Development and Validation of a Nomogram to Predict Overall Survival in Stage I–III Colorectal Cancer Patients after Radical Resection with Normal Preoperative Serum Carcinoembryonic Antigen
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
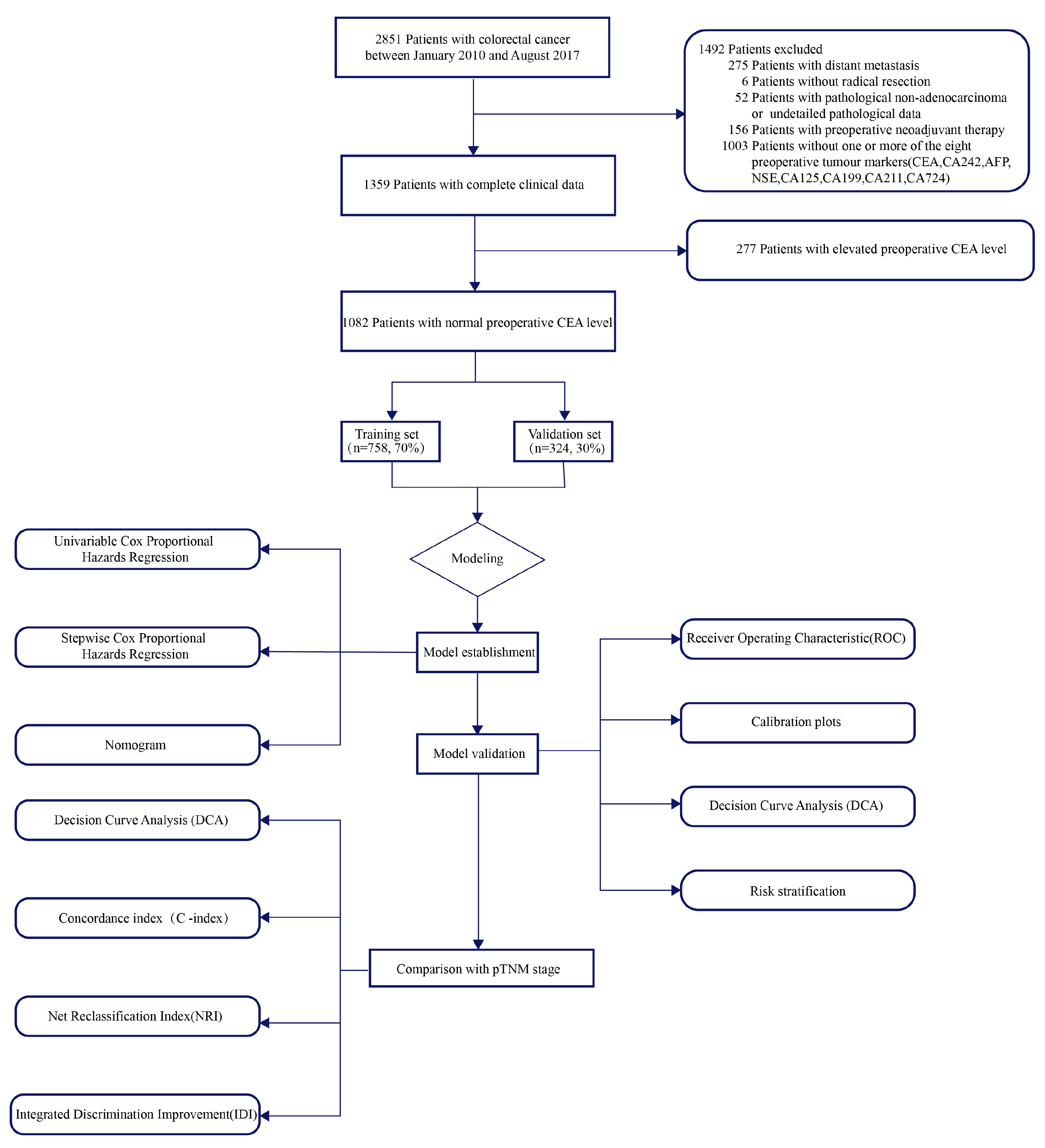
2.1. Research Population
2.2. The Detection of Tumor Markers
2.3. Follow-Up Study
2.4. Data Analysis
3. Results
3.1. Clinicopathologic Characteristics and Comparison of OS Based on Preoperative Tumor Markers
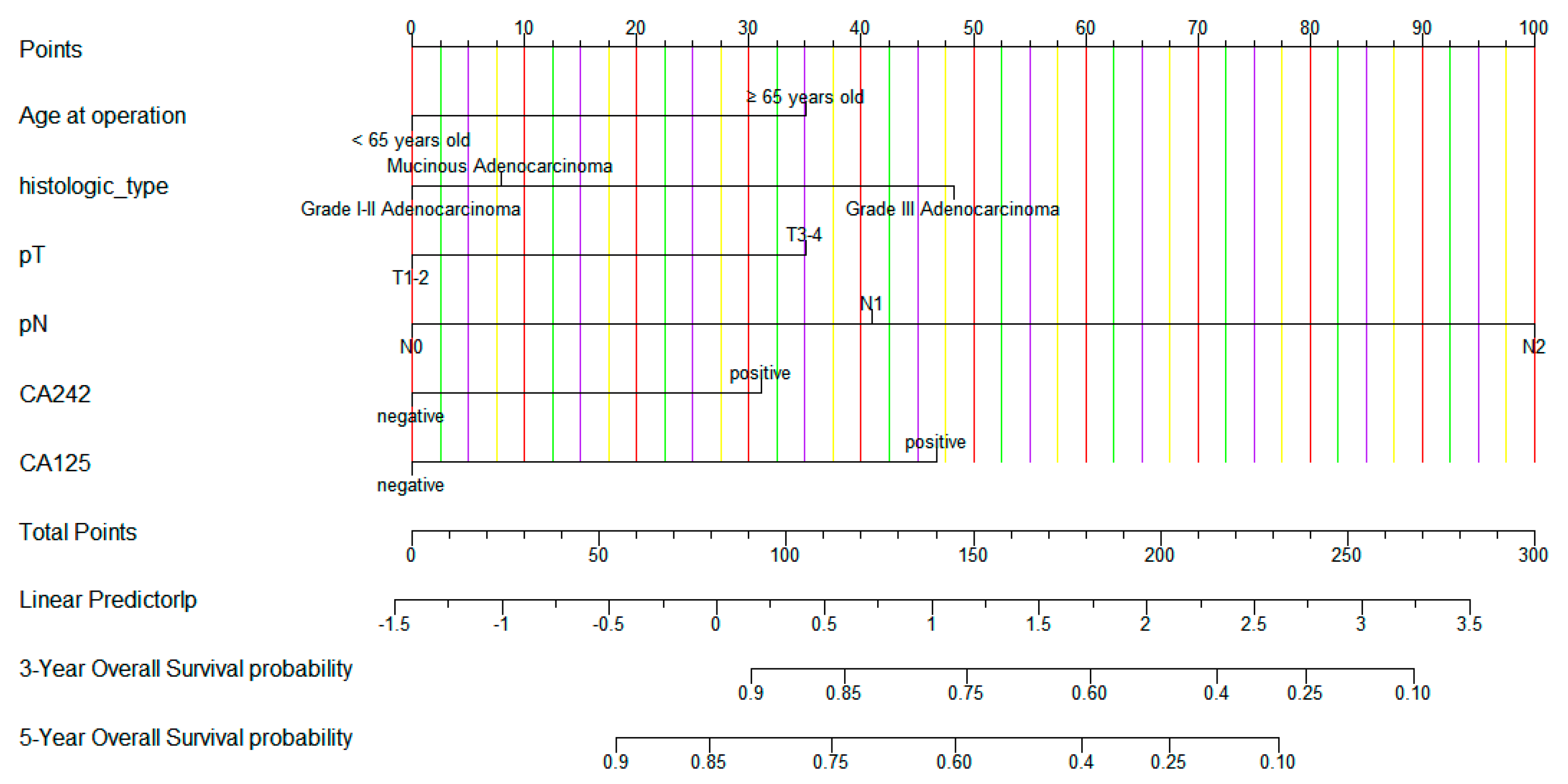
3.2. Nomogram Variables Screening
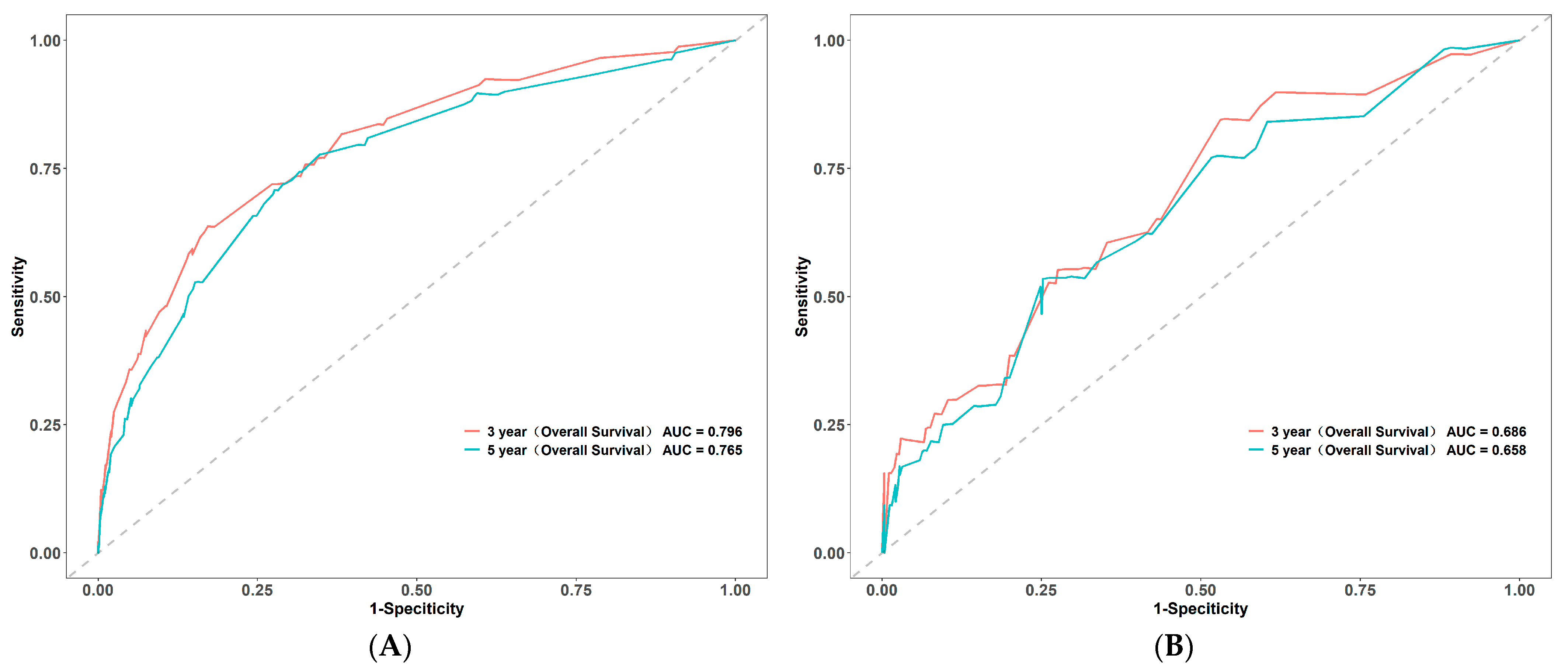
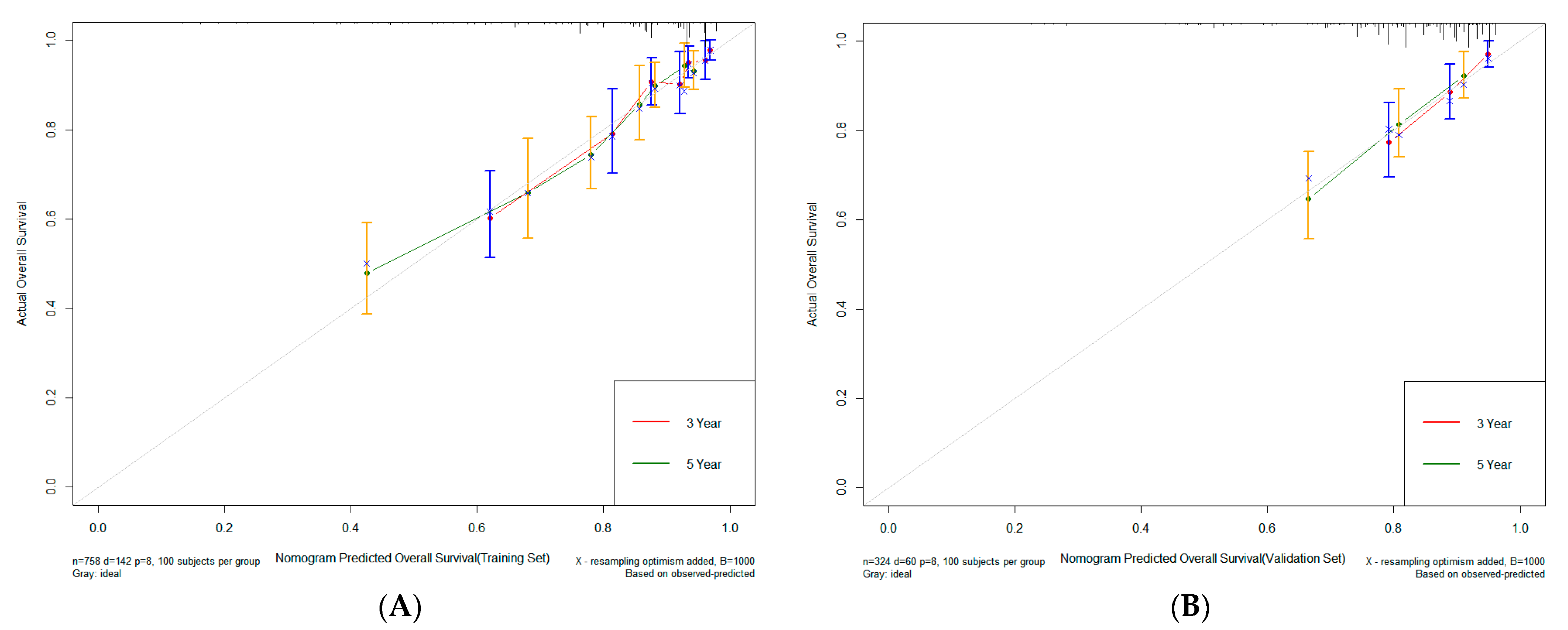
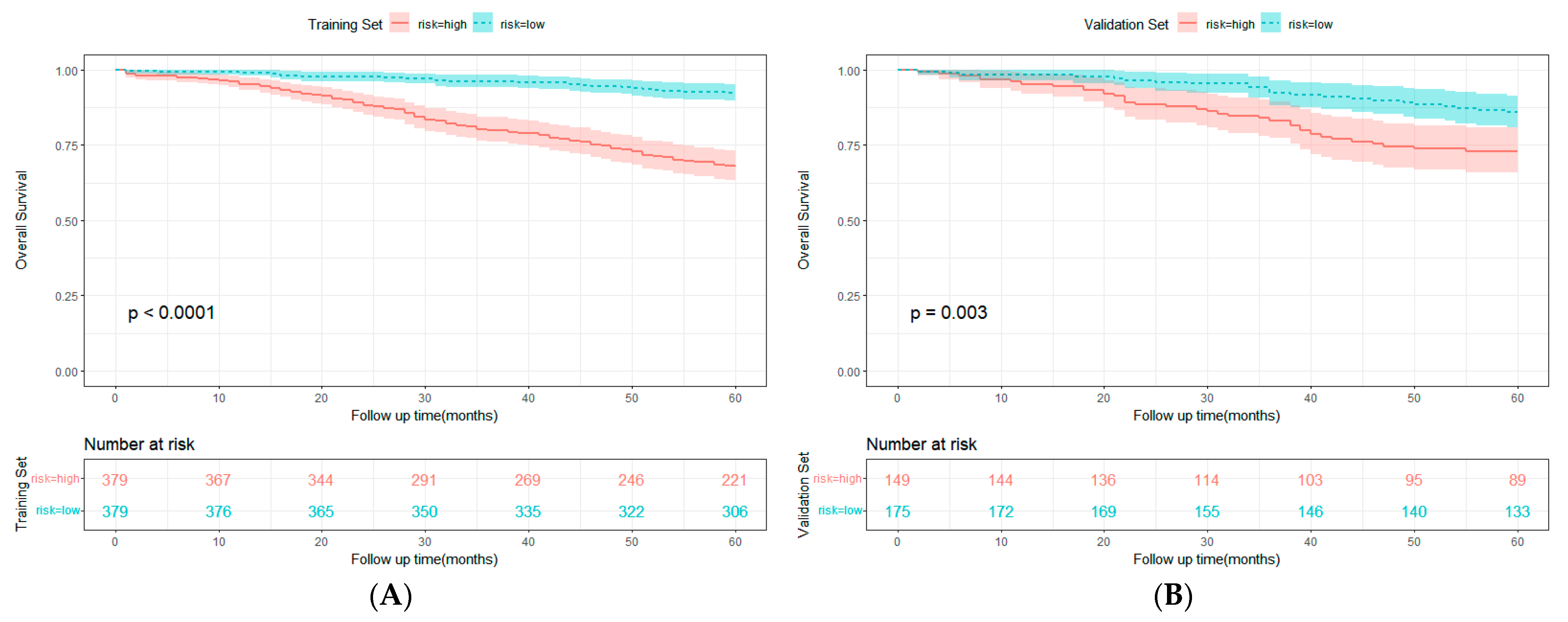
3.3. Construction and Validation of Nomogram
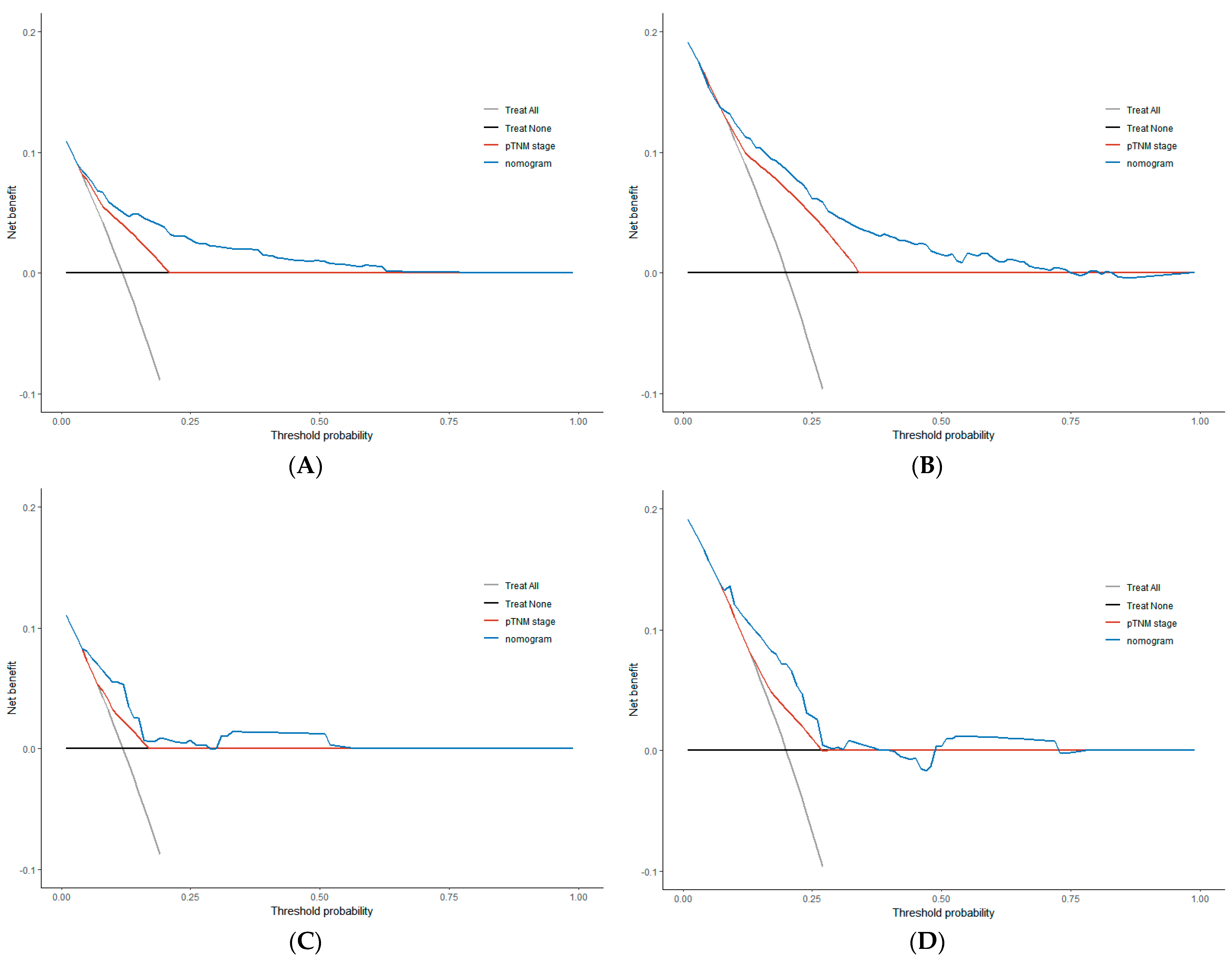
3.4. Comparison of Clinical Value between Nomogram and TNM Stage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Takagawa, R.; Fujii, S.; Ohta, M.; Nagano, Y.; Kunisaki, C.; Yamagishi, S.; Osada, S.; Ichikawa, Y.; Shimada, H. Preoperative serum carcinoembryonic antigen level as a predictive factor of recurrence after curative resection of colorectal cancer. Ann. Surg. Oncol. 2008, 15, 3433–3439. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Lim, S.B.; Kim, D.Y.; Jung, K.H.; Hong, Y.S.; Chang, H.J.; Choi, H.S.; Jeong, S.Y. Carcinoembryonic antigen as a predictor of pathologic response and a prognostic factor in locally advanced rectal cancer patients treated with preoperative chemoradiotherapy and surgery. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Baqar, A.R.; Wilkins, S.; Staples, M.; Angus Lee, C.H.; Oliva, K.; McMurrick, P. The role of preoperative CEA in the management of colorectal cancer: A cohort study from two cancer centres. Int. J. Surg. 2019, 64, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Carpelan-Holmstrom, M.; Haglund, C.; Kuusela, P.; Jarvinen, H.; Roberts, P.J. Preoperative serum levels of CEA and CA 242 in colorectal cancer. Br. J. Cancer 1995, 71, 868–872. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carpelan-Holmstrom, M.; Haglund, C.; Lundin, J.; Jarvinen, H.; Roberts, P. Pre-operative serum levels of CA 242 and CEA predict outcome in colorectal cancer. Eur. J. Cancer 1996, 32A, 1156–1161. [Google Scholar] [CrossRef]
- Huang, S.H.; Tsai, W.S.; You, J.F.; Hung, H.Y.; Yeh, C.Y.; Hsieh, P.S.; Chiang, S.F.; Lai, C.C.; Chiang, J.M.; Tang, R.; et al. Preoperative Carcinoembryonic Antigen as a Poor Prognostic Factor in Stage I-III Colorectal Cancer After Curative-Intent Resection: A Propensity Score Matching Analysis. Ann. Surg. Oncol. 2019, 26, 1685–1694. [Google Scholar] [CrossRef]
- Saito, G.; Sadahiro, S.; Kamata, H.; Miyakita, H.; Okada, K.; Tanaka, A.; Suzuki, T. Monitoring of Serum Carcinoembryonic Antigen Levels after Curative Resection of Colon Cancer: Cutoff Values Determined according to Preoperative Levels Enhance the Diagnostic Accuracy for Recurrence. Oncology 2017, 92, 276–282. [Google Scholar] [CrossRef]
- Ramphal, W.; Boeding, J.R.E.; van Iwaarden, M.; Schreinemakers, J.M.J.; Rutten, H.J.T.; Crolla, R.; Gobardhan, P.D. Serum carcinoembryonic antigen to predict recurrence in the follow-up of patients with colorectal cancer. Int. J. Biol. Mark. 2019, 34, 60–68. [Google Scholar] [CrossRef]
- Huh, J.W.; Kim, C.H.; Lim, S.W.; Kim, H.R.; Kim, Y.J. Factors predicting long-term survival in colorectal cancer patients with a normal preoperative serum level of carcinoembryonic antigen. J. Cancer Res. Clin. Oncol. 2013, 139, 1449–1455. [Google Scholar] [CrossRef]
- Beom, S.H.; Shin, S.J.; Kim, C.G.; Kim, J.H.; Hur, H.; Min, B.S.; Lee, K.Y.; Kim, N.K.; Ahn, J.B. Clinical Significance of Preoperative Serum Carcinoembryonic Antigen Within the Normal Range in Colorectal Cancer Patients Undergoing Curative Resection. Ann. Surg. Oncol. 2020, 27, 2774–2783. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Liu, H.S.; Hu, T.; Zhang, Z.J.; He, X.W.; Mo, T.W.; Wen, X.F.; Lan, P.; Lian, L.; Wu, X.R. Elevated preoperative CA125 is associated with poor survival in patients with metastatic colorectal cancer undergoing primary tumor resection: A retrospective cohort study. Gastroenterol. Rep. 2022, 10, goac020. [Google Scholar] [CrossRef] [PubMed]
- Hall, N.R.; Finan, P.J.; Stephenson, B.M.; Purves, D.A.; Cooper, E.H. The role of CA-242 and CEA in surveillance following curative resection for colorectal cancer. Br. J. Cancer 1994, 70, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, H.; Pang, X.; Mao, Y.; Yi, X.; Li, C.; Lei, M.; Cheng, X.; Liang, L.; Wu, J.; et al. Preoperative serum CA19-9 should be routinely measured in the colorectal patients with preoperative normal serum CEA: A multicenter retrospective cohort study. BMC Cancer 2022, 22, 962. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Nomura, T.; Fukushima, Y.; Morimoto, T.; Hiraoka, N.; Shibata, N. Does serum CA19-9 play a practical role in the management of patients with colorectal cancer? Dis. Colon Rectum 2004, 47, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Ayude, D.; Rodríguez-Berrocal, F.J.; Ayude, J.; Blanco-Prieto, S.; Vázquez-Iglesias, L.; Vázquez-Cedeira, M.; Páez de la Cadena, M. Preoperative serum CA 72.4 as prognostic factor of recurrence and death, especially at TNM stage II, for colorectal cancer. BMC Cancer 2013, 13, 543. [Google Scholar] [CrossRef]
- Luo, H.; Shen, K.; Li, B.; Li, R.; Wang, Z.; Xie, Z. Clinical significance and diagnostic value of serum NSE, CEA, CA19-9, CA125 and CA242 levels in colorectal cancer. Oncol. Lett. 2020, 20, 742–750. [Google Scholar] [CrossRef]
- Ren, F.; Weng, W.; Zhang, Q.; Tan, C.; Xu, M.; Zhang, M.; Wang, L.; Sheng, W.; Ni, S.; Huang, D. Clinicopathological features and prognosis of AFP-producing colorectal cancer: A single-center analysis of 20 cases. Cancer Manag. Res. 2019, 11, 4557–4567. [Google Scholar] [CrossRef]
- Chen, J.; Wu, L.; Sun, Y.; Luo, C.; Chen, X.; Wu, L.; Ding, J.; Pan, G.; Han, C.; Wu, Z.; et al. Diagnostic value and clinical significance of circulating miR-650 and CA211 in detecting of gastric carcinoma. Oncol. Lett. 2020, 20, 254. [Google Scholar] [CrossRef]
- Cao, H.; Zhu, L.; Li, L.; Wang, W.; Niu, X. Serum CA724 has no diagnostic value for gastrointestinal tumors. Clin. Exp. Med. 2023, 23, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Kumada, T.; Tada, T.; Niinomi, T.; Ito, T.; Kaneoka, Y.; Maeda, A. Prognostic significance of a combination of pre- and post-treatment tumor markers for hepatocellular carcinoma curatively treated with hepatectomy. J. Hepatol. 2012, 57, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, K.; Zhang, D.; Pang, X.; Pu, H.; Lei, M.; Fan, B.; Lv, J.; You, D.; Li, Z.; et al. Prediction models of colorectal cancer prognosis incorporating perioperative longitudinal serum tumor markers: A retrospective longitudinal cohort study. BMC Med. 2023, 21, 63. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.R.; Landmann, R.G.; Kattan, M.W.; Gonen, M.; Shia, J.; Chou, J.; Paty, P.B.; Guillem, J.G.; Temple, L.K.; Schrag, D.; et al. Individualized prediction of colon cancer recurrence using a nomogram. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 380–385. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef]
- Iasonos, A.; Schrag, D.; Raj, G.V.; Panageas, K.S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 1364–1370. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, Y.; Seaberg, E.C.; Becker, J.T. Quantifying diagnostic accuracy improvement of new biomarkers for competing risk outcomes. Biostatistics 2020, 23, 666–682. [Google Scholar] [CrossRef]
- Primrose, J.N.; Perera, R.; Gray, A.; Rose, P.; Fuller, A.; Corkhill, A.; George, S.; Mant, D. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: The FACS randomized clinical trial. JAMA 2014, 311, 263–270. [Google Scholar] [CrossRef]
- Becerra, A.Z.; Probst, C.P.; Tejani, M.A.; Aquina, C.T.; González, M.G.; Hensley, B.J.; Noyes, K.; Monson, J.R.; Fleming, F.J. Evaluating the Prognostic Role of Elevated Preoperative Carcinoembryonic Antigen Levels in Colon Cancer Patients: Results from the National Cancer Database. Ann. Surg. Oncol. 2016, 23, 1554–1561. [Google Scholar] [CrossRef]
- Jo, Y.; Lee, J.H.; Cho, E.S.; Lee, H.S.; Shin, S.J.; Park, E.J.; Baik, S.H.; Lee, K.Y.; Kang, J. Clinical Significance of Early Carcinoembryonic Antigen Change in Patients with Nonmetastatic Colorectal Cancer. Front. Oncol. 2022, 12, 739614. [Google Scholar] [CrossRef]
- Hara, M.; Kanemitsu, Y.; Hirai, T.; Komori, K.; Kato, T. Negative serum carcinoembryonic antigen has insufficient accuracy for excluding recurrence from patients with Dukes C colorectal cancer: Analysis with likelihood ratio and posttest probability in a follow-up study. Dis. Colon Rectum 2008, 51, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.C.; Lin, J.K.; Lin, C.C.; Wang, H.S.; Yang, S.H.; Jiang, J.K.; Lan, Y.T.; Lin, T.C.; Li, A.F.; Chen, W.S.; et al. Carbohydrate antigen 19-9 is a valuable prognostic factor in colorectal cancer patients with normal levels of carcinoembryonic antigen and may help predict lung metastasis. Int. J. Color. Dis. 2012, 27, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Cohen, A.M.; Urmacher, C. Usefulness of carcinoembryonic antigen monitoring despite normal preoperative values in node-positive colon cancer patients. Dis. Colon Rectum 1993, 36, 1063–1068. [Google Scholar] [CrossRef]
- Kuang, J.; Gong, Y.; Xie, H.; Yan, L.; Huang, S.; Gao, F.; Tang, S.; Gan, J. The prognostic value of preoperative serum CA724 for CEA-normal colorectal cancer patients. PeerJ 2020, 8, e8936. [Google Scholar] [CrossRef] [PubMed]
- Paku, M.; Uemura, M.; Kitakaze, M.; Miyoshi, N.; Takahashi, H.; Mizushima, T.; Doki, Y.; Eguchi, H. Clinical Significance of Preoperative and Postoperative Serum CEA and Carbohydrate Antigen 19-9 Levels in Patients Undergoing Curative Resection of Locally Recurrent Rectal Cancer. Dis. Colon Rectum 2023, 66, 392–400. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Sheng, N.; Yan, L.; Chen, H.; Gong, J.; He, Z.; Zheng, K.; Chen, Z.; Wang, Y.; Tan, G.; et al. The difference in prognosis of stage II and III colorectal cancer based on preoperative serum tumor markers. J. Cancer 2019, 10, 3757–3766. [Google Scholar] [CrossRef] [PubMed]
- Stiksma, J.; Grootendorst, D.C.; van der Linden, P.W. CA 19-9 as a marker in addition to CEA to monitor colorectal cancer. Clin. Color. Cancer 2014, 13, 239–244. [Google Scholar] [CrossRef]
- Shibutani, M.; Maeda, K.; Nagahara, H.; Ohtani, H.; Sakurai, K.; Toyokawa, T.; Kubo, N.; Tanaka, H.; Muguruma, K.; Ohira, M.; et al. Significance of CEA and CA19-9 combination as a prognostic indicator and for recurrence monitoring in patients with stage II colorectal cancer. Anticancer Res. 2014, 34, 3753–3758. [Google Scholar]
- Rosen, D.G.; Wang, L.; Atkinson, J.N.; Yu, Y.; Lu, K.H.; Diamandis, E.P.; Hellstrom, I.; Mok, S.C.; Liu, J.; Bast, R.C., Jr. Potential markers that complement expression of CA125 in epithelial ovarian cancer. Gynecol. Oncol. 2005, 99, 267–277. [Google Scholar] [CrossRef]
- Streppel, M.M.; Vincent, A.; Mukherjee, R.; Campbell, N.R.; Chen, S.H.; Konstantopoulos, K.; Goggins, M.G.; Van Seuningen, I.; Maitra, A.; Montgomery, E.A. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum. Pathol. 2012, 43, 1755–1763. [Google Scholar] [CrossRef]
- Theriault, C.; Pinard, M.; Comamala, M.; Migneault, M.; Beaudin, J.; Matte, I.; Boivin, M.; Piche, A.; Rancourt, C. MUC16 (CA125) regulates epithelial ovarian cancer cell growth, tumorigenesis and metastasis. Gynecol. Oncol. 2011, 121, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Gaetje, R.; Winnekendonk, D.W.; Scharl, A.; Kaufmann, M. Ovarian cancer antigen CA 125 enhances the invasiveness of the endometriotic cell line EEC 145. J. Soc. Gynecol. Investig. 1999, 6, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Rump, A.; Morikawa, Y.; Tanaka, M.; Minami, S.; Umesaki, N.; Takeuchi, M.; Miyajima, A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J. Biol. Chem. 2004, 279, 9190–9198. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Q.; Li, Y.; Chen, C.; Peng, C.W.; Liu, S.P.; Liu, Y. Preoperative serum carbohydrate antigen 125 level is an independent negative prognostic marker for overall survival in colorectal cancer. Med. Oncol. 2011, 28, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Q.; Chen, C.; Wang, F.B.; Peng, C.W.; Li, Y. Preoperative serum carcinoembryonic antigen, carbohydrate antigen19-9 and carbohydrate antigen 125 as prognostic factors for recurrence-free survival in colorectal cancer. Asian Pac. J. Cancer Prev. APJCP 2011, 12, 1251–1256. [Google Scholar] [PubMed]
- Huo, Y.R.; Huang, Y.; Liauw, W.; Zhao, J.; Morris, D.L. Prognostic Value of Carcinoembryonic Antigen (CEA), AFP, CA19-9 and CA125 for Patients with Colorectal Cancer with Peritoneal Carcinomatosis Treated by Cytoreductive Surgery and Intraperitoneal Chemotherapy. Anticancer Res. 2016, 36, 1041–1049. [Google Scholar] [PubMed]
- Huang, C.J.; Jiang, J.K.; Chang, S.C.; Lin, J.K.; Yang, S.H. Serum CA125 concentration as a predictor of peritoneal dissemination of colorectal cancer in men and women. Medicine 2016, 95, e5177. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, J.; Du, F.; Zhang, L.; Li, D.; Huang, H.; Tian, T.; Liu, Y.; Zhang, L.; Liu, Y.; et al. Prognostic Inflammatory Index Based on Preoperative Peripheral Blood for Predicting the Prognosis of Colorectal Cancer Patients. Cancers 2021, 13, 3. [Google Scholar] [CrossRef]
- Liu, H.; Lv, L.; Qu, Y.; Zheng, Z.; Zhao, J.; Liu, B.; Zhang, D.; Wang, H.; Zhang, J. Prediction of cancer-specific survival and overall survival in middle-aged and older patients with rectal adenocarcinoma using a nomogram model. Transl. Oncol. 2021, 14, 100938. [Google Scholar] [CrossRef]
| Clinicopathological Features | Overall | Training Set | Validation Set | p Value |
|---|---|---|---|---|
| (n = 1082) | (n = 758) | (n = 324) | ||
| Sex | 0.633 a | |||
| Male | 628 (58.0) | 444 (58.6) | 184 (56.8) | |
| Female | 454 (42.0) | 314 (41.4) | 140 (43.2) | |
| Age (median (IQR)) | 65 (57, 75) | 65 (57, 75) | 65.5 (58, 74.2) | 0.583 b |
| Age | 0.728 a | |||
| <65 | 518 (47.9) | 366 (48.3) | 152 (46.9) | |
| ≥65 | 564 (52.1) | 392 (51.7) | 172 (53.1) | |
| Tumor Location | 0.749 a | |||
| Right Colon | 227 (21.0) | 158 (20.8) | 69 (21.3) | |
| Left Colon | 328 (30.3) | 235 (31.0) | 93 (28.7) | |
| Rectum | 527 (48.7) | 365 (48.2) | 162 (50.0) | |
| Histologic type | 0.254 a | |||
| Grade I–II Adenocarcinoma | 810 (74.9) | 566 (74.7) | 244 (75.3) | |
| Grade III Adenocarcinoma | 102 (9.4) | 78 (10.3) | 24 (7.4) | |
| Mucinous Adenocarcinoma | 170 (15.7) | 114 (15.0) | 56 (17.3) | |
| pT stage | 0.026 a* | |||
| T1 | 82 (7.6) | 48 (6.3) | 34 (10.5) | |
| T2 | 216 (20) | 144 (19) | 72 (22.2) | |
| T3 | 604 (55.8) | 442 (58.3) | 162 (50) | |
| T4 | 180 (16.6) | 124 (16.4) | 56 (17.3) | |
| pN stage | 0.951 a | |||
| N0 | 654 (60.4) | 460 (60.7) | 194 (59.9) | |
| N1 | 267 (24.7) | 185 (24.4) | 82 (25.3) | |
| N2 | 161 (14.9) | 113 (14.9) | 48 (14.8) | |
| pTNM stage | 0.180 a | |||
| I | 248 (22.9) | 164 (21.6) | 84 (25.9) | |
| II | 406 (37.5) | 296 (39.1) | 110 (34.0) | |
| III | 428 (39.6) | 298 (39.3) | 130 (40.1) | |
| Perineural/Vascular invasion | 0.510 a | |||
| No | 998 (92.2) | 696 (91.8) | 302 (93.2) | |
| Yes | 84 (7.8) | 62 (8.2) | 22 (6.8) | |
| CA242 | 0.589 a | |||
| Negative | 936 (86.5) | 659 (86.9) | 277 (85.5) | |
| Positive | 146 (13.5) | 99 (13.1) | 47 (14.5) | |
| AFP | 0.704 a | |||
| Negative | 1061 (98.1) | 742 (97.9) | 319 (98.5) | |
| Positive | 21 (1.9) | 16 (2.1) | 5 (1.5) | |
| NSE | 0.428 a | |||
| Negative | 989 (91.4) | 689 (90.9) | 300 (92.6) | |
| Positive | 93 (8.6) | 69 (9.1) | 24 (7.4) | |
| CA125 | 1.000 a | |||
| Negative | 1037 (95.8) | 726 (95.8) | 311 (96.0) | |
| Positive | 45 (4.2) | 32 (4.2) | 13 (4.0) | |
| CA19-9 | 0.681 a | |||
| Negative | 999 (92.3) | 702 (92.6) | 297 (91.7) | |
| Positive | 83 (7.7) | 56 (7.4) | 27 (8.3) | |
| CA211 | 0.483 a | |||
| Negative | 851 (78.7) | 601 (79.3) | 250 (77.2) | |
| Positive | 231 (21.3) | 157 (20.7) | 74 (22.8) | |
| CA724 | 0.828 a | |||
| Negative | 980 (90.6) | 688 (90.8) | 292 (90.1) | |
| Positive | 102 (9.4) | 70 (9.2) | 32 (9.9) | |
| 5-year OS | 1.000 a | |||
| other | 880 (81.3) | 616 (81.3) | 264 (81.5) | |
| event | 202 (18.7) | 142 (18.7) | 60 (18.5) | |
| 5-year DFS | 1.000 a | |||
| other | 827 (76.4) | 579 (76.4) | 248 (76.5) | |
| event | 255 (23.6) | 179 (23.6) | 76 (23.5) | |
| Follow-up, Median (Q1, Q3) | 75 (49, 101) | 75.5 (49.2, 101) | 75 (41.8, 99.2) | 0.596 b |
| Clinicopathological Features | HR | 95%CI | p Value | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Sex | ||||
| Male | Reference | |||
| Female | 0.720 | 0.509 | 1.018 | 0.063 |
| Age | ||||
| <65 | Reference | |||
| ≥65 | 1.438 | 1.028 | 2.012 | 0.034 * |
| Tumor Location | ||||
| Right Colon | Reference | |||
| Left Colon | 0.522 | 0.328 | 0.830 | 0.006 * |
| Rectum | 0.753 | 0.509 | 1.115 | 0.157 |
| Histologic type | ||||
| Grade I–II Adenocarcinoma | Reference | |||
| Grade III Adenocarcinoma | 3.282 | 2.170 | 4.962 | 0.000 * |
| Mucinous Adenocarcinoma | 1.891 | 1.238 | 2.888 | 0.003 * |
| pT stage | ||||
| T1–T2 | Reference | |||
| T3–T4 | 2.728 | 1.643 | 4.527 | 0.000 * |
| pN stage | ||||
| N0 | Reference | |||
| N1 | 2.514 | 1.670 | 3.784 | 0.000 * |
| N2 | 6.121 | 4.104 | 9.129 | 0.000 * |
| Perineural/Vascular invasion | ||||
| No | Reference | |||
| Yes | 2.707 | 1.758 | 4.170 | 0.000 * |
| CA242 | ||||
| Negative | Reference | |||
| Positive | 2.109 | 1.409 | 3.156 | 0.000 * |
| AFP | ||||
| Negative | Reference | |||
| Positive | 0.639 | 0.158 | 2.579 | 0.529 |
| NSE | ||||
| Negative | Reference | |||
| Positive | 1.234 | 0.722 | 2.107 | 0.442 |
| CA125 | ||||
| Negative | Reference | |||
| Positive | 2.707 | 1.531 | 4.789 | 0.001 * |
| CA19-9 | ||||
| Negative | Reference | |||
| Positive | 1.886 | 1.121 | 3.174 | 0.017 * |
| CA211 | ||||
| Negative | Reference | |||
| Positive | 1.636 | 1.135 | 2.358 | 0.008 * |
| CA724 | ||||
| Negative | Reference | |||
| Positive | 1.808 | 1.127 | 2.902 | 0.014 * |
| Clinicopathological Features | HR | 95%CI | p Value | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Age | ||||
| <65 | Reference | |||
| ≥65 | 1.798 | 1.265 | 2.554 | 0.001 * |
| Tumor Location | ||||
| Right Colon | Reference | |||
| Left Colon | 0.652 | 0.402 | 1.057 | 0.083 |
| Rectum | 1.081 | 0.715 | 1.636 | 0.711 |
| Histologic type | ||||
| Grade I–II Adenocarcinoma | Reference | |||
| Grade III Adenocarcinoma | 2.282 | 1.483 | 3.510 | 0.000 * |
| Mucinous Adenocarcinoma | 1.128 | 0.716 | 1.778 | 0.602 |
| pT stage | ||||
| T1–T2 | Reference | |||
| T3–T4 | 1.958 | 1.157 | 3.313 | 0.012 * |
| pN stage | ||||
| N0 | Reference | |||
| N1 | 2.102 | 1.382 | 3.197 | 0.001 * |
| N2 | 5.714 | 3.739 | 8.731 | 0.000 * |
| CA242 | ||||
| Negative | Reference | |||
| Positive | 1.686 | 1.119 | 2.540 | 0.013 * |
| CA125 | ||||
| Negative | Reference | |||
| Positive | 2.133 | 1.179 | 3.859 | 0.012 * |
| Index | Training Set | Validation Set | ||||
|---|---|---|---|---|---|---|
| Estimate | 95%CI | p Value | Estimate | 95%CI | p Value | |
| NRI (vs. pTNM stage) | 0 | |||||
| For 3-year OS | 0.432 | 0.251–0.601 | 0.375 | 0.193–0.572 | ||
| For 5-year OS | 0.332 | 0.192–0.481 | 0.442 | 0.241–0.655 | ||
| IDI (vs. pTNM stage) | ||||||
| For 3-year OS | 0.111 | 0.061–0.197 | 0.000 * | 0.076 | 0.030–0.231 | 0.000 * |
| For 5-year OS | 0.103 | 0.062–0.162 | 0.000 * | 0.068 | 0.035–0.180 | 0.000 * |
| C-index (OS | ||||||
| The nomogram) | 0.748 | 0.706–0.791 | 0.702 | 0.643–0.761 | ||
| The pTNM stage | 0.668 | 0.629–0.706 | 0.593 | 0.528–0.658 | ||
| Change | 0.080 | 0.051–0.094 | 0.000 * | 0.109 | 0.040–0.199 | 0.007 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, X.; Wang, H.; Lu, Y.; Chen, Y.; Liu, Y.; Huang, S. Development and Validation of a Nomogram to Predict Overall Survival in Stage I–III Colorectal Cancer Patients after Radical Resection with Normal Preoperative Serum Carcinoembryonic Antigen. Cancers 2023, 15, 5643. https://doi.org/10.3390/cancers15235643
Dai X, Wang H, Lu Y, Chen Y, Liu Y, Huang S. Development and Validation of a Nomogram to Predict Overall Survival in Stage I–III Colorectal Cancer Patients after Radical Resection with Normal Preoperative Serum Carcinoembryonic Antigen. Cancers. 2023; 15(23):5643. https://doi.org/10.3390/cancers15235643
Chicago/Turabian StyleDai, Xuan, Haoran Wang, Yaqi Lu, Yan Chen, Yun Liu, and Shiyong Huang. 2023. "Development and Validation of a Nomogram to Predict Overall Survival in Stage I–III Colorectal Cancer Patients after Radical Resection with Normal Preoperative Serum Carcinoembryonic Antigen" Cancers 15, no. 23: 5643. https://doi.org/10.3390/cancers15235643
APA StyleDai, X., Wang, H., Lu, Y., Chen, Y., Liu, Y., & Huang, S. (2023). Development and Validation of a Nomogram to Predict Overall Survival in Stage I–III Colorectal Cancer Patients after Radical Resection with Normal Preoperative Serum Carcinoembryonic Antigen. Cancers, 15(23), 5643. https://doi.org/10.3390/cancers15235643





