease Severity Score (PSDSS)
4.2.3. The Biological Score of CRC-PM (BIOSCOPE)
5. Prevention and Treatment
5.1. High-Risk Factors and Prevention
5.1.1. High-Risk Factors
5.1.2. Prevention
5.2. Treatments
5.2.1. Systemic Therapy
Chemotherapy
Targeted Therapy
Immunotherapy
5.2.2. Regional Therapy
5.2.2.1. CRS
5.2.2.2. IPC
HIPEC
EPIC, SPIC
Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC)
5.2.2.3. Intraperitoneal Biotherapy
ITs
Catumaxomab
Immunostimulant
Vaccines
Cell Therapy
ICIs
Targeted Therapy
5.2.3. Radiation and Photodynamic Therapy
Radiation Therapy (RT)
Photodynamic Therapy (PDT)
6. New Developments
6.1. Patient-Derived Tumor Organoid (PDTO)
6.2. Liquid Biopsy
6.3. Drug Delivery Technology
6.4. Radspherin for Short-Range Radiation
7. Conclusions
7.1. Current Status
7.2. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Liu, Q.; Yu, W.; Ma, Y.; Zhu, J.; Lian, P.; Cai, S.; Li, Q.; Li, X. Prognostic value of distant metastasis sites and surgery in stage IV colorectal cancer: A population-based study. Int. J. Color. Dis. 2018, 33, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.A.; Seymour, M.T.; Saltz, L.; Punt, C.J.A.; Koopman, M.; Tournigand, C.; et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: An analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef]
- Segelman, J.; Granath, F.; Holm, T.; Machado, M.; Mahteme, H.; Martling, A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 2012, 99, 699–705. [Google Scholar] [CrossRef]
- Sadeghi, B.; Arvieux, C.; Glehen, O.; Beaujard, A.C.; Rivoire, M.; Baulieux, J.; Fontaumard, E.; Brachet, A.; Caillot, J.L.; Faure, J.L.; et al. Peritoneal carcinomatosis from non-gynecologic malignancies: Results of the EVOCAPE 1 multicentric prospective study. Cancer 2000, 88, 358–363. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Carcinoma of the colon--prognosis and operative choice. Curr. Probl. Surg. 1981, 18, 753–802. [Google Scholar] [CrossRef]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Sloothen, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef]
- Ceelen, W.P.; Bracke, M.E. Peritoneal minimal residual disease in colorectal cancer: Mechanisms, prevention, and treatment. Lancet Oncol. 2009, 10, 72–79. [Google Scholar] [CrossRef]
- Sleeman, J.; Steeg, P.S. Cancer metastasis as a therapeutic target. Eur. J. Cancer 2010, 46, 1177–1180. [Google Scholar] [CrossRef]
- Pandya, P.; Orgaz, J.L.; Sanz-Moreno, V. Actomyosin contractility and collective migration: May the force be with you. Curr. Opin. Cell Biol. 2017, 48, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Becker, T.M.; Chua, W.; Ng, W.L.; de Souza, P.; Spring, K.J. Circulating tumour cells and the epithelial mesenchymal transition in colorectal cancer. J. Clin. Pathol. 2014, 67, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Observations concerning cancer spread within the peritoneal cavity and concepts supporting an ordered pathophysiology. Cancer Res. Treat. 1996, 82, 79–100. [Google Scholar] [CrossRef]
- Mikuła-Pietrasik, J.; Sosińska, P.; Maksin, K.; Kucińska, M.G.; Piotrowska, H.; Murias, M.; Woźniak, A.; Szpurek, D.; Książek, K. Colorectal cancer-promoting activity of the senescent peritoneal mesothelium. Oncotarget 2015, 6, 29178–29195. [Google Scholar] [CrossRef]
- Heldin, P.; Kolliopoulos, C.; Lin, C.Y.; Heldin, C.H. Involvement of hyaluronan and CD44 in cancer and viral infections. Cell Signal 2020, 65, 109427. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.M.; Jayne, D.G.; O’Leary, R.; Morrison, E.E.; Guillou, P.J. Tumour-induced apoptosis in human mesothelial cells: A mechanism of peritoneal invasion by Fas Ligand/Fas interaction. Br. J. Cancer 2004, 90, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Vansaun, M.N.; Shim, J.H.; Matrisian, L.M.; Gorden, D.L. Increased metastases are associated with inflammation and matrix metalloproteinase-9 activity at incision sites in a murine model of peritoneal dissemination of colorectal cancer. J. Surg. Res. 2013, 180, 252–259. [Google Scholar] [CrossRef]
- Kataoka, H.; Tanaka, H.; Nagaike, K.; Uchiyama, S.; Itoh, H. Role of cancer cell-stroma interaction in invasive growth of cancer cells. Hum. Cell 2003, 16, 1–14. [Google Scholar] [CrossRef]
- Kim, T.D.; Song, K.S.; Li, G.; Choi, H.; Park, H.D.; Lim, K.; Hwang, B.D.; Yoon, W.H. Activity and expression of urokinase-type plasminogen activator and matrix metalloproteinases in human colorectal cancer. BMC Cancer 2006, 6, 211. [Google Scholar] [CrossRef][Green Version]
- Nataraj, N.B.; Marrocco, I.; Yarden, Y. Roles for growth factors and mutations in metastatic dissemination. Biochem. Soc. Trans. 2021, 49, 1409–1423. [Google Scholar] [CrossRef]
- Manalo, D.J.; Rowan, A.; Lavoie, T.; Natarajan, L.; Kelly, B.D.; Ye, S.Q.; Garcia, J.G.; Semenza, G.L. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 2005, 105, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Shweiki, D.; Itin, A.; Soffer, D.; Keshet, E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992, 359, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Lin, Y.; Zhang, H.; Liu, C.; Cheng, Z.; Yang, X.; Zhang, J.; Xiao, Y.; Sang, N.; Qian, X.; et al. Reprogramming of lipid metabolism in cancer-associated fibroblasts potentiates migration of colorectal cancer cells. Cell Death Dis. 2020, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Chen, D.; Cai, J.; Yuan, Z.; Huang, B.; Li, Y.; Wang, H.; Luo, Q.; Kuang, Y.; Liang, W.; et al. Enhancing cancer-associated fibroblast fatty acid catabolism within a metabolically challenging tumor microenvironment drives colon cancer peritoneal metastasis. Mol. Oncol. 2021, 15, 1391–1411. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Li, Y.; Huang, M.; Tang, G.; Xie, Y.; Chen, D.; Hu, Y.; Yu, T.; Cai, J.; Yuan, Z.; et al. Metabolomics reveals that CAF-derived lipids promote colorectal cancer peritoneal metastasis by enhancing membrane fluidity. Int. J. Biol. Sci. 2022, 18, 1912–1932. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef]
- Schneider, M.A.; Eden, J.; Pache, B.; Laminger, F.; Lopez-Lopez, V.; Steffen, T.; Hübner, M.; Kober, F.; Roka, S.; Campos, P.C.; et al. Mutations of RAS/RAF Proto-oncogenes Impair Survival After Cytoreductive Surgery and HIPEC for Peritoneal Metastasis of Colorectal Origin. Ann. Surg. 2018, 268, 845–853. [Google Scholar] [CrossRef]
- Graf, W.; Cashin, P.H.; Ghanipour, L.; Enblad, M.; Botling, J.; Terman, A.; Birgisson, H. Prognostic Impact of BRAF and KRAS Mutation in Patients with Colorectal and Appendiceal Peritoneal Metastases Scheduled for CRS and HIPEC. Ann. Surg. Oncol. 2020, 27, 293–300. [Google Scholar] [CrossRef]
- Baratti, D.; Kusamura, S.; Niger, M.; Perrone, F.; Milione, M.; Cattaneo, L.; Guaglio, M.; Bartolini, V.; Pietrantonio, F.; Deraco, M. Prognostic Impact of Primary Side and RAS/RAF Mutations in a Surgical Series of Colorectal Cancer with Peritoneal Metastases. Ann. Surg. Oncol. 2021, 28, 3332–3342. [Google Scholar] [CrossRef]
- Stein, M.K.; Williard, F.W.; Xiu, J.; Tsao, M.W.; Martin, M.G.; Deschner, B.W.; Dickson, P.V.; Glazer, E.S.; Yakoub, D.; Shibata, D.; et al. Comprehensive tumor profiling reveals unique molecular differences between peritoneal metastases and primary colorectal adenocarcinoma. J. Surg. Oncol. 2020, 121, 1320–1328. [Google Scholar] [CrossRef]
- Ubink, I.; van Eden, W.J.; Snaebjornsson, P.; Kok, N.F.M.; van Kuik, J.; van Grevenstein, W.M.U.; Laclé, M.M.; Sanders, J.; Fijneman, R.J.A.; Elias, S.G.; et al. Histopathological and molecular classification of colorectal cancer and corresponding peritoneal metastases. Br. J. Surg. 2018, 105, e204–e211. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Nosho, K.; Kirkner, G.J.; Kawasaki, T.; Meyerhardt, J.A.; Loda, M.; Giovannucci, E.L.; Fuchs, C.S. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut 2009, 58, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Flood, M.P.; Jain, A.; Mitchell, C.; Hewitt, C.; Ramsay, R.; Michael, M.; Heriot, A.G.; Tie, J. The impact of molecular and mismatch repair status on the survival outcomes of surgically treated patients with colorectal peritoneal metastases. Eur. J. Surg. Oncol. 2022, 48, 2218–2225. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.G.; Goscinski, M.A.; Dueland, S.; Steigen, S.E.; Hofsli, E.; Torgunrud, A.; Lund-Iversen, M.; Dagenborg, V.J.; Flatmark, K.; Sorbye, H. Impact of KRAS, BRAF and microsatellite instability status after cytoreductive surgery and HIPEC in a national cohort of colorectal peritoneal metastasis patients. Br. J. Cancer 2022, 126, 726–735. [Google Scholar] [CrossRef]
- He, K.; Wang, Y.; Zhong, Y.; Pan, X.; Si, L.; Lu, J. KRAS Codon 12 Mutation is Associated with More Aggressive Invasiveness in Synchronous Metastatic Colorectal Cancer (mCRC): Retrospective Research. OncoTargets Ther. 2020, 13, 12601–12613. [Google Scholar] [CrossRef]
- Arjona-Sanchez, A.; Rodriguez-Ortiz, L.; Baratti, D.; Schneider, M.A.; Gutiérrez-Calvo, A.; García-Fadrique, A.; Tuynman, J.B.; Cascales-Campos, P.A.; Martín, V.C.; Morales, R.; et al. RAS Mutation Decreases Overall Survival After Optimal Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy of Colorectal Peritoneal Metastasis: A Modification Proposal of the Peritoneal Surface Disease Severity Score. Ann. Surg. Oncol. 2019, 26, 2595–2604. [Google Scholar] [CrossRef]
- Breuer, E.; Hebeisen, M.; Schneider, M.A.; Roth, L.; Pauli, C.; Frischer-Ordu, K.; Eden, J.; Pache, B.; Steffen, T.; Hübner, M.; et al. Site of Recurrence and Survival After Surgery for Colorectal Peritoneal Metastasis. J. Natl. Cancer Inst. 2021, 113, 1027–1035. [Google Scholar] [CrossRef]
- Tran, B.; Kopetz, S.; Tie, J.; Gibbs, P.; Jiang, Z.Q.; Lieu, C.H.; Agarwal, A.; Maru, D.M.; Sieber, O.; Desai, J. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 2011, 117, 4623–4632. [Google Scholar] [CrossRef]
- Kim, C.G.; Ahn, J.B.; Jung, M.; Beom, S.H.; Kim, C.; Kim, J.H.; Heo, S.J.; Park, H.S.; Kim, J.H.; Kim, N.K.; et al. Effects of microsatellite instability on recurrence patterns and outcomes in colorectal cancers. Br. J. Cancer 2016, 115, 25–33. [Google Scholar] [CrossRef]
- Tonello, M.; Baratti, D.; Sammartino, P.; Di Giorgio, A.; Robella, M.; Sassaroli, C.; Framarini, M.; Valle, M.; Macrì, A.; Graziosi, L.; et al. Microsatellite and RAS/RAF Mutational Status as Prognostic Factors in Colorectal Peritoneal Metastases Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Ann. Surg. Oncol. 2022, 29, 3405–3417. [Google Scholar] [CrossRef]
- Christensen, T.D.; Palshof, J.A.; Larsen, F.O.; Poulsen, T.S.; Høgdall, E.; Pfeiffer, P.; Jensen, B.V.; Yilmaz, M.K.; Nielsen, D. Associations between primary tumor RAS, BRAF and PIK3CA mutation status and metastatic site in patients with chemo-resistant metastatic colorectal cancer. Acta Oncol. 2018, 57, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Peerenboom, R.; Dhiman, A.; Witmer, H.D.D.; Spurr, L.F.; Polite, B.; Eng, O.S.; Shergill, A.; Turaga, K.K. PI3K Pathway Alterations in Peritoneal Metastases are Associated with Earlier Recurrence in Patients with Colorectal Cancer Undergoing Optimal Cytoreductive Surgery. Ann. Surg. Oncol. 2023, 30, 3114–3122. [Google Scholar] [CrossRef] [PubMed]
- Mlecnik, B.; Bindea, G.; Kirilovsky, A.; Angell, H.K.; Obenauf, A.C.; Tosolini, M.; Church, S.E.; Maby, P.; Vasaturo, A.; Angelova, M.; et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci. Transl. Med. 2016, 8, 327ra326. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, R.; Chatila, W.K.; Lipsyc, M.D.; Hechtman, J.F.; Cercek, A.; Sanchez-Vega, F.; Jayakumaran, G.; Middha, S.; Zehir, A.; Donoghue, M.T.A.; et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell 2018, 33, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Siesing, C.; Petersson, A.; Ulfarsdottir, T.; Chattopadhyay, S.; Nodin, B.; Eberhard, J.; Brändstedt, J.; Syk, I.; Gisselsson, D.; Jirström, K. Delineating the intra-patient heterogeneity of molecular alterations in treatment-naïve colorectal cancer with peritoneal carcinomatosis. Mod. Pathol. 2022, 35, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Diep, C.B.; Teixeira, M.R.; Thorstensen, L.; Wiig, J.N.; Eknaes, M.; Nesland, J.M.; Giercksky, K.E.; Johansson, B.; Lothe, R.A. Genome characteristics of primary carcinomas, local recurrences, carcinomatoses, and liver metastases from colorectal cancer patients. Mol. Cancer 2004, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Kleivi, K.; Lind, G.E.; Diep, C.B.; Meling, G.I.; Brandal, L.T.; Nesland, J.M.; Myklebost, O.; Rognum, T.O.; Giercksky, K.E.; Skotheim, R.I.; et al. Gene expression profiles of primary colorectal carcinomas, liver metastases, and carcinomatoses. Mol. Cancer 2007, 6, 2. [Google Scholar] [CrossRef][Green Version]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Song, N.; Pogue-Geile, K.L.; Gavin, P.G.; Yothers, G.; Kim, S.R.; Johnson, N.L.; Lipchik, C.; Allegra, C.J.; Petrelli, N.J.; O'Connell, M.J.; et al. Clinical Outcome From Oxaliplatin Treatment in Stage II/III Colon Cancer According to Intrinsic Subtypes: Secondary Analysis of NSABP C-07/NRG Oncology Randomized Clinical Trial. JAMA Oncol. 2016, 2, 1162–1169. [Google Scholar] [CrossRef]
- Laoukili, J.; Constantinides, A.; Wassenaar, E.C.E.; Elias, S.G.; Raats, D.A.E.; van Schelven, S.J.; van Wettum, J.; Volckmann, R.; Koster, J.; Huitema, A.D.R.; et al. Peritoneal metastases from colorectal cancer belong to Consensus Molecular Subtype 4 and are sensitised to oxaliplatin by inhibiting reducing capacity. Br. J. Cancer 2022, 126, 1824–1833. [Google Scholar] [CrossRef]
- Becht, E.; de Reyniès, A.; Giraldo, N.A.; Pilati, C.; Buttard, B.; Lacroix, L.; Selves, J.; Sautès-Fridman, C.; Laurent-Puig, P.; Fridman, W.H. Immune and Stromal Classification of Colorectal Cancer Is Associated with Molecular Subtypes and Relevant for Precision Immunotherapy. Clin. Cancer Res. 2016, 22, 4057–4066. [Google Scholar] [CrossRef] [PubMed]
- Trinh, A.; Trumpi, K.; De Sousa, E.M.F.; Wang, X.; de Jong, J.H.; Fessler, E.; Kuppen, P.J.; Reimers, M.S.; Swets, M.; Koopman, M.; et al. Practical and Robust Identification of Molecular Subtypes in Colorectal Cancer by Immunohistochemistry. Clin. Cancer Res. 2017, 23, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Barriuso, J.; Nagaraju, R.T.; Belgamwar, S.; Chakrabarty, B.; Burghel, G.J.; Schlecht, H.; Foster, L.; Kilgour, E.; Wallace, A.J.; Braun, M.; et al. Early Adaptation of Colorectal Cancer Cells to the Peritoneal Cavity Is Associated with Activation of “Stemness” Programs and Local Inflammation. Clin. Cancer Res. 2021, 27, 1119–1130. [Google Scholar] [CrossRef]
- van Baal, J.; van Noorden, C.J.F.; Nieuwland, R.; Van de Vijver, K.K.; Sturk, A.; van Driel, W.J.; Kenter, G.G.; Lok, C.A.R. Development of Peritoneal Carcinomatosis in Epithelial Ovarian Cancer: A Review. J. Histochem. Cytochem. 2018, 66, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Kastelein, A.W.; Vos, L.M.C.; de Jong, K.H.; van Baal, J.; Nieuwland, R.; van Noorden, C.J.F.; Roovers, J.W.R.; Lok, C.A.R. Embryology, anatomy, physiology and pathophysiology of the peritoneum and the peritoneal vasculature. Semin. Cell Dev. Biol. 2019, 92, 27–36. [Google Scholar] [CrossRef]
- Seebauer, C.T.; Brunner, S.; Glockzin, G.; Piso, P.; Ruemmele, P.; Schlitt, H.J.; Geissler, E.K.; Fichtner-Feigl, S.; Kesselring, R. Peritoneal carcinomatosis of colorectal cancer is characterized by structural and functional reorganization of the tumor microenvironment inducing senescence and proliferation arrest in cancer cells. Oncoimmunology 2016, 5, e1242543. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, C.; Xu, X.; Ge, X.; Ding, K.; Zheng, S.; Wang, J.; Sun, L. An Efficient Prognostic Immune Scoring System For Colorectal Cancer Patients With Peritoneal Metastasis. Oncoimmunology 2021, 10, 1901464. [Google Scholar] [CrossRef] [PubMed]
- Pesce, S.; Belgrano, V.; Greppi, M.; Carlomagno, S.; Squillario, M.; Barla, A.; Della Chiesa, M.; Di Domenico, S.; Mavilio, D.; Moretta, L.; et al. Different Features of Tumor-Associated NK Cells in Patients With Low-Grade or High-Grade Peritoneal Carcinomatosis. Front. Immunol. 2019, 10, 1963. [Google Scholar] [CrossRef]
- Dohan, A.; Hobeika, C.; Najah, H.; Pocard, M.; Rousset, P.; Eveno, C. Preoperative assessment of peritoneal carcinomatosis of colorectal origin. J. Visc. Surg. 2018, 155, 293–303. [Google Scholar] [CrossRef]
- Marin, D.; Catalano, C.; Baski, M.; Di Martino, M.; Geiger, D.; Di Giorgio, A.; Sibio, S.; Passariello, R. 64-Section multi-detector row CT in the preoperative diagnosis of peritoneal carcinomatosis: Correlation with histopathological findings. Abdom. Imaging 2010, 35, 694–700. [Google Scholar] [CrossRef]
- Martin, D.R.; Danrad, R.; Herrmann, K.; Semelka, R.C.; Hussain, S.M. Magnetic resonance imaging of the gastrointestinal tract. Top. Magn. Reson. Imaging 2005, 16, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Low, R.N.; Semelka, R.C.; Worawattanakul, S.; Alzate, G.D. Extrahepatic abdominal imaging in patients with malignancy: Comparison of MR imaging and helical CT in 164 patients. J. Magn. Reson. Imaging 2000, 12, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, B.D.; Aschoff, P.; Schwenzer, N.; Fenchel, M.; Koenigsrainer, I.; Falch, C.; Bruecher, B.; Claussen, C.D.; Koenigsrainer, A.; Pfannenberg, C.; et al. Peritoneal carcinomatosis: Comparison of dynamic contrast-enhanced magnetic resonance imaging with surgical and histopathologic findings. Abdom. Imaging 2012, 37, 834–842. [Google Scholar] [CrossRef]
- Low, R.N. Magnetic resonance imaging in the oncology patient: Evaluation of the extrahepatic abdomen. Semin. Ultrasound CT MRI 2005, 26, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Low, R.N. Diffusion-weighted MR imaging for whole body metastatic disease and lymphadenopathy. Magn. Reson. Imaging Clin. N. Am. 2009, 17, 245–261. [Google Scholar] [CrossRef] [PubMed]
- van 't Sant, I.; Engbersen, M.P.; Bhairosing, P.A.; Lambregts, D.M.J.; Beets-Tan, R.G.H.; van Driel, W.J.; Aalbers, A.G.J.; Kok, N.F.M.; Lahaye, M.J. Diagnostic performance of imaging for the detection of peritoneal metastases: A meta-analysis. Eur. Radiol. 2020, 30, 3101–3112. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, H.; Meng, X.; Shen, Y.; Hu, D.; Kamel, I.; Li, Z. Histological grades of rectal cancer: Whole-volume histogram analysis of apparent diffusion coefficient based on reduced field-of-view diffusion-weighted imaging. Quant. Imaging Med. Surg. 2020, 10, 243–256. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, W.; Fu, C.; Yan, X.; Stemmer, A.; Tong, T.; Cai, G. Diagnostic value of whole-body MRI with diffusion-weighted sequence for detection of peritoneal metastases in colorectal malignancy. Cancer Biol. Med. 2018, 15, 165–170. [Google Scholar] [CrossRef]
- Fujii, S.; Matsusue, E.; Kanasaki, Y.; Kanamori, Y.; Nakanishi, J.; Sugihara, S.; Kigawa, J.; Terakawa, N.; Ogawa, T. Detection of peritoneal dissemination in gynecological malignancy: Evaluation by diffusion-weighted MR imaging. Eur. Radiol. 2008, 18, 18–23. [Google Scholar] [CrossRef]
- van ‘t Sant, I.; van Eden, W.J.; Engbersen, M.P.; Kok, N.F.M.; Woensdregt, K.; Lambregts, D.M.J.; Shanmuganathan, S.; Beets-Tan, R.G.H.; Aalbers, A.G.J.; Lahaye, M.J. Diffusion-weighted MRI assessment of the peritoneal cancer index before cytoreductive surgery. Br. J. Surg. 2019, 106, 491–498. [Google Scholar] [CrossRef]
- Low, R.N.; Barone, R.M.; Lucero, J. Comparison of MRI and CT for predicting the Peritoneal Cancer Index (PCI) preoperatively in patients being considered for cytoreductive surgical procedures. Ann. Surg. Oncol. 2015, 22, 1708–1715. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Balasubramaniam, R. Role of Imaging in Peritoneal Surface Malignancies. Indian J. Surg. Oncol. 2016, 7, 441–452. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, S.J.; Lee, S.W. Diagnostic accuracy of (18)F-FDG PET/CT for detection of peritoneal carcinomatosis; a systematic review and meta-analysis. Br. J. Radiol. 2018, 91, 20170519. [Google Scholar] [CrossRef] [PubMed]
- Liberale, G.; Lecocq, C.; Garcia, C.; Muylle, K.; Covas, A.; Deleporte, A.; Hendlisz, A.; Bouazza, F.; El Nakadi, I.; Flamen, P. Accuracy of FDG-PET/CT in Colorectal Peritoneal Carcinomatosis: Potential Tool for Evaluation of Chemotherapeutic Response. Anticancer Res. 2017, 37, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Chong, G.O.; Jeong, S.Y.; Lee, Y.H.; Lee, H.J.; Lee, S.W.; Han, H.S.; Hong, D.G.; Lee, Y.S. The ability of whole-body SUVmax in F-18 FDG PET/CT to predict suboptimal cytoreduction during primary debulking surgery for advanced ovarian cancer. J. Ovarian Res. 2019, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, B.; Schwenzer, N.F.; Gatidis, S.; Koenigsrainer, I.; Koenigsrainer, A.; Beckert, S.; Mueller, M.; Claussen, C.D.; Pfannenberg, C. Assessment of relapse in patients with peritoneal carcinomatosis after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy using F-18-FDG-PET/CT. Rofo 2014, 186, 359–366. [Google Scholar] [CrossRef]
- Zhao, L.; Pang, Y.; Luo, Z.; Fu, K.; Yang, T.; Zhao, L.; Sun, L.; Wu, H.; Lin, Q.; Chen, H. Role of [(68)Ga]Ga-DOTA-FAPI-04 PET/CT in the evaluation of peritoneal carcinomatosis and comparison with [(18)F]-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1944–1955. [Google Scholar] [CrossRef]
- Jónsdóttir, B.; Ripoll, M.A.; Bergman, A.; Silins, I.; Poromaa, I.S.; Ahlström, H.; Stålberg, K. Validation of (18)F-FDG PET/MRI and diffusion-weighted MRI for estimating the extent of peritoneal carcinomatosis in ovarian and endometrial cancer—A pilot study. Cancer Imaging 2021, 21, 34. [Google Scholar] [CrossRef]
- Moradi, F.; Iagaru, A.; McConathy, J. Clinical Applications of PET/MR Imaging. Radiol. Clin. N. Am. 2021, 59, 853–874. [Google Scholar] [CrossRef]
- Bogdanovic, B.; Solari, E.L.; Villagran Asiares, A.; McIntosh, L.; van Marwick, S.; Schachoff, S.; Nekolla, S.G. PET/MR Technology: Advancement and Challenges. Semin. Nucl. Med. 2022, 52, 340–355. [Google Scholar] [CrossRef]
- Kim, B.C.; Bae, J.H.; Park, S.M.; Won, D.Y.; Lee, I.K. Is ascites CEA a risk factor for peritoneal carcinomatosis in colorectal cancer?: A long-term follow-up study. Int. J. Color. Dis. 2020, 35, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Kim, D.H.; Gorden, D.L.; Lee, Y.S.; Sung, N.Y.; Park, G.S.; Kim, H.J.; Kang, W.K.; Park, J.K.; Ahn, C.H.; et al. Prognostic value of CEA and CA 19-9 tumor markers combined with cytology from peritoneal fluid in colorectal cancer. Ann. Surg. Oncol. 2009, 16, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Kozman, M.A.; Fisher, O.M.; Rebolledo, B.J.; Parikh, R.; Valle, S.J.; Arrowaili, A.; Alzahrani, N.; Liauw, W.; Morris, D.L. CEA to peritoneal carcinomatosis index (PCI) ratio is prognostic in patients with colorectal cancer peritoneal carcinomatosis undergoing cytoreduction surgery and intraperitoneal chemotherapy: A retrospective cohort study. J. Surg. Oncol. 2018, 117, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Hasbahceci, M.; Malya, F.U.; Kunduz, E.; Guzel, M.; Unver, N.; Akcakaya, A. Use of serum and peritoneal CEA and CA19-9 in prediction of peritoneal dissemination and survival of gastric adenocarcinoma patients: Are they prognostic factors? Ann. R. Coll. Surg. Engl. Home 2018, 100, 257–266. [Google Scholar] [CrossRef]
- Kanellos, I.; Zacharakis, E.; Kanellos, D.; Pramateftakis, M.G.; Betsis, D. Prognostic significance of CEA levels and positive cytology in peritoneal washings in patients with colorectal cancer. Color. Dis. 2006, 8, 436–440. [Google Scholar] [CrossRef]
- Huang, C.J.; Jiang, J.K.; Chang, S.C.; Lin, J.K.; Yang, S.H. Serum CA125 concentration as a predictor of peritoneal dissemination of colorectal cancer in men and women. Medicine 2016, 95, e5177. [Google Scholar] [CrossRef]
- Huo, Y.R.; Huang, Y.; Liauw, W.; Zhao, J.; Morris, D.L. Prognostic Value of Carcinoembryonic Antigen (CEA), AFP, CA19-9 and CA125 for Patients with Colorectal Cancer with Peritoneal Carcinomatosis Treated by Cytoreductive Surgery and Intraperitoneal Chemotherapy. Anticancer Res. 2016, 36, 1041–1049. [Google Scholar]
- Hugen, N.; van de Velde, C.J.H.; de Wilt, J.H.W.; Nagtegaal, I.D. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann. Oncol. 2014, 25, 651–657. [Google Scholar] [CrossRef]
- Bosanquet, D.C.; Harris, D.A.; Evans, M.D.; Beynon, J. Systematic review and meta-analysis of intraoperative peritoneal lavage for colorectal cancer staging. Br. J. Surg. 2013, 100, 853–862. [Google Scholar] [CrossRef]
- Passot, G.; Dumont, F.; Goéré, D.; Arvieux, C.; Rousset, P.; Regimbeau, J.M.; Elias, D.; Villeneuve, L.; Glehen, O. Multicentre study of laparoscopic or open assessment of the peritoneal cancer index (BIG-RENAPE). Br. J. Surg. 2018, 105, 663–667. [Google Scholar] [CrossRef]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Goéré, D.; Sourrouille, I.; Gelli, M.; Benhaim, L.; Faron, M.; Honoré, C. Peritoneal Metastases from Colorectal Cancer: Treatment Principles and Perspectives. Surg. Oncol. Clin. N. Am. 2018, 27, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Goéré, D.; Souadka, A.; Faron, M.; Cloutier, A.S.; Viana, B.; Honoré, C.; Dumont, F.; Elias, D. Extent of colorectal peritoneal carcinomatosis: Attempt to define a threshold above which HIPEC does not offer survival benefit: A comparative study. Ann. Surg. Oncol. 2015, 22, 2958–2964. [Google Scholar] [CrossRef] [PubMed]
- Goéré, D.; Malka, D.; Tzanis, D.; Gava, V.; Boige, V.; Eveno, C.; Maggiori, L.; Dumont, F.; Ducreux, M.; Elias, D. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann. Surg. 2013, 257, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Vassos, N.; Piso, P. Metastatic Colorectal Cancer to the Peritoneum: Current Treatment Options. Curr. Treat. Options Oncol. 2018, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, J.; Lowy, A.M.; Markman, M.; Chua, T.; Pelz, J.; Baratti, D.; Baumgartner, J.M.; Berri, R.; Bretcha-Boix, P.; Deraco, M.; et al. The American Society of Peritoneal Surface Malignancies (ASPSM) Multiinstitution Evaluation of the Peritoneal Surface Disease Severity Score (PSDSS) in 1,013 Patients with Colorectal Cancer with Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2014, 21, 4195–4201. [Google Scholar] [CrossRef]
- Enblad, M.; Graf, W.; Birgisson, H. Risk factors for appendiceal and colorectal peritoneal metastases. Eur. J. Surg. Oncol. 2018, 44, 997–1005. [Google Scholar] [CrossRef]
- Quere, P.; Facy, O.; Manfredi, S.; Jooste, V.; Faivre, J.; Lepage, C.; Bouvier, A.M. Epidemiology, Management, and Survival of Peritoneal Carcinomatosis from Colorectal Cancer: A Population-Based Study. Dis. Colon Rectum 2015, 58, 743–752. [Google Scholar] [CrossRef]
- Mo, T.W.; Zhang, Z.J.; Chen, Y.L.; Huang, J.H.; Su, D.; Song, W.L.; Hu, J.C.; He, X.W. Risk factors for metachronous peritoneal carcinomatosis after radical resection for patients with nonmetastatic pT3-4 colon cancer. J. Surg. Oncol. 2022, 126, 757–771. [Google Scholar] [CrossRef]
- Honoré, C.; Gelli, M.; Francoual, J.; Benhaim, L.; Elias, D.; Goéré, D. Ninety percent of the adverse outcomes occur in 10% of patients: Can we identify the populations at high risk of developing peritoneal metastases after curative surgery for colorectal cancer? Int. J. Hyperth. 2017, 33, 505–510. [Google Scholar] [CrossRef]
- Hansen, E.; Wolff, N.; Knuechel, R.; Ruschoff, J.; Hofstaedter, F.; Taeger, K. Tumor cells in blood shed from the surgical field. Arch. Surg. 1995, 130, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Management of peritoneal-surface malignancy: The surgeon’s role. Langenbeck Arch. Surg. 1999, 384, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Peritoneum as the first-line of defense in carcinomatosis. J. Surg. Oncol. 2007, 95, 93–96. [Google Scholar] [CrossRef] [PubMed]
- van Grevenstein, W.M.; Hofland, L.J.; van Rossen, M.E.; van Koetsveld, P.M.; Jeekel, J.; van Eijck, C.H. Inflammatory cytokines stimulate the adhesion of colon carcinoma cells to mesothelial monolayers. Dig. Dis. Sci. 2007, 52, 2775–2783. [Google Scholar] [CrossRef] [PubMed]
- Zeamari, S.; Roos, E.; Stewart, F.A. Tumour seeding in peritoneal wound sites in relation to growth-factor expression in early granulation tissue. Eur. J. Cancer 2004, 40, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Jiang, P.; Yamauchi, K.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Moossa, A.R.; Bouvet, M.; Hoffman, R.M. Real-time imaging of tumor-cell shedding and trafficking in lymphatic channels. Cancer Res. 2007, 67, 8223–8228. [Google Scholar] [CrossRef] [PubMed]
- Verwaal, V.J.; Bruin, S.; Boot, H.; van Slooten, G.; van Tinteren, H. 8-year follow-up of randomized trial: Cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann. Surg. Oncol. 2008, 15, 2426–2432. [Google Scholar] [CrossRef]
- Glehen, O.; Mithieux, F.; Osinsky, D.; Beaujard, A.C.; Freyer, G.; Guertsch, P.; Francois, Y.; Peyrat, P.; Panteix, G.; Vignal, J.; et al. Surgery combined with peritonectomy procedures and intraperitoneal chemohyperthermia in abdominal cancers with peritoneal carcinomatosis: A phase II study. J. Clin. Oncol. 2003, 21, 799–806. [Google Scholar] [CrossRef]
- Elias, D.; Goéré, D.; Di Pietrantonio, D.; Boige, V.; Malka, D.; Kohneh-Shahri, N.; Dromain, C.; Ducreux, M. Results of systematic second-look surgery in patients at high risk of developing colorectal peritoneal carcinomatosis. Ann. Surg. 2008, 247, 445–450. [Google Scholar] [CrossRef]
- Sammartino, P.; Sibio, S.; Biacchi, D.; Cardi, M.; Accarpio, F.; Mingazzini, P.; Rosati, M.S.; Cornali, T.; Di Giorgio, A. Prevention of Peritoneal Metastases from Colon Cancer in High-Risk Patients: Preliminary Results of Surgery plus Prophylactic HIPEC. Gastroenterol. Res. Pract. 2012, 2012, 141585. [Google Scholar] [CrossRef]
- Klaver, C.E.; Musters, G.D.; Bemelman, W.A.; Punt, C.J.; Verwaal, V.J.; Dijkgraaf, M.G.; Aalbers, A.G.; van der Bilt, J.D.; Boerma, D.; Bremers, A.J.; et al. Adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with colon cancer at high risk of peritoneal carcinomatosis; the COLOPEC randomized multicentre trial. BMC Cancer 2015, 15, 428. [Google Scholar] [CrossRef]
- Klaver, C.E.L.; Wisselink, D.D.; Punt, C.J.A.; Snaebjornsson, P.; Crezee, J.; Aalbers, A.G.J.; Brandt, A.; Bremers, A.J.A.; Burger, J.W.A.; Fabry, H.F.J.; et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): A multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 2019, 4, 761–770. [Google Scholar] [CrossRef]
- Goéré, D.; Glehen, O.; Quenet, F.; Guilloit, J.M.; Bereder, J.M.; Lorimier, G.; Thibaudeau, E.; Ghouti, L.; Pinto, A.; Tuech, J.J.; et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): A randomised, phase 3 study. Lancet Oncol. 2020, 21, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef] [PubMed]
- Franko, J.; Shi, Q.; Goldman, C.D.; Pockaj, B.A.; Nelson, G.D.; Goldberg, R.M.; Pitot, H.C.; Grothey, A.; Alberts, S.R.; Sargent, D.J. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: A pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J. Clin. Oncol. 2012, 30, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Glockzin, G.; Zeman, F.; Croner, R.S.; Königsrainer, A.; Pelz, J.; Ströhlein, M.A.; Rau, B.; Arnold, D.; Koller, M.; Schlitt, H.J.; et al. Perioperative Systemic Chemotherapy, Cytoreductive Surgery, and Hyperthermic Intraperitoneal Chemotherapy in Patients With Colorectal Peritoneal Metastasis: Results of the Prospective Multicenter Phase 2 COMBATAC Trial. Clin. Color. Cancer 2018, 17, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Glockzin, G.; Rochon, J.; Arnold, D.; Lang, S.A.; Klebl, F.; Zeman, F.; Koller, M.; Schlitt, H.J.; Piso, P. A prospective multicenter phase II study evaluating multimodality treatment of patients with peritoneal carcinomatosis arising from appendiceal and colorectal cancer: The COMBATAC trial. BMC Cancer 2013, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Jiang, Y.; Liang, J.; Pei, W.; Zhou, Z. Neoadjuvant chemotherapy followed by hyperthermic intraperitoneal chemotherapy for patients with colorectal peritoneal metastasis: A retrospective study of its safety and efficacy. World J. Surg. Oncol. 2021, 19, 151. [Google Scholar] [CrossRef] [PubMed]
- Beal, E.W.; Suarez-Kelly, L.P.; Kimbrough, C.W.; Johnston, F.M.; Greer, J.; Abbott, D.E.; Pokrzywa, C.; Raoof, M.; Lee, B.; Grotz, T.E.; et al. Impact of Neoadjuvant Chemotherapy on the Outcomes of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Multi-Institutional Retrospective Review. J. Clin. Med. 2020, 9, 748. [Google Scholar] [CrossRef] [PubMed]
- Rovers, K.P.; Bakkers, C.; Simkens, G.; Burger, J.W.A.; Nienhuijs, S.W.; Creemers, G.M.; Thijs, A.M.J.; Brandt-Kerkhof, A.R.M.; Madsen, E.V.E.; Ayez, N.; et al. Perioperative systemic therapy and cytoreductive surgery with HIPEC versus upfront cytoreductive surgery with HIPEC alone for isolated resectable colorectal peritoneal metastases: Protocol of a multicentre, open-label, parallel-group, phase II–III, randomised, superiority study (CAIRO6). BMC Cancer 2019, 19, 390. [Google Scholar] [CrossRef]
- Rovers, K.P.; Bakkers, C.; Nienhuijs, S.W.; Burger, J.W.A.; Creemers, G.M.; Thijs, A.M.J.; Brandt-Kerkhof, A.R.M.; Madsen, E.V.E.; van Meerten, E.; Tuynman, J.B.; et al. Perioperative Systemic Therapy vs Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Alone for Resectable Colorectal Peritoneal Metastases: A Phase 2 Randomized Clinical Trial. JAMA Surg. 2021, 156, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Bakkers, C.; Rovers, K.P.; Rijken, A.; Nienhuijs, S.W.; de Hingh, I. ASO Author Reflections: Patient-Reported Outcomes of the CAIRO6 Phase II Trial. Ann. Surg. Oncol. 2023, 30, 2689–2690. [Google Scholar] [CrossRef] [PubMed]
- Rovers, K.P.; Bakkers, C.; de Hingh, I. New Insights on the Treatment of Colorectal Peritoneal Metastases From the CAIRO6 Trial-Reply. JAMA Surg. 2022, 157, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Flood, M.P.; Kong, J.C.H.; Wilson, K.; Mohan, H.; Waters, P.S.; McCormick, J.J.; Warrier, S.K.; Tie, J.; Ramsay, R.; Michael, M.; et al. The Impact of Neoadjuvant Chemotherapy on the Surgical Management of Colorectal Peritoneal Metastases: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2022, 29, 6619–6631. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, A.; Brandl, A.; Wakama, S.; Sako, S.; Ishibashi, H.; Mizumoto, A.; Takao, N.; Ichinose, M.; Motoi, S.; Liu, Y.; et al. Effect of oxaliplatin-based chemotherapy on chemosensitivity in patients with peritoneal metastasis from colorectal cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: Proof-of-concept study. BJS Open 2021, 5, zraa075. [Google Scholar] [CrossRef]
- Rovers, K.P.; Bakkers, C.; van Erning, F.N.; Burger, J.W.A.; Nienhuijs, S.W.; Simkens, G.; Creemers, G.M.; Hemmer, P.H.J.; Punt, C.J.A.; Lemmens, V.; et al. Adjuvant Systemic Chemotherapy vs Active Surveillance Following Up-front Resection of Isolated Synchronous Colorectal Peritoneal Metastases. JAMA Oncol. 2020, 6, e202701. [Google Scholar] [CrossRef]
- Cashin, P.H.; Esquivel, J.; Larsen, S.G.; Liauw, W.; Alzahrani, N.A.; Morris, D.L.; Kepenekian, V.; Sourrouille, I.; Dumont, F.; Tuech, J.J.; et al. Perioperative chemotherapy in colorectal cancer with peritoneal metastases: A global propensity score matched study. EClinicalMedicine 2023, 55, 101746. [Google Scholar] [CrossRef]
- Maillet, M.; Glehen, O.; Lambert, J.; Goere, D.; Pocard, M.; Msika, S.; Passot, G.; Elias, D.; Eveno, C.; Sabaté, J.M.; et al. Early Postoperative Chemotherapy After Complete Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy for Isolated Peritoneal Carcinomatosis of Colon Cancer: A Multicenter Study. Ann. Surg. Oncol. 2016, 23, 863–869. [Google Scholar] [CrossRef]
- Razenberg, L.G.; van Gestel, Y.R.; Lemmens, V.E.; de Hingh, I.H.; Creemers, G.J. Bevacizumab in Addition to Palliative Chemotherapy for Patients With Peritoneal Carcinomatosis of Colorectal Origin: A Nationwide Population-Based Study. Clin. Color. Cancer 2016, 15, e41–e46. [Google Scholar] [CrossRef]
- Shida, D.; Yoshida, T.; Tanabe, T.; Tsukamoto, S.; Ochiai, H.; Kanemitsu, Y. Prognostic Impact of R0 Resection and Targeted Therapy for Colorectal Cancer with Synchronous Peritoneal Metastasis. Ann. Surg. Oncol. 2018, 25, 1646–1653. [Google Scholar] [CrossRef]
- Bai, L.; Wang, F.; Li, Z.Z.; Ren, C.; Zhang, D.S.; Zhao, Q.; Lu, Y.X.; Wang, D.S.; Ju, H.Q.; Qiu, M.Z.; et al. Chemotherapy plus bevacizumab versus chemotherapy plus cetuximab as first-line treatment for patients with metastatic colorectal cancer: Results of a registry-based cohort analysis. Medicine 2016, 95, e4531. [Google Scholar] [CrossRef] [PubMed]
- Gremonprez, F.; Descamps, B.; Izmer, A.; Vanhove, C.; Vanhaecke, F.; De Wever, O.; Ceelen, W. Pretreatment with VEGF(R)-inhibitors reduces interstitial fluid pressure, increases intraperitoneal chemotherapy drug penetration, and impedes tumor growth in a mouse colorectal carcinomatosis model. Oncotarget 2015, 6, 29889–29900. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ceelen, W.; Van Nieuwenhove, Y.; Putte, D.V.; Pattyn, P. Neoadjuvant chemotherapy with bevacizumab may improve outcome after cytoreduction and hyperthermic intraperitoneal chemoperfusion (HIPEC) for colorectal carcinomatosis. Ann. Surg. Oncol. 2014, 21, 3023–3028. [Google Scholar] [CrossRef] [PubMed]
- Eveno, C.; Passot, G.; Goéré, D.; Soyer, P.; Gayat, E.; Glehen, O.; Elias, D.; Pocard, M. Bevacizumab doubles the early postoperative complication rate after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal carcinomatosis of colorectal origin. Ann. Surg.Oncol. 2014, 21, 1792–1800. [Google Scholar] [CrossRef]
- Willaert, W.; Van Der Speeten, K.; Liberale, G.; Ceelen, W. BEV-IP: Perioperative chemotherapy with bevacizumab in patients undergoing cytoreduction and intraperitoneal chemoperfusion for colorectal carcinomatosis. BMC Cancer 2015, 15, 980. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siena, S.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Fakih, M.; Elez, E.; et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2021, 22, 779–789. [Google Scholar] [CrossRef]
- Xu, J.; Kim, T.W.; Shen, L.; Sriuranpong, V.; Pan, H.; Xu, R.; Guo, W.; Han, S.W.; Liu, T.; Park, Y.S.; et al. Results of a Randomized, Double-Blind, Placebo-Controlled, Phase III Trial of Trifluridine/Tipiracil (TAS-102) Monotherapy in Asian Patients with Previously Treated Metastatic Colorectal Cancer: The TERRA Study. J. Clin. Oncol. 2018, 36, 350–358. [Google Scholar] [CrossRef]
- Kuboki, Y.; Nishina, T.; Shinozaki, E.; Yamazaki, K.; Shitara, K.; Okamoto, W.; Kajiwara, T.; Matsumoto, T.; Tsushima, T.; Mochizuki, N.; et al. TAS-102 plus bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (C-TASK FORCE): An investigator-initiated, open-label, single-arm, multicentre, phase 1/2 study. Lancet Oncol. 2017, 18, 1172–1181. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Yilmaz, M.; Möller, S.; Zitnjak, D.; Krogh, M.; Petersen, L.N.; Poulsen, L.; Winther, S.B.; Thomsen, K.G.; Qvortrup, C. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: An investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 412–420. [Google Scholar] [CrossRef]
- Koch, J.; Mönch, D.; Maaß, A.; Mangold, A.; Gužvić, M.; Mürdter, T.; Leibold, T.; Dahlke, M.H.; Renner, P. Pharmacologic Targeting of MMP2/9 Decreases Peritoneal Metastasis Formation of Colorectal Cancer in a Human Ex Vivo Peritoneum Culture Model. Cancers 2022, 14, 3760. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Barraud, S.; Tougeron, D.; Villeneuve, L.; Eveno, C.; Bayle, A.; Parc, Y.; Pocard, M.; André, T.; Cohen, R. Immune checkpoint inhibitors for patients with isolated peritoneal carcinomatosis from dMMR/MSI-H colorectal cancer, a BIG-RENAPE collaboration. Dig. Liver Dis. 2023, 55, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Parikh, M.S.; Johnson, P.; Romanes, J.P.; Freitag, H.E.; Spring, M.E.; Garcia-Henriquez, N.; Monson, J.R.T. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Systematic Review. Dis. Colon Rectum 2022, 65, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Peritonectomy procedures. Ann. Surg. 1995, 221, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O. A real-world, population-based study of the outcomes of patients with metastatic colorectal cancer to the peritoneum treated with or without cytoreductive surgery. Int. J. Color. Dis. 2020, 35, 719–725. [Google Scholar] [CrossRef]
- Lundy, M.E.; Moaven, O.; Perry, K.C.; Mangieri, C.W.; Valenzuela, C.D.; Russell, G.B.; Bordelon, R.; Shen, P.; Votanopoulos, K.I.; Levine, E.A. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Management of Colorectal Cancer with Peritoneal Dissemination: 30 Years of Experience at a Single Institution. J. Am. Coll. Surg. 2022, 234, 546–556. [Google Scholar] [CrossRef]
- Elias, D.; Gilly, F.; Boutitie, F.; Quenet, F.; Bereder, J.M.; Mansvelt, B.; Lorimier, G.; Dubè, P.; Glehen, O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: Retrospective analysis of 523 patients from a multicentric French study. J. Clin. Oncol. 2010, 28, 63–68. [Google Scholar] [CrossRef]
- Glehen, O.; Kwiatkowski, F.; Sugarbaker, P.H.; Elias, D.; Levine, E.A.; De Simone, M.; Barone, R.; Yonemura, Y.; Cavaliere, F.; Quenet, F.; et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A multi-institutional study. J. Clin. Oncol. 2004, 22, 3284–3292. [Google Scholar] [CrossRef]
- Yonemura, Y.; Canbay, E.; Ishibashi, H. Prognostic factors of peritoneal metastases from colorectal cancer following cytoreductive surgery and perioperative chemotherapy. Sci. World J. 2013, 2013, 978394. [Google Scholar] [CrossRef]
- Ihemelandu, C.; Sugarbaker, P.H. Management for Peritoneal Metastasis of Colonic Origin: Role of Cytoreductive Surgery and Perioperative Intraperitoneal Chemotherapy: A Single Institution’s Experience During Two Decades. Ann. Surg. Oncol. 2017, 24, 898–905. [Google Scholar] [CrossRef]
- Hall, B.; Padussis, J.; Foster, J.M. Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy in the Management of Colorectal Peritoneal Metastasis. Surg. Clin. N. Am. 2017, 97, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H.; Chang, D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann. Surg. Oncol. 1999, 6, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, V.; Tan, S.; Kong, J.; Pham, T.; Michael, M.; Ramsay, R.; Warrier, S.; Heriot, A. Prognostic factors influencing survival in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for isolated colorectal peritoneal metastases: A systematic review and meta-analysis. Color. Dis. 2020, 22, 1482–1495. [Google Scholar] [CrossRef] [PubMed]
- Cashin, P.H.; Dranichnikov, F.; Mahteme, H. Cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy treatment of colorectal peritoneal metastases: Cohort analysis of high volume disease and cure rate. J. Surg. Oncol. 2014, 110, 203–206. [Google Scholar] [CrossRef]
- Liberale, G.; Bourgeois, P.; Larsimont, D.; Moreau, M.; Donckier, V.; Ishizawa, T. Indocyanine green fluorescence-guided surgery after IV injection in metastatic colorectal cancer: A systematic review. Eur. J. Surg. Oncol. 2017, 43, 1656–1667. [Google Scholar] [CrossRef] [PubMed]
- Baiocchi, G.L.; Gheza, F.; Molfino, S.; Arru, L.; Vaira, M.; Giacopuzzi, S. Indocyanine green fluorescence-guided intraoperative detection of peritoneal carcinomatosis: Systematic review. BMC Surg. 2020, 20, 158. [Google Scholar] [CrossRef]
- Hernot, S.; van Manen, L.; Debie, P.; Mieog, J.S.D.; Vahrmeijer, A.L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019, 20, e354–e367. [Google Scholar] [CrossRef]
- Boogerd, L.S.F.; Hoogstins, C.E.S.; Schaap, D.P.; Kusters, M.; Handgraaf, H.J.M.; van der Valk, M.J.M.; Hilling, D.E.; Holman, F.A.; Peeters, K.; Mieog, J.S.D.; et al. Safety and effectiveness of SGM-101, a fluorescent antibody targeting carcinoembryonic antigen, for intraoperative detection of colorectal cancer: A dose-escalation pilot study. Lancet Gastroenterol. Hepatol. 2018, 3, 181–191. [Google Scholar] [CrossRef]
- Schaap, D.P.; de Valk, K.S.; Deken, M.M.; Meijer, R.P.J.; Burggraaf, J.; Vahrmeijer, A.L.; Kusters, M. Carcinoembryonic antigen-specific, fluorescent image-guided cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for metastatic colorectal cancer. Br. J. Surg. 2020, 107, 334–337. [Google Scholar] [CrossRef]
- de Gooyer, J.M.; Elekonawo, F.M.K.; Bremers, A.J.A.; Boerman, O.C.; Aarntzen, E.; de Reuver, P.R.; Nagtegaal, I.D.; Rijpkema, M.; de Wilt, J.H.W. Multimodal CEA-targeted fluorescence and radioguided cytoreductive surgery for peritoneal metastases of colorectal origin. Nat. Commun. 2022, 13, 2621. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Sugarbaker, P.H. Peritoneal-plasma barrier. Cancer Treat. Res. 1996, 82, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.D.; McPartland, S.; Detelich, D.; Saif, M.W. Chemotherapy for intraperitoneal use: A review of hyperthermic intraperitoneal chemotherapy and early post-operative intraperitoneal chemotherapy. J. Gastrointest. Oncol. 2016, 7, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Speyer, J.L.; Sugarbaker, P.H.; Collins, J.M.; Dedrick, R.L.; Klecker, R.W., Jr.; Myers, C.E. Portal levels and hepatic clearance of 5-fluorouracil after intraperitoneal administration in humans. Cancer Res. 1981, 41, 1916–1922. [Google Scholar] [PubMed]
- de Bree, E.; Michelakis, D.; Stamatiou, D.; Romanos, J.; Zoras, O. Pharmacological principles of intraperitoneal and bidirectional chemotherapy. Pleura Peritoneum 2017, 2, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Elias, D.; Benizri, E.; Di Pietrantonio, D.; Menegon, P.; Malka, D.; Raynard, B. Comparison of two kinds of intraperitoneal chemotherapy following complete cytoreductive surgery of colorectal peritoneal carcinomatosis. Ann. Surg. Oncol. 2007, 14, 509–514. [Google Scholar] [CrossRef]
- Elias, D.; Lefevre, J.H.; Chevalier, J.; Brouquet, A.; Marchal, F.; Classe, J.M.; Ferron, G.; Guilloit, J.M.; Meeus, P.; Goéré, D.; et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J. Clin. Oncol. 2009, 27, 681–685. [Google Scholar] [CrossRef]
- Franko, J.; Ibrahim, Z.; Gusani, N.J.; Holtzman, M.P.; Bartlett, D.L.; Zeh, H.J., 3rd. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer 2010, 116, 3756–3762. [Google Scholar] [CrossRef]
- Quenet, F.; Goéré, D.; Mehta, S.S.; Roca, L.; Dumont, F.; Hessissen, M.; Saint-Aubert, B.; Elias, D. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann. Surg. 2011, 254, 294–301. [Google Scholar] [CrossRef]
- Cashin, P.H.; Graf, W.; Nygren, P.; Mahteme, H. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis: Prognosis and treatment of recurrences in a cohort study. Eur. J. Surg. Oncol. 2012, 38, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Votanopoulos, K.I.; Swett, K.; Blackham, A.U.; Ihemelandu, C.; Shen, P.; Stewart, J.H.; Levine, E.A. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from rectal cancer. Ann. Surg. Oncol. 2013, 20, 1088–1092. [Google Scholar] [CrossRef]
- Navez, J.; Remue, C.; Leonard, D.; Bachmann, R.; Kartheuser, A.; Hubert, C.; Coubeau, L.; Komuta, M.; Van den Eynde, M.; Zech, F.; et al. Surgical Treatment of Colorectal Cancer with Peritoneal and Liver Metastases Using Combined Liver and Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Report from a Single-Centre Experience. Ann. Surg. Oncol. 2016, 23, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Ba, M.; Chen, C.; Long, H.; Gong, Y.; Wu, Y.; Lin, K.; Tu, Y.; Zhang, B.; Wu, W. Cytoreductive surgery and HIPEC for malignant ascites from colorectal cancer—A randomized study. Medicine 2020, 99, e21546. [Google Scholar] [CrossRef] [PubMed]
- Birgisson, H.; Enblad, M.; Artursson, S.; Ghanipour, L.; Cashin, P.; Graf, W. Patients with colorectal peritoneal metastases and high peritoneal cancer index may benefit from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2020, 46, 2283–2291. [Google Scholar] [CrossRef] [PubMed]
- Levine, E.A. The randomized trial of cytoreductive surgery with hyperthermic intraperitoneal chemoperfusion: What it does and does not tell us. Ann. Surg. Oncol. 2008, 15, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Bonnay, M.; Puizillou, J.M.; Antoun, S.; Demirdjian, S.; El, O.A.; Pignon, J.P.; Drouard-Troalen, L.; Ouellet, J.F.; Ducreux, M. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: Pharmacokinetics and tissue distribution. Ann. Oncol. 2002, 13, 267–272. [Google Scholar] [CrossRef]
- Elias, D.; Sideris, L.; Pocard, M.; Edè, C.; Ben Hassouna, D.; Ducreux, M.; Boige, V.; Côté, J.F.; Lasser, P. Efficacy of intraperitoneal chemohyperthermia with oxaliplatin in colorectal peritoneal carcinomatosis. Preliminary results in 24 patients. Ann. Oncol. 2004, 15, 781–785. [Google Scholar] [CrossRef]
- Ceelen, W. HIPEC with oxaliplatin for colorectal peritoneal metastasis: The end of the road? Eur. J. Surg. Oncol. 2019, 45, 400–402. [Google Scholar] [CrossRef]
- Klempner, S.J.; Ryan, D.P. HIPEC for colorectal peritoneal metastases. Lancet Oncol. 2021, 22, 162–164. [Google Scholar] [CrossRef]
- Baratti, D.; Kusamura, S.; Azmi, N.; Guaglio, M.; Montenovo, M.; Deraco, M. Colorectal Peritoneal Metastases Treated by Perioperative Systemic Chemotherapy and Cytoreductive Surgery With or Without Mitomycin C-Based HIPEC: A Comparative Study Using the Peritoneal Surface Disease Severity Score (PSDSS). Ann. Surg. Oncol. 2020, 27, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.; Serrano, A.; Manzanedo, I.; Pérez-Viejo, E.; González-Moreno, S.; González-Bayón, L.; Arjona-Sánchez, A.; Torres, J.; Ramos, I.; Barrios, M.E.; et al. GECOP-MMC: Phase IV randomized clinical trial to evaluate the efficacy of hyperthermic intraperitoneal chemotherapy (HIPEC) with mytomicin-C after complete surgical cytoreduction in patients with colon cancer peritoneal metastases. BMC Cancer 2022, 22, 536. [Google Scholar] [CrossRef] [PubMed]
- Charrier, T.; Passot, G.; Peron, J.; Maurice, C.; Gocevska, S.; Quénet, F.; Eveno, C.; Pocard, M.; Goere, D.; Elias, D.; et al. Cytoreductive Surgery Combined with Hyperthermic Intraperitoneal Chemotherapy with Oxaliplatin Increases the Risk of Postoperative Hemorrhagic Complications: Analysis of Predictive Factors. Ann. Surg. Oncol. 2016, 23, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- Spiegelberg, J.; Neeff, H.; Holzner, P.; Runkel, M.; Fichtner-Feigl, S.; Glatz, T. Comparison of hyperthermic intraperitoneal chemotherapy regimens for treatment of peritoneal-metastasized colorectal cancer. World J. Gastrointest. Oncol. 2020, 12, 903–917. [Google Scholar] [CrossRef] [PubMed]
- Delhorme, J.B.; Sauvinet, G.; Séverac, F.; Diab, S.; Liu, D.; Rohr, S.; Romain, B.; Brigand, C. Peritoneal Metastases of Colorectal Origin Treated with Complete Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy: The Efficiency of Mitomycin C. Ann. Surg. Oncol. 2022, 29, 7568–7576. [Google Scholar] [CrossRef]
- Zeng, L.; Liao, Q.; Zhao, Q.; Jiang, S.; Yang, X.; Tang, H.; He, Q.; Yang, X.; Fang, S.; He, J.; et al. Raltitrexed as a synergistic hyperthermia chemotherapy drug screened in patient-derived colorectal cancer organoids. Cancer Biol. Med. 2021, 18, 750–762. [Google Scholar] [CrossRef]
- Qiu, C.; Li, Y.; Liang, X.; Qi, Y.; Chen, Y.; Meng, X.; Zheng, H.; Xu, Y.; Cai, S.; Cai, G.; et al. A study of peritoneal metastatic xenograft model of colorectal cancer in the treatment of hyperthermic intraperitoneal chemotherapy with Raltitrexed. Biomed. Pharmacother. 2017, 92, 149–156. [Google Scholar] [CrossRef]
- Gong, Q.; Song, C.; Wang, X.; Wang, R.; Cai, G.; Liang, X.; Liu, J. Hyperthermic intraperitoneal chemotherapy with recombinant mutant human TNF-α and raltitrexed in mice with colorectal-peritoneal carcinomatosis. Exp. Biol. Med. 2020, 245, 542–551. [Google Scholar] [CrossRef]
- Sardi, A.; Jimenez, W.; Nieroda, C.; Sittig, M.; Shankar, S.; Gushchin, V. Melphalan: A promising agent in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2014, 21, 908–914. [Google Scholar] [CrossRef]
- Sipok, A.; Sardi, A.; Nieroda, C.; King, M.C.; Sittig, M.; Gushchin, V. Comparison of Survival in Patients with Isolated Peritoneal Carcinomatosis from Colorectal Cancer Treated with Cytoreduction and Melphalan or Mitomycin-C as Hyperthermic Intraperitoneal Chemotherapy Agent. Int. J. Surg. Oncol. 2018, 2018, 1920276. [Google Scholar] [CrossRef]
- Lemoine, L.; Thijssen, E.; Carleer, R.; Geboers, K.; Sugarbaker, P.; van der Speeten, K. Body surface area-based vs concentration-based perioperative intraperitoneal chemotherapy after optimal cytoreductive surgery in colorectal peritoneal surface malignancy treatment: COBOX trial. J. Surg. Oncol. 2019, 119, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Helderman, R.; Bokan, B.; van Bochove, G.G.W.; Rodermond, H.M.; Thijssen, E.; Marchal, W.; Torang, A.; Löke, D.R.; Franken, N.A.P.; Kok, H.P.; et al. Elevated temperatures and longer durations improve the efficacy of oxaliplatin- and mitomycin C-based hyperthermic intraperitoneal chemotherapy in a confirmed rat model for peritoneal metastasis of colorectal cancer origin. Front. Oncol. 2023, 13, 1122755. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.E.; Tiesi, G.; Razavi, R.; Wang, C.C. A phase I trial of thermal sensitization using induced oxidative stress in the context of HIPEC. Ann. Surg. Oncol. 2013, 20, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, S.; Padilla-Valverde, D.; Villarejo-Campos, P.; Martín-Fernández, J.; García-Rojo, M.; Rodríguez-Martínez, M. Experimental development of an intra-abdominal chemohyperthermia model using a closed abdomen technique and a PRS-1.0 Combat CO2 recirculation system. Surgery 2014, 155, 719–725. [Google Scholar] [CrossRef]
- Gómez-Sanz, R.; Ovejero-Merino, E.; Lasa-Unzúe, I.; López-García, A.; Marcos-Hernández, R.; Mínguez-García, J.; García-Moreno Nisa, F.; Mendoza-Moreno, F.; Díez-Alonso, M.; Ortega, M.A.; et al. Hyperthermic Intraperitoneal Chemotherapy and Recirculation with CO(2): A Safe Technique. J. Clin. Med. 2022, 11, 6152. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.C.V.; Kusamura, S.; Azmi, N.; Fumagalli, L.; Piccioni, F.; Valenza, F.; Baratti, D.; Guaglio, M.; Cavalleri, A.; Garrone, G.; et al. Hemodynamic and respiratory implications of high intra-abdominal pressure during HIPEC. Eur. J. Surg. Oncol. 2020, 46, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Kusamura, S.; Azmi, N.; Fumagalli, L.; Baratti, D.; Guaglio, M.; Cavalleri, A.; Garrone, G.; Battaglia, L.; Barretta, F.; Deraco, M. Phase II randomized study on tissue distribution and pharmacokinetics of cisplatin according to different levels of intra-abdominal pressure (IAP) during HIPEC (NCT02949791). Eur. J. Surg. Oncol. 2021, 47, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Feng, Q.; Zhang, J.; Zhou, H.; Jiang, Z.; Liu, Z.; Zheng, Z.; Chen, H.; Wang, Z.; Liang, J.; et al. High-grade postoperative complications affect survival outcomes of patients with colorectal Cancer peritoneal metastases treated with Cytoreductive surgery and Hyperthermic Intraperitoneal chemotherapy. BMC Cancer 2021, 21, 41. [Google Scholar] [CrossRef]
- Elias, D.; Viganò, L.; Orsi, F.; Scorsetti, M.; Comito, T.; Lerut, J.; Cosola, D.; Torzilli, G. New Perspectives in the Treatment of Colorectal Metastases. Liver Cancer 2016, 6, 90–98. [Google Scholar] [CrossRef]
- Assaf, D.; Mor, E.; Laks, S.; Zohar, N.; Benvenisti, H.; Hazzan, D.; Segev, L.; Akopyan, O.K.; Shacham-Shmueli, E.; Margalit, O.; et al. The pattern of peritoneal colorectal metastasis predicts survival after cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. Eur. J. Surg. Oncol. 2022, 48, 197–203. [Google Scholar] [CrossRef]
- Hentzen, J.; Rovers, K.P.; Kuipers, H.; van der Plas, W.Y.; Been, L.B.; Hoogwater, F.J.H.; van Ginkel, R.J.; Hemmer, P.H.J.; van Dam, G.M.; de Hingh, I.; et al. Impact of Synchronous Versus Metachronous Onset of Colorectal Peritoneal Metastases on Survival Outcomes After Cytoreductive Surgery (CRS) with Hyperthermic Intraperitoneal Chemotherapy (HIPEC): A Multicenter, Retrospective, Observational Study. Ann. Surg. Oncol. 2019, 26, 2210–2221. [Google Scholar] [CrossRef] [PubMed]
- Bong, T.S.H.; Tan, G.H.C.; Chia, C.; Soo, K.C.; Teo, M.C.C. Preoperative platelet-lymphocyte ratio is an independent prognostic marker and superior to carcinoembryonic antigen in colorectal peritoneal carcinomatosis patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int. J. Clin. Oncol. 2017, 22, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, A.; Rotolo, S.; Cintoni, M.; Rinninella, E.; Pulcini, G.; Schena, C.A.; Ferracci, F.; Grassi, F.; Raoul, P.; Moroni, R.; et al. The prognostic value of skeletal muscle index on clinical and survival outcomes after cytoreduction and HIPEC for peritoneal metastases from colorectal cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2022, 48, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Laks, S.; Schtrechman, G.; Adileh, M.; Ben-Yaacov, A.; Purim, O.; Ivanov, V.; Aderka, D.; Shacham-Shmueli, E.; Halpern, N.; Goren, S.; et al. Repeat Cytoreductive Surgery and Intraperitoneal Chemotherapy for Colorectal Cancer Peritoneal Recurrences is Safe and Efficacious. Ann. Surg. Oncol. 2021, 28, 5330–5338. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- Bijelic, L.; Yan, T.D.; Sugarbaker, P.H. Failure analysis of recurrent disease following complete cytoreduction and perioperative intraperitoneal chemotherapy in patients with peritoneal carcinomatosis from colorectal cancer. Ann. Surg. Oncol. 2007, 14, 2281–2288. [Google Scholar] [CrossRef]
- Chua, T.C.; Morris, D.L.; Esquivel, J. Impact of the peritoneal surface disease severity score on survival in patients with colorectal cancer peritoneal carcinomatosis undergoing complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2010, 17, 1330–1336. [Google Scholar] [CrossRef]
- Cavaliere, F.; De Simone, M.; Virzì, S.; Deraco, M.; Rossi, C.R.; Garofalo, A.; Di Filippo, F.; Giannarelli, D.; Vaira, M.; Valle, M.; et al. Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: Italian multicenter study S.I.T.I.L.O. Eur. J. Surg. Oncol. 2011, 37, 148–154. [Google Scholar] [CrossRef]
- Cashin, P.H.; Graf, W.; Nygren, P.; Mahteme, H. Intraoperative hyperthermic versus postoperative normothermic intraperitoneal chemotherapy for colonic peritoneal carcinomatosis: A case-control study. Ann. Oncol. 2012, 23, 647–652. [Google Scholar] [CrossRef]
- Kuijpers, A.M.; Mirck, B.; Aalbers, A.G.; Nienhuijs, S.W.; de Hingh, I.H.; Wiezer, M.J.; van Ramshorst, B.; van Ginkel, R.J.; Havenga, K.; Bremers, A.J.; et al. Cytoreduction and HIPEC in the Netherlands: Nationwide long-term outcome following the Dutch protocol. Ann. Surg. Oncol. 2013, 20, 4224–4230. [Google Scholar] [CrossRef]
- Huang, C.Q.; Feng, J.P.; Yang, X.J.; Li, Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from colorectal cancer: A case-control study from a Chinese center. J. Surg. Oncol. 2014, 109, 730–739. [Google Scholar] [CrossRef] [PubMed]
- van Oudheusden, T.R.; Braam, H.J.; Nienhuijs, S.W.; Wiezer, M.J.; van Ramshorst, B.; Luyer, M.D.; Lemmens, V.E.; de Hingh, I.H. Cytoreduction and hyperthermic intraperitoneal chemotherapy: A feasible and effective option for colorectal cancer patients after emergency surgery in the presence of peritoneal carcinomatosis. Ann. Surg. Oncol. 2014, 21, 2621–2626. [Google Scholar] [CrossRef] [PubMed]
- Baratti, D.; Kusamura, S.; Iusco, D.; Bonomi, S.; Grassi, A.; Virzì, S.; Leo, E.; Deraco, M. Postoperative complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy affect long-term outcome of patients with peritoneal metastases from colorectal cancer: A two-center study of 101 patients. Dis. Colon. Rectum 2014, 57, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Razenberg, L.G.; van Gestel, Y.R.; Creemers, G.J.; Verwaal, V.J.; Lemmens, V.E.; de Hingh, I.H. Trends in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of synchronous peritoneal carcinomatosis of colorectal origin in the Netherlands. Eur. J. Surg. Oncol. 2015, 41, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Simkens, G.A.; van Oudheusden, T.R.; Luyer, M.D.; Nienhuijs, S.W.; Nieuwenhuijzen, G.A.; Rutten, H.J.; de Hingh, I.H. Serious Postoperative Complications Affect Early Recurrence After Cytoreductive Surgery and HIPEC for Colorectal Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2015, 22, 2656–2662. [Google Scholar] [CrossRef]
- Teo, M.C.; Ching Tan, G.H.; Lim, C.; Chia, C.S.; Tham, C.K.; Soo, K.C. Colorectal peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: The experience of a tertiary Asian center. Asian J. Surg. 2015, 38, 65–73. [Google Scholar] [CrossRef]
- Passot, G.; Vaudoyer, D.; Villeneuve, L.; Kepenekian, V.; Beaujard, A.C.; Bakrin, N.; Cotte, E.; Gilly, F.N.; Glehen, O. What made hyperthermic intraperitoneal chemotherapy an effective curative treatment for peritoneal surface malignancy: A 25-year experience with 1,125 procedures. J. Surg. Oncol. 2016, 113, 796–803. [Google Scholar] [CrossRef]
- Schneider, M.A.; Eshmuminov, D.; Lehmann, K. Major Postoperative Complications Are a Risk Factor for Impaired Survival after CRS/HIPEC. Ann. Surg. Oncol. 2017, 24, 2224–2232. [Google Scholar] [CrossRef]
- Baratti, D.; Kusamura, S.; Iusco, D.; Cotsoglou, C.; Guaglio, M.; Battaglia, L.; Virzì, S.; Mazzaferro, V.; Deraco, M. Should a History of Extraperitoneal Disease Be a Contraindication to Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Cancer Peritoneal Metastases? Dis. Colon. Rectum 2018, 61, 1026–1034. [Google Scholar] [CrossRef]
- Tonello, M.; Ortega-Perez, G.; Alonso-Casado, O.; Torres-Mesa, P.; Guiñez, G.; Gonzalez-Moreno, S. Peritoneal carcinomatosis arising from rectal or colonic adenocarcinoma treated with cytoreductive surgery (CRS) hyperthermic intraperitoneal chemotherapy (HIPEC): Two different diseases. Clin. Transl. Oncol. 2018, 20, 1268–1273. [Google Scholar] [CrossRef]
- Burnett, A.; Lecompte, M.A.; Trabulsi, N.; Dubé, P.; Gervais, M.K.; Trilling, B.; Cloutier, A.S.; Sideris, L. Peritoneal carcinomatosis index predicts survival in colorectal patients undergoing HIPEC using oxaliplatin: A retrospective single-arm cohort study. World J. Surg. Oncol. 2019, 17, 83. [Google Scholar] [CrossRef]
- Narasimhan, V.; Das, A.; Warrier, S.; Lynch, C.; McCormick, J.; Tie, J.; Michael, M.; Ramsay, R.; Heriot, A. Evaluation of cytoreductive surgery and HIPEC for peritoneal surface malignancies: Analysis of 384 consecutive cases. Langenbecks Arch. Surg. 2019, 404, 527–539. [Google Scholar] [CrossRef]
- Larentzakis, A.; O'Dwyer, S.T.; Becker, J.; Shuweihdi, F.; Aziz, O.; Selvasekar, C.R.; Fulford, P.; Renehan, A.G.; Wilson, M. Referral pathways and outcome of patients with colorectal peritoneal metastasis (CRPM). Eur. J. Surg. Oncol. 2019, 45, 2310–2315. [Google Scholar] [CrossRef]
- Solaini, L.; D'Acapito, F.; Passardi, A.; Framarini, M.; Tauceri, F.; Di Pietrantonio, D.; Frassineti, G.L.; Casadei Gardini, A.; Cucchetti, A.; Cavaliere, D.; et al. Cytoreduction plus hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis in colorectal cancer patients: A single-center cohort study. World J. Surg. Oncol. 2019, 17, 58. [Google Scholar] [CrossRef]
- Solomon, D.; DeNicola, N.L.; Feferman, Y.; Bekhor, E.; Reppucci, M.L.; Feingold, D.; Aycart, S.N.; Magge, D.R.; Golas, B.J.; Labow, D.M.; et al. More Synchronous Peritoneal Disease but Longer Survival in Younger Patients with Carcinomatosis from Colorectal Cancer Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2019, 26, 845–851. [Google Scholar] [CrossRef]
- Wong, J.S.M.; Tan, G.H.C.; Chia, C.S.; Ong, J.; Ng, W.Y.; Teo, M.C.C. The importance of synchronicity in the management of colorectal peritoneal metastases with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J. Surg. Oncol. 2020, 18, 10. [Google Scholar] [CrossRef]
- Manzanedo, I.; Pereira, F.; Cascales-Campos, P.; Muñoz-Casares, C.; Asensio, E.; Torres-Melero, J.; Prada-Villaverde, A.; Caravaca-García, I.; Gutiérrez-Calvo, A.; Vaqué, J.; et al. Treatment of Peritoneal Surface Malignancies by Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Spain: Results of the National Registry of the Spanish Group of Peritoneal Oncologic Surgery (REGECOP). J. Clin. Med. 2023, 12, 3774. [Google Scholar] [CrossRef]
- Pazdur, R.; Lassere, Y.; Soh, L.T.; Ajani, J.A.; Bready, B.; Soo, E.; Sugarman, S.; Patt, Y.; Abbruzzese, J.L.; Levin, B. Phase II trial of docetaxel (Taxotere) in metastatic colorectal carcinoma. Ann. Oncol. 1994, 5, 468–470. [Google Scholar] [CrossRef]
- Murono, K.; Nagata, H.; Ishimaru, K.; Emoto, S.; Kaneko, M.; Hiyoshi, M.; Sasaki, K.; Otani, K.; Shuno, Y.; Nishikawa, T.; et al. Safety of intraperitoneal paclitaxel combined with conventional chemotherapy for colorectal cancer with peritoneal carcinomatosis: A phase I trial. Cancer Chemother. Pharmacol. 2019, 83, 145–150. [Google Scholar] [CrossRef]
- Murono, K.; Nozawa, H.; Nagata, H.; Ishimaru, K.; Sonoda, H.; Emoto, S.; Kaneko, M.; Sasaki, K.; Otani, K.; Kawai, K.; et al. Efficacy of intraperitoneally administered paclitaxel for colorectal cancer with peritoneal metastases. Int. J. Color. Dis. 2020, 35, 1945–1949. [Google Scholar] [CrossRef]
- Park, S.Y.; Choi, G.S.; Park, J.S.; Kim, H.J.; Yang, C.S.; Kim, J.G.; Kang, B.W. Efficacy of Early Postoperative Intraperitoneal Chemotherapy After Complete Surgical Resection of Peritoneal Metastasis from Colorectal Cancer: A Case-Control Study from a Single Center. Ann. Surg. Oncol. 2016, 23, 2266–2273. [Google Scholar] [CrossRef] [PubMed]
- Cashin, P.H.; Mahteme, H.; Spång, N.; Syk, I.; Frödin, J.E.; Torkzad, M.; Glimelius, B.; Graf, W. Cytoreductive surgery and intraperitoneal chemotherapy versus systemic chemotherapy for colorectal peritoneal metastases: A randomised trial. Eur. J. Cancer 2016, 53, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.H.; Ong, W.S.; Chia, C.S.; Tham, C.K.; Soo, K.C.; Teo, M.C. Does early post-operative intraperitoneal chemotherapy (EPIC) for patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) make a difference? Int. J. Hyperth. 2016, 32, 281–288. [Google Scholar] [CrossRef]
- Huang, Y.; Alzahrani, N.A.; Liauw, W.; Soudy, H.; Alzahrani, A.M.; Morris, D.L. Early postoperative intraperitoneal chemotherapy is associated with survival benefit for appendiceal adenocarcinoma with peritoneal dissemination. Eur. J. Surg. Oncol. 2017, 43, 2292–2298. [Google Scholar] [CrossRef] [PubMed]
- Soucisse, M.L.; Liauw, W.; Hicks, G.; Morris, D.L. Early postoperative intraperitoneal chemotherapy for lower gastrointestinal neoplasms with peritoneal metastasis: A systematic review and critical analysis. Pleura Peritoneum 2019, 4, 20190007. [Google Scholar] [CrossRef] [PubMed]
- Dakwar, G.R.; Shariati, M.; Willaert, W.; Ceelen, W.; De Smedt, S.C.; Remaut, K. Nanomedicine-based intraperitoneal therapy for the treatment of peritoneal carcinomatosis—Mission possible? Adv. Drug Deliv. Rev. 2017, 108, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Alyami, M.; Hübner, M.; Grass, F.; Bakrin, N.; Villeneuve, L.; Laplace, N.; Passot, G.; Glehen, O.; Kepenekian, V. Pressurised intraperitoneal aerosol chemotherapy: Rationale, evidence, and potential indications. Lancet Oncol. 2019, 20, e368–e377. [Google Scholar] [CrossRef]
- Raoof, M.; Dellinger, T. ASO Author Reflections: Defining the Role of PIPAC in the Treatment of Peritoneal Metastases. Ann. Surg. Oncol. 2022, 29, 186–187. [Google Scholar] [CrossRef]
- Dumont, F.; Passot, C.; Raoul, J.L.; Kepenekian, V.; Lelièvre, B.; Boisdron-Celle, M.; Hiret, S.; Senellart, H.; Pein, F.; Blanc-Lapierre, A.; et al. A phase I dose-escalation study of oxaliplatin delivered via a laparoscopic approach using pressurised intraperitoneal aerosol chemotherapy for advanced peritoneal metastases of gastrointestinal tract cancers. Eur. J. Cancer 2020, 140, 37–44. [Google Scholar] [CrossRef]
- Kim, G.; Tan, H.L.; Sundar, R.; Lieske, B.; Chee, C.E.; Ho, J.; Shabbir, A.; Babak, M.V.; Ang, W.H.; Goh, B.C.; et al. PIPAC-OX: A Phase I Study of Oxaliplatin-Based Pressurized Intraperitoneal Aerosol Chemotherapy in Patients with Peritoneal Metastases. Clin. Cancer Res. 2021, 27, 1875–1881. [Google Scholar] [CrossRef]
- de Jong, L.A.W.; van Erp, N.P.; Bijelic, L. Pressurized Intraperitoneal Aerosol Chemotherapy: The Road from Promise to Proof. Clin. Cancer Res. 2021, 27, 1830–1832. [Google Scholar] [CrossRef]
- Graversen, M.; Detlefsen, S.; Bjerregaard, J.K.; Fristrup, C.W.; Pfeiffer, P.; Mortensen, M.B. Prospective, single-center implementation and response evaluation of pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastasis. Ther. Adv. Med. Oncol. 2018, 10, 1758835918777036. [Google Scholar] [CrossRef]
- Sgarbura, O.; Hübner, M.; Alyami, M.; Eveno, C.; Gagnière, J.; Pache, B.; Pocard, M.; Bakrin, N.; Quénet, F. Oxaliplatin use in pressurized intraperitoneal aerosol chemotherapy (PIPAC) is safe and effective: A multicenter study. Eur. J. Surg. Oncol. 2019, 45, 2386–2391. [Google Scholar] [CrossRef]
- Ellebæk, S.B.; Graversen, M.; Detlefsen, S.; Lundell, L.; Fristrup, C.W.; Pfeiffer, P.; Mortensen, M.B. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC)-directed treatment of peritoneal metastasis in end-stage colo-rectal cancer patients. Pleura Peritoneum 2020, 5, 20200109. [Google Scholar] [CrossRef]
- Alyami, M.; Mercier, F.; Siebert, M.; Bonnot, P.E.; Laplace, N.; Villeneuve, L.; Passot, G.; Glehen, O.; Bakrin, N.; Kepenekian, V. Unresectable peritoneal metastasis treated by pressurized intraperitoneal aerosol chemotherapy (PIPAC) leading to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2021, 47, 128–133. [Google Scholar] [CrossRef]
- Ploug, M.; Graversen, M.; Pfeiffer, P.; Mortensen, M.B. Bidirectional treatment of peritoneal metastasis with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) and systemic chemotherapy: A systematic review. BMC Cancer 2020, 20, 105. [Google Scholar] [CrossRef]
- De Simone, M.; Vaira, M.; Argenziano, M.; Berchialla, P.; Pisacane, A.; Cinquegrana, A.; Cavalli, R.; Borsano, A.; Robella, M. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with Oxaliplatin, Cisplatin, and Doxorubicin in Patients with Peritoneal Carcinomatosis: An Open-Label, Single-Arm, Phase II Clinical Trial. Biomedicines 2020, 8, 102. [Google Scholar] [CrossRef]
- Taibi, A.; Sgarbura, O.; Hübner, M.; Bardet, S.M.; Alyami, M.; Bakrin, N.; Durand Fontanier, S.; Eveno, C.; Gagniere, J.; Pache, B.; et al. Feasibility and Safety of Oxaliplatin-Based Pressurized Intraperitoneal Aerosol Chemotherapy With or Without Intraoperative Intravenous 5-Fluorouracil and Leucovorin for Colorectal Peritoneal Metastases: A Multicenter Comparative Cohort Study. Ann. Surg. Oncol. 2022, 29, 5243–5251. [Google Scholar] [CrossRef]
- Siebert, M.; Alyami, M.; Mercier, F.; Gallice, C.; Villeneuve, L.; Laplace, N.; Passot, G.; Bakrin, N.; Glehen, O.; Kepenekian, V. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) in association with systemic chemotherapy and bevacizumab, evaluation of safety and feasibility. A single center comparative study. Eur. J. Surg. Oncol. 2021, 47, 139–142. [Google Scholar] [CrossRef]
- Willaert, W.; Van de Sande, L.; Van Daele, E.; Van De Putte, D.; Van Nieuwenhove, Y.; Pattyn, P.; Ceelen, W. Safety and preliminary efficacy of electrostatic precipitation during pressurized intraperitoneal aerosol chemotherapy (PIPAC) for unresectable carcinomatosis. Eur. J. Surg. Oncol. 2019, 45, 2302–2309. [Google Scholar] [CrossRef]
- Graversen, M.; Detlefsen, S.; Ellebaek, S.B.; Fristrup, C.; Pfeiffer, P.; Mortensen, M.B. Pressurized IntraPeritoneal Aerosol Chemotherapy with one minute of electrostatic precipitation (ePIPAC) is feasible, but the histological tumor response in peritoneal metastasis is insufficient. Eur. J. Surg. Oncol. 2020, 46, 155–159. [Google Scholar] [CrossRef]
- Kreitman, R.J. Recombinant toxins for the treatment of cancer. Curr. Opin. Mol. Ther. 2003, 5, 44–51. [Google Scholar]
- Andersson, Y.; Engebraaten, O.; Fodstad, Ø. Synergistic anti-cancer effects of immunotoxin and cyclosporin in vitro and in vivo. Br. J. Cancer 2009, 101, 1307–1315. [Google Scholar] [CrossRef]
- Thorgersen, E.B.; Asvall, J.; Frøysnes, I.S.; Schjalm, C.; Larsen, S.G.; Dueland, S.; Andersson, Y.; Fodstad, Ø.; Mollnes, T.E.; Flatmark, K. Increased Local Inflammatory Response to MOC31PE Immunotoxin After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2021, 28, 5252–5262. [Google Scholar] [CrossRef]
- Frøysnes, I.S.; Andersson, Y.; Larsen, S.G.; Davidson, B.; Øien, J.T.; Olsen, K.H.; Giercksky, K.E.; Julsrud, L.; Fodstad, Ø.; Dueland, S.; et al. Novel Treatment with Intraperitoneal MOC31PE Immunotoxin in Colorectal Peritoneal Metastasis: Results From the ImmunoPeCa Phase 1 Trial. Ann. Surg. Oncol. 2017, 24, 1916–1922. [Google Scholar] [CrossRef]
- Frøysnes, I.S.; Andersson, Y.; Larsen, S.G.; Davidson, B.; Øien, J.T.; Julsrud, L.; Fodstad, Ø.; Dueland, S.; Flatmark, K. ImmunoPeCa trial: Long-term outcome following intraperitoneal MOC31PE immunotoxin treatment in colorectal peritoneal metastasis. Eur. J. Surg. Oncol. 2021, 47, 134–138. [Google Scholar] [CrossRef]
- Seimetz, D.; Lindhofer, H.; Bokemeyer, C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3) as a targeted cancer immunotherapy. Cancer Treat. Rev. 2010, 36, 458–467. [Google Scholar] [CrossRef]
- Ströhlein, M.A.; Lordick, F.; Rüttinger, D.; Grützner, K.U.; Schemanski, O.C.; Jäger, M.; Lindhofer, H.; Hennig, M.; Jauch, K.W.; Peschel, C.; et al. Immunotherapy of peritoneal carcinomatosis with the antibody catumaxomab in colon, gastric, or pancreatic cancer: An open-label, multicenter, phase I/II trial. Onkologie 2011, 34, 101–108. [Google Scholar] [CrossRef]
- Knödler, M.; Körfer, J.; Kunzmann, V.; Trojan, J.; Daum, S.; Schenk, M.; Kullmann, F.; Schroll, S.; Behringer, D.; Stahl, M.; et al. Randomised phase II trial to investigate catumaxomab (anti-EpCAM × anti-CD3) for treatment of peritoneal carcinomatosis in patients with gastric cancer. Br. J. Cancer 2018, 119, 296–302. [Google Scholar] [CrossRef]
- Lee, S.J.; Yang, H.; Kim, W.R.; Lee, Y.S.; Lee, W.S.; Kong, S.J.; Lee, H.J.; Kim, J.H.; Cheon, J.; Kang, B.; et al. STING activation normalizes the intraperitoneal vascular-immune microenvironment and suppresses peritoneal carcinomatosis of colon cancer. J. Immunother. Cancer 2021, 9, e002195. [Google Scholar] [CrossRef]
- Miller, A.M.; Lemke-Miltner, C.D.; Blackwell, S.; Tomanek-Chalkley, A.; Gibson-Corely, K.N.; Coleman, K.L.; Weiner, G.J.; Chan, C.H.F. Intraperitoneal CMP-001: A Novel Immunotherapy for Treating Peritoneal Carcinomatosis of Gastrointestinal and Pancreaticobiliary Cancer. Ann. Surg. Oncol. 2021, 28, 1187–1197. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, H.; Li, Y.; Jayakumar, P.; Liao, H.; Huang, H.; Billiar, T.R.; Deng, M. Intraperitoneal injection of class A TLR9 agonist enhances anti-PD-1 immunotherapy in colorectal peritoneal metastases. JCI Insight 2022, 7, e160063. [Google Scholar] [CrossRef]
- Liu, Z.; Ravindranathan, R.; Kalinski, P.; Guo, Z.S.; Bartlett, D.L. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat. Commun. 2017, 8, 14754. [Google Scholar] [CrossRef] [PubMed]
- Chon, H.J.; Lee, W.S.; Yang, H.; Kong, S.J.; Lee, N.K.; Moon, E.S.; Choi, J.; Han, E.C.; Kim, J.H.; Ahn, J.B.; et al. Tumor Microenvironment Remodeling by Intratumoral Oncolytic Vaccinia Virus Enhances the Efficacy of Immune-Checkpoint Blockade. Clin. Cancer Res. 2019, 25, 1612–1623. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lee, W.S.; Kim, C.W.; Lee, S.J.; Yang, H.; Kong, S.J.; Ning, J.; Yang, K.M.; Kang, B.; Kim, W.R.; et al. Oncolytic vaccinia virus reinvigorates peritoneal immunity and cooperates with immune checkpoint inhibitor to suppress peritoneal carcinomatosis in colon cancer. J. Immunother. Cancer 2020, 8, e000857. [Google Scholar] [CrossRef] [PubMed]
- Tate, S.J.; Van de Sande, L.; Ceelen, W.P.; Torkington, J.; Parker, A.L. The Feasibility of Pressurised Intraperitoneal Aerosolised Virotherapy (PIPAV) to Administer Oncolytic Adenoviruses. Pharmaceutics 2021, 13, 2043. [Google Scholar] [CrossRef] [PubMed]
- Alkayyal, A.A.; Tai, L.H.; Kennedy, M.A.; de Souza, C.T.; Zhang, J.; Lefebvre, C.; Sahi, S.; Ananth, A.A.; Mahmoud, A.B.; Makrigiannis, A.P.; et al. NK-Cell Recruitment Is Necessary for Eradication of Peritoneal Carcinomatosis with an IL12-Expressing Maraba Virus Cellular Vaccine. Cancer Immunol. Res. 2017, 5, 211–221. [Google Scholar] [CrossRef]
- Lauer, U.M.; Schell, M.; Beil, J.; Berchtold, S.; Koppenhöfer, U.; Glatzle, J.; Königsrainer, A.; Möhle, R.; Nann, D.; Fend, F.; et al. Phase I Study of Oncolytic Vaccinia Virus GL-ONC1 in Patients with Peritoneal Carcinomatosis. Clin. Cancer Res. 2018, 24, 4388–4398. [Google Scholar] [CrossRef]
- Katz, S.C.; Point, G.R.; Cunetta, M.; Thorn, M.; Guha, P.; Espat, N.J.; Boutros, C.; Hanna, N.; Junghans, R.P. Regional CAR-T cell infusions for peritoneal carcinomatosis are superior to systemic delivery. Cancer Gene Ther. 2016, 23, 142–148. [Google Scholar] [CrossRef]
- Xiao, L.; Cen, D.; Gan, H.; Sun, Y.; Huang, N.; Xiong, H.; Jin, Q.; Su, L.; Liu, X.; Wang, K.; et al. Adoptive Transfer of NKG2D CAR mRNA-Engineered Natural Killer Cells in Colorectal Cancer Patients. Mol. Ther. 2019, 27, 1114–1125. [Google Scholar] [CrossRef]
- Kumagai, Y.; Futoh, Y.; Miyato, H.; Ohzawa, H.; Yamaguchi, H.; Saito, S.; Kurashina, K.; Hosoya, Y.; Lefor, A.K.; Sata, N.; et al. Effect of Systemic or Intraperitoneal Administration of Anti-PD-1 Antibody for Peritoneal Metastases from Gastric Cancer. In Vivo 2022, 36, 1126–1135. [Google Scholar] [CrossRef]
- Si, X.; Ji, G.; Ma, S.; Xu, Y.; Zhao, J.; Huang, Z.; Zhang, Y.; Song, W.; Tang, Z. Biodegradable Implants Combined with Immunogenic Chemotherapy and Immune Checkpoint Therapy for Peritoneal Metastatic Carcinoma Postoperative Treatment. ACS Biomater. Sci. Eng. 2020, 6, 5281–5289. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Ji, G.; Ma, S.; Chen, H.; Shi, Z.; Zhang, Y.; Tang, Z.; Song, W.; Chen, X. Comprehensive evaluation of biopolymer immune implants for peritoneal metastasis carcinoma therapy. J. Control. Release 2023, 353, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.; Luetkens, T.; Gog, C.; Killing, B.; Arnold, D.; Hinke, A.; Stahl, M.; Freier, W.; Rüssel, J.; Atanackovic, D.; et al. Intraperitoneal bevacizumab for control of malignant ascites due to advanced-stage gastrointestinal cancers: A multicentre double-blind, placebo-controlled phase II study—AIO SUP-0108. Eur. J. Cancer 2016, 63, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, N.; Maebayashi, T.; Hata, M.; Okada, M. The role of palliative radiation therapy in treating pleural or peritoneal disseminated tumors: 22 cases and a review of the literature. Ann. Palliat. Med. 2020, 9, 2586–2591. [Google Scholar] [CrossRef] [PubMed]
- Chandler, C.S.; Bell, M.M.; Chung, S.K.; Veach, D.R.; Fung, E.K.; Punzalan, B.; Burnes Vargas, D.; Patel, M.; Xu, H.; Guo, H.F.; et al. Intraperitoneal Pretargeted Radioimmunotherapy for Colorectal Peritoneal Carcinomatosis. Mol. Cancer Ther. 2022, 21, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Rondon, A.; Schmitt, S.; Briat, A.; Ty, N.; Maigne, L.; Quintana, M.; Membreno, R.; Zeglis, B.M.; Navarro-Teulon, I.; Pouget, J.P.; et al. Pretargeted radioimmunotherapy and SPECT imaging of peritoneal carcinomatosis using bioorthogonal click chemistry: Probe selection and first proof-of-concept. Theranostics 2019, 9, 6706–6718. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, C.; Senge, M.O.; Arnaut, L.G.; Gomes-da-Silva, L.C. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 188308. [Google Scholar] [CrossRef]
- Gu, B.; Wang, B.; Li, X.; Feng, Z.; Ma, C.; Gao, L.; Yu, Y.; Zhang, J.; Zheng, P.; Wang, Y.; et al. Photodynamic therapy improves the clinical efficacy of advanced colorectal cancer and recruits immune cells into the tumor immune microenvironment. Front. Immunol. 2022, 13, 1050421. [Google Scholar] [CrossRef]
- Almerie, M.Q.; Gossedge, G.; Wright, K.E.; Jayne, D.G. Treatment of peritoneal carcinomatosis with photodynamic therapy: Systematic review of current evidence. Photodiagn. Photodyn. Ther. 2017, 20, 276–286. [Google Scholar] [CrossRef]
- Pinto, A.; Pocard, M. Photodynamic therapy and photothermal therapy for the treatment of peritoneal metastasis: A systematic review. Pleura Peritoneum 2018, 3, 20180124. [Google Scholar] [CrossRef] [PubMed]
- Ubink, I.; Bolhaqueiro, A.C.F.; Elias, S.G.; Raats, D.A.E.; Constantinides, A.; Peters, N.A.; Wassenaar, E.C.E.; de Hingh, I.; Rovers, K.P.; van Grevenstein, W.M.U.; et al. Organoids from colorectal peritoneal metastases as a platform for improving hyperthermic intraperitoneal chemotherapy. Br. J. Surg. 2019, 106, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, S.D.; Sasikumar, S.; Moaven, O.; Sivakumar, H.; Shen, P.; Levine, E.A.; Soker, S.; Skardal, A.; Votanopoulos, K.I. Personalized Identification of Optimal HIPEC Perfusion Protocol in Patient-Derived Tumor Organoid Platform. Ann. Surg. Oncol. 2020, 27, 4950–4960. [Google Scholar] [CrossRef]
- Leick, K.M.; Kazarian, A.G.; Rajput, M.; Tomanek-Chalkley, A.; Miller, A.; Shrader, H.R.; McCarthy, A.; Coleman, K.L.; Kasi, P.M.; Chan, C.H.F. Peritoneal Cell-Free Tumor DNA as Biomarker for Peritoneal Surface Malignancies. Ann. Surg. Oncol. 2020, 27, 5065–5071. [Google Scholar] [CrossRef]
- López-Rojo, I.; Olmedillas-López, S.; Villarejo Campos, P.; Domínguez Prieto, V.; Barambio Buendía, J.; Cortés Guiral, D.; García-Arranz, M.; García-Olmo, D. Liquid biopsy in peritoneal fluid and plasma as a prognostic factor in advanced colorectal and appendiceal tumors after complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Ther. Adv. Med. Oncol. 2020, 12, 1758835920981351. [Google Scholar] [CrossRef] [PubMed]
- Van't Erve, I.; Rovers, K.P.; Constantinides, A.; Bolhuis, K.; Wassenaar, E.C.; Lurvink, R.J.; Huysentruyt, C.J.; Snaebjornsson, P.; Boerma, D.; van den Broek, D.; et al. Detection of tumor-derived cell-free DNA from colorectal cancer peritoneal metastases in plasma and peritoneal fluid. J. Pathol. Clin. Res. 2021, 7, 203–208. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, W.; Liu, D.; Qin, Q.; Grady, W.M.; Fichera, A.; Wang, H.; Hou, T.; Lv, X.; Li, C.; et al. Peritoneal cell-free DNA as a sensitive biomarker for detection of peritoneal metastasis in colorectal cancer: A prospective diagnostic study: A prospective diagnostic study. Clin. Epigenet. 2023, 15, 65. [Google Scholar] [CrossRef]
- Fan, R.; Tong, A.; Li, X.; Gao, X.; Mei, L.; Zhou, L.; Zhang, X.; You, C.; Guo, G. Enhanced antitumor effects by docetaxel/LL37-loaded thermosensitive hydrogel nanoparticles in peritoneal carcinomatosis of colorectal cancer. Int. J. Nanomed. 2015, 10, 7291–7305. [Google Scholar] [CrossRef]
- Xu, S.; Fan, H.; Yin, L.; Zhang, J.; Dong, A.; Deng, L.; Tang, H. Thermosensitive hydrogel system assembled by PTX-loaded copolymer nanoparticles for sustained intraperitoneal chemotherapy of peritoneal carcinomatosis. Eur. J. Pharm. Biopharm. 2016, 104, 251–259. [Google Scholar] [CrossRef]
- Hyldbakk, A.; Fleten, K.G.; Snipstad, S.; Åslund, A.K.O.; Davies, C.L.; Flatmark, K.; Mørch, Y. Intraperitoneal administration of cabazitaxel-loaded nanoparticles in peritoneal metastasis models. Nanomedicine 2023, 48, 102656. [Google Scholar] [CrossRef]
- Fleten, K.G.; Hyldbakk, A.; Einen, C.; Benjakul, S.; Strand, B.L.; Davies, C.L.; Mørch, Ý.; Flatmark, K. Alginate Microsphere Encapsulation of Drug-Loaded Nanoparticles: A Novel Strategy for Intraperitoneal Drug Delivery. Mar. Drugs 2022, 20, 744. [Google Scholar] [CrossRef]
- Göhler, D.; Große, S.; Bellendorf, A.; Falkenstein, T.A.; Ouaissi, M.; Zieren, J.; Stintz, M.; Giger-Pabst, U. Hyperthermic intracavitary nanoaerosol therapy (HINAT) as an improved approach for pressurised intraperitoneal aerosol chemotherapy (PIPAC): Technical description, experimental validation and first proof of concept. Beilstein J. Nanotechnol. 2017, 8, 2729–2740. [Google Scholar] [CrossRef]
- Minnaert, A.K.; Dakwar, G.R.; Benito, J.M.; García Fernández, J.M.; Ceelen, W.; De Smedt, S.C.; Remaut, K. High-Pressure Nebulization as Application Route for the Peritoneal Administration of siRNA Complexes. Macromol. Biosci. 2017, 17, 1700024. [Google Scholar] [CrossRef]
- Shariati, M.; Zhang, H.; Van de Sande, L.; Descamps, B.; Vanhove, C.; Willaert, W.; Ceelen, W.; De Smedt, S.C.; Remaut, K. High Pressure Nebulization (PIPAC) Versus Injection for the Intraperitoneal Administration of mRNA Complexes. Pharm. Res. 2019, 36, 126. [Google Scholar] [CrossRef]
- Yuan, Z.; Fan, G.; Wu, H.; Liu, C.; Zhan, Y.; Qiu, Y.; Shou, C.; Gao, F.; Zhang, J.; Yin, P.; et al. Photodynamic therapy synergizes with PD-L1 checkpoint blockade for immunotherapy of CRC by multifunctional nanoparticles. Mol. Ther. 2021, 29, 2931–2948. [Google Scholar] [CrossRef]
- Abuzar, S.M.; Park, E.J.; Seo, Y.; Lee, J.; Baik, S.H.; Hwang, S.J. Preparation and Evaluation of Intraperitoneal Long-Acting Oxaliplatin-Loaded Multi-Vesicular Liposomal Depot for Colorectal Cancer Treatment. Pharmaceutics 2020, 12, 736. [Google Scholar] [CrossRef]
- Larsen, S.G.; Graf, W.; Mariathasan, A.B.; Sørensen, O.; Spasojevic, M.; Goscinski, M.A.; Selboe, S.; Lundstrøm, N.; Holtermann, A.; Revheim, M.E.; et al. First experience with (224)Radium-labeled microparticles (Radspherin®) after CRS-HIPEC for peritoneal metastasis in colorectal cancer (a phase 1 study). Front. Med. 2023, 10, 1070362. [Google Scholar] [CrossRef]
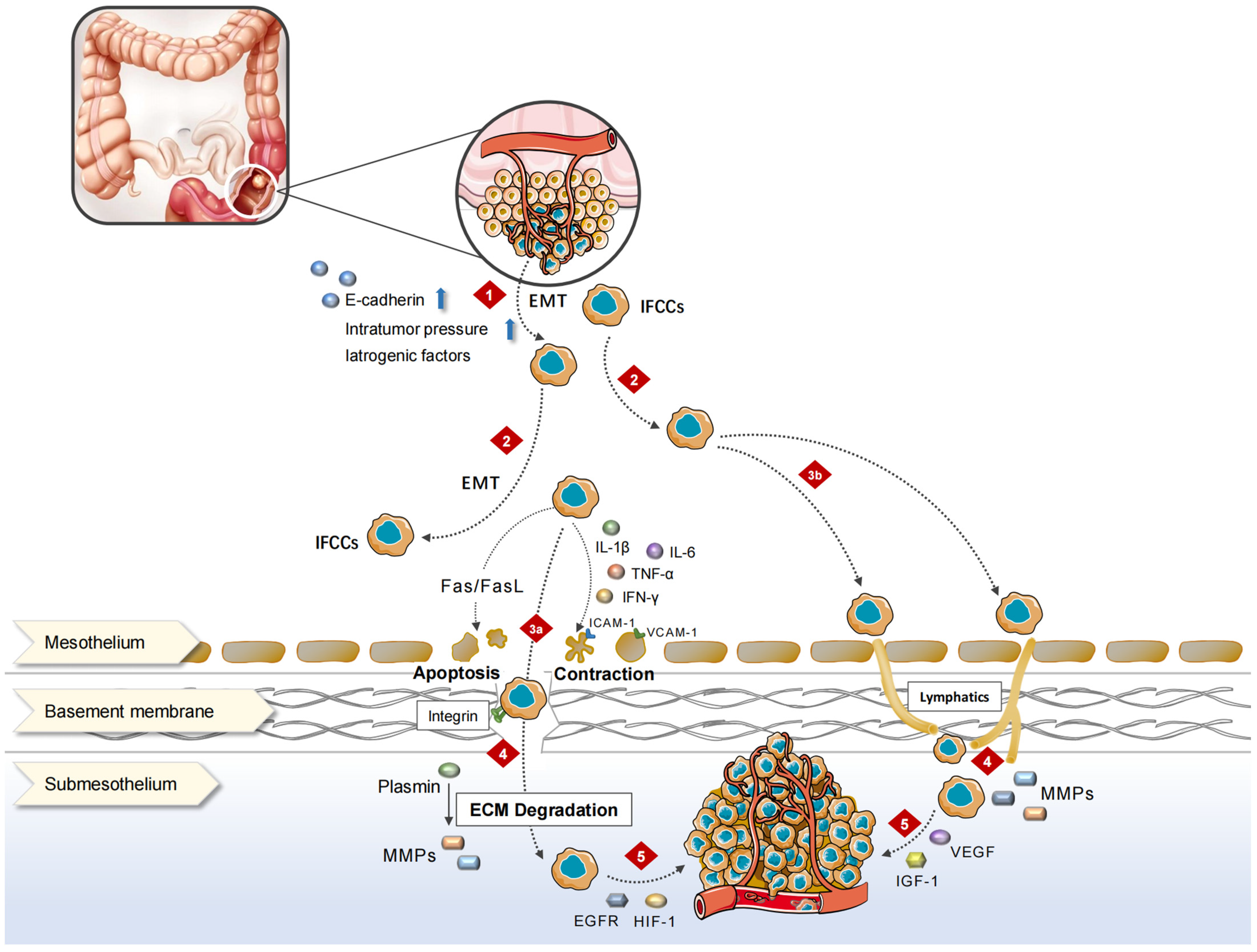
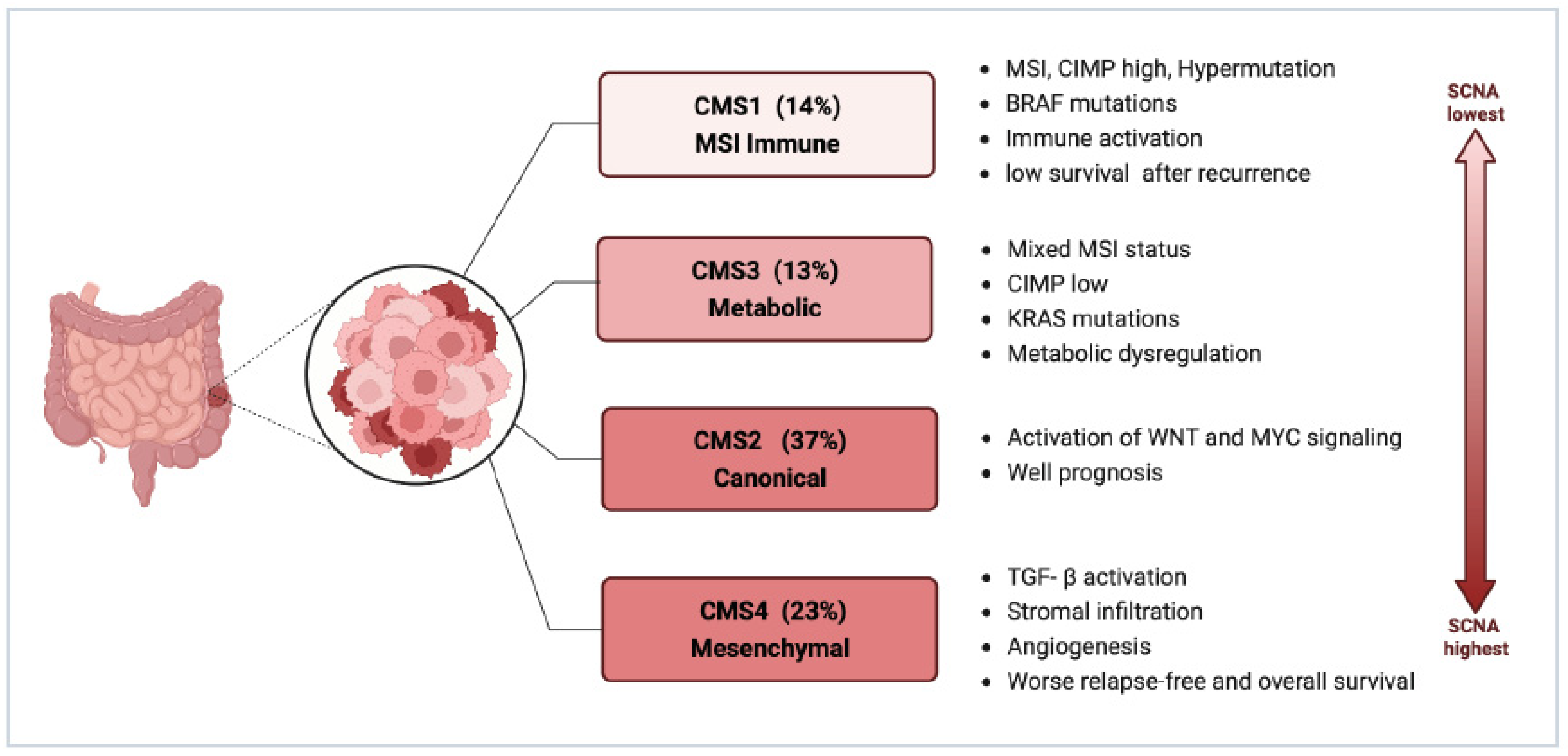
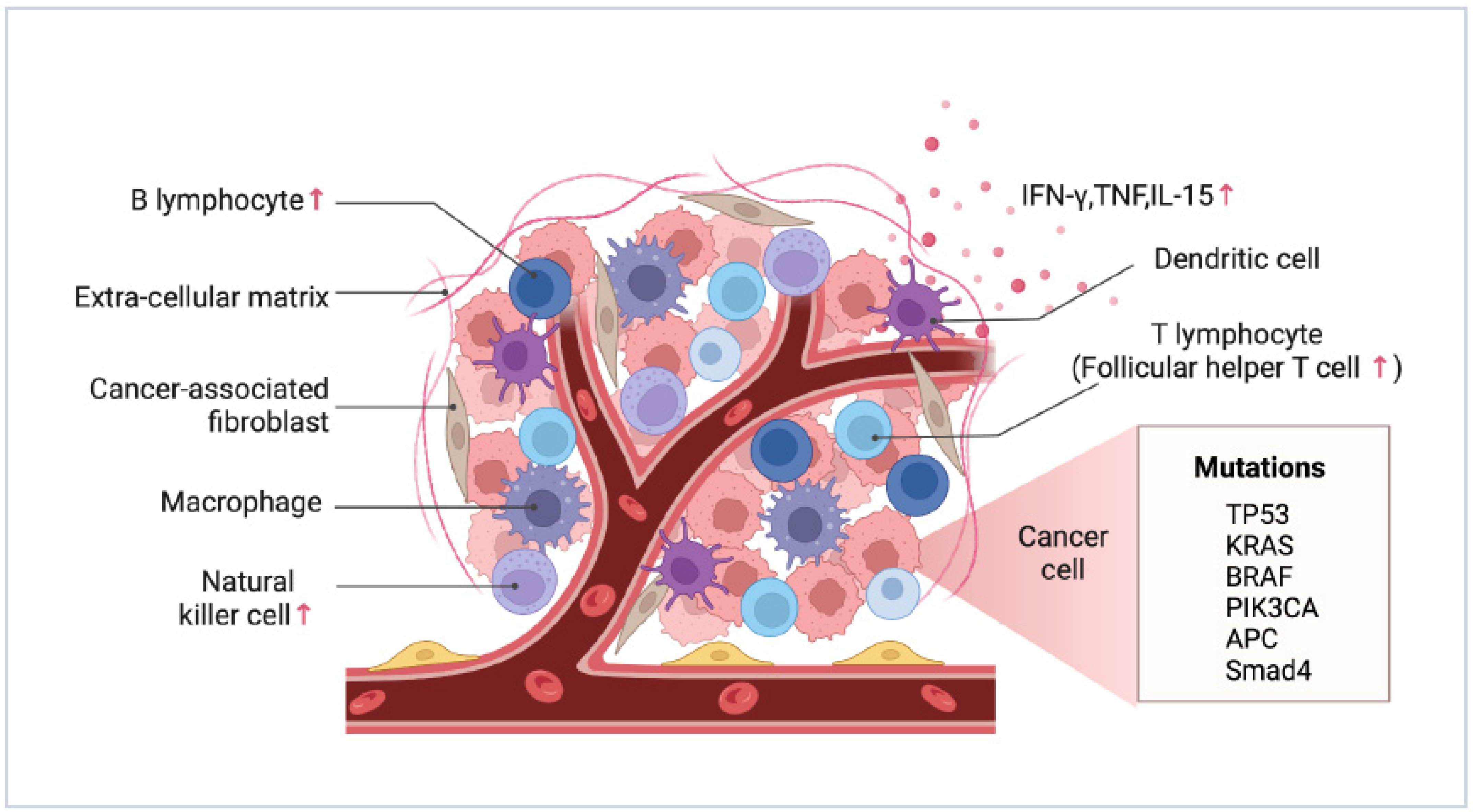
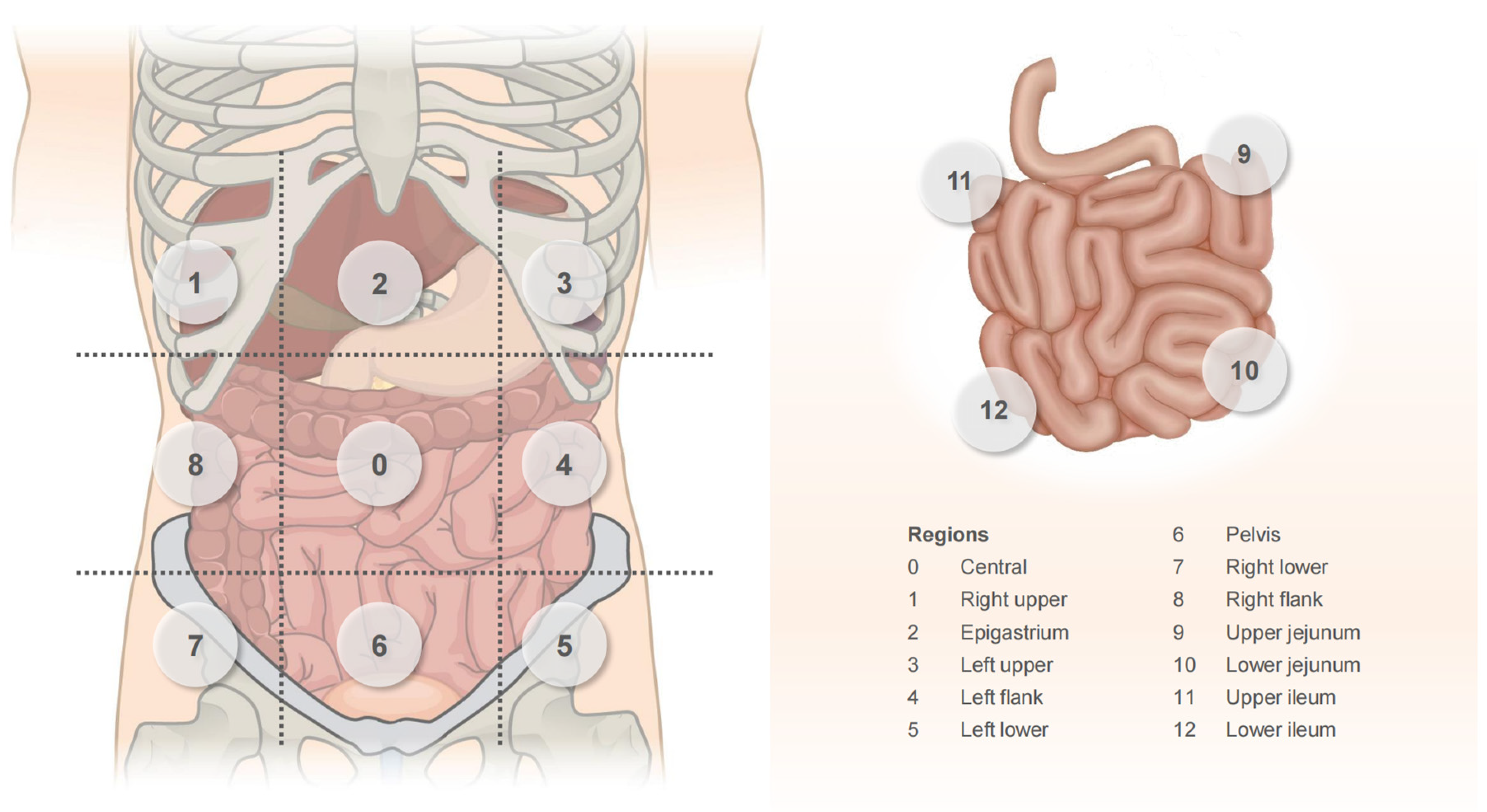
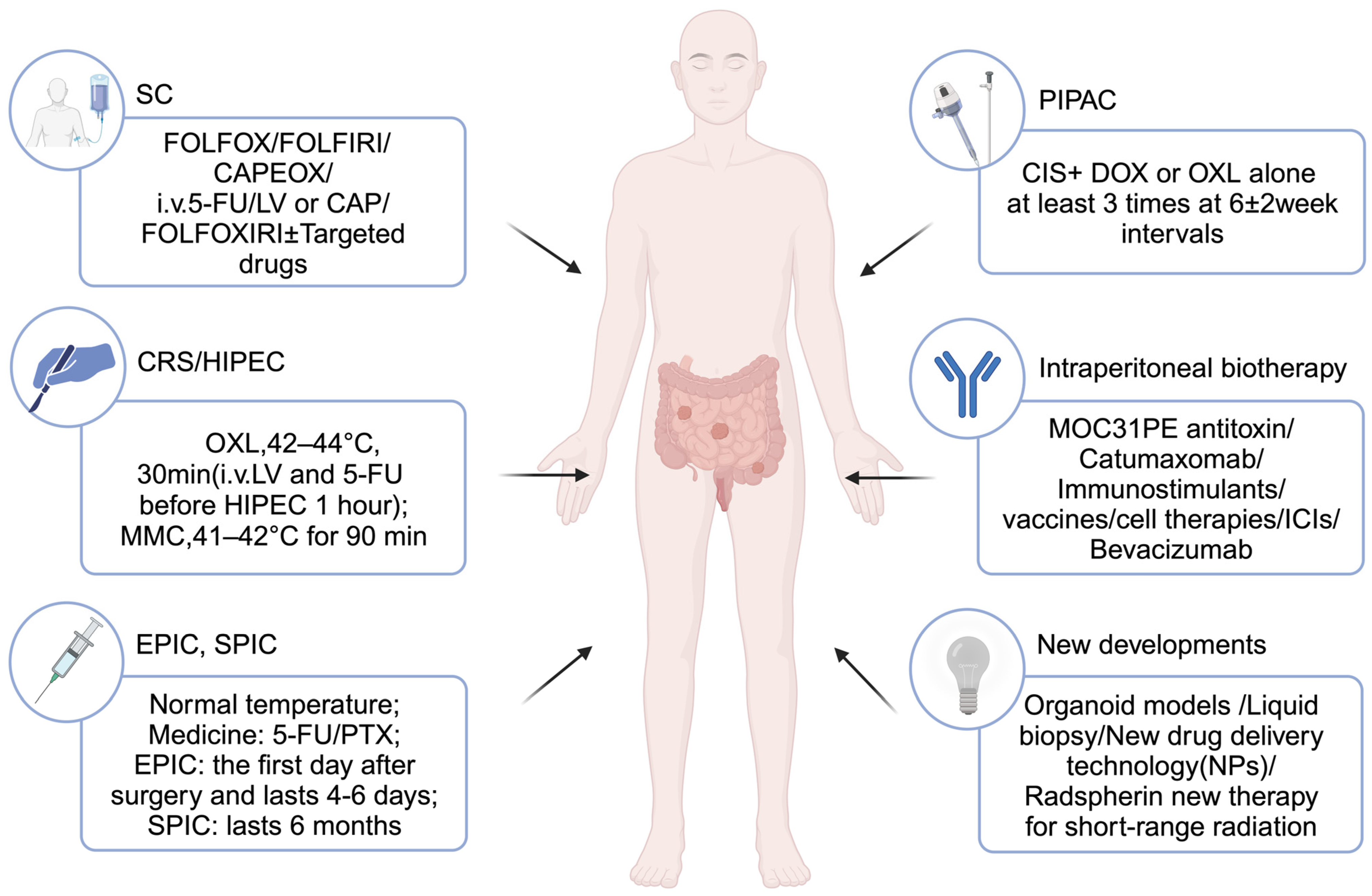
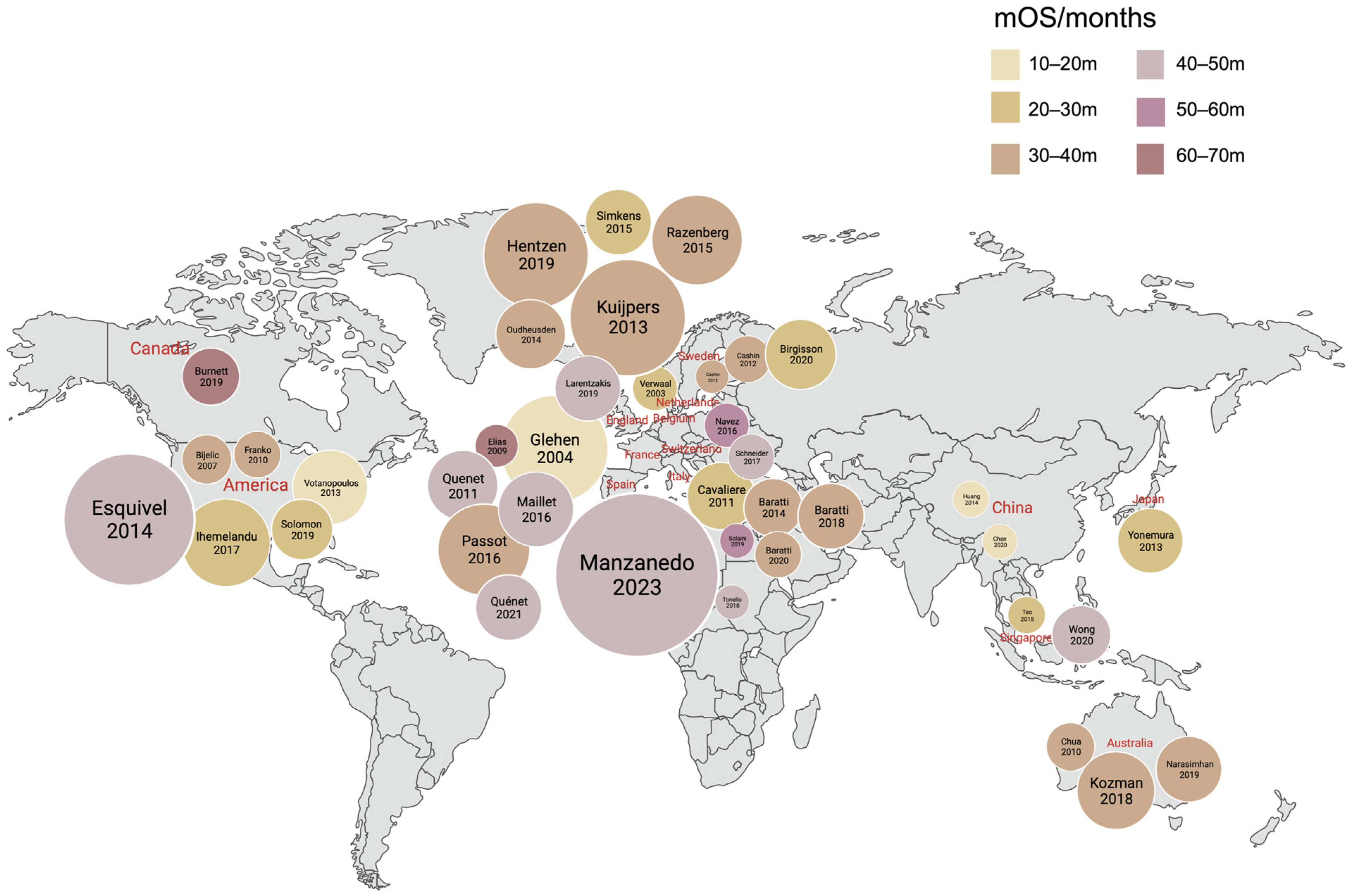
| Evaluation Indicators | Score |
|---|---|
| Clinical symptoms | |
| None | 0 |
| Mild Weight loss < 10%, mild abdominal pain, some ascites | 1 |
| Severe Weight loss > 10%, persistent abdominal pain, bowel obstruction, symptomatic ascites | 6 |
| PCI score | |
| <10 | 1 |
| 10~20 | 3 |
| >20 | 7 |
| Histopathological features | |
| G1, G2, N−, L−, V− | 1 |
| G2, N+ and/or L+ and/or V+ | 3 |
| G3, Signet ring | 9 |
| Evaluation Indicators | Score |
|---|---|
| PCI score | |
| 1–10 | 0 |
| 11–20 | 2 |
| 21–30 | 4 |
| G stage | |
| G1 | 0 |
| G2 | 0 |
| G3 | 2 |
| N stage | |
| N0 | 0 |
| N1 | 2 |
| N2 | 3 |
| RAS/BRAF status | |
| Wild type | 0 |
| NRAS mutation | 0 |
| KRAS mutation | 1 |
| BRAF mutation | 3 |
| Grade | Diameter of Residual Tumor |
|---|---|
| CC0 | No residual tumor |
| CC1 | <0.25 cm |
| CC2 | 0.25~2.5 cm |
| CC3 | >2.5 cm |
| Grade | Residual Tumor Visible to the Naked Eye | Microscopic Margins |
|---|---|---|
| R0 | no | negative |
| R1 | no | positive |
| R2 | yes | |
| R2a | residual lesion < 5 mm | |
| R2b | 5 mm < residual lesion < 2 cm | |
| R2c | residual lesion > 2 cm |
| Author, Year, Region | Type | Treatment Arms (Number of Points) | HIPEC Regimen | Overall Survival (Months or Rate) | Morbidity (%) | Mortality (%) | References |
|---|---|---|---|---|---|---|---|
| Verwaal, 2003/2008, Netherlands | RCT | CRS + HIPEC (n = 54) vs. SC (n = 51) | Open, 41–42 °C, 90 min, MMC 35 mg/m2 | 22.3 m vs. 12.6 m (p = 0.032) | NR | 8 | [8,107] |
| Elias, 2007, France | Cohort | CRS + HIPEC (n = 23) vs. CRS + EPIC (n = 23) | Open, 43 °C, 30 min, OXL, 460 mg/m2 | 5 y: 54% vs. 28% (p = 0.22) | 47.8 vs.56.5 | 0 vs. 8.7 | [167] |
| Elias, 2009, France | Cohort | CRS + HIPEC + SC (n = 48) vs. SC (n = 48) | Open, 43 °C, 30 min, OXL 460 mg/m2 | 62.7 m vs. 23.9 m (p < 0.05) 2 y: 81.0% vs. 65.0% (p < 0.05) 5 y: 51.0% vs. 13.0% (p < 0.05) | NR | NR | [168] |
| Franko, 2010, America | Cohort | CRS + HIPEC (n = 67) vs. SC (n = 38) | Close, 42 °C, 100 min, MMC 40 mg/3L | 34.7 m vs.16.8 m (p < 0.001) | NR | NR | [169] |
| Quenet, 2011, France | Cohort | CRS + HIPEC (OXL + IRI, n = 103 vs. OXL, n = 43) | Open, 43 °C, 30 min, OXL 300–460 ± IRI 200 mg/m2 | 47.0 m vs. 40.83 m (p = 0.94) | 52.4 vs. 34.9 (p = 0.05) | 4.9 vs.2.3 (p = 0.67) | [170] |
| Cashin, 2012, Sweden | Cohort | CRS + HIPEC (n = 69) vs. CRS + SPIC (n = 57) | Open, 41–42 °C, 30–90 min, MMC 30/OXL 360–460 ± IRI 360 mg/m2 | 34 m vs. 25 m (p = 0.047) | 40.6 vs. 29.8 (p = 0.02) | 4.3 vs. 3.5 (p = 0.98) | [171] |
| Votanopoulos, 2013, America | Cohort | CRS + HIPEC (RC-PM, n = 13 vs. CC-PM, n = 204) | NR | 14.6 m vs. 17.3 m 3 y: 28.2% vs. 25.1% (p = 0.644) | 51 vs. 46 (p = 0.78) | 5.7 vs. 0 (p = 1.0) | [172] |
| Goéré, 2015, France | Cohort | CRS + HIPEC (n = 139) vs. SC (n = 41) | OXL ± IRI | 3 y: 52% vs. 7% (p < 0.0001) | 44.6 vs. 12.2 (p = 0.003) | 5.8 vs. 4.9 (p > 0.999) | [93] |
| Navez, 2016, Belgium | Cohort | CRS + HIPEC (n = 52) vs. CRS + HIPEC + LS (n = 25) | Close, 41–42 °C, 30–90 min, MMC 35/OXL 460 mg/m2 | 59.2 m vs. 27.5 m (p = 0.06) | 15.4 vs. 32.0 (p = 0.15) | 0 vs. 4.0 (p = 0.21) | [173] |
| Klaver, 2019, Netherlands | RCT | Adjuvant HIPEC+ SC (n = 102) vs. SC (n = 102) | Close/Open, 42–43 °C, 30 min, OXL 460 mg/m2 | 93% vs.94.1% (p = 0.82) | 14 (HIPEC group) | NR | [112] |
| Chen, 2020, China | RCT | CRS + HIPEC (n = 14) vs. HIPEC + dCRS (n = 14) | Close, 41–42 °C, 90 min OXL 125/RTX 3/5-FU 175 mg/m2 | 14.5 m vs. 14.3 m (p > 0.05) | 21.4 | NR | [174] |
| Birgisson, 2020, Sweden | Cohort | CRS + HIPEC (PCI ≤ 20, n = 112 vs. PCI > 20, n = 45) vs. open-close/debulking (n = 44) | Close, 42 °C, 30 min, OXL 460 mg/m2 | 33 m vs. 20 m vs. 9 m (p < 0.001) | 77 vs. 58 vs. 91 (p = 0.08) | 1 vs. 4 vs. 30 (p < 0.01) | [175] |
| Beal, 2020, America | Cohort | NAC + CRS + HIPEC (n = 196) vs. CRS + HIPEC (n = 102) | OXL/MMC, 30–120 min | 32.7 m vs. 22.0 m (p = 0.044) | 22.4 vs. 16.7 (p = 0.650) | 1.5 vs. 2.9 (p = 0.411) | [119] |
| Quénet, 2021, France | RCT | CRS + HIPEC (n = 133) vs. CRS (n = 132) | Close/Open, 43 °C OXL 360–460 mg/m2, 30 min | 41.7 m vs. 41.2 m (p = 0.99) | 60 d: 26 vs. 15 (p = 0.035) | 59 vs. 61 | [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, W.; Geng, Y.; Hu, W. Peritoneal Metastasis: A Dilemma and Challenge in the Treatment of Metastatic Colorectal Cancer. Cancers 2023, 15, 5641. https://doi.org/10.3390/cancers15235641
Xia W, Geng Y, Hu W. Peritoneal Metastasis: A Dilemma and Challenge in the Treatment of Metastatic Colorectal Cancer. Cancers. 2023; 15(23):5641. https://doi.org/10.3390/cancers15235641
Chicago/Turabian StyleXia, Wei, Yiting Geng, and Wenwei Hu. 2023. "Peritoneal Metastasis: A Dilemma and Challenge in the Treatment of Metastatic Colorectal Cancer" Cancers 15, no. 23: 5641. https://doi.org/10.3390/cancers15235641
APA StyleXia, W., Geng, Y., & Hu, W. (2023). Peritoneal Metastasis: A Dilemma and Challenge in the Treatment of Metastatic Colorectal Cancer. Cancers, 15(23), 5641. https://doi.org/10.3390/cancers15235641





