Treatment Sequencing and Outcome of Chronic Lymphocytic Leukemia Patients Treated at Fondazione Policlinico Universitario Agostino Gemelli IRCCS: A Thirty-Year Single-Center Experience
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
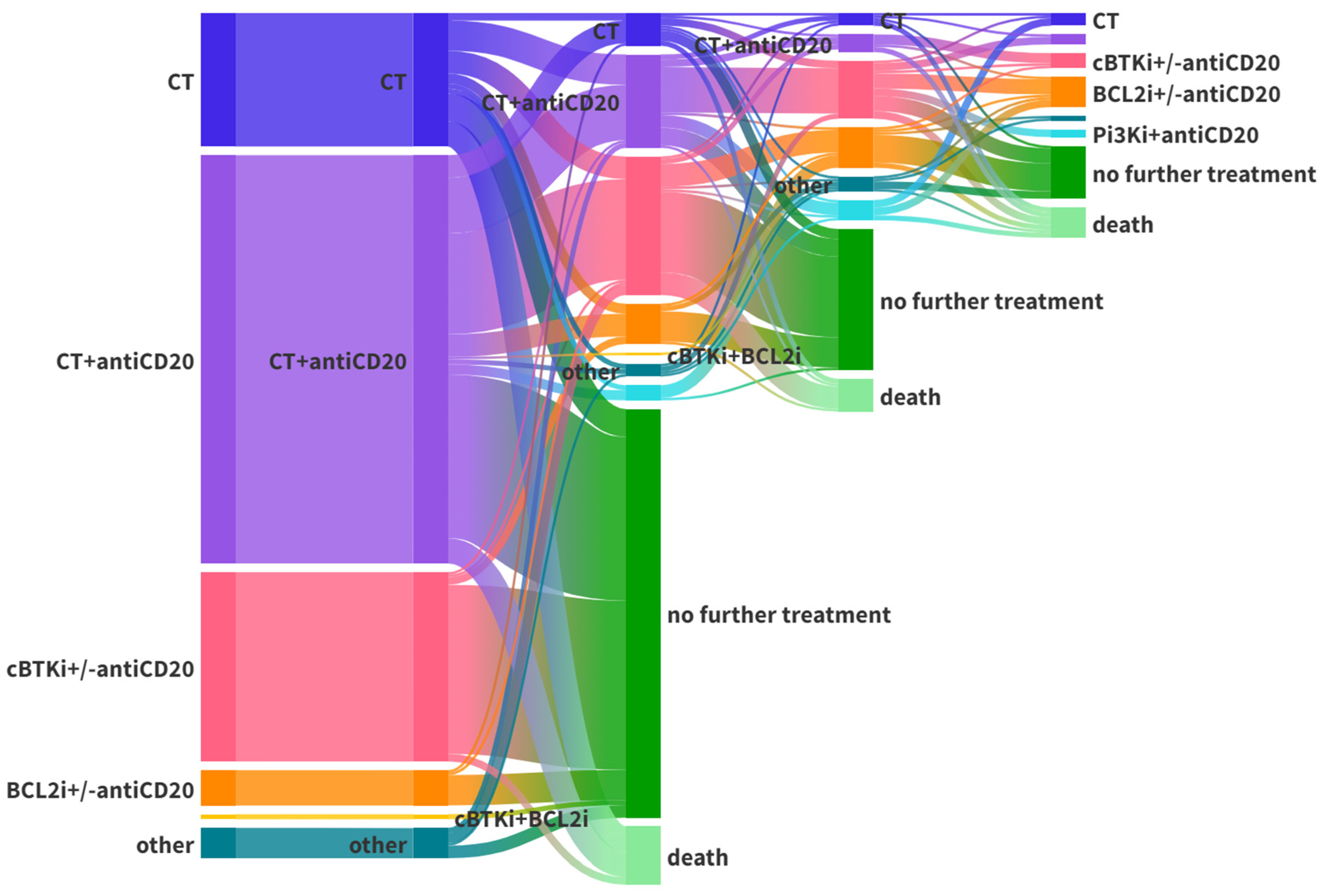
3.1. Treated Cohort
3.2. cBTKi- and/or BCL2i-Exposed
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review 1975–2007; National Cancer Institute: Bethesda, MD, USA, 2018; pp. 4–5.
- Shadman, M. Diagnosis and Treatment of Chronic Lymphocytic Leukemia: A Review. JAMA 2023, 329, 918–932. [Google Scholar] [CrossRef] [PubMed]
- Munir, T.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Barr, P.M.; Reddy, N.M.; Coutre, S.; Tam, C.; Mulligan, S.; Jaeger, U.; et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am. J. Hematol. 2019, 94, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, W.; Pluta, A.; Wach, M.; Lysak, D.; Simkovic, M.; Kriachok, I.; Illés, A.; De La Serna, J.; Dolan, S.; Campbell, P.; et al. Acalabrutinib versus rituximab plus idelalisib or bendamustine in relapsed/refractory chronic lymphocytic leukemia: ASCEND results at 4 years of follow-up. Hemasphere 2022, 6, e801. [Google Scholar] [CrossRef]
- Byrd, J.C.; Wierda, W.G.; Schuh, A.; Devereux, S.; Chaves, J.M.; Brown, J.R.; Hillmen, P.; Martin, P.; Awan, F.; Stephens, D.; et al. Acalabrutinib Monotherapy in Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia: Updated Phase 2 Results. Blood 2020, 135, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Eichhorst, B.; Hillmen, P.; Jurczak, W.; Kaźmierczak, M.; Lamanna, N.; O’Brien, S.; Tam, C.; Qiu, L.; Zhou, K.; et al. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2022, 388, 319–332. [Google Scholar] [CrossRef]
- Jones, J.A.; Mato, A.R.; Wierda, W.G.; Davids, M.S.; Choi, M.; Cheson, B.D.; Furman, R.; Lamanna, N.; Barr, P.; Zhou, L.; et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: An interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 2018, 19, 65–75. [Google Scholar] [CrossRef]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Coutre, S.; Seymour, J.F.; Munir, T.; Puvvada, S.; Wendtner, C.; Roberts, A.; Jurczak, W.; et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: A multicentre, open-label, phase 2 study. Lancet Oncol. 2016, 17, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Gerecitano, J.; Robak, T.; De La Serna, J.; et al. Venetoclax–Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2018, 378, 1107–1120. [Google Scholar] [CrossRef]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.F.; D’Rozario, J.; Owen, C.J.; Assouline, S.; Lamanna, N.; Robak, T.; De La Serna, J.; Jaeger, U.; et al. Enduring undetectable MRD and updated outcomes in relapsed/refractory CLL after fixed-duration venetoclax-rituximab. Blood 2022, 140, 839–850. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.M.; Ding, W.; Bartlett, N.; Brander, D.; Barr, P.; Rogers, K.; et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N. Engl. J. Med. 2018, 379, 2517–2528. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Wang, X.V.; Kay, N.E.; Hanson, C.A.; O’Brien, S.; Barrientos, J.; Jelinek, D.; Braggio, E.; Leis, J.; Zhang, C.; et al. Ibrutinib–Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2019, 381, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Barr, P.M.; Owen, C.; Robak, T.; Tedeschi, A.; Bairey, O.; Burger, J.A.; Hillmen, P.; Coutre, S.; Dearden, C.; Grosicki, S.; et al. Up to 8-year follow-up from RESONATE-2: First-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022, 6, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Barr, P.M.; Robak, T.; Owen, C.; Tedeschi, A.; Bairey, O.; Bartlett, N.L.; Burger, J.; Hillmen, P.; Coutre, S.; Devereux, S.; et al. Sustained efficacy and detailed clinical follow-up of first-line ibrutinib treatment in older patients with chronic lymphocytic leukemia: Extended phase 3 results from RESONATE-2. Haematologica 2018, 103, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia 2022, 36, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): A randomised, controlled, phase 3 trial. Lancet 2020, 395, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Robak, T.; Ghia, P.; Kahl, B.S.; Walker, P.; Janowski, W.; Simpson, D.; Shadman, M.; Ganly, P.; Laurenti, L.; et al. Zanubrutinib monotherapy for patients with treatment-naïve chronic lymphocytic leukemia and 17p deletion. Haematologica 2021, 106, 2354–2363. [Google Scholar] [CrossRef]
- Tam, C.S.; Brown, J.R.; Kahl, B.S.; Ghia, P.; Giannopoulos, K.; Jurczak, W.; Šimkovič, M.; Shadman, M.; Österborg, A.; Laurenti, L.; et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): A randomised, controlled, phase 3 trial. Lancet Oncol. 2022, 23, 1031–1043. [Google Scholar] [CrossRef]
- Fischer, K.; Al-Sawaf, O.; Bahlo, J.; Fink, A.M.; Tandon, M.; Dixon, M.; Robrecht, S.; Warburton, S.; Humphrey, K.; Samoylova, O.; et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2019, 380, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Al-Sawaf, O.; Zhang, C.; Tong, L.; Liao, M.Z.; Panchal, A.; Robrecht, S.; Ching, T.; Tandon, M.; Fink, A.M.; Tausch, E.; et al. Minimal Residual Disease Dynamics after Venetoclax-Obinutuzumab Treatment: Extended Off-Treatment Follow-up From the Randomized CLL14 Study. J. Clin. Oncol. 2021, 39, 4049–4060. [Google Scholar] [CrossRef]
- Furman, R.R.; Sharman, J.P.; Coutre, S.E.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.; Zelenetz, A.; Kipps, T.; Flinn, I.; et al. Idelalisib and Rituximab in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2014, 370, 997–1007. [Google Scholar] [CrossRef]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Hillmen, P.; Keating, M.; Monserrat, E.; Chiorazzi, N.; Silgenbauer, S.; et al. Special Report iwCLL Guidelines for Diagnosis, Indications for Treatment, Response Assessment, and Supportive Management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef]
- Al-Sawaf, O.; Robrecht, S.; Zhang, C.; Olivieri, S.; Chang, Y.M.; Fink, A.M.; Tausch, E.; Schneider, C.; Ritgen, M.; Kreuzer, K.A.; et al. Venetoclax-Obinutuzumab for previously untreated chronic lymphocytic leukemia: 6-year results of the randomized CLL14 study. Hemasphere 2023, 7, 103–105. [Google Scholar] [CrossRef]
- Eichhorst, B.; Niemann, C.U.; Kater, A.P.; Fürstenau, M.; von Tresckow, J.; Zhang, C.; Robrecht, S.; Gregor, M.; Juliusson, G.; Thornton, P.; et al. First-Line Venetoclax Combinations in Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2023, 388, 1739–1754. [Google Scholar] [CrossRef]
- International CLL-IPI Working Group. An international Prognostic Index for Patients with Chronic Lymphocytic Leukaemia (CLL-IPI): A Meta-Analysis of Individual Patient Data. Lancet Oncol. 2016, 17, 779–790. [Google Scholar] [CrossRef]
- Da Cunha-Bang, C.; Christiansen, I.; Niemann, C.U. The CLL-IPI applied in a population-based cohort. Blood Am. Soc. Hematol. 2016, 128, 2181–2183. [Google Scholar] [CrossRef]
- Gentile, M.; Shanafelt, T.D.; Mauro, F.R.; Reda, G.; Rossi, D.; Laurenti, L.; Del Principe, M.; Cutrona, G.; Angeletti, I.; Coscia, M.; et al. Predictive value of the CLL-IPI in CLL patients receiving chemo-immunotherapy as first-line treatment. Eur. J. Haematol. 2018, 101, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Allan, J.N.; Shanafelt, T.; Wiestner, A.; Moreno, C.; O’Brien, S.M.; Li, J.; Krigsfeld, G.; Dean, J.P.; Ahn, I.E. Long-term efficacy of first-line ibrutinib treatment for chronic lymphocytic leukaemia in patients with TP53 aberrations: A pooled analysis from four clinical trials. Br. J. Haematol. 2022, 196, 947–953. [Google Scholar] [CrossRef]
- Ahn, I.E.; Tian, X.; Wiestner, A. Ibrutinib for Chronic Lymphocytic Leukemia with TP53 Alterations. N. Engl. J. Med. 2020, 383, 498–500. [Google Scholar] [CrossRef]
- Tam, C.S.; Allan, J.N.; Siddiqi, T.; Kipps, T.J.; Jacobs, R.; Opat, S.; Barr, P.; Tedeschi, A.; Trentin, L.; Bannerji, R.; et al. Fixed-duration ibrutinib plus venetoclax for first-line treatment of CLL: Primary analysis of the CAPTIVATE FD cohort. Blood 2022, 139, 3278–3289. [Google Scholar] [CrossRef]
- Wierda, W.G.; Allan, J.N.; Siddiqi, T.; Kipps, T.J.; Opat, S.; Tedeschi, A.; Badoux, X.C.; Kuss, B.J.; Jackson, S.; Moreno, C.; et al. Ibrutinib Plus Venetoclax for First-Line Treatment of Chronic Lymphocytic Leukemia: Primary Analysis Results From the Minimal Residual Disease Cohort of the Randomized Phase II CAPTIVATE Study. J. Clin. Oncol. 2021, 39, 3853–3865. [Google Scholar] [CrossRef]
- Kater, A.P.; Owen, C.; Moreno, C.; Follows, G.; Munir, T.; Levin, M.D.; Benjamini, O.; Janssens, A.; Osterborg, A.; Robak, T.; et al. Fixed-Duration Ibrutinib-Venetoclax in Patients with Chronic Lymphocytic Leukemia and Comorbidities. NEJM Evid. 2022, 1, EVIDoa2200006. [Google Scholar] [CrossRef]
- Mato, A.R.; Hess, L.M.; Chen, Y.; Abada, P.B.; Konig, H.; Pagel, J.M.; Walgren, R. Outcomes for Patients with Chronic Lymphocytic Leukemia (CLL) Previously Treated With Both a Covalent BTK and BCL2 Inhibitor in the United States: A Real-World Database Study. Clin. Lymphoma Myeloma Leuk. 2023, 23, 57–67. [Google Scholar] [CrossRef]
- Eyre, T.A.; Hess, L.M.; Sugihara, T.; He, D.; Khanal, M.; Pagel, J.M.; Walgren, R.; Abada, P.B.; Konig, H.; Roeker, L.; et al. Clinical outcomes among patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) who received treatment with a covalent BTK and BCL2 inhibitor in the United States: A real-world database study. Leuk. Lymphoma 2023, 64, 1005–1016. [Google Scholar] [CrossRef]
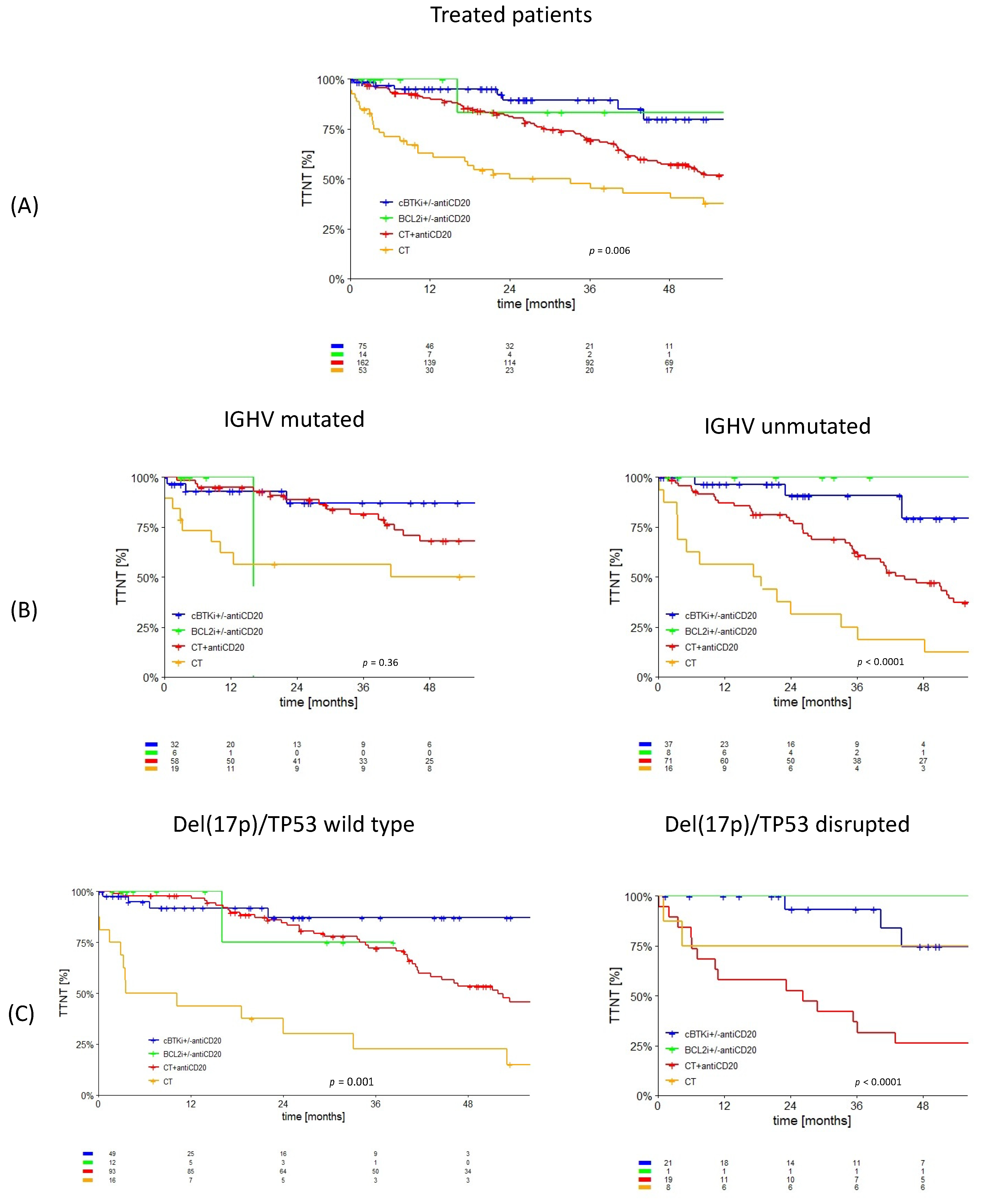

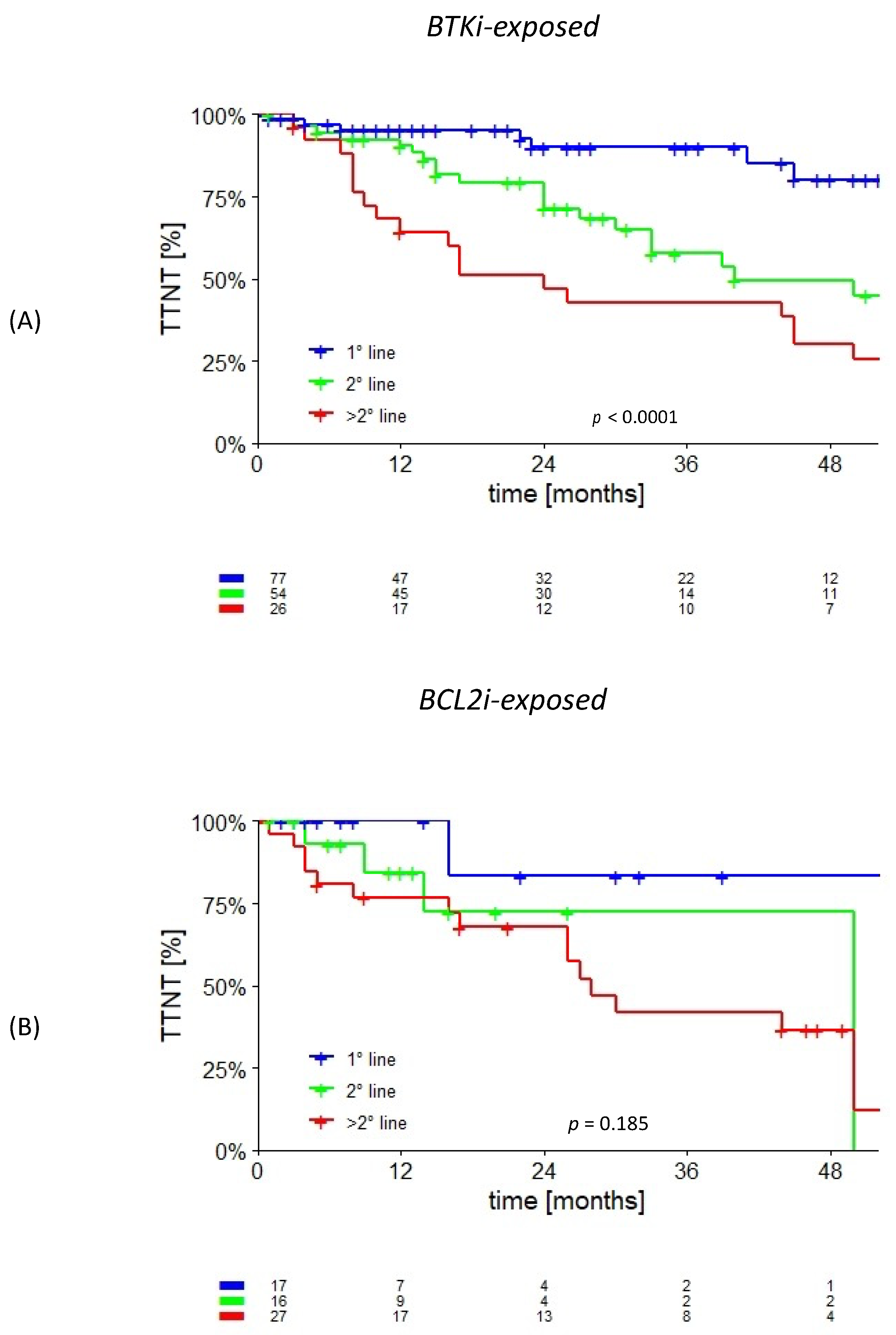
| Entire Cohort (n = 637) | Treated Cohort (n = 318) | ||
|---|---|---|---|
| Age, years (median, IQR) | 64 (56–72) | 65 (58–72) | |
| Gender, n (%) | Male | 369 (57.9%) | 201 (63.2%) |
| Female | 268 (42.1%) | 117 (36.8%) | |
| FISH analysis, n (%) | Deletion 17p | 30/475 (6.3%) | 28/279 (10.0%) |
| Deletion 13q | 202/470 (43.0%) | 123/276 (44.6%) | |
| Deletion 11q | 42/470 (8.9%) | 36/276 (13.0%) | |
| Trisomy 12 | 72/470 (15.3%) | 43/276 (15.6%) | |
| TP53, n (%) | Mutated | 46 (7.2%) | 42 (13.2%) |
| Unmutated | 280 (44.0%) | 190 (59.7%) | |
| Missing | 311 (48.8%) | 86 (27.0%) | |
| Del (17p) and/or TP53 mut, n (%) | 59 (9.3%) | 53 (16.7%) | |
| IGHV, n (%) | Mutated | 246 (38.6%) | 118 (37.1%) |
| Unmutated | 170 (26.7%) | 137 (43.1%) | |
| Missing | 221 (34.7%) | 63 (19.8%) | |
| First-line treatment, n (%) | No | 319 (50.1%) | / |
| Yes | 318 (49.9%) | 318 (100%) | |
| Time to Treatment, months (median, IQR) | / | 24 (7–51) | |
| cBTKi- and BCL2i-naïve, n (%) | / | 127 (39.9%) | |
| cBTKi-exposed, n (%) | / | 157 (49.4%) | |
| BCL2i-exposed, n (%) | / | 60 (18.9%) | |
| BCL2i-exposed cBTKi-naïve, n (%) | / | 34 (10.7%) | |
| cBTKi- and BCL2i- exposed, n (%) | / | 26 (8.2%) | |
| CIT, cBTKi-, and BCL2i-exposed, n (%) | / | 17 (5.3%) | |
| First-line Treatment, n (%) cBTKi +/− antiCD20 BCL2i +/− antiCD20 cBTKi+BCL2i CT+antiCD20 CT Other | / | 75 (23.6%) 14 (4.4%) 2 (0.6%) 162 (50.9%) 53 (16.7%) 12 (3.8%) | |
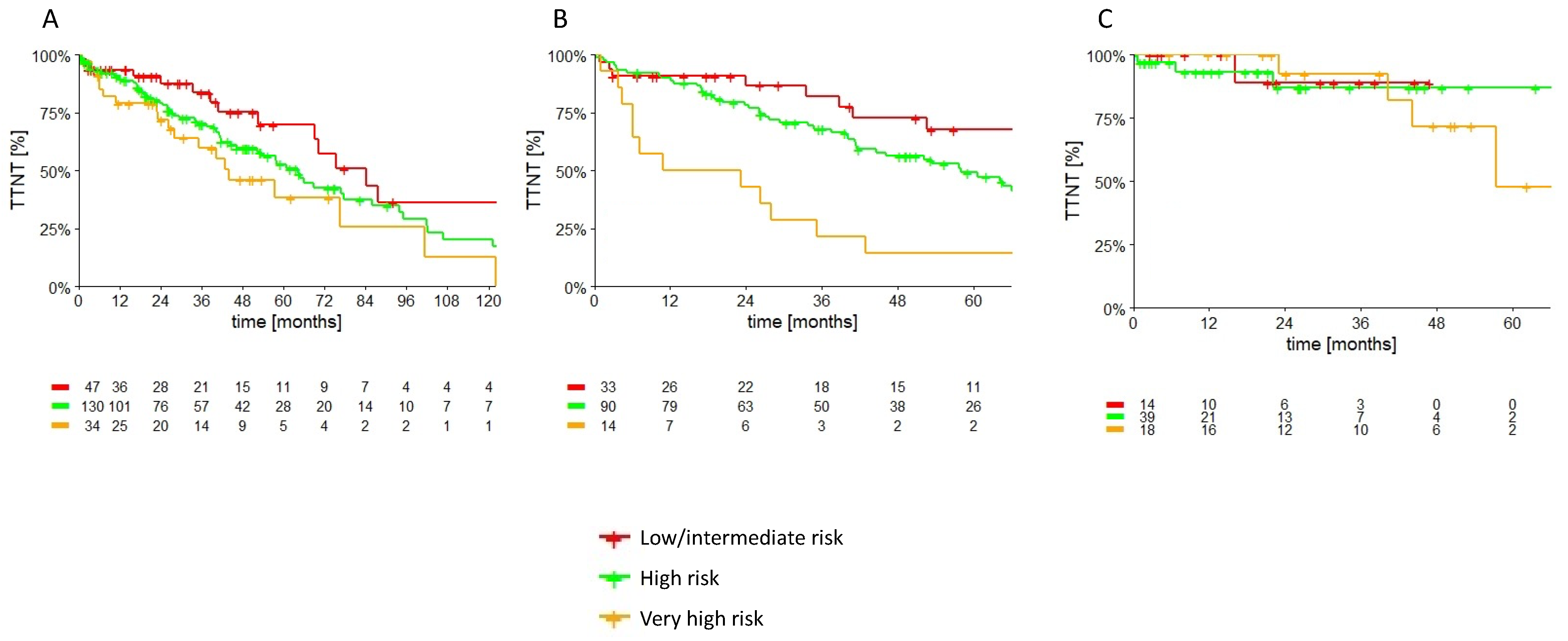
| Treated Cohort | p | CT +/− anti-CD20 Cohort | p | Inhibitors Cohort | p | |
|---|---|---|---|---|---|---|
| Low/intermediate risk CLL-IPI at treatment 3-year TTNT | 83.8% (95% CI: 73.4–94.2) | 0.052 | 82.2% (95% CI: 67.7–96.7) | <0.001 | 88.9% (95% CI: 68.3–100.0) | 0.88 |
| High risk CLL-IPI at treatment 3-year TTNT | 70.3% (95% CI: 61.3–79.3) | 67.9% (95% CI: 57.9–77.9) | 87.0% (95% CI: 72.5–100.0) | |||
| Very high risk CLL-IPI at treatment 3-year TTNT | 59.8% (95% CI: 41.8–77.8) | 21.4% (95% CI: 0.0–43.0) | 92.3% (95% CI: 77.8–100.0) |
| Cohort | Median Age (Years) | CT +/− antiCD20, n (%) | cBTKi +/− antiCD20, n (%) | BCL2 +/− antiCD20, n (%) | cBTKi+BCL2, n (%) | PI3Ki, n (%) | Other, n (%) | Total |
|---|---|---|---|---|---|---|---|---|
| Frontline treatment | ||||||||
| 1992–2013 | 63 | 68 (94.4) | 0 | 0 | 0 | 0 | 4 (5.6) | 72 |
| 2014–2019 | 69 | 105 (77.2) | 25 (18.4) | 2 (1.5) | 0 | 0 | 4 (2.9) | 136 |
| 2020–2023 | 70 | 41 (37.7) | 50 (45.9) | 12 (11.0) | 2 (1.8) | 0 | 4 (3.7) | 109 |
| Relapsed/refractory treatment | ||||||||
| 1992–2013 | 62 | 14 (70.0) | 0 | 0 | 0 | 0 | 6 (30.0) | 20 |
| 2014–2019 | 69 | 38 (30.6) | 50 (40.3) | 16 (12.9) | 1 (0.8) | 16 (12.9) | 3 (2.4) | 124 |
| 2020–2023 | 69 | 18 (20.7) | 32 (36.8) | 30 (34.5) | 0 | 1 (1.1) | 6 (6.9) | 87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Innocenti, I.; Fresa, A.; Tomasso, A.; Tarnani, M.; De Padua, L.; Benintende, G.; Pasquale, R.; Galli, E.; Morelli, F.; Giannarelli, D.; et al. Treatment Sequencing and Outcome of Chronic Lymphocytic Leukemia Patients Treated at Fondazione Policlinico Universitario Agostino Gemelli IRCCS: A Thirty-Year Single-Center Experience. Cancers 2023, 15, 5592. https://doi.org/10.3390/cancers15235592
Innocenti I, Fresa A, Tomasso A, Tarnani M, De Padua L, Benintende G, Pasquale R, Galli E, Morelli F, Giannarelli D, et al. Treatment Sequencing and Outcome of Chronic Lymphocytic Leukemia Patients Treated at Fondazione Policlinico Universitario Agostino Gemelli IRCCS: A Thirty-Year Single-Center Experience. Cancers. 2023; 15(23):5592. https://doi.org/10.3390/cancers15235592
Chicago/Turabian StyleInnocenti, Idanna, Alberto Fresa, Annamaria Tomasso, Michela Tarnani, Laura De Padua, Giulia Benintende, Raffaella Pasquale, Eugenio Galli, Francesca Morelli, Diana Giannarelli, and et al. 2023. "Treatment Sequencing and Outcome of Chronic Lymphocytic Leukemia Patients Treated at Fondazione Policlinico Universitario Agostino Gemelli IRCCS: A Thirty-Year Single-Center Experience" Cancers 15, no. 23: 5592. https://doi.org/10.3390/cancers15235592
APA StyleInnocenti, I., Fresa, A., Tomasso, A., Tarnani, M., De Padua, L., Benintende, G., Pasquale, R., Galli, E., Morelli, F., Giannarelli, D., Autore, F., & Laurenti, L. (2023). Treatment Sequencing and Outcome of Chronic Lymphocytic Leukemia Patients Treated at Fondazione Policlinico Universitario Agostino Gemelli IRCCS: A Thirty-Year Single-Center Experience. Cancers, 15(23), 5592. https://doi.org/10.3390/cancers15235592







