The Role of Apolipoproteins in the Commonest Cancers: A Review
Abstract
Simple Summary
Abstract
1. Introduction
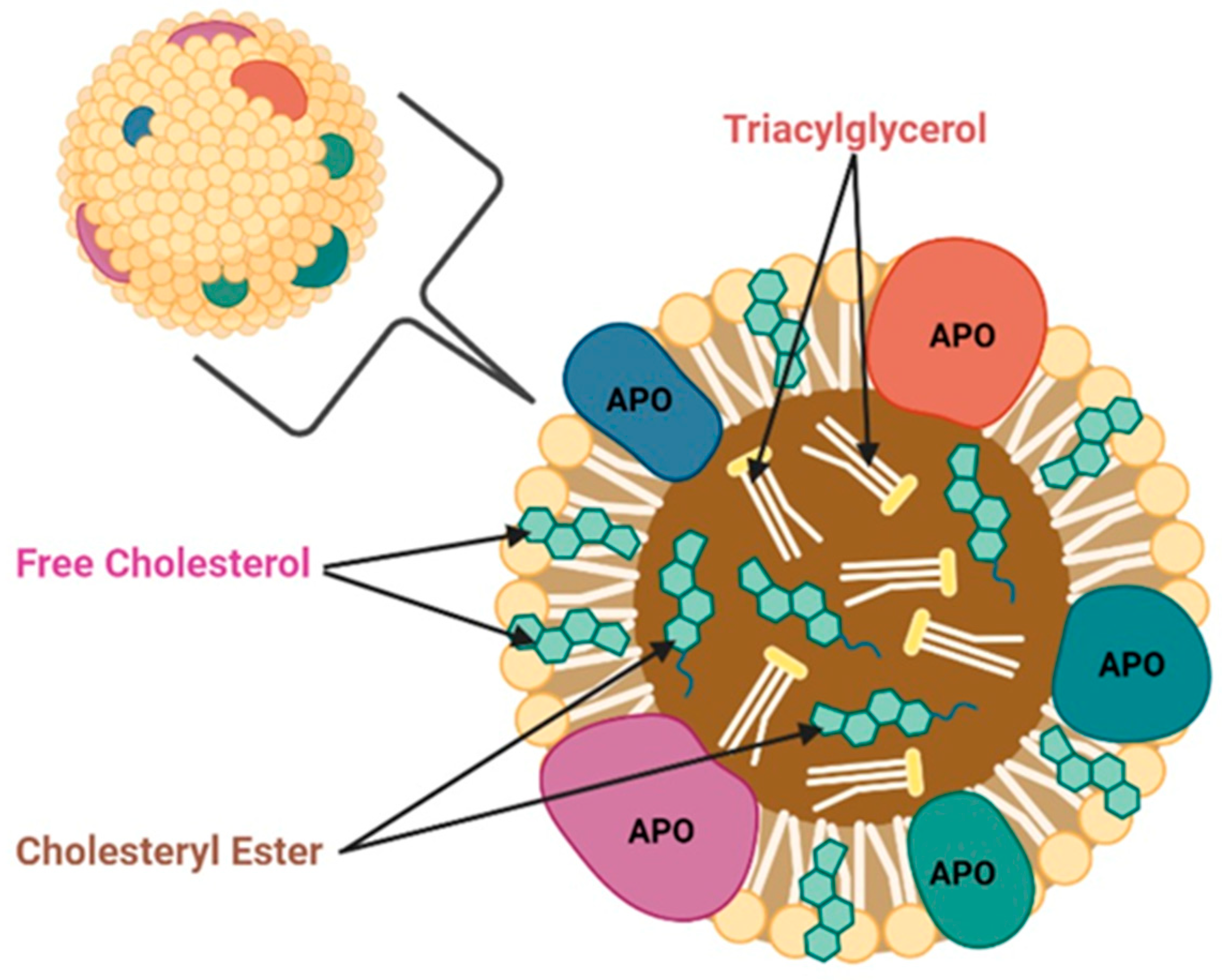
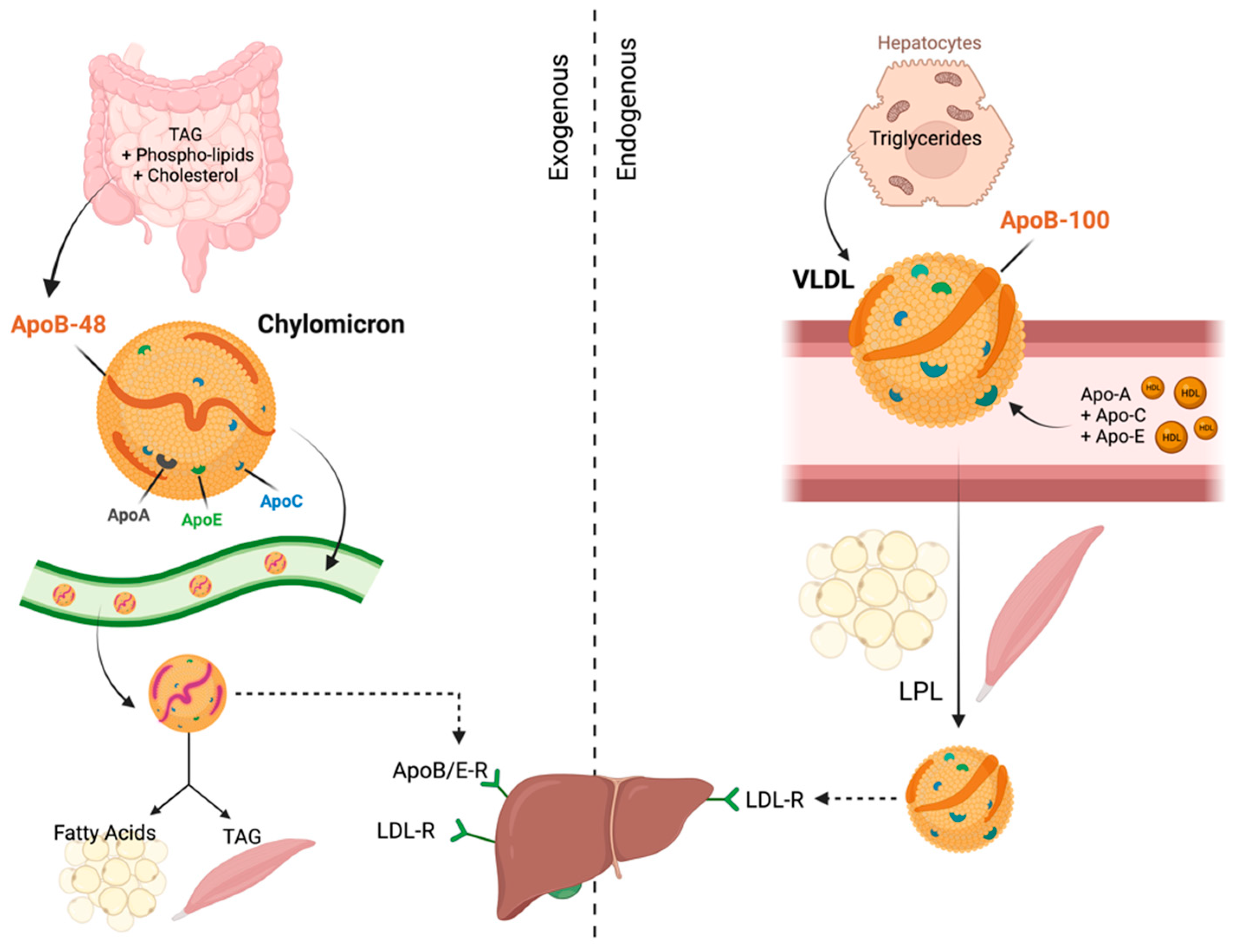
2. Functions of Apolipoproteins
3. Functions of Apolipoproteins in Normal Cells
4. Role of Apolipoproteins in Cancer
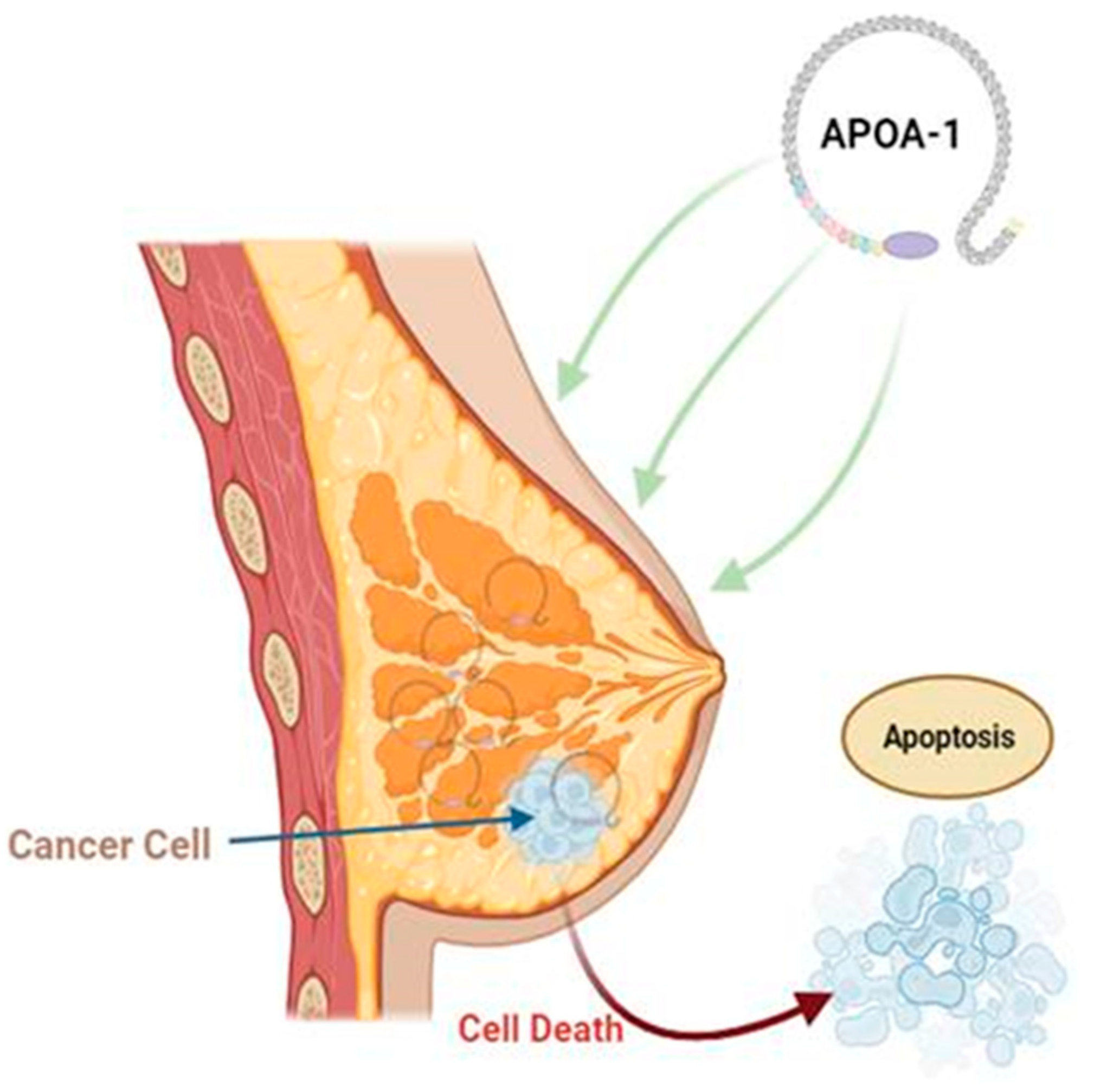
5. Role of Apolipoproteins in Breast Cancer
6. Apolipoproteins in Gynecological Cancers
7. Apolipoproteins in Lung Cancer
8. Apolipoproteins in Colorectal Cancer (CRC)
9. Apolipoproteins in Pancreatic Cancer
10. Apolipoproteins in Hepatic Cancer
11. Apolipoproteins in Prostate Cancer
12. Apolipoproteins in Gastric Cancer
13. Apolipoproteins in Thyroid Cancer
14. Inhibitors and Mimetic Peptides of Apolipoprotein
15. Future Research Directions
16. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Mahley, R.W.; Innerarity, T.L.; Rall, S.C.; Weisgraber, K.H. Plasma lipoproteins: Apolipoprotein structure and function. J. Lipid Res. 1984, 25, 1277–1294. [Google Scholar] [CrossRef]
- Gursky, O.; Dldc, C. Energetic-structure-function of lipoproteins and their protein components. In Recent Research Developments in Proteins; Transworld Research Network: Trivandrum, India, 1994; pp. 43–51. [Google Scholar]
- Cham, B.E. Importance of apolipoproteins in lipid metabolism. Chem. Interact. 1978, 20, 263–277. [Google Scholar] [CrossRef]
- Camejo, G.; Suárez, Z.M.; Muñoz, V. The apo-lipoproteins of human plasma high density lipoprotein: A study of their lipid binding capacity and interaction with lipid monolayers. Biochim. Biophys Acta 1970, 218, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.C.; Pownall, H.J.; Gotto, A.M. The plasma lipoproteins: Structure and metabolism. Annu. Rev. Biochem. 1978, 47, 751–777. [Google Scholar] [CrossRef]
- von Zychlinski, A.; Williams, M.; McCormick, S.; Kleffmann, T. Absolute quantification of apolipoproteins and associated proteins on human plasma lipoproteins. J. Proteom. 2014, 106, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yi, J.; Li, W.; Zheng, X.; Liu, J.; Wang, J.; Du, G. Apolipoproteins and cancer. Cancer Med. 2019, 8, 7032–7043. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, J.-M.; Zhang, D.; Zhang, Q.; Peng, B.; Xu, L.; Tang, H. ApoPred: Identification of Apolipoproteins and Their Subfamilies With Multifarious Features. Front. Cell Dev. Biol. 2021, 8, 621144. [Google Scholar] [CrossRef] [PubMed]
- Semenkovich, C.F. 213—Disorders of Lipid Metabolism. In Goldman’s Cecil Medicine, 24th ed.; Goldman, L., Schafer, A.I., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2012; pp. 1346–1354. [Google Scholar]
- Lu, Y.; Cui, X.; Zhang, L.; Wang, X.; Xu, Y.; Qin, Z.; Liu, G.; Wang, Q.; Tian, K.; Lim, K.S.; et al. The Functional Role of Lipoproteins in Atherosclerosis: Novel Directions for Diagnosis and Targeting Therapy. Aging Dis. 2022, 13, 491–520. [Google Scholar] [CrossRef]
- Dominiczak, M.H.; Caslake, M.J. Apolipoproteins: Metabolic role and clinical biochemistry applications. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2011, 48, 498–515. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. A receptor-mediated pathway for cholesterol homeostasis. Science 1986, 232, 34–47. [Google Scholar] [CrossRef]
- Beisiegel, U.; Weber, W.; Ihrke, G.; Herz, J.; Stanley, K.K. The LDL–receptor–related protein, LRP, is an apolipoprotein E-binding protein. Nature 1989, 341, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, A.; Miettinen, H.E.; Krieger, M. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocr. Rev. 2003, 24, 357–387. [Google Scholar] [CrossRef]
- Powell, L.M.; Wallis, S.C.; Pease, R.J.; Edwards, Y.H.; Knott, T.J.; Scott, J. A novel form of tissue-specific RNA processing produces apolipoprotein-B48 in intestine. Cell 1987, 50, 831–840. [Google Scholar] [CrossRef]
- Dominiczak, M.H. Lipoproteins in Health & Disease; DBetteridge, J., Illingworth, R., Shepherd, J., Eds.; De Gruyter: Berlin, Germany, 2000; Volume 38, p. 677. [Google Scholar]
- Eisenberg, S.; Sehayek, E. Remnant particles and their metabolism. Baillière’s Clin. Endocrinol. Metab. 1995, 9, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Bayly, G.R. Chapter 37—Lipids and disorders of lipoprotein metabolism. In Clinical Biochemistry: Metabolic and Clinical Aspects, 3rd ed.; Marshall, W.J., Lapsley, M., Day, A., Ayling, R., Eds.; Churchill Livingstone: London, UK, 2014; pp. 702–736. [Google Scholar]
- Cohn, J.S.; Marcoux, C.; Davignon, J. Detection, quantification, and characterization of potentially atherogenic triglyceriderich remnant lipoproteins. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2474–2486. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, S.; Bilheimer, D.B.; Levy, R.I.; Lindgren, F.T. On the metabolic conversion of human plasma very low density lipoprotein to low density lipoprotein. Biochim. Biophys Acta 1973, 326, 361–377. [Google Scholar] [CrossRef]
- Bruce, C.; Chouinard, R.A., Jr.; Tall, A.R. Plasma lipid transfer proteins, high-density lipoproteins, and reverse cholesterol transport. Annu. Rev. Nutr. 1998, 18, 297–330. [Google Scholar] [CrossRef]
- Bruckert, E.; Hansel, B. HDL-c is a powerful lipid predictor of cardiovascular diseases. Int. J. Clin. Pract. 2007, 61, 1905–1913. [Google Scholar] [CrossRef]
- Florentin, M.; Liberopoulos, E.N.; Wierzbicki, A.S.; Mikhailidis, D.P. Multiple actions of high-density lipoprotein. Curr. Opin. Cardiol. 2008, 23, 370–378. [Google Scholar] [CrossRef]
- Dieplinger, H.; Zechner, R.; Kostner, G.M. The in vitro formation of HDL2 during the action of LCAT: The role of triglyceride-rich lipoproteins. J. Lipid Res. 1985, 26, 273–282. [Google Scholar] [CrossRef]
- Acton, S.; Rigotti, A.; Landschulz, K.T.; Xu, S.; Hobbs, H.H.; Krieger, M. Identification of Scavenger Receptor SR-BI as a High Density Lipoprotein Receptor. Science 1996, 271, 518–520. [Google Scholar] [CrossRef]
- Wolska, A.; Dunbar, R.L.; Freeman, L.A.; Ueda, M.; Amar, M.J.; Sviridov, D.O.; Remaley, A.T. Apolipoprotein C-II: New findings related to genetics, biochemistry, and role in triglyceride metabolism. Atherosclerosis 2017, 267, 49–60. [Google Scholar] [CrossRef]
- Tabas, I.; Williams, K.J.; Borén, J. Subendothelial Lipoprotein Retention as the Initiating Process in Atherosclerosis. Circulation 2007, 116, 1832–1844. [Google Scholar] [CrossRef] [PubMed]
- Baenke, F.; Peck, B.; Miess, H.; Schulze, A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Model. Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef]
- Mineo, C.; Shaul, P.W. Novel biological functions of high-density lipoprotein cholesterol. Circ. Res. 2012, 111, 1079–1090. [Google Scholar] [CrossRef]
- Yang, D.-D.; Chen, Z.-H.; Wang, D.-S.; Yu, H.-E.; Lu, J.-H.; Xu, R.-H.; Zeng, Z.-L. Prognostic value of the serum apolipoprotein B to apolipoprotein A-I ratio in metastatic colorectal cancer patients. J. Cancer 2020, 11, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, L. Apolipoprotein: Prospective biomarkers in digestive tract cancer. Transl. Cancer Res. 2020, 9, 3712–3720. [Google Scholar] [CrossRef]
- Hamrita, B.; Nasr, H.B.; Gabbouj, S.; Bouaouina, N.; Chouchane, L.; Chahed, K. Apolipoprotein A1 −75 G/A and +83 C/T polymorphisms: Susceptibility and prognostic implications in breast cancer. Mol. Biol. Rep. 2011, 38, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- His, M.; Zelek, L.; Deschasaux, M.; Pouchieu, C.; Kesse-Guyot, E.; Hercberg, S.; Galan, P.; Latino-Martel, P.; Blacher, J.; Touvier, M. Prospective associations between serum biomarkers of lipid metabolism and overall, breast and prostate cancer risk. Eur. J. Epidemiol. 2014, 29, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-X.; Yuan, Q.; Min, Y.-L.; He, Y.; Xu, Q.-H.; Li, B.; Shi, W.-Q.; Lin, Q.; Li, Q.-H.; Zhu, P.-W.; et al. Apolipoprotein A1 and B as risk factors for development of intraocular metastasis in patients with breast cancer. Cancer Manag. Res. 2019, 11, 2881–2888. [Google Scholar] [CrossRef]
- Nouri, M.; Mohsenpour, M.A.; Katsiki, N.; Ghobadi, S.; Jafari, A.; Faghih, S.; Banach, M.; Mazidi, M. Effect of Serum Lipid Profile on the Risk of Breast Cancer: Systematic Review and Meta-Analysis of 1,628,871 Women. J. Clin. Med. 2022, 11, 4503. [Google Scholar] [CrossRef] [PubMed]
- Cedó, L.; García-León, A.; Baila-Rueda, L.; Santos, D.; Grijalva, V.; Martínez-Cignoni, M.R.; Carbó, J.M.; Metso, J.; López-Vilaró, L.; Zorzano, A.; et al. ApoA-I mimetic administration, but not increased apoA-I-containing HDL, inhibits tumour growth in a mouse model of inherited breast cancer. Sci. Rep. 2016, 6, 36387. [Google Scholar] [CrossRef] [PubMed]
- Sawada, M.; de Fátima Mello Santana, M.; Reis, M.; de Assis, S.I.S.; Pereira, L.A.; Santos, D.R.; Nunes, V.S.; Correa-Giannella, M.L.C.; Gebrim, L.H.; Passarelli, M. Increased plasma lipids in triple-negative breast cancer and impairment in HDL functionality in advanced stages of tumors. Sci. Rep. 2023, 13, 8998. [Google Scholar] [CrossRef]
- Melvin, J.C.; Garmo, H.; Holmberg, L.; Hammar, N.; Walldius, G.; Jungner, I.; Lambe, M.; Van Hemelrijck, M. Glucose and lipoprotein biomarkers and breast cancer severity using data from the Swedish AMORIS cohort. BMC Cancer 2017, 17, 246. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Guo, F.; Zhao, W.; Zhan, Y.; Liu, C.; Fan, Y.; Wang, J. Identification of Apolipoprotein C-I Peptides as a Potential Biomarker and its Biological Roles in Breast Cancer. Experiment 2016, 22, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Yue, L.; Zhang, J.; Ma, S.; Zhao, W.; Guo, F.; Fan, Y.; Yang, H.; Liu, Q.; Zhang, D.; et al. Diagnostic and prognostic significance of serum apolipoprotein C-I in triple-negative breast cancer based on mass spectrometry. Cancer Biol. Ther. 2016, 17, 635–647. [Google Scholar] [CrossRef]
- Opstal-van Winden, A.W.; Beijnen, J.H.; Loof, A.; van Heerde, W.L.; Vermeulen, R.; Peeters, P.H.; van Gils, C.H. Search for breast cancer biomarkers in fractionated serum samples by protein profiling with SELDI-TOF MS. J. Clin. Lab. Anal. 2012, 26, 1–9. [Google Scholar] [CrossRef]
- Sánchez, L.M.; Díez-Itza, I.; Vizoso, F.; López-Otín, C. Cholesterol and apolipoprotein D in gross cystic disease of the breast. Clin. Chem. 1992, 38, 695–698. [Google Scholar] [CrossRef]
- Lea, O.A.; Kvinnsland, S.; Thorsen, T. Progesterone-binding cyst protein in human breast tumor cytosol. Cancer Res. 1987, 47, 6189–6192. [Google Scholar]
- Díez-Itza, I.; Vizoso, F.; Merino, A.M.; Sánchez, L.M.; Tolivia, J.; Fernández, J.; Ruibal, A.; López-Otín, C. Expression and prognostic significance of apolipoprotein D in breast cancer. Am. J. Pathol. 1994, 144, 310–320. [Google Scholar]
- Wu, M.; Li, Q.; Wang, H. Identification of Novel Biomarkers Associated with the Prognosis and Potential Pathogenesis of Breast Cancer via Integrated Bioinformatics Analysis. Technol. Cancer Res. Treat. 2021, 20, 1533033821992081. [Google Scholar] [CrossRef]
- Sarjeant, J.M.; Lawrie, A.; Kinnear, C.; Yablonsky, S.; Leung, W.; Massaeli, H.; Prichett, W.; Veinot, J.P.; Rassart, E.; Rabinovitch, M. Apolipoprotein D inhibits platelet-derived growth factor-BB-induced vascular smooth muscle cell proliferated by preventing translocation of phosphorylated extracellular signal regulated kinase 1/2 to the nucleus. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2172–2177. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Luo, G. Apolipoproteins, as the carrier proteins for lipids, are involved in the development of breast cancer. Clin. Transl. Oncol. 2020, 22, 1952–1962. [Google Scholar] [CrossRef]
- Moysich, K.B.; Freudenheim, J.L.; Baker, J.A.; Ambrosone, C.B.; Bowman, E.D.; Schisterman, E.F.; Vena, J.; Shields, P.G. Apolipoprotein E genetic polymorphism, serum lipoproteins, and breast cancer risk. Mol. Carcinog. 2000, 27, 2–9. [Google Scholar] [CrossRef]
- Cibeira, G.H.; Giacomazzi, J.; Aguiar, E.; Schneider, S.; Ettrich, B.; DE Souza, C.I.; Camey, S.; Caleffi, M.; Weber, B.; Ashton-Prolla, P.; et al. Apolipoprotein E genetic polymorphism, serum lipoprotein levels and breast cancer risk: A case-control study. Mol. Clin. Oncol. 2014, 2, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Menzel, H.-J.; Sarmanova, J.; Soucek, P.; Berberich, R.; Grünewald, K.; Haun, M.; Kraft, H.-G. Association of NQO1 polymorphism with spontaneous breast cancer in two independent populations. Br. J. Cancer 2004, 90, 1989–1994. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.-W.; Chen, D.-R.; Wu, C.-T.; Aouizerat, B.-E.; Chen, F.-N.; Hung, S.-J.; Wang, S.-H.; Wei, M.-F.; Chang, C.-S. Influences of apolipoprotein E polymorphism on the risk for breast cancer and HER2/neu status in Taiwan. Breast Cancer Res. Treat. 2005, 90, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.J.; Hou, M.F.; Tsai, S.M.; Kao, J.T.; Wu, S.H.; Hou, L.A.; Tsai, L.Y. Association between the apolipoprotein E genotypes and breast cancer patients in Taiwanese. Breast Cancer Res. Treat. 2006, 98, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Surekha, D.; Vishnupriya, S.; Sailaja, K.; Rao, D.N.; Raghunadharao, D. Influence of Apolipoprotein E Gene Polymorphism on the Risk for Breast Cancer. Int. J. Hum. Genet. 2008, 8, 277–282. [Google Scholar] [CrossRef]
- Porrata-Doria, T.; Matta, J.L.; Acevedo, S.F. Apolipoprotein E Allelic Frequency Altered in Women with Earlyonset Breast Cancer. Breast Cancer Basic Clin. Res. 2010, 4, 43–48. [Google Scholar] [CrossRef]
- Niemi, M.; Kervinen, K.; Kiviniemi, H.; Lukkarinen, O.; Kyllönen, A.-P.; Apaja-Sarkkinen, M.; Savolainen, M.J.; I Kairaluoma, M.; Kesäniemi, Y.A. Apolipoprotein E phenotype, cholesterol and breast and prostate cancer. J. Epidemiol. Community Health 2000, 54, 938–939. [Google Scholar] [CrossRef] [PubMed]
- Yaylim, I.; Bozkurt, N.; Yilmaz, H.; Isbir, T.; Isik, N.; Arikan, S. The apolipoprotein E epsilon 4 allele is not a risk factor for Turkish breast cancer patients. Cancer Genet. Cytogenet. 2003, 146, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Saadat, M. Apolipoprotein E (APOE) Polymorphisms and Susceptibility to Breast Cancer: A Meta-Analysis. Cancer Res. Treat. 2012, 44, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Zunarelli, E.; Nicoll, J.; Migaldi, M.; Trentini, G. Apolipoprotein E polymorphism and breast carcinoma: Correlation with cell proliferation indices and clinical outcome. Breast Cancer Res. Treat. 2000, 63, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.; Moore, K.; Phillips, L.; Boyle, F.M.; Marsh, D.J.; Baxter, R.C. Novel serum protein biomarker panel revealed by mass spectrometry and its prognostic value in breast cancer. Breast Cancer Res. 2014, 16, R63. [Google Scholar] [CrossRef]
- Pettingale, K.W.; E Tee, D. Serum protein changes in breast cancer: A prospective study. J. Clin. Pathol. 1977, 30, 1048–1052. [Google Scholar] [CrossRef]
- Koltai, T. Clusterin: A key player in cancer chemoresistance and its inhibition. OncoTargets Ther. 2014, 7, 447–456. [Google Scholar] [CrossRef]
- Yom, C.K.; Woo, H.-Y.; Min, S.Y.; Kang, S.Y.; Kim, H.S. Clusterin Overexpression and Relapse-Free Survival in Breast Cancer. Anticancer. Res. 2009, 29, 3909–3912. [Google Scholar]
- So, A.; Sinnemann, S.; Huntsman, D.; Fazli, L.; Gleave, M. Knockdown of the cytoprotective chaperone, clusterin, chemosensitizes human breast cancer cells both in vitro and in vivo. Mol. Cancer Ther. 2005, 4, 1837–1849. [Google Scholar] [CrossRef]
- Redondo, M.; Téllez, T.; Roldan, M.J.; Serrano, A.; García-Aranda, M.; Gleave, M.E.; Hortas, M.L.; Morell, M. Anticlusterin treatment of breast cancer cells increases the sensitivities of chemotherapy and tamoxifen and counteracts the inhibitory action of dexamethasone on chemotherapy-induced cytotoxicity. Breast Cancer Res. 2007, 9, R86. [Google Scholar] [CrossRef]
- Li, J.; Jia, L.; Zhao, P.; Jiang, Y.; Zhong, S.; Chen, D. Stable knockdown of clusterin by vector-based RNA interference in a human breast cancer cell line inhibits tumour cell invasion and metastasis. J. Int. Med. Res. 2012, 40, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-A.A.; Klopfer, E.I.; Ray, P.E. Human apolipoprotein L1 (ApoL1) in cancer and chronic kidney disease. FEBS Lett. 2012, 586, 947–955. [Google Scholar] [CrossRef]
- Chidiac, M.; Fayyad-Kazan, M.; Daher, J.; Poelvoorde, P.; Bar, I.; Maenhaut, C.; Delrée, P.; Badran, B.; Vanhamme, L. ApolipoproteinL1 is expressed in papillary thyroid carcinomas. Pathol.-Res. Pract. 2016, 212, 631–635. [Google Scholar] [CrossRef]
- Chu, J.; Li, N.; Li, F. A risk score staging system based on the expression of seven genes predicts the outcome of bladder cancer. Oncol. Lett. 2018, 16, 2091–2096. [Google Scholar] [CrossRef]
- Johanneson, B.; McDonnell, S.K.; Karyadi, D.M.; Quignon, P.; McIntosh, L.; Riska, S.M.; FitzGerald, L.M.; Johnson, G.; Deutsch, K.; Williams, G.; et al. Family-based association analysis of 42 hereditary prostate cancer families identifies the Apolipoprotein L3 region on chromosome 22q12 as a risk locus. Hum. Mol. Genet. 2010, 19, 3852–3862. [Google Scholar] [CrossRef]
- Apasu, J.E.; Schuette, D.; LaRanger, R.; Steinle, J.A.; Nguyen, L.D.; Grosshans, H.K.; Zhang, M.; Cai, W.L.; Yan, Q.; Robert, M.E.; et al. Neuronal calcium sensor 1 (NCS1) promotes motility and metastatic spread of breast cancer cells in vitro and in vivo. FASEB J. 2019, 33, 4802–4813. [Google Scholar] [CrossRef] [PubMed]
- Grosshans, H.K.; Fischer, T.T.; Steinle, J.A.; Brill, A.L.; Ehrlich, B.E. Neuronal Calcium Sensor 1 is up-regulated in response to stress to promote cell survival and motility in cancer cells. Mol. Oncol. 2020, 14, 1134–1151. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, C.; Obinata, H.; Kumaraswamy, S.B.; Galvani, S.; Ahnström, J.; Sevvana, M.; Egerer-Sieber, C.; Muller, Y.A.; Hla, T.; Nielsen, L.B.; et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA 2011, 108, 9613–9618. [Google Scholar] [CrossRef]
- Zhou, Y.; Yao, S.; Yu, M.; Wei, J.; Fang, Q.; Xu, N.; Luo, G. The effects and possible mechanism of action of apolipoprotein M on the growth of breast cancer cells. Mol. Biol. Rep. 2021, 49, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Timms, J.F.; Arslan-Low, E.; Kabir, M.; Worthington, J.; Camuzeaux, S.; Sinclair, J.; Szaub, J.; Afrough, B.; Podust, V.N.; Fourkala, E.; et al. Discovery of serum biomarkers of ovarian cancer using complementary proteomic profiling strategies. Proteom.–Clin. Appl. 2014, 8, 982–993. [Google Scholar] [CrossRef]
- Mangaraj, M.; Nanda, R.; Panda, S. Apolipoprotein A-I: A Molecule of Diverse Function. Indian J. Clin. Biochem. 2015, 31, 253–259. [Google Scholar] [CrossRef]
- Clarke, C.H.; Yip, C.; Badgwell, D.; Fung, E.T.; Coombes, K.R.; Zhang, Z.; Lu, K.H.; Bast, R.C., Jr. Proteomic biomarkers apolipoprotein A1, truncated transthyretin and connective tissue activating protein III enhance the sensitivity of CA125 for detecting early stage epithelial ovarian cancer. Gynecol Oncol. 2011, 122, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chao, S.; Guo, B. Integrated weighted gene coexpression network analysis reveals biomarkers associated with prognosis of high-grade serous ovarian cancer. J. Clin. Lab. Anal. 2022, 36, e24165. [Google Scholar] [CrossRef]
- Kristjansdottir, B.; Partheen, K.; Fung, E.T.; Marcickiewicz, J.; Yip, C.; Brännström, M.; Sundfeldt, K. Ovarian cyst fluid is a rich proteome resource for detection of new tumor biomarkers. Clin. Proteom. 2012, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Rassart, E.; Bedirian, A.; Do Carmo, S.; Guinard, O.; Sirois, J.; Terrisse, L.; Milne, R. Apolipoprotein D. Biochim. Biophys. Acta 2000, 1482, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.; González, L.; Merino, A.; Vizoso, F. Expression and clinical significance of apolipoprotein D in epithelial ovarian carcinomas. Gynecol. Oncol. 2000, 76, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-W.; Bae, S.M.; Kim, I.-W.; Liu, H.-B.; Bang, H.J.; Chaturvedi, P.K.; Battogtokh, G.; Lim, H.; Ahn, W.S. Multiplexed bead-based immunoassay of four serum biomarkers for diagnosis of ovarian cancer. Oncol. Rep. 2012, 28, 585–591. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Pohl, G.; Wang, T.-L.; Morin, P.J.; Risberg, B.; Kristensen, G.B.; Yu, A.; Davidson, B.; Shih, I.-M. Apolipoprotein E is required for cell proliferation and survival in ovarian cancer. Cancer Res 2005, 65, 331–337. [Google Scholar] [CrossRef]
- Podzielinski, I.; Saunders, B.A.; Kimbler, K.D.; Branscum, A.J.; Fung, E.T.; DePriest, P.D.; van Nagell, J.R.; Ueland, F.R.; Baron, A.T. Apolipoprotein concentrations are elevated in malignant ovarian cyst fluids suggesting that lipoprotein metabolism is dysregulated in epithelial ovarian cancer. Cancer Investig. 2013, 31, 258–272. [Google Scholar] [CrossRef]
- Yang, G.F.; Li, X.M.; Xie, D. Overexpression of clusterin in ovarian cancer is correlated with impaired survival. Int. J. Gynecol. Cancer 2009, 19, 1342–1346. [Google Scholar] [CrossRef]
- Hough, C.D.; A Sherman-Baust, C.; Pizer, E.S.; Montz, F.J.; Im, D.D.; Rosenshein, N.B.; Cho, K.; Riggins, G.J.; Morin, P.J. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 2000, 60, 6281–6287. [Google Scholar]
- Kang, K.N.; Koh, E.Y.; Jang, J.Y.; Kim, C.W. Multiple biomarkers are more accurate than a combination of carbohydrate antigen 125 and human epididymis protein 4 for ovarian cancer screening. Obstet. Gynecol. Sci. 2022, 65, 346–354. [Google Scholar] [CrossRef]
- Keeratichamroen, S.; Subhasitanont, P.; Chokchaichamnankit, D.; Weeraphan, C.; Saharat, K.; Sritana, N.; Kantathavorn, N.; Wiriyaukaradecha, K.; Sricharunrat, T.; Paricharttanakul, N.M.; et al. Identification of potential cervical cancer serum biomarkers in Thai patients. Oncol. Lett. 2020, 19, 3815–3826. [Google Scholar] [CrossRef] [PubMed]
- Harima, Y.; Ikeda, K.; Utsunomiya, K.; Komemushi, A.; Kanno, S.; Shiga, T.; Tanigawa, N. Apolipoprotein C-II is a potential serum biomarker as a prognostic factor of locally advanced cervical cancer after chemoradiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Lee, J.K.; Lee, N.W.; Jung, H.H.; Kim, S.H.; Lee, K.W. Microarray analysis of normal cervix, carcinoma in situ, and invasive cervical cancer: Identification of candidate genes in pathogenesis of invasion in cervical cancer. Int. J. Gynecol. Cancer 2008, 18, 1051–1059. [Google Scholar] [CrossRef]
- Lin, Q.; Lu, Y.; Lu, R.; Chen, Y.; Wang, L.; Lu, J.; Ye, X. Assessing Metabolic Risk Factors for LVSI in Endometrial Cancer: A Cross-Sectional Study. Ther. Clin. Risk Manag. 2022, 18, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Rojo, J.V.; González, L.O.; Lamelas, M.L.; Merino, A.; Vizoso, F. Apolipoprotein D expression in endometrial carcinomas. Acta Obstet. Gynecol. Scand. 2001, 80, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.M.; Çolak, Y.; Bojesen, S.E.; Nordestgaard, B.G. Low high-density lipoprotein and increased risk of several cancers: 2 population-based cohort studies including 116,728 individuals. J. Hematol. Oncol. 2020, 13, 129. [Google Scholar] [CrossRef]
- Borgquist, S.; Butt, T.; Almgren, P.; Shiffman, D.; Stocks, T.; Orho-Melander, M.; Manjer, J.; Melander, O. Apolipoproteins, lipids and risk of cancer. Int. J. Cancer 2016, 138, 2648–2656. [Google Scholar] [CrossRef]
- Zabłocka-Słowińska, K.; Płaczkowska, S.; Skórska, K.; Prescha, A.; Pawełczyk, K.; Porębska, I.; Kosacka, M.; Grajeta, H. Oxidative stress in lung cancer patients is associated with altered serum markers of lipid metabolism. PLoS ONE 2019, 14, e0215246. [Google Scholar] [CrossRef]
- Dong, Y.; Haocheng, W.A.N.G.; Dongfeng, S.H.A.N.; Zhang, L.; Zhuang, Y.U. Correlation between Pretreatment Serum Apolipoprotein Level and Prognosis of Small Cell Lung Cancer Patients. Chin. J. Lung Cancer 2020, 23, 845–851. [Google Scholar]
- Guilbaud, E.; Barouillet, T.; Ilie, M.; Borowczyk, C.; Ivanov, S.; Sarrazy, V.; Vaillant, N.; Ayrault, M.; Castiglione, A.; Rignol, G.; et al. Cholesterol efflux pathways hinder KRAS-driven lung tumor progenitor cell expansion. Cell Stem Cell 2023, 30, 800–817.e9. [Google Scholar] [CrossRef]
- Zamanian-Daryoush, M.; Lindner, D.; Tallant, T.C.; Wang, Z.; Buffa, J.; Klipfell, E.; Parker, Y.; Hatala, D.; Parsons-Wingerter, P.; Rayman, P.; et al. The cardioprotective protein apolipoprotein A1 promotes potent antitumorigenic effects. J. Biol. Chem. 2013, 288, 21237–21252. [Google Scholar] [CrossRef]
- Gordon, E.M.; Figueroa, D.M.; Barochia, A.V.; Yao, X.; Levine, S.J. High-density Lipoproteins and Apolipoprotein A-I: Potential New Players in the Prevention and Treatment of Lung Disease. Front. Pharmacol. 2016, 7, 323. [Google Scholar] [CrossRef]
- Vantaggiato, L.; Shaba, E.; Cameli, P.; Bergantini, L.; d’Alessandro, M.; Carleo, A.; Montuori, G.; Bini, L.; Bargagli, E.; Landi, C. BAL Proteomic Signature of Lung Adenocarcinoma in IPF Patients and Its Transposition in Serum Samples for Less Invasive Diagnostic Procedures. Int. J. Mol. Sci. 2023, 24, 925. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yang, H.; Duan, X.; Li, L.; Sun, L.; Li, Q.; Zhang, J. Apolipoproteins as Differentiating and Predictive Markers for Assessing Clinical Outcomes in Patients with Small Cell Lung Cancer. Yonsei Med. J. 2016, 57, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.I.; Kwon, O.-R.; Kang, K.N.; Shin, Y.S.; Shin, H.S.; Yeon, E.H.; Kwon, K.Y.; Hwang, I.; Jeon, Y.K.; Kim, Y.; et al. Diagnostic Value of Combining Tumor and Inflammatory Markers in Lung Cancer. J. Cancer Prev. 2016, 21, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Dowling, P.; O’Driscoll, L.; Meleady, P.; Henry, M.; Roy, S.; Ballot, J.; Moriarty, M.; Crown, J.; Clynes, M. 2-D difference gel electrophoresis of the lung squamous cell carcinoma versus normal sera demonstrates consistent alterations in the levels of ten specific proteins. Electrophoresis 2007, 28, 4302–4310. [Google Scholar] [CrossRef]
- Borlak, J.; Länger, F.; Chatterji, B. Serum proteome mapping of EGF transgenic mice reveal mechanistic biomarkers of lung cancer precursor lesions with clinical significance for human adenocarcinomas. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2018, 1864, 3122–3144. [Google Scholar] [CrossRef]
- Deng, W.; Liu, H.; Luo, S.; Clarke, J.; Glass, C.; Su, L.; Lin, L.; Christiani, D.C.; Wei, Q. APOB Genotypes and CDH13 Haplotypes in the Cholesterol-Related Pathway Genes Predict Non–Small Cell Lung Cancer Survival. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1204–1213. [Google Scholar] [CrossRef]
- Tavazoie, M.F.; Pollack, I.; Tanqueco, R.; Ostendorf, B.N.; Reis, B.S.; Gonsalves, F.C.; Kurth, I.; Andreu-Agullo, C.; Derbyshire, M.L.; Posada, J.; et al. LXR/ApoE Activation Restricts Innate Immune Suppression in Cancer. Cell 2018, 172, 825–840.e18. [Google Scholar] [CrossRef] [PubMed]
- El-Bahrawy, A.H.; Tarhuni, A.; Kim, H.; Subramaniam, V.; Benslimane, I.; Elmajeed, Z.Y.A.; Okpechi, S.C.; Ghonim, M.A.; Hemeida, R.A.M.; Abo-yousef, A.M.; et al. ApoE deficiency promotes colon inflammation and enhances the inflammatory potential of oxidized-LDL and TNF-α in primary colon epithelial cells. Biosci. Rep. 2016, 36, e00388. [Google Scholar]
- Trost, Z.; Marc, J.; Sok, M.; Cerne, D. Increased Apolipoprotein E Gene Expression and Protein Concentration in Lung Cancer Tissue Do Not Contribute to the Clinical Assessment of Non-small Cell Lung Cancer Patients. Arch. Med Res. 2008, 39, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Yeo, I.J.; Kim, K.C.; Han, S.B.; Hong, J.T. Corrigendum: Inhibition of Lung Tumor Development in ApoE Knockout Mice via Enhancement of TREM-1 Dependent NK Cell Cytotoxicity. Front. Immunol. 2021, 12, 840856. [Google Scholar] [CrossRef]
- Su, W.P.; Chen, Y.T.; Lai, W.W.; Lin, C.C.; Yan, J.J.; Su, W.C. Apolipoprotein E expression promotes lung adenocarcinoma proliferation and migration and as a potential survival marker in lung cancer. Lung Cancer 2011, 71, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Pietrowska, M.; Jelonek, K.; Michalak, M.; Roś, M.; Rodziewicz, P.; Chmielewska, K.; Polański, K.; Polańska, J.; Gdowicz-Kłosok, A.; Giglok, M.; et al. Identification of serum proteome components associated with progression of non-small cell lung cancer. Acta Biochim. Pol. 2014, 61, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Beecken, W.-D.C.; Ringel, E.M.; Babica, J.; Oppermann, E.; Jonas, D.; Blaheta, R.A. Plasmin-clipped β2-glycoprotein-I inhibits endothelial cell growth by down-regulating cyclin A, B and D1 and up-regulating p21 and p27. Cancer Lett. 2010, 296, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Luo, G.; Jiang, B.; Yu, M.; Feng, Y.; Wang, M.; Xu, N.; Zhang, X. Apolipoprotein M promotes proliferation and invasion in non-small cell lung cancers via up-regulating S1PR1 and activating the ERK1/2 and PI3K/AKT signaling pathways. Bioche Biophy Re Commun. 2018, 501, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, J.; Iurlaro, R.; El Mjiyad, N.; Díez-Pérez, J.; Gabaldón, T.; Muñoz-Pinedo, C. Apolipoprotein L2 contains a BH3-like domain but it does not behave as a BH3-only protein. Cell Death Dis. 2014, 5, e1275. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Goh, F.Y.; Betts, R.J.; Kemeny, D.M.; Tam, J.; Bay, B.H.; Wong, W.F. A novel anti-apoptotic role for apolipoprotein L2 in IFN-γ-induced cytotoxicity in human bronchial epithelial cells. J. Cell. Physiol. 2011, 226, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; He, M.; Song, M. Serum lipid profiles and risk of colorectal cancer: A prospective cohort study in the UK Biobank. Br. J. Cancer 2020, 124, 663–670. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, J.; Ma, Y.; Chen, H. Apolipoproteins: New players in cancers. Front. Pharmacol. 2022, 13, 1051280. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Chen, Z.; Yang, L.; Xiong, W.; Yang, H.; Xu, K.; Zhai, E.; Ding, L.; He, Y.; Song, X. Apolipoprotein C1 (APOC1) promotes tumor progression via MAPK signaling pathways in colorectal cancer. Cancer Manag. Res. 2019, 11, 4917–4930. [Google Scholar] [CrossRef]
- Kopylov, A.T.; Stepanov, A.A.; Malsagova, K.A.; Soni, D.; Kushlinsky, N.E.; Enikeev, D.V.; Potoldykova, N.V.; Lisitsa, A.V.; Kaysheva, A.L. Revelation of Proteomic Indicators for Colorectal Cancer in Initial Stages of Development. Molecules 2020, 25, 619. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Y.; Qiu, Y.; Xuan, W. Analysis of the Relationship between the Expression Level of TTR and APOH and Prognosis in Patients with Colorectal Cancer Metastasis Based on Bioinformatics. Contrast Media Mol. Imaging 2022, 2022, 1121312. [Google Scholar] [CrossRef]
- Mu, Q.; Luo, G.; Wei, J. Apolipoprotein M promotes growth and inhibits apoptosis of colorectal cancer cells through upregulation of ribosomal protein S27a. EXCLI J. 2021, 20, 145–159. [Google Scholar]
- Li, M.; Wang, Z.; Zhu, L.; Shui, Y.; Zhang, S.; Guo, W. Down-regulation of RBP4 indicates a poor prognosis and correlates with immune cell infiltration in hepatocellular carcinoma. Biosci. Rep. 2021, 41, BSR20210328. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, D.; Yang, C.; Yang, W.; Zhao, J.; Zhou, Y.; Jin, X.; Wu, H. TRIM15 promotes the invasion and metastasis of pancreatic cancer cells by mediating APOA1 ubiquitination and degradation. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2021, 1867, 166213. [Google Scholar] [CrossRef]
- Florea, G.; Tudorache, I.F.; Fuior, E.V.; Ionita, R.; Dumitrescu, M.; Fenyo, I.M.; Bivol, V.G.; Gafencu, A.V. Apolipoprotein A-II, a Player in Multiple Processes and Diseases. Biomedicines 2022, 10, 1578. [Google Scholar] [CrossRef]
- Hayasaki, A.; Murata, Y.; Usui, M.; Hibi, T.; Fujii, T.; Iizawa, Y.; Kato, H.; Tanemura, A.; Azumi, Y.; Kuriyama, N.; et al. Clinical Significance of Plasma Apolipoprotein-AII Isoforms as a Marker of Pancreatic Exocrine Disorder for Patients with Pancreatic Adenocarcinoma Undergoing Chemoradiotherapy, Paying Attention to Pancreatic Morphological Changes. BioMed Res. Int. 2019, 2019, 5738614. [Google Scholar] [CrossRef]
- Felix, K.; Honda, K.; Nagashima, K.; Kashiro, A.; Takeuchi, K.; Kobayashi, T.; Hinterkopf, S.; Gaida, M.M.; Dang, H.; Brindl, N.; et al. Noninvasive risk stratification of intraductal papillary mucinous neoplasia with malignant potential by serum apolipoprotein-A2-isoforms. Int. J. Cancer 2021, 150, 881–894. [Google Scholar] [CrossRef]
- Peng, H.; Pan, S.; Yan, Y.; Brand, R.E.; Petersen, G.M.; Chari, S.T.; Lai, L.A.; Eng, J.K.; Brentnall, T.A.; Chen, R. Systemic Proteome Alterations Linked to Early Stage Pancreatic Cancer in Diabetic Patients. Cancers 2020, 12, 1534. [Google Scholar] [CrossRef]
- Qian, Y.; Gong, Y.; Zou, X.; Liu, Y.; Chen, Y.; Wang, R.; Dai, Z.; Tasiheng, Y.; Lin, X.; Wang, X. Aberrant APOBEC3C expression induces characteristic genomic instability in pancreatic ductal adenocarcinoma. Oncogenesis 2022, 11, 35. [Google Scholar] [CrossRef]
- Kemp, S.B.; Samantha, B.; Steele, N.G. Apolipoprotein E Promotes Immune Suppression in Pancreatic Cancer through NF-κB-Mediated Production of CXCL1. Cancer Res. 2021, 81, 4305–4318. [Google Scholar] [CrossRef] [PubMed]
- Miao, G.; Zhuo, D.; Han, X.; Yao, W.; Liu, C.; Liu, H.; Cao, H.; Sun, Y.; Chen, Z.; Feng, T. From degenerative disease to malignant tumors: Insight to the function of ApoE. BioMedicine 2023, 158, 114127. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, H.C.; Ren, R.L.; Du, S.X. Apolipoprotein E2 inhibits mitochondrial apoptosis in pancreatic cancer cells through ERK1/2/CREB/BCL-2 signaling. Hepatobiliary Pancreat Dis. Int. 2023, 22, 179–189. [Google Scholar] [CrossRef]
- Du, S.; Wang, H.; Cai, J.; Ren, R.; Zhang, W.; Wei, W.; Shen, X. Apolipoprotein E2 modulates cell cycle function to promote proliferation in pancreatic cancer cells via regulation of the c-Myc–p21Waf1signalling pathway. Biochem. Cell Biol. 2020, 98, 191–202. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, X.; Chen, L.; Liu, Y. The role and function of CLU in cancer biology and therapy. Clin. Exp. Med. 2022, 23, 1375–1391. [Google Scholar] [CrossRef] [PubMed]
- Shaashua, L.; Ben-Shmuel, A.; Pevsner-Fischer, M.; Friedman, G.; Levi-Galibov, O.; Nandakumar, S.; Barki, D.; Nevo, R.; Brown, L.E.; Zhang, W.; et al. BRCA mutational status shapes the stromal microenvironment of pancreatic cancer linking clusterin expression in cancer associated fibroblasts with HSF1 signaling. Nat. Commun. 2022, 13, 6213. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tang, W.; Azizian, A.; Gaedcke, J.; Ströbel, P.; Wang, L.; Cawley, H.; Ohara, Y.; Valenzuela, P.; Zhang, L.; et al. Dysregulation of HNF1B/Clusterin axis enhances disease progression in a highly aggressive subset of pancreatic cancer patients. Carcinogenesis 2022, 43, 1198–1210. [Google Scholar] [CrossRef]
- Amada, K.; Hijiya, N.; Ikarimoto, S.; Yanagihara, K.; Hanada, T.; Hidano, S.; Kurogi, S.; Tsukamoto, Y.; Nakada, C.; Kinoshita, K.; et al. Involvement of clusterin expression in the refractory response of pancreatic cancer cells to a MEK inhibitor. Cancer Sci. 2023, 114, 2189–2202. [Google Scholar] [CrossRef]
- Lin, J.; Xu, Z.; Xie, J.; Deng, X.; Jiang, L.; Chen, H.; Peng, C.; Li, H.; Zhang, J.; Shen, B. Oncogene APOL1 promotes proliferation and inhibits apoptosis via activating NOTCH1 signaling pathway in pancreatic cancer. Cell Death Dis. 2021, 12, 760. [Google Scholar] [CrossRef] [PubMed]
- Gumilas, N.S.A.; Harini, I.M.; Ernawati, D.A. Potential of Apolipoprotein A1 (ApoA1) for Detecting Liver Cirrhosis and Hepato-cellular Carcinoma. Asian Pac. J. Cancer Prev. 2022, 23, 2001–2008. [Google Scholar] [CrossRef]
- Shaglouf, L.H.F.; Ranjpour, M.; Wajid, S.; Tandon, R.; Vasudevan, K.R.; Jain, S.K. Elevated expression of ISY1, APOA-1, SYNE1, MTG1, and MMP10 at HCC initiation: HCC specific protein network involving interactions of key regulators of lipid metabolism, EGFR signaling, MAPK, and splicing pathways. Protoplasma 2022, 260, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-M.; Chen, Z.-H.; Zhao, L.-Y.; Zhao, J.-Y.; Rong, D.-L.; Ma, X.-K.; Ruan, D.-Y.; Lin, J.-X.; Qi, J.-J.; Hu, P.-S.; et al. Prognostic Value of Serum Apolipoprotein B to Apolipoprotein A-I Ratio in Hepatocellular Carcinoma Patients Treated with Transcatheter Arterial Chemoembolization: A Propensity Score-Matched Analysis. Oncol. Res. Treat 2021, 44, 450–468. [Google Scholar] [CrossRef]
- Ieda, A.; Wada, M.; Moriyasu, Y.; Okuno, Y.; Zaima, N.; Moriyama, T. Ellagic Acid Suppresses ApoB Secretion and Enhances ApoA-1 Secretion from Human Hepatoma Cells, HepG2. Molecules 2021, 26, 3885. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.K.; Haruehanroengra, P.; Irani, S.; Wang, T.; Ansari, A.; Sheng, J.; Hussain, M.M. Novel efficacious microRNA-30c analogs reduce apolipoprotein B secretion in human hepatoma and primary hepatocyte cells. J. Biol. Chem. 2022, 298, 101813. [Google Scholar] [CrossRef]
- Chen, C.; Yi, W.; Zeng, Z.-F.; Wang, Q.-X.; Jiang, W.; Gao, Y.-H.; Chang, H. Serum apolipoprotein B to apolipoprotein A-I ratio is an independent predictor of liver metastasis from locally advanced rectal cancer in patients receiving neoadjuvant chemoradiotherapy plus surgery. BMC Cancer 2022, 22, 7. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, D.; Sima, X.; Fu, Y. Levels of pretreatment serum lipids predict responses to PD-1 inhibitor treatment in ad-vanced intrahepatic cholangiocarcinoma. Int. Immunopharmacol. 2023, 115, 109687. [Google Scholar] [CrossRef]
- Wang, X.; Gong, Y.; Deng, T.; Zhang, L.; Liao, X.; Han, C.; Yang, C.; Huang, J.; Wang, Q.; Song, X.; et al. Diagnostic and prognostic significance of mRNA expressions of apolipoprotein A and C family genes in hepatitis B virus-related hepatocellular carcinoma. J. Cell. Biochem. 2019, 120, 18246–18265. [Google Scholar] [CrossRef]
- Chang, T.-T.; Ho, C.-H. Plasma proteome atlas for differentiating tumor stage and post-surgical prognosis of hepatocellular carcinoma and cholangiocarcinoma. PLoS ONE 2020, 15, e0238251. [Google Scholar] [CrossRef]
- Lu, C.; Luo, X.; Xing, C. Construction of a novel mRNA-miRNA-lncRNA network and identification of potential regula-tory axis associated with prognosis in colorectal cancer liver metastases. Aging 2021, 13, 14968–14988. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Zheng, Z.; Liu, H.; Zhang, Y.; Kang, J.; Kong, X.; Rong, D.; Sun, G.; Sun, G.; Liu, L.; et al. Inhibition of APOC1 promotes the transformation of M2 into M1 macrophages via the ferroptosis pathway and enhances anti-PD1 immunotherapy in hepatocellular carcinoma based on single-cell RNA sequencing. Redox Biol. 2022, 56, 102463. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Luan, X. Diagnostic performance of clusterin in hepatocellular carcinoma: A meta-analysis. Int. J. Biol. Markers 2022, 37, 404–411. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Zhou, W.; Zhang, S.; Chu, P.; Lin, F.; Wang, H.L. Diagnostic value of clusterin immunostaining in hepatocellular carcinoma. Diagn. Pathol. 2020, 15, 127. [Google Scholar] [CrossRef] [PubMed]
- Patarat, R.; Riku, S.; Kunadirek, P.; Chuaypen, N.; Tangkijvanich, P.; Mutirangura, A.; Puttipanyalears, C. The expression of FLNA and CLU in PBMCs as a novel screening marker for hepatocellular carcinoma. Sci. Rep. 2021, 11, 14838. [Google Scholar] [CrossRef]
- Fu, N.; Du, H.; Li, D.; Lu, Y.; Li, W.; Wang, Y.; Kong, L.; Du, J.; Zhao, S.; Ren, W.; et al. Clusterin contributes to hepatitis C virus-related hepatocellular carcinoma by regulating autophagy. Life Sci. 2020, 256, 117911. [Google Scholar] [CrossRef]
- Zheng, W.; Yao, M.; Wu, M.; Yang, J.; Yao, D.; Wang, L. Secretory clusterin promotes hepatocellular carcinoma progression by facilitating cancer stem cell properties via AKT/GSK-3β/β-catenin axis. J. Transl. Med. 2020, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-Z.; Fan, Y.-D.; Wang, J.; Xu, L.-F.; Liu, C.; Huang, T. Identifying the role of apolipoprotein A-I in prostate cancer. Asian J. Androl. 2021, 23, 400. [Google Scholar] [CrossRef]
- Malik, G.; Ward, M.D.; Gupta, S.K.; Trosset, M.W.; Grizzle, W.E.; Adam, B.-L.; Diaz, J.I.; Semmes, O.J. Serum Levels of an Isoform of Apolipoprotein A-II as a Potential Marker for Prostate Cancer. Clin. Cancer Res. 2005, 11, 1073–1085. [Google Scholar] [CrossRef]
- Yamamoto-Ishikawa, K.; Suzuki, H.; Nezu, M.; Kamiya, N.; Imamoto, T.; Komiya, A.; Sogawa, K.; Tomonaga, T.; Nomura, F.; Ichikawa, T. The isolation and identification of apolipoprotein C-I in hormone-refractory prostate cancer using surface-enhanced laser desorption/ionization time-of-flight mass spectrometry. Asian J. Androl. 2009, 11, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Su, W.P.; Sun, L.N.; Yang, S.L.; Zhao, H.; Zeng, T.Y.; Wu, W.Z.; Wang, D. Apolipoprotein C1 pro-motes prostate cancer cell proliferation in vitro. J. Biochem. Mol. Toxicol. 2018, 32, e22158. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.; Horsfall, D.; Stahl, J.; Vivekanandan, S.; Ricciardelli, C.; Stapleton, A.; Scardino, P.; Neufing, P.; Tilley, W.D. Apolipoprotein-D: A novel cellular marker for HGPIN and prostate cancer. Prostate 2003, 58, 103–108. [Google Scholar] [CrossRef]
- Bancaro, N.; Calì, B.; Troiani, M.; Elia, A.R.; Arzola, R.A.; Attanasio, G.; Lai, P.; Crespo, M.; Gurel, B.; Pereira, R.; et al. Apolipoprotein E induces pathogenic senescent-like myeloid cells in prostate cancer. Cancer Cell 2023, 41, 602–619.e11. [Google Scholar] [CrossRef] [PubMed]
- Trougakos, I.P.; So, A.; Jansen, B.; Gleave, M.E.; Gonos, E.S. Silencing expression of the clusterin/apolipoprotein j gene in human cancer cells using small interfering RNA induces spontaneous apoptosis, reduced growth ability, and cell sensitization to genotoxic and oxidative stress. Cancer Res. 2004, 64, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Wu, H.; Qu, K.; Sun, Q.; Li, F.; Shi, C.; Li, Y.; Xiong, X.; Qin, Q.; Yu, T.; et al. Identification of serum proteins AHSG, FGA and APOA-I as diagnostic biomarkers for gastric cancer. Clin. Proteom. 2018, 15, 18. [Google Scholar] [CrossRef]
- Yu, L.; Lai, Q.; Feng, Q.; Li, Y.; Feng, J.; Xu, B. Serum Metabolic Profiling Analysis of Chronic Gastritis and Gastric Cancer by Untargeted Metabolomics. Front. Oncol. 2021, 11, 636917. [Google Scholar] [CrossRef]
- Dwivedi, S.; Hernández-Montes, G.; Montaño, L.F.; Rendón-Huerta, E.P. Chromosomally Unstable Gastric Cancers Overexpressing Claudin-6 Disclose Cross-Talk between HNF1A and HNF4A, and Upregulated Cholesterol Metabolism. Int. J. Mol. Sci. 2022, 23, 13977. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Jiang, H. Diagnostic value of apolipoprotein C-I, transthyretin and apolipoprotein C-III in gastric cancer. Oncol. Lett. 2019, 17, 3227–3232. [Google Scholar] [CrossRef]
- Cohen, M.; Yossef, R.; Erez, T.; Kugel, A.; Welt, M.; Karpasas, M.M.; Bones, J.; Rudd, P.M.; Taieb, J.; Boissin, H.; et al. Serum apolipoproteins C-I and C-III are reduced in stomach cancer patients: Results from MALDI-based peptidome and immuno-based clinical assays. PLoS ONE 2011, 6, e14540. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Ren, L.; Wu, J.; Li, W.; Zheng, X.; Du, G.; Wang, J. Apolipoprotein C1 (APOC1) as a novel diagnostic and prognostic biomarker for gastric cancer. Ann. Transl. Med. 2019, 7, 380. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Z.; Xu, E.; Shen, X.; Wang, X.; Li, Z.; Yu, H.; Chen, K.; Hu, Q.; Xia, X.; et al. Apolipoprotein C-II induces EMT to promote gastric cancer peritoneal metastasis via PI3K/AKT/mTOR pathway. Clin. Transl. Med. 2021, 11, e522. [Google Scholar] [CrossRef]
- Liu, C.; Pan, C.; Liang, Y. Screening and identification of serum proteomic biomarkers for gastric adeno-carcinoma. Exp. Ther. Med. 2012, 3, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Liu, Y. ZNF460 mediates epithelial-mesenchymal transition to promote gastric cancer progression by transactivating APOC1 expression. Exp. Cell Res. 2023, 422, 113452. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, K.; Tanaka, F.; Zhang, X.; Mimori, K.; Kamohara, Y.; Inoue, H.; Sawada, T.; Hirakawa, K.; Mori, M. Clinical significance of ApoE expression in human gastric cancer. Oncol. Rep. 1994, 20, 1313–1319. [Google Scholar] [CrossRef]
- Oue, N.; Hamai, Y.; Mitani, Y. Gene expression profile of gastric carcinoma: Identification of genes and tags potentially in-volved in invasion, metastasis, and carcinogenesis by serial analysis of gene expression. Cancer Res. 2004, 64, 2397–2405. [Google Scholar] [CrossRef]
- Liu, W.; Liu, B.; Cai, Q.; Li, J.; Chen, X.; Zhu, Z. Proteomic identification of serum biomarkers for gastric cancer using multi-dimensional liquid chromatography and 2D differential gel electrophoresis. Clin. Chim. Acta 2012, 413, 1098–1106. [Google Scholar] [CrossRef]
- Li, D.; Zhou, L.; Ma, C.; Chen, W.; Zhang, Y.; Yu, S.; Wang, D.; Zou, Y.; Wu, J.; Qiu, L. Comparative analysis of the serum proteome profiles of thyroid cancer: An initial focus on the lipid profile. Oncol. Lett. 2019, 18, 3349–3357. [Google Scholar] [CrossRef]
- Li, D.; Wu, J.; Liu, Z.; Qiu, L.; Zhang, Y. Novel circulating protein biomarkers for thyroid cancer determined through data-independent acquisition mass spectrometry. PeerJ 2020, 8, e9507. [Google Scholar] [CrossRef] [PubMed]
- Valledor, A.F.; Hsu, L.-C.; Ogawa, S.; Sawka-Verhelle, D.; Karin, M.; Glass, C.K. Activation of liver X receptors and retinoid X receptors prevents bacterial-induced macrophage apoptosis. Proc. Natl. Acad. Sci. USA 2004, 101, 17813–17818. [Google Scholar] [CrossRef]
- Feng, J.; Zhao, F.; Sun, J.; Lin, B.; Zhao, L.; Liu, Y.; Jin, Y.; Li, S.; Li, A.; Wei, Y. Alterations in the gut microbiota and metabolite profiles of thyroid carcinoma patients. Int. J. Cancer 2018, 144, 2728–2745. [Google Scholar] [CrossRef]
- Li, L.-R.; Song, J.-L.; Liu, H.-Q.; Chen, C. Metabolic syndrome and thyroid Cancer: Risk, prognosis, and mechanism. Discov. Oncol. 2023, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Huang, Y.; Sadeghi, F.; Feychting, M.; Hammar, N.; Fang, F.; Zhang, Z.; Liu, Q. Carbohydrate, Lipid, and Apolipoprotein Biomarkers in Blood and Risk of Thyroid Cancer: Findings from the AMORIS Cohort. Cancers 2023, 15, 520. [Google Scholar] [CrossRef] [PubMed]
- Giusti, L.; Iacconi, P.; Ciregia, F.; Giannaccini, G.; Donatini, G.L.; Basolo, F.; Miccoli, P.; Pinchera, A.; Lucacchini, A. Fine-needle aspiration of thyroid nodules: Proteomic analysis To identify cancer biomarkers. J. Proteome Res. 2008, 7, 4079–4088. [Google Scholar] [CrossRef]
- Abdullah, M.I.; Lee, C.C.; Junit, S.M.; Ng, K.L.; Hashim, O.H. Tissue and serum samples of patients with papillary thyroid cancer with and without benign background demonstrate different altered expression of proteins. PeerJ 2016, 4, e2450. [Google Scholar] [CrossRef]
- Ma, M.; Wang, M.; Zhang, Z.; Lin, B.; Sun, Z.; Guan, H.; Lv, W.; Li, J. Apolipoprotein A1 is negatively associated with male papillary thyroid cancer patients: A cross-sectional study of single academic center in China. BMC Endocr. Disord. 2021, 21, 69. [Google Scholar] [CrossRef]
- Smith, A.; Galli, M. Molecular signatures of medullary thyroid carcinoma by matrix-assisted laser desorp-tion/ionisation mass spectrometry imaging. J. Proteom. 2019, 191, 114–123. [Google Scholar] [CrossRef]
- Jung, K.Y.; Ahn, H.Y.; Han, S.K.; Park, Y.J.; Cho, B.Y.; Moon, M.K. Association between thyroid function and lipid profiles, apolipoproteins, and high-density lipoprotein function. J. Clin. Lipidol. 2017, 11, 1347–1353. [Google Scholar] [CrossRef]
- Revilla, G.; Pons, M.d.P.; Baila-Rueda, L.; García-León, A.; Santos, D.; Cenarro, A.; Magalhaes, M.; Blanco, R.M.; Moral, A.; Pérez, J.I.; et al. Cholesterol and 27-hydroxycholesterol promote thyroid carcinoma aggressiveness. Sci. Rep. 2019, 9, 10260. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Shi, L.; Liu, Q. Discovery and identification of potential biomarkers of papillary thyroid carcinoma. Mol. Cancer 2009, 8, 79. [Google Scholar] [CrossRef]
- Wang, J.-X.; Dong, R.; Liu, Q.-L.; Yang, S.-B.; Fan, Y.-X.; Zhang, Q.; Yang, F.-Q.; Wu, P.; Yu, J.-K.; Zheng, S. Detection and identification of specific serum biomarkers in papillary thyroid cancer. Chin. J. Oncol. 2009, 31, 265–268. [Google Scholar]
- Jeong, S.; Kim, I.-K.; Kim, H.; Choi, M.J.; Lee, J.; Jo, Y.S. Liver X Receptor β Related to Tumor Progression and Ribosome Gene Expression in Papillary Thyroid Cancer. Endocrinol. Metab. 2020, 35, 656–668. [Google Scholar] [CrossRef]
- Ruchong, P.; Haiping, T.; Xiang, W. A Five-Gene Prognostic Nomogram Predicting Disease-Free Survival of Differentiated Thyroid Cancer. Dis. Markers 2021, 2021, 5510780. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, J.; Zhao, R.-H.; Zhang, W.-J.; Wu, J.-F.; Xue, G. APOE Is a Prognostic Biomarker and Correlates with Immune Infiltrates in Papillary Thyroid Carcinoma. J. Cancer 2022, 13, 1652–1663. [Google Scholar] [CrossRef]
- Xue, G.; Lin, X.; Wu, J.-F.; Pei, D.; Wang, D.-M.; Zhang, J.; Zhang, W.-J. Identification of key genes of papillary thyroid carcinoma by integrated bioinformatics analysis. Biosci. Rep. 2020, 40, BSR20201555. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shen, Y.; Ye, B.; Hu, H.; Fan, C.; Wang, T.; Zheng, Y.; Lv, J.; Ma, Y.; Xiang, M. Gene expression differences between thyroid carcinoma, thyroid adenoma and normal thyroid tissue. Oncol. Rep. 2018, 40, 3359–3369. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhao, H. Ferroptosis-Related APOE, BCL3 and ALOX5AP Gene Polymorphisms are Associated with the Risk of Thyroid Cancer. Pharmacogenomics Pers. Med. 2022, 15, 157–165. [Google Scholar] [CrossRef]
- Nan, B.Y.; Xiong, G.F.; Zhao, Z.R.; Gu, X.; Huang, X.S. Comprehensive Identification of Potential Crucial Genes and miRNA-mRNA Regulatory Networks in Papillary Thyroid Cancer. Biomed. Res. Int. 2021, 2021, 6752141. [Google Scholar] [CrossRef]
- Huang, J.; Sun, W.; Wang, Z.; Lv, C.; Zhang, T.; Zhang, D.; Dong, W.; Shao, L.; He, L.; Ji, X.; et al. FTO suppresses glycolysis and growth of papillary thyroid cancer via decreasing stability of APOE mRNA in an N6-methyladenosine-dependent manner. J. Exp. Clin. Cancer Res. 2022, 41, 42. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Feng, W.; Xiong, C.; Lv, Y. Integrated bioinformatics analysis of the association between apolipoprotein E expression and patient prognosis in papillary thyroid carcinoma. Oncol. Lett. 2020, 19, 2295–2305. [Google Scholar] [CrossRef]
- Ito, Y.; Takano, T.; Miyauchi, A. Apolipoprotein E expression in anaplastic thyroid carcinoma. Oncology 2006, 71, 388–393. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in cancer therapy: A novel approach to reversing drug resistance. Mol. Cancer 2022, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, H.; Wu, A.; Peng, Y.; Shu, G.; Yin, G. Functions of N6-methyladenosine and its role in cancer. Mol. Cancer 2019, 18, 176. [Google Scholar] [CrossRef]
- Delk, S.C.; Chattopadhyay, A.; Escola-Gil, J.C.; Fogelman, A.M.; Reddy, S.T. Apolipoprotein mimetics in cancer. Semin. Cancer Biol. 2021, 73, 158–168. [Google Scholar] [CrossRef]
- Su, F.; Kozak, K.R.; Imaizumi, S.; Gao, F. Apolipoprotein A-I (apoA-I) and apoA-I mimetic peptides inhibit tumor development in a mouse model of ovarian cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 19997–20002. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.T.; Lu, H. Anti-tumorigenic and Platinum-Sensitizing Effects of Apolipoprotein A1 and Apolipo-protein A1 Mimetic Peptides in Ovarian Cancer. Front. Pharmacol. 2018, 9, 1524. [Google Scholar] [CrossRef]
- Neyen, C.; Mukhopadhyay, S.; Gordon, S.; Hagemann, T. An apolipoprotein A-I mimetic targets scavenger receptor A on tumor-associated macro-phages: A prospective anticancer treatment? Oncoimmunology 2013, 2, e24461. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Vasquez, S.X.; Su, F.; Roberts, S.; Shah, N.; Grijalva, V.; Imaizumi, S.; Chattopadhyay, A.; Ganapathy, E.; Meriwether, D.; et al. L-5F, an apolipoprotein A-I mimetic, inhibits tumor angiogenesis by suppressing VEGF/basic FGF signaling pathways. Integr. Biol. 2011, 3, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chattopadhyay, A.; Navab, M. Apolipoprotein A-I mimetic peptides inhibit expression and activity of hypoxia-inducible fac-tor-1α in human ovarian cancer cell lines and a mouse ovarian cancer model. J. Pharmacol. Exp. Ther. 2012, 342, 255–362. [Google Scholar] [CrossRef]
- Peng, M.; Zhang, Q.; Liu, Y. Apolipoprotein A-I Mimetic Peptide L-4F Suppresses Granulocytic-Myeloid-Derived Sup-pressor Cells in Mouse Pancreatic Cancer. Front. Pharmacol. 2020, 11, 576. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Zhang, Q.; Cheng, Y.; Fu, S.; Yang, H.; Guo, X.; Zhang, J.; Wang, L.; Zhang, L.; Xue, Z.; et al. Apolipoprotein A-I mimetic peptide 4F suppresses tumor-associated macrophages and pancreatic cancer progression. Oncotarget 2017, 8, 99693–99706. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, E.; Su, F.; Meriwether, D.; Devarajan, A.; Grijalva, V.; Gao, F.; Chattopadhyay, A.; Anantharamaiah, G.; Navab, M.; Fogelman, A.M.; et al. D-4F, an apoA-I mimetic peptide, inhibits proliferation and tumorigenicity of epithelial ovarian cancer cells by upregulating the antioxidant enzyme MnSOD. Int. J. Cancer 2011, 130, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Ou, J.; Ackerman, A.W.; Oldham, K.T.; Pritchard, K.A., Jr. L-4F, an apolipoprotein A-1 mimetic, restores nitric oxide and superoxide anion balance in low-density lipoprotein-treated endothelial cells. Circulation 2003, 107, 1520–1524. [Google Scholar] [CrossRef][Green Version]
- Behrend, L.; Henderson, G.; Zwacka, R.M. Reactive oxygen species in oncogenic transformation. Biochem. Soc. Trans. 2003, 31, 1441–1444. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Yang, X.; Mukherjee, P.; Sulaiman, D.; Fogelman, H.R.; Grijalva, V.; Dubinett, S.; Wasler, T.C.; Paul, M.K.; Salehi-Rad, R.; et al. Treating the Intestine with Oral ApoA-I Mimetic Tg6F Reduces Tumor Burden in Mouse Models of Metastatic Lung Cancer. Sci. Rep. 2018, 8, 9032. [Google Scholar] [CrossRef]
- Wong, E.; Goldberg, T. Mipomersen (kynamro): A novel antisense oligonucleotide inhibitor for the management of homozygous familial hypercholesterolemia. Pharm. Ther. 2014, 39, 119–122. [Google Scholar]
- Tani, S.; Nagao, K.; Hirayama, A. Association between urinary albumin excretion and low-density lipoprotein heterogeneity following treatment of type 2 diabetes patients with the dipeptidyl peptidase-4 inhibitor, vildagliptin: A pilot study. Am. J. Cardiovasc. Drugs 2013, 13, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Furuta, S.; Yamashita, S.; Hashimoto, H.; Yano, W.; Inoue, N.; Kato, N.; Kaku, K. Dipeptidyl peptidase 4 inhibitor anagliptin ameliorates hypercholesterolemia in hypercho-lesterolemic mice through inhibition of intestinal cholesterol transport. J. Diabetes Investig. 2018, 9, 1261–1269. [Google Scholar] [CrossRef]
- Komatsu, T.; Sakurai, T.; Wolska, A.; Amar, M.J.; Sakurai, A.; Vaisman, B.L.; Sviridov, D.; Demosky, S.; Pryor, M.; Ikewaki, K.; et al. Apolipoprotein C-II Mimetic Peptide Promotes the Plasma Clearance of Triglycer-ide-Rich Lipid Emulsion and the Incorporation of Fatty Acids into Peripheral Tissues of Mice. J. Nutr. Metab. 2019, 2019, 7078241. [Google Scholar] [CrossRef]
- Wolska, A.; Lo, L.; Sviridov, D.O.; Pourmousa, M.; Pryor, M.; Ghosh, S.S.; Kakkar, R.; Davidson, M.; Wilson, S.; Pastor, R.W.; et al. A dual apolipoprotein C-II mimetic-apolipoprotein C-III antagonist peptide lowers plasma triglycerides. Sci. Transl. Med. 2020, 12, eaaw7905. [Google Scholar] [CrossRef]
- Sakurai, T.; Sakurai, A.; Vaisman, B.L.; Amar, M.J.; Liu, C.; Gordon, S.M.; Drake, S.K.; Pryor, M.; Sampson, M.L.; Yang, L.; et al. Creation of Apolipoprotein C-II (ApoC-II) Mutant Mice and Correction of Their Hypertri-glyceridemia with an ApoC-II Mimetic Peptide. J. Pharmacol. Exp. Ther. 2016, 356, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C. Investigating the mechanism of action and potential efficacy of apolipoprotein E mimetics as therapeutic agents for the treatment of metastatic prostate cancer. Ph.D. Thesis, National University of Ireland, Galway, Ireland, 2017. [Google Scholar]
- Périchon, B.; Garcia, A. Anti-Infective Properties of Anti-Cancer Cationic Peptides containing Survivin or Apolipoprotein E Sequences. J. Biotechnol. Biomed. 2019, 2, 161–168. [Google Scholar] [CrossRef]
- Bhattacharjee, P.S.; Huq, T.S.; Mandal, T.K.; Graves, R.A.; Muniruzzaman, S.; Clement, C.; McFerrin, H.E.; Hill, J.M. A Novel Peptide Derived from Human Apolipoprotein E Is an Inhibitor of Tumor Growth and Ocular Angiogenesis. PLoS ONE 2011, 6, e15905. [Google Scholar] [CrossRef] [PubMed]

| Type of Cancer | APOA | APOB | APOC | APOD | APOE | APOH | APOL | APOM | APOJ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A4 | C1 | C2 | C3 | E2 | E4 | L1 | L3 | ||||||
| Cervical cancer | ↓88 | ↓90 | |||||||||||||
| Breast cancer | ↓32 | ↑34 | ↓39 | ↓39 | ↑39 | ↓45 | ↑59 | ↓73 | ↑72 | ||||||
| Lung cancer | ↓13 | ↑26 | ↓29 | ||||||||||||
| Pancreatic cancer | ↓14 | ↓21 | ↑44 | ↑55 | ↑62 | ↑78 | |||||||||
| Nasopharyngeal cancer | ↓15 | ||||||||||||||
| Hepatocellular cancer | ↓15 | ↑24 | ↑28 | ↓35 | ↑48 | ↑74 | |||||||||
| Colorectal cancer | ↓15 | ↓35 | ↑43 | ↓52 | ↑60 | ↓66 | ↓69 | ↑75 | |||||||
| Esophageal carcinoma | ↓16 | ↑25 | |||||||||||||
| Renal cancer | ↓16 | ↓22 | ↑41 | ↑58 | ↑76 | ||||||||||
| Gastric cancer | ↓17 | ↓31 | ↓32 | ↑42 | ↑45 | ↑77 | |||||||||
| Bladder cancer | ↑18 | ↑23 | ↑27 | ↑37 | ↑47 | ↑63 | |||||||||
| Thyroid cancer | ↓19 | ||||||||||||||
| Ovarian cancer | ↓74 | ↓74 | ↓74 | ↑77 | ↑77 | ↑83 | |||||||||
| Cholangiocarcinoma | ↓24 | ||||||||||||||
| Squamous lung cancer | ↑29 | ↑13 | ↑61 | ||||||||||||
| Papillary thyroid cancer | ↓30 | ↑64 | |||||||||||||
| Duodenal adenoma carcinoma | ↓34 | ||||||||||||||
| Lung NSCLC | ↓40 | ↓7 | ↑67 | ||||||||||||
| Prostatic cancer | ↑51 | ↑56 | ↑65 | ↑73 | |||||||||||
| Acute lymphoblastic leukemia | ↓53 | ||||||||||||||
| Melanoma | ↓54 | ||||||||||||||
| Acute myeloid leukemia | ↑59 | ||||||||||||||
| Larynx cancer | ↑70 | ||||||||||||||
| Apolipoproteins in Breast Cancer | ||||||
|---|---|---|---|---|---|---|
| Level | Effect | Mechanism | Clinical Implications | Prognostic Value | Source | |
| APOA1 [31,32] | Downregulated | Cancer cell apoptosis. | Binding of C1QBP to APOA, inhibiting its expression and APOA’s antioxidation ability. | Potential anti-tumor therapeutic target. | Reduced expression led to poor prognosis. | Unclear |
| APOB [34] | Upregulated | Increases metastasis. | Mutation of tumor suppressor and proto-oncogenes. | Potential prognostic marker. | Increased metastasis. | Unclear |
| APOC1 [39,40] | Downregulated | Anti-tumor | Inhibit cell proliferation (in vitro) and inhibit tumor growth (in vivo). | Potential diagnostic tool for differentiating between triple-negative and non-triple-negative breast cancer, as well as for early detection. | When levels decrease, there is an associated increase in tumor growth. | Hepatic |
| APOD [42,45,46] | Downregulated | Anti-tumor | Reduced suppression of MAPK pathway. | Potential therapeutic target. | Lower levels are associated with a higher risk of metastasis. | Localized |
| APOE [47,48,49,50,51,52,53,54] | Controversial | Inhibit proliferation. | High-affinity interaction with heparin and proteoglycan. | Potential prognostic biomarker. | Linked to metastasis. | Unclear |
| APOH [59] | Upregulated | Inhibiting apoptosis. | Unclear | Potential prognostic marker. | Linked to metastasis. | Unclear |
| APOJ [62] | Upregulated | Inhibiting apoptosis. | Initiate tumorigenesis | Potential prognostic marker and therapeutic target. | Linked to metastasis. | Unclear |
| APOL3 [70,71] | Unclear | Unclear | Regulates NCS-1, which is responsible for initiating cell metastasis and survival. | Potential prognostic marker. | Needs further research. | Unclear |
| APOM [72,73] | Downregulated | Reduces metastasis. | Stabilize sphingosine-1-phosphate. | Potential prognostic marker and therapeutic target. | Protective | Liver |
| (a) Apolipoproteins in ovarian cancer | ||||||
| Level | Effect | Mechanism | Clinical Implications | Prognostic Value | Source | |
| APOA1 [74,75] | Downregulated | Anti-tumor | Alters macrophage polarization towards anti-tumor M1 phenotype and inhibits angiogenesis via downregulation of MMP-9 expression. | Potential therapeutic target when induced and potential biomarker. | Associated with improved prognosis owing to protective properties. | Intestines |
| APOB [77,78] | Upregulated | Promotes tumor growth. | Unknown | Potential prognostic and diagnostic marker. | Linked to higher tumor grades. | Unclear |
| APOC3 [78] | Upregulated | Tumor metastasis. | Inhibit lipoprotein lipase and hepatic lipase. | Potential diagnostic biomarker. | Promotes malignancy. | Liver and small intestine. |
| APOD [80] | No significant correlation. | Improved survival. | Inhibition of tumor growth. | Potential therapeutic and prognostic target. | Linked to better prognosis when tumor is larger than 1 cm. | Unclear |
| APOE [81,82,83] | Upregulated | Vital for tumor growth. | Prevent cell arrest in G2 phase and apoptosis. | Potential prognostic biomarker. | Better survival when expressed. | Unclear |
| APOJ [84] | Upregulated | Unclear | Unknown | Diagnostic and predictive. | Linked to poor prognosis. | Unclear |
| (b) Apolipoproteins in cervical cancer | ||||||
| Level | Effect | Mechanism | Clinical Implications | Prognostic Value | Source | |
| APOA1 [87] | Downregulated | Unclear | Anti-inflammatory, antioxidant, and anti-apoptotic. | Potential biomarker. | Decreased levels are associated with advancing stages of cancer. | Liver and small intestine. |
| APOC2 [88] | Downregulated in cervical cancer that caused death. | Promotes tumor growth. | Dysregulation of lipoprotein lipase. | Potential prognostic factor. | Lower levels had poorer prognosis with treatment. | Liver and intestine. |
| APOD [89] | Downregulated | Protective | Inhibit osteopontin-induced malignancy. | Potential therapeutic and diagnostic target. | Lower levels linked to poor prognosis. | Unclear |
| Apolipoproteins in Lung Cancer | ||||||
|---|---|---|---|---|---|---|
| Level | Effect | Mechanism | Clinical Implications | Prognostic Value | Source | |
| APOA1 [15,16,75,93,94,96,97,98,100] | Controversial | Anti-tumorigenic roles. | Convert cancer-linked macrophages to anti-tumor M1 phenotype, inhibit neo-angiogenesis. | Potential therapeutic target. | Linked to better prognosis. | Hepatic |
| APOA2 [15,16,101] | Unclear | Inflammatory marker. | Unclear | Potential diagnostic marker for early-stage lung cancer. | Requires further research. | Hepatic |
| APOA4 [102,103] | Variable. Upregulated in squamous cell carcinomas. Downregulated in adenocarcinomas. | Unclear | Unclear | Potential diagnostic marker. | Requires further research. | Intestinal, Tumor |
| APOB [93,94,104] | Variable | Controversial | Regulates cholesterol transport and metabolism. | Potential diagnostic or prognostic marker. | Controversial, may vary with tumor type. | Hepatic |
| APOC3 [100] | Downregulated | Unclear | Unclear | Potential diagnostic or predictive marker. | Increased levels linked to recurrence. | Hepatic |
| APOE [105,106,107,108,109] | Upregulated | Supports the proliferation and metastasis of lung cancer cells. | Associated with increased oxidative stress. | Potential diagnostic marker. | Linked to increased complications. | Hepatic, Unclear |
| APOH [103,110,111] | Upregulated | Inhibits angiogenesis. | Suppression of endothelial cell growth. | Potential therapeutic target. | Requires further research. | Unclear |
| APOM [72,103,112] | Downregulated in AAH. Upregulated in NSCLC. | Increased apoptosis and tumor suppression. Cell proliferation, invasion, and tumor development. | Carrier for sphingosine-1-phosphate, which inhibits ceramide, leading to cell proliferation. Inducing sphingosine-1-phosphate, which activates the ERK1/2 and PI3K/AKT signaling pathways. | Potential therapeutic target. | Requires further research. | Hepatic, Tumor |
| APOL2 [114] | Upregulated | Unclear | Possibly through anti-apoptotic properties. | Potential diagnostic or prognostic marker and therapeutic target. | Requires further research. | Unclear |
| Apolipoproteins in Colorectal Cancer | ||||||
|---|---|---|---|---|---|---|
| Level | Effect | Mechanism | Clinical Implications | Prognostic Value | Source | |
| APOA [7,15,30,115,116] | Upregulated | Possible anti-tumor impact. | Increases cholesterol efflux, damaging invasion distribution. | Potential therapeutic target. | Favorable factor in metastatic colorectal cancer. | Hepatic Synthesis |
| APOB [30,31,35,116] | Upregulated in primary CRC and with liver metastasis. | Controversial role. | May act as a lipid carrier, inactivating mutations in APOB gene, or other factors may affect its expression. | Potential therapeutic target. | Controversial, may vary with tumor stage and location. | Systemic and Hepatic |
| APOC1 [43,116,117] | Upregulated | Promotes cell proliferation and migration. | P38-MAPK signaling pathway activation. | Potential diagnostic or prognostic marker. | Requires further research. | Cancer Cells |
| APOD [7,116,118] | Downregulated in advanced stages. | Inversely connected with tumor advancement. | Reacts to oxidative stress, enhances tumor suppression through apoptosis. | Early diagnostic marker. | Linked to worse prognosis in advanced stages. | Systemic and Cancer Cells |
| APOE [31,116,118] | Upregulated in CRC. | Controversial role, both as a protective factor and promoter of cancer growth. | May affect intracellular adhesion and junctions, PI3K/Akt/mTOR pathway. | Potential therapeutic target. | Controversial, may vary with tumor stage and location. | Unknown |
| APOH [119] | Upregulated | Associated with worse prognosis. | Underlying mechanisms unknown. | Requires further research. | Linked to worse prognosis. | Unknown |
| APOJ [7,75,79] | Upregulated in colon cancer. | Promotes colorectal carcinogenesis, metastasis, and tumor invasion. | Activated by stress, pro- and anti-apoptotic activities. | Potential diagnostic or prognostic marker. | Requires further research on mechanisms. | Cancer Cells |
| APOM [7,69,120] | Controversial | Controversial role. | May inhibit EMT or promote cell proliferation and invasion through the RPS27A-MDM2-p53 pathway. | Potential diagnostic or prognostic marker. | Controversial, may vary with tumor stage and grade. | Systemic and Cancer Cells |
| Apolipoproteins in Pancreatic Cancer | ||||||
|---|---|---|---|---|---|---|
| Level | Effect | Mechanism | Clinical Implications | Prognostic Value | Source | |
| APOA [31,122,123,124,125,126] | Upregulated in early stages and downregulated in later stages. | Inhibits tumor progression. | Underlying mechanisms unknown. | Potential therapeutic target, and prognostic and diagnostic marker. | Low levels of APOA1 are linked to worse prognosis, while high levels of APOA4 indicate worse prognosis. | Tumor cells |
| APOC [7,31] | Upregulated | APOC1 induces apoptosis while APOC2 promotes cell proliferation and invasion. | Requires further research. | Potential therapeutic target and prognostic marker. | High levels of APOC1 are linked to worse survival. | Tumor cells |
| APOE1 [128,129] | Upregulated | Inhibits apoptosis of malignant cells. | Activation of the NF-κB signaling pathway and production of CXCL1. | Potential therapeutic target and prognostic marker. | High levels are linked to worse prognosis. | Tumor cells |
| APOE2 [130,131] | Upregulated | Prevents mitochondrial apoptosis and promotes tumor growth. | Increased expression of BCL-2 through activating ERK1/2/CREB signaling cascade. | Potential prognostic marker and therapeutic target for PC. | Requires further research. | Tumor cells |
| APOL1 [135,136] | Controversial | Complex role; can both induce and inhibit proliferation and apoptosis. | Can inhibit proliferation and induce apoptosis by activating the NOTCH1 pathway. | Potential therapeutic target. | Requires further research. | Human pancreatic ductal adenocarcinomal cell lines. |
| APOJ [132,134] | Further research is needed | Complex role as it regulates a diversity of pathways. | Modulates a variety of signaling pathways such as ERK, AKT, and NF-κB. | Potential target for treatment. | Requires further research. | Tumor cells |
| Apolipoproteins in Hepatic Cancer | ||||||
|---|---|---|---|---|---|---|
| Level | Effect | Mechanism | Clinical Implications | Prognostic Value | Source | |
| APOA1 [137,138,139,140] | Upregulated in early stages of hepatic cancer. | Suppresses immunity and promotes cell proliferation. | Requires further research. | Potential therapeutic target and prognostic marker. | Predictor of liver metastasis and resistance to PD-1 inhibitor treatment. | Hepatic |
| APOB [31,142,143] | Upregulated in hepatic cancer and liver metastasis. | Requires further research. | Unknown | Potential therapeutic target and prognostic marker. | Predictor of liver metastasis and resistance to PD-1 inhibitor treatment. | Hepatic |
| APOC [144,145,146,147] | Upregulated in hepatic cancer and liver metastasis. | APOC1 can promote immune evasion and angiogenesis2 of tumor cells. | APOC1 can trigger the transformation of tumor-associated macrophages (TAMs) into M2-like cells. | Potential prognostic marker. | APOC1 and APOC4 are associated with overall survival, APOC3 is associated with both overall survival and recurrence-free survival. | Hepatic and tumor cells. |
| APOJ [150,151,152] | Controversial | Promotes autophagy and metastasis. | Upregulation of NF-κB and Beclin1, promotion of ER stress, and activation of PERK and ATF6 signaling pathways. | Potential therapeutic target. | Linked with resistance to sorafenib/doxorubicin. | Hepatic |
| Apolipoproteins in Prostate Cancer | ||||||
|---|---|---|---|---|---|---|
| Level | Effect | Mechanism | Clinical Implications | Prognostic Value | Source | |
| APOA [153] | Upregulated, increases as disease progresses. | Enhances cell proliferation, invasiveness, and resistance to therapeutics. | MYC regulates APOA expression. | Potential prognostic marker. | High levels of APOA1 indicate disease progression. | Tumor cells |
| APOC [155,156] | Upregulated | APOC1 inhibits apoptosis and promotes proliferation. | APOC1 promotes survivin/phosphor-Rb/p21 pathway, reducing caspase-3, inhibiting apoptosis. | Potential therapeutic target and prognostic marker. | Elevated APOC1 levels are linked to worse prognosis. | Tumor cells |
| APOD [157] | Upregulated | Requires further research. | Requires further research. | Potential cellular marker. | Tumor cells | |
| APOE [158] | Upregulated | Inhibits immune reaction against tumor cells. | Binds TREM2 on neutrophils, inducing senescence. | Potential therapeutic target and prognostic marker. | Elevated expression is linked to poor prognosis. | Tumor cells |
| APOL3 [69] | Increases hereditary prostate cancer susceptibility. | Suggests the role of 22q locus in sporadic prostate cancer. | Potential risk estimator. | Requires further research. | Patient samples | |
| APOJ [159] | Upregulated | Inhibits apoptosis. Helps tumor cells evade the effects of androgen ablation and chemotherapeutic agents. | Requires further research. | Potential target to increase chemosensitivity. | Requires further research. | Tumor cells |
| Apolipoproteins in Gastric Cancer | ||||||
|---|---|---|---|---|---|---|
| Level | Effect | Mechanism | Clinical Implications | Prognostic Value | Source | |
| APOA1 [160,161] | Controversial | Increases tumor burden. | Requires further research. | After gastrectomy, APOA-1 significantly decreases. May be used to differentiate between chronic gastritis and gastric cancer. | Circulating APOA-1 levels reflect tumor burden. | Tumor cells |
| APOA2 [162] | Upregulated in high-claudin-6 gastric cancers. | Affects cholesterol metabolism. | Requires further research. | Effective prognostics marker. | Tumor cells | |
| APOC1-3 [163,164,165,166,167,168] | Controversial | Inhibit apoptosis and promotes proliferation. | CD36-mediated PI3K/AKT/mTOR signaling pathway. | Potential therapeutic target and prognostic marker. | Elevated APOC1 levels are linked to worse prognosis. APOC2 is elevated in peritoneal metastasis. | Unknown |
| APOE [169,170] | Upregulated | Cancer development and progression. | Requires further research. | Elevated levels are associated with increased risk of muscular invasion. | Shorter survival time in patients with elevated APOE. | |
| APOJ [171] | Upregulated | Tumor progression and metastasis. | Requires further research. | Potential therapeutic target. | Requires further research. | |
| Apolipoproteins in Thyroid Cancer | ||||||
|---|---|---|---|---|---|---|
| Level | Effect | Mechanism | Clinical Implications | Prognostic Value | Source | |
| APOA [15,16,172,173,174,175,176,180] | Downregulated | Dysregulated lipid profile and altered gut microbiota symbiosis. | Regulates lipid proteomic profiles. LXR/RXR activation pathway. | Potential diagnostic or prognostic marker. | APOA1 linked to smaller tumor size. Reduced APOA1 levels linked to worse prognosis and aggressive tumor characteristics. | Hepatic, Intestinal |
| APOB [15,16,183] | Downregulated | Unclear | Unclear, hypothesized to be through lipid pathways. | Potential diagnostic or prognostic marker. | Reduced APOB linked with more aggressive tumors. | Hepatic |
| APOC [184,185,186] | Controversial | Unclear, possibly leads to tumor progression. | Orphan nuclear hormone receptor superfamily receptors bind to HRE RXRalpha and T3Rbeta LXRβ overexpression. | Potential prognostic biomarker. | APOC decreases as cancer stage increases. APOC linked to poor tumor characteristics. | Hepatic, possibly tumor. |
| APOD [187] | Downregulated in DTC. | Increased tumor proliferation. | P53 tumor suppressor family. | Potential prognostic marker. | Linked to higher risk score and recurrence. | Unclear |
| APOE [181,186,188,189,190,191,192,193] | Upregulated | Tumor progression. | Programmed cell death and tumor modulation. Modulating the inflammatory response. | Potential prognostic biomarker. Possible therapeutic target. | Linked to overall survival. | Hepatic, Tumor |
| APOL1 [67] | Upregulated | Unclear | Apoptosis and autophagy. | Potential diagnostic marker and therapeutic target. | Requires further research. | Unclear |
| APO | Therapeutic Agent | Effect | Mechanism of Action |
|---|---|---|---|
| APO Mimetics | |||
| APOA1 | D-4F | Decreases cancer proliferation. | Eliminates oxidized lipids. Limits inflammatory responses. Upregulates MnSOD [1,2]. |
| L-4F | Represses tumor angiogenesis, tumorigenicity of cell and inflammation. | Represses HIF-1α. Reduces interleukins and ROS [3,4]. | |
| L-5F | Represses tumor angiogenesis. | Inhibits VEGF and bFGF signaling pathways [5]. Suppresses intracellular levels of HIF-1α [6]. | |
| Tg6F | Reduces MDSC in jejunum and lung. | Alters expression of Notch and Spp1 [7]. | |
| APOC2 | 18A-CII | Regains lipolysis to normal levels in APOC-II deficient patients [8]. | |
| D6PV [9] | Decreases TG levels. | ||
| C-II-a [10] | |||
| APOE | COG112 | Anti-inflammatory and reduces cell cycle progression [11,12]. | Hinders signaling for PRR. |
| OP449 | |||
| APOEdp | Inhibit tumor growth and restrict ocular angiogenesis [13] | ||
| APOJ | G* | Decreases tumorigenesis [14]. | Decreases pro-tumorigenic lipids [14]. |
| APO Inhibitors | |||
| APOB-100 | Mipomersen | Decreases levels of newly synthesized APOB-100. | Triggers activation of RNase H and inhibits microsomal triglyceride transfer protein [15]. |
| APOC2 | DPP4 Inhibitor [16] | Decreases APOC-II levels. | |
| Anagliptin [17] | Decreases APOC-II mRNA expression. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, N.M.; Al-Hail, M.K.; Mohamed, Y.; Al Saady, R.; Mohsen, S.; Zar, A.; Al-Mansoori, L.; Pedersen, S. The Role of Apolipoproteins in the Commonest Cancers: A Review. Cancers 2023, 15, 5565. https://doi.org/10.3390/cancers15235565
Darwish NM, Al-Hail MK, Mohamed Y, Al Saady R, Mohsen S, Zar A, Al-Mansoori L, Pedersen S. The Role of Apolipoproteins in the Commonest Cancers: A Review. Cancers. 2023; 15(23):5565. https://doi.org/10.3390/cancers15235565
Chicago/Turabian StyleDarwish, Nour M., Mooza Kh. Al-Hail, Youssef Mohamed, Rafif Al Saady, Sara Mohsen, Amna Zar, Layla Al-Mansoori, and Shona Pedersen. 2023. "The Role of Apolipoproteins in the Commonest Cancers: A Review" Cancers 15, no. 23: 5565. https://doi.org/10.3390/cancers15235565
APA StyleDarwish, N. M., Al-Hail, M. K., Mohamed, Y., Al Saady, R., Mohsen, S., Zar, A., Al-Mansoori, L., & Pedersen, S. (2023). The Role of Apolipoproteins in the Commonest Cancers: A Review. Cancers, 15(23), 5565. https://doi.org/10.3390/cancers15235565







