Association between Immune Checkpoint Inhibitor Treatment Outcomes and Body Composition Factors in Metastatic Renal Cell Carcinoma Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Body Composition Analysis
2.3. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Association between Body Composition Markers and Objective Responses
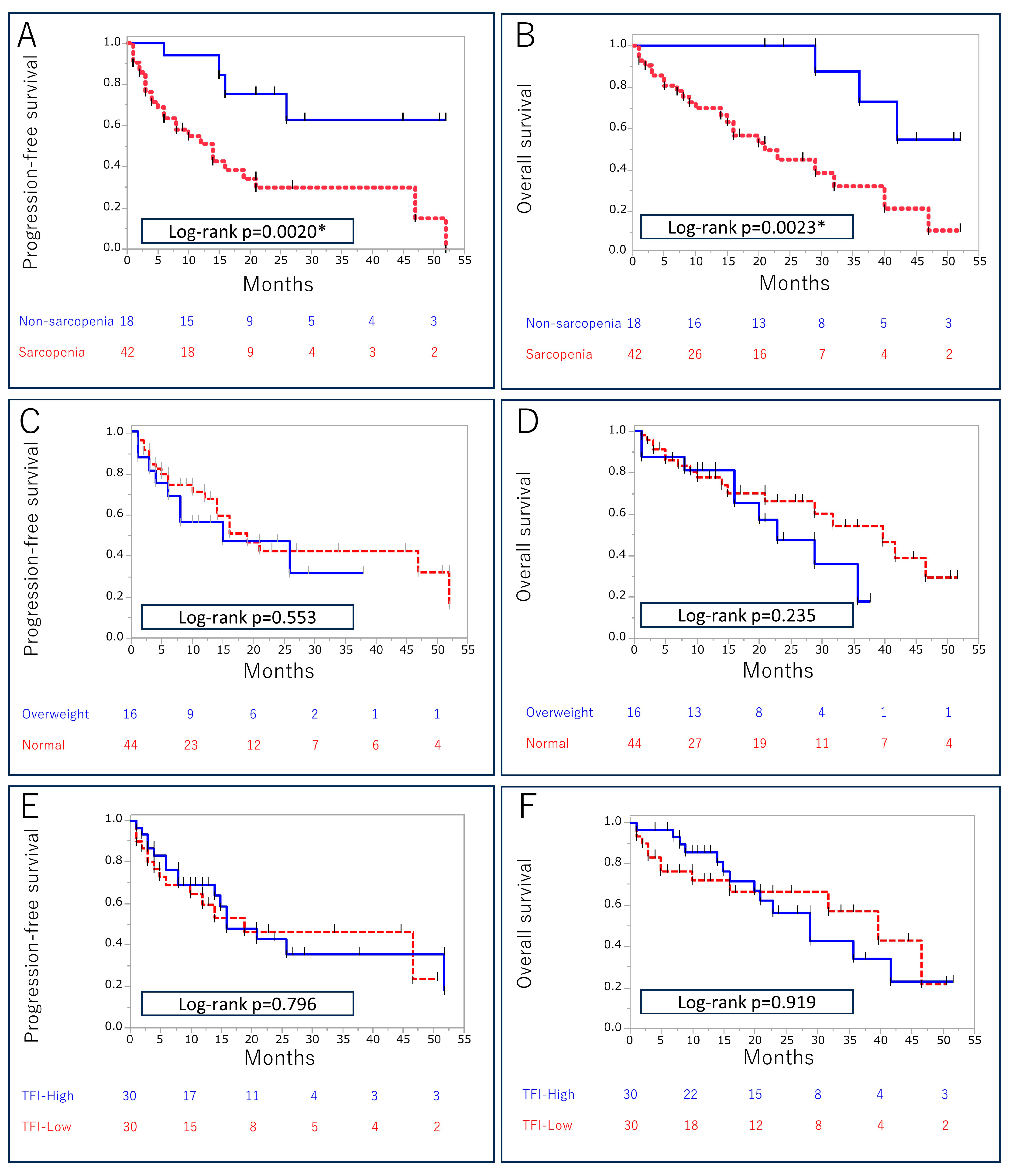
3.3. Association between Body Composition Markers and Survival Outcomes
3.4. Association between Body Composition Markers and irAEs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Motzer, R.J.; Jonasch, E.; Boyle, S.; Carlo, M.I.; Manley, B.; Agarwal, N.; Alva, A.; Beckermann, K.; Choueiri, T.K.; Costello, B.A.; et al. NCCN Guidelines Insights: Kidney Cancer, Version 1. J. Natl. Compr. Cancer Netw. 2020, 18, 1160–1170. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Motzer, R.J.; Rini, B.I.; McDermott, D.F.; Aren Frontera, O.; Hammers, H.J.; Carducci, M.A.; Salman, P.; Escudier, B.; Beuselinck, B.; Amin, A.; et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: Extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019, 20, 1370–1385. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Mori, K.; Katayama, S.; Mostafaei, H.; Quhal, F.; Laukhtina, E.; Rajwa, P.; Motlagh, R.S.; Aydh, A.; König, F.; et al. Hematological prognosticators in metastatic renal cell cancer treated with immune checkpoint inhibitors: A meta-analysis. Immunotherapy 2022, 14, 709–725. [Google Scholar] [CrossRef]
- Kijima, T.; Kubo, T.; Nishihara, D.; Nukui, A.; Hirohashi, Y.; Torigoe, T.; Kamai, T. Cancer immunohistogram representing cancer-immunity cycle by immunohistochemistry predicts the efficacy of immune checkpoint inhibitors in urological cancer patients. Sci. Rep. 2022, 12, 10617. [Google Scholar] [CrossRef]
- Motzer, R.J.; Choueiri, T.K.; McDermott, D.F.; Powles, T.; Vano, Y.-A.; Gupta, S.; Yao, J.; Han, C.; Ammar, R.; Papillon-Cavanagh, S.; et al. Biomarker analysis from CheckMate 214: Nivolumab plus ipilimumab versus sunitinib in renal cell carcinoma. J. Immunother. Cancer 2022, 10, e004316. [Google Scholar] [CrossRef]
- Lennon, H.; Sperrin, M.; Badrick, E.; Renehan, A.G. The Obesity Paradox in Cancer: A Review. Curr. Oncol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.; Massari, F.; Myint, Z.W.; Iacovelli, R.; Pichler, M.; Basso, U.; Kopecky, J.; Kucharz, J.; Buti, S.; Salfi, A.; et al. Clinico-Pathological Features Influencing the Prognostic Role of Body Mass Index in Patients with Advanced Renal Cell Carcinoma Treated by Immuno-Oncology Combinations (ARON-1). Clin. Genitourin. Cancer 2023, 21, e309–e319.e1. [Google Scholar] [CrossRef] [PubMed]
- Vrieling, A.; Kampman, E.; Knijnenburg, N.C.; Mulders, P.F.; Sedelaar, J.M.; Baracos, V.E.; Kiemeney, L.A. Body Composition in Relation to Clinical Outcomes in Renal Cell Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2018, 4, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.J.; Olsen, T.A.; Goyal, S.; Liu, Y.; Evans, S.T.; Magod, B.; Brown, J.T.; Yantorni, L.; Russler, G.A.; Caulfield, S.; et al. Body Composition Variables as Radiographic Biomarkers of Clinical Outcomes in Metastatic Renal Cell Carcinoma Patients Receiving Immune Checkpoint Inhibitors. Front. Oncol. 2021, 11, 707050. [Google Scholar] [CrossRef] [PubMed]
- Ged, Y.; Sanchez, A.; Patil, S.; Knezevic, A.; Stein, E.; Petruzella, S.; Weiss, K.; Duzgol, C.; Chaim, J.; Akin, O.; et al. Associations between Pretreatment Body Composition Features and Clinical Outcomes among Patients with Metastatic Clear Cell Renal Cell Carcinoma Treated with Immune Checkpoint Blockade. Clin. Cancer Res. 2022, 28, 5180–5189. [Google Scholar] [CrossRef] [PubMed]
- McManus, H.D.; Zhang, D.; Schwartz, F.R.; Wu, Y.; Infield, J.; Ho, E.; Armstrong, A.J.; George, D.J.; Kruse, D.; Gupta, R.T.; et al. Relationship between Pretreatment Body Composition and Clinical Outcomes in Patients with Metastatic Renal Cell Carcinoma Receiving First-Line Ipilimumab Plus Nivolumab. Clin. Genitourin. Cancer 2023. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.A.; Dubois, S.; Miljkovic, M.D.; Conlon, K.C. IL-15 in the Combination Immunotherapy of Cancer. Front. Immunol. 2020, 11, 868. [Google Scholar] [CrossRef]
- Park, S.-Y.; Hwang, B.-O.; Song, N.-Y. The role of myokines in cancer: Crosstalk between skeletal muscle and tumor. BMB Rep. 2023, 56, 365–373. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Calarota, S.A.; Vidali, F.; MacDonald, T.T.; Corazza, G.R. Role of IL-15 in immune-mediated and infectious diseases. Cytokine Growth Factor Rev. 2011, 22, 19–33. [Google Scholar] [CrossRef]
- Reyes, A.F.; Goldusky, J.; Bhimalli, P.; Marzo, A.L.; Schneider, J.R. Tracking fluorescently labeled IL-15 and anti-PD-1 in the tumor microenvironment and draining lymph nodes. J. Immunol. Methods 2022, 505, 113253. [Google Scholar] [CrossRef]
- Stravokefalou, V.; Stellas, D.; Karaliota, S.; Nagy, B.A.; Valentin, A.; Bergamaschi, C.; Dimas, K.; Pavlakis, G.N. Heterodimeric IL-15 (hetIL-15) reduces circulating tumor cells and metastasis formation improving chemotherapy and surgery in 4T1 mouse model of TNBC. Front. Immunol. 2022, 13, 1014802. [Google Scholar] [CrossRef]
- Miljkovic, M.D.; Dubois, S.P.; Müller, J.R.; Bryant, B.R.; Ma, E.; Conlon, K.C.; Waldmann, T.A. Interleukin-15 augments NK cell–mediated ADCC of alemtuzumab in patients with CD52+ T-cell malignancies. Blood Adv. 2023, 7, 384–394. [Google Scholar] [CrossRef]
- Wrangle, J.M.; Velcheti, V.; Patel, M.R.; Garrett-Mayer, E.; Hill, E.G.; Ravenel, J.G.; Miller, J.S.; Farhad, M.; Anderton, K.; Lindsey, K.; et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: A non-randomised, open-label, phase 1b trial. Lancet Oncol. 2018, 19, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Felices, M.; Chu, S.; Kodal, B.; Bendzick, L.; Ryan, C.; Lenvik, A.; Boylan, K.; Wong, H.; Skubitz, A.; Miller, J.; et al. IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol. Oncol. 2017, 145, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Kanzleiter, T.; Rath, M.; Görgens, S.W.; Jensen, J.; Tangen, D.S.; Kolnes, A.J.; Kolnes, K.J.; Lee, S.; Eckel, J.; Schürmann, A.; et al. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem. Biophys. Res. Commun. 2014, 450, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Neill, T.; Schaefer, L.; Iozzo, R.V. Decorin as a multivalent therapeutic agent against cancer. Adv. Drug Deliv. Rev. 2016, 97, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Ostrand-Rosenberg, S. Myeloid-derived suppressor cells: More mechanisms for inhibiting antitumor immunity. Cancer Immunol. Immunother. 2010, 59, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Limagne, E.; Richard, C.; Thibaudin, M.; Fumet, J.-D.; Truntzer, C.; Lagrange, A.; Favier, L.; Coudert, B.; Ghiringhelli, F. Tim-3/galectin-9 pathway and mMDSC control primary and secondary resistances to PD-1 blockade in lung cancer patients. OncoImmunology 2019, 8, e1564505. [Google Scholar] [CrossRef] [PubMed]
- Serafini, P.; Mgebroff, S.; Noonan, K.; Borrello, I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res 2008, 68, 5439–5449. [Google Scholar] [CrossRef]
- Mendes, M.C.S.; Pimentel, G.D.; O Costa, F.; Carvalheira, J.B.C. Molecular and neuroendocrine mechanisms of cancer cachexia. J. Endocrinol. 2015, 226, R29–R43. [Google Scholar] [CrossRef]
- Patel, H.J.; Patel, B.M. TNF-α and cancer cachexia: Molecular insights and clinical implications. Life Sci. 2017, 170, 56–63. [Google Scholar] [CrossRef]
- Lu, C.; Talukder, A.; Savage, N.M.; Singh, N.; Liu, K. JAK-STAT-mediated chronic inflammation impairs cytotoxic T lymphocyte activation to decrease anti-PD-1 immunotherapy efficacy in pancreatic cancer. OncoImmunology 2017, 6, e1291106. [Google Scholar] [CrossRef]
- Wu, B.; Sun, X.; Gupta, H.B.; Yuan, B.; Li, J.; Ge, F.; Chiang, H.-C.; Zhang, X.; Zhang, C.; Zhang, D.; et al. Adipose PD-L1 Modulates PD-1/PD-L1 Checkpoint Blockade Immunotherapy Efficacy in Breast Cancer. OncoImmunology 2018, 7, e1500107. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.W.; Dixit, V.D. Adipose tissue as an immunological organ. Obesity 2015, 23, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, L.; Xia, Q.; Yuan, Y.; Mi, Y. The impact of metformin use on survival in kidney cancer patients with diabetes: A meta-analysis. Int. Urol. Nephrol. 2017, 49, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Keizman, D.; Ish-Shalom, M.; Sella, A.; Gottfried, M.; Maimon, N.; Peer, A.; Hammers, H.; Eisenberger, M.A.; Sinibaldi, V.; Neiman, V.; et al. Metformin Use and Outcome of Sunitinib Treatment in Patients with Diabetes and Metastatic Renal Cell Carcinoma. Clin. Genitourin. Cancer 2016, 14, 420–425. [Google Scholar] [CrossRef]
- Eskelinen, T.; Veitonmäki, T.; Kotsar, A.; Tammela, T.L.J.; Pöyhönen, A.; Murtola, T.J. Improved renal cancer prognosis among users of drugs targeting renin-angiotensin system. Cancer Causes Control. 2022, 33, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Roeland, E.J.; Bohlke, K.; Baracos, V.E.; Smith, T.J.; Loprinzi, C.L.; Bruera, E.; del Fabbro, E.; Dixon, S.; Fallon, M.; Herrstedt, J.; et al. Cancer Cachexia: ASCO Guideline Rapid Recommendation Update. J. Clin. Oncol. 2023, 41, 4178–4179. [Google Scholar] [CrossRef] [PubMed]
- de van der Schueren, M.; Laviano, A.; Blanchard, H.; Jourdan, M.; Arends, J.; Baracos, V. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: Current evidence and guidance for design of future trials. Ann. Oncol. 2018, 29, 1141–1153. [Google Scholar] [CrossRef]
- Garcia, V.R.; López-Briz, E.; Sanchis, R.C.; Perales, J.L.G.; Bort-Martí, S. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst. Rev. 2013, 2019, CD004310. [Google Scholar] [CrossRef]
- Yavuzsen, T.; Davis, M.P.; Walsh, D.; LeGrand, S.; Lagman, R. Systematic review of the treatment of cancer-associated anorexia and weight loss. J. Clin. Oncol. 2005, 23, 8500–8511. [Google Scholar] [CrossRef]
- Solheim, T.S.; Laird, B.J.; Balstad, T.R.; Stene, G.B.; Bye, A.; Johns, N.; Pettersen, C.H.; Fallon, M.; Fayers, P.; Fearon, K.; et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 778–788. [Google Scholar] [CrossRef]
- Kijima, T.; Fukushima, H.; Kusuhara, S.; Tanaka, H.; Yoshida, S.; Yokoyama, M.; Ishioka, J.; Matsuoka, Y.; Numao, N.; Sakai, Y.; et al. Association between the Occurrence and Spectrum of Immune-Related Adverse Events and Efficacy of Pembrolizumab in Asian Patients with Advanced Urothelial Cancer: Multicenter Retrospective Analyses and Systematic Literature Review. Clin. Genitourin. Cancer 2021, 19, 208–216.e1. [Google Scholar] [CrossRef] [PubMed]
- Ueki, H.; Hara, T.; Okamura, Y.; Bando, Y.; Terakawa, T.; Furukawa, J.; Harada, K.; Nakano, Y.; Fujisawa, M. Association between sarcopenia based on psoas muscle index and the response to nivolumab in metastatic renal cell carcinoma: A retrospective study. Investig. Clin. Urol. 2022, 63, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, N.; Uchino, J.; Hirai, S.; Katayama, Y.; Yoshimura, A.; Okura, N.; Tanimura, K.; Harita, S.; Imabayashi, T.; Chihara, Y.; et al. Association of Sarcopenia with and Efficacy of Anti-PD-1/PD-L1 Therapy in Non-Small-Cell Lung Cancer. J. Clin. Med. 2019, 8, 450. [Google Scholar] [CrossRef] [PubMed]
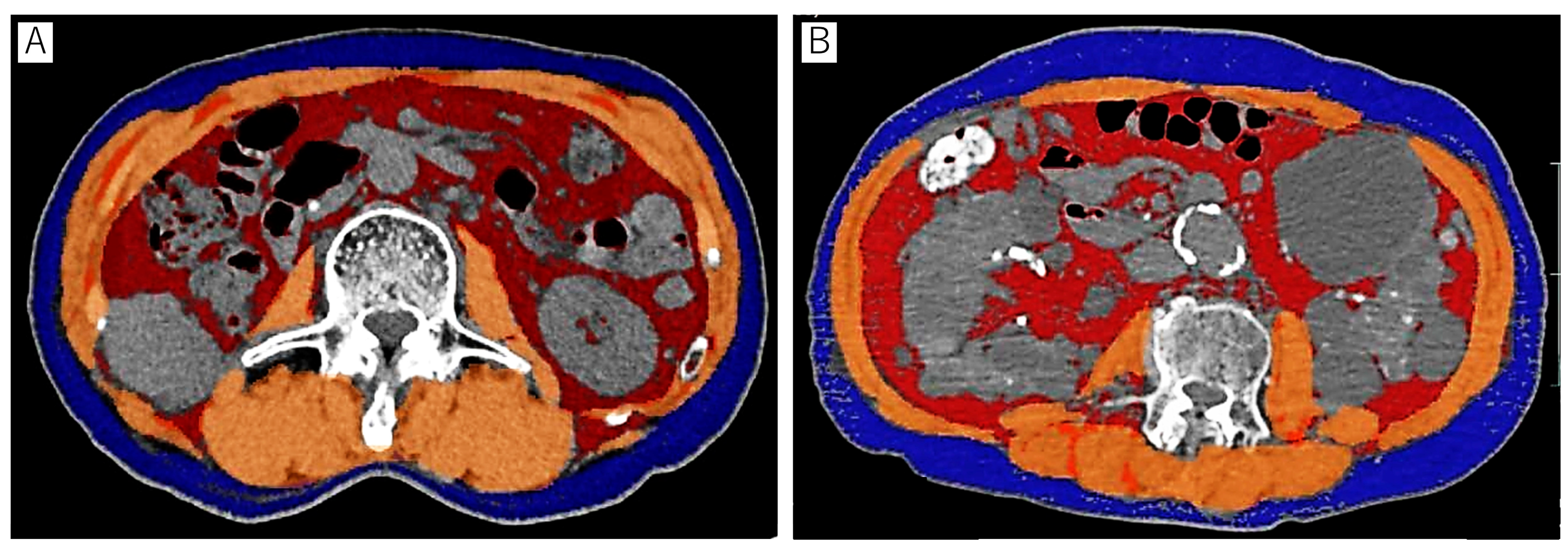
| Overall | |
|---|---|
| n = 60 | |
| Age (years), median (IQR) | 71 (63–75) |
| Sex, n (%) | |
| Male | 46 (77) |
| Female | 14 (23) |
| ICI-based therapy, n (%) | |
| ICI combination | 34 (57) |
| ICIs + TKIs | 26 (43) |
| Prior nephrectomy, n (%) | |
| Yes | 27 (45) |
| No | 33 (55) |
| ECOG PS, n (%) | |
| ≤1 | 38 (63) |
| ≥2 | 22 (37) |
| Clinical T stage, n (%) | |
| ≤2 | 20 (33) |
| ≥3 | 40 (67) |
| Histology, n (%) | |
| Clear cell carcinoma | 44 (73) |
| Non-clear cell carcinoma | 10 (17) |
| Unknown | 6 (10) |
| IMDC risk score, n (%) | |
| Intermediate | 38 (63) |
| Poor | 22 (37) |
| Site of metastasis, n (%) | |
| Lymph nodes | 20 (33) |
| Lung | 35 (58) |
| Liver | 6 (10) |
| Other organs | 28 (47) |
| NLR, median (IQR) | 3.52 (2.29–4.84) |
| PLR, median (IQR) | 188 (133–244) |
| CRP (mg/dL), median (IQR) | 0.81 (0.08–5.96) |
| BMI (kg/m2), median (IQR) | 22.9 (20.9–25.2) |
| SMI (kg/m2), median (IQR) | 41.1 (37.1–49.6) |
| SFI (kg/m2), median (IQR) | 31.7 (20.7–51.3) |
| VFI (kg/m2), median (IQR) | 36.1 (19.1–56.8) |
| TFI (kg/m2), median (IQR) | 80.2 (42.7–103) |
| Sarcopenia | Non-Sarcopenia | p-Value | |
|---|---|---|---|
| n = 42 | n = 18 | ||
| Age (years), median (IQR) | 72 (64–75) | 69 (62–73) | 0.428 |
| Sex, n (%) | 0.0455 * | ||
| Male | 29 (69) | 17 (94) | |
| Female | 13 (31) | 1 (6) | |
| ICI-based therapy, n (%) | 0.779 | ||
| ICI combination | 23 (55) | 11 (61) | |
| ICIs + TKIs | 19 (45) | 7 (39) | |
| Prior nephrectomy, n (%) | 0.778 | ||
| Yes | 18 (43) | 9 (50) | |
| No | 24 (57) | 9 (50) | |
| ECOG PS, n (%) | 0.773 | ||
| ≤1 | 25 (60) | 12 (67) | |
| ≥2 | 17 (40) | 6 (33) | |
| Clinical T stage, n (%) | 0.766 | ||
| ≤2 | 15 (36) | 5 (28) | |
| ≥3 | 27 (64) | 13 (72) | |
| Histology, n (%) | 0.0417 * | ||
| Clear cell carcinoma | 27 (64) | 17 (94) | |
| Non-clear cell carcinoma | 10 (24) | 0 | |
| Unknown | 5 (12) | 1(6) | |
| IMDC risk score, n (%) | 0.155 | ||
| Intermediate | 24 (57) | 14 (78) | |
| Poor | 18 (43) | 4 (22) | |
| Site of metastasis, n (%) | |||
| Lymph nodes | 14 (33) | 6 (33) | 1.000 |
| Lung | 25 (60) | 10 (56) | 0.783 |
| Liver | 5 (12) | 1 (6) | 0.658 |
| Other organs | 23 (55) | 5 (28) | 0.0893 |
| NLR, median (IQR) | 3.89 (2.78–5.64) | 2.56 (1.93–3.86) | 0.0209 * |
| PLR, median (IQR) | 195 (145–307) | 171 (112–196) | 0.0330 * |
| CRP (mg/dL), median (IQR) | 1.16 (0.06–6.19) | 0.34 (0.14–2.79) | 0.237 |
| BMI (kg/m2), median (IQR) | 22.7 (19.9–24.5) | 24.4 (22.2–28.4) | 0.0062 * |
| SMI (kg/m2), median (IQR) | 39.3 (35.5–41.9) | 51.4 (46.1–55.3) | <0.0001 * |
| SFI (kg/m2), median (IQR) | 29.8 (14.5–51.6) | 33.6 (25.7–51.5) | 0.297 |
| VFI (kg/m2), median (IQR) | 30.8 (15.7–54.3) | 53.2 (35.3–73.9) | 0.0262 * |
| TFI (kg/m2), median (IQR) | 72.7 (38.2–100) | 93 (64.5–127) | 0.0407 * |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variable Category | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Age (continuous) | 0.96 (0.93–0.99) | 0.0210 * | ||
| Sex Female vs. male (ref) | 1.83 (0.76–5.47) | 0.191 | ||
| ECOG PS 2 vs. 0–1 (ref) | 1.64 (0.77–3.41) | 0.190 | ||
| IMDC risk Poor vs. intermediate (ref) | 2.79 (1.32–6.00) | <0.0077 * | 2.61 (1.22–5.67) | 0.0136 * |
| Liver metastases Yes vs. no (ref) | 2.25 (0.66–5.85) | 0.175 | ||
| NLR (continuous) | 1.20 (1.08–1.32) | 0.0011 * | ||
| PLR (continuous) | 1.003 (1.000–1.004) | 0.0275 * | ||
| BMI Overweight vs. normal (ref) | 0.79 (0.37–1.83) | 0.566 | ||
| SFI Low vs. high (ref) | 0.81 (0.39–1.66) | 0.553 | ||
| VFI Low vs. high (ref) | 0.87 (0.42–1.82) | 0.717 | ||
| TFI Low vs. high (ref) | 1.10 (0.52–2.29) | 0.799 | ||
| Sarcopenia Yes vs. no (ref) | 4.52 (1.75–15.4) | 0.0010 * | 4.31 (1.65–14.8) | 0.0018 * |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variable Category | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Age (continuous) | 0.97 (0.93–1.00) | 0.0728 | ||
| Sex Female vs. male (ref) | 1.03 (0.37–2.42) | 0.953 | ||
| ECOG PS 2 vs. 0–1 (ref) | 1.84 (0.82–4.05) | 0.136 | ||
| IMDC risk Poor vs. intermediate (ref) | 3.37 (1.52–7.80) | 0.0029 * | 3.30 (1.48–7.76) | 0.0037 * |
| Liver metastases Yes vs. no (ref) | 2.35 (0.68–6.27) | 0.158 | ||
| NLR (continuous) | 1.18 (1.05–1.30) | 0.0058 * | ||
| PLR (continuous) | 1.002 (0.99–1.004) | 0.0527 | ||
| BMI Overweight vs. normal (ref) | 1.65 (0.68–3.38) | 0.254 | ||
| SFI Low vs. high (ref) | 1.05 (0.47–2.30) | 0.899 | ||
| VFI Low vs. high (ref) | 1.20 (0.55–2.65) | 0.636 | ||
| TFI Low vs. high (ref) | 1.04 (0.48–2.30) | 0.919 | ||
| Sarcopenia Yes vs. no (ref) | 5.45 (1.86–23.2) | 0.0010 * | 5.44 (1.83–23.4) | 0.0013 * |
| All-Grade irAEs, n(%) | p-Value | irAEs ≥ Grade 3, n (%) | p-Value | |
|---|---|---|---|---|
| Sarcopenia | ||||
| Yes (n = 42) | 20 (48%) | 0.0463 * | 12 (29%) | 0.144 |
| No (n = 18) | 14 (78%) | 9 (50%) | ||
| BMI | ||||
| Overweight (n = 16) | 9 (56%) | 1.000 | 6 (38%) | 1.000 |
| Normal (n = 44) | 25 (57%) | 15 (34%) | ||
| TFI | ||||
| High (n = 30) | 18 (60%) | 0.795 | 12 (40%) | 0.589 |
| Low (n = 30) | 16 (53%) | 9 (30%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takei, K.; Kijima, T.; Okubo, N.; Kurashina, R.; Kokubun, H.; Uematsu, T.; Betsunoh, H.; Yashi, M.; Kamai, T. Association between Immune Checkpoint Inhibitor Treatment Outcomes and Body Composition Factors in Metastatic Renal Cell Carcinoma Patients. Cancers 2023, 15, 5591. https://doi.org/10.3390/cancers15235591
Takei K, Kijima T, Okubo N, Kurashina R, Kokubun H, Uematsu T, Betsunoh H, Yashi M, Kamai T. Association between Immune Checkpoint Inhibitor Treatment Outcomes and Body Composition Factors in Metastatic Renal Cell Carcinoma Patients. Cancers. 2023; 15(23):5591. https://doi.org/10.3390/cancers15235591
Chicago/Turabian StyleTakei, Kohei, Toshiki Kijima, Naoya Okubo, Ryo Kurashina, Hidetoshi Kokubun, Toshitaka Uematsu, Hironori Betsunoh, Masahiro Yashi, and Takao Kamai. 2023. "Association between Immune Checkpoint Inhibitor Treatment Outcomes and Body Composition Factors in Metastatic Renal Cell Carcinoma Patients" Cancers 15, no. 23: 5591. https://doi.org/10.3390/cancers15235591
APA StyleTakei, K., Kijima, T., Okubo, N., Kurashina, R., Kokubun, H., Uematsu, T., Betsunoh, H., Yashi, M., & Kamai, T. (2023). Association between Immune Checkpoint Inhibitor Treatment Outcomes and Body Composition Factors in Metastatic Renal Cell Carcinoma Patients. Cancers, 15(23), 5591. https://doi.org/10.3390/cancers15235591







