Influence of C60 Nanofilm on the Expression of Selected Markers of Mesenchymal–Epithelial Transition in Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
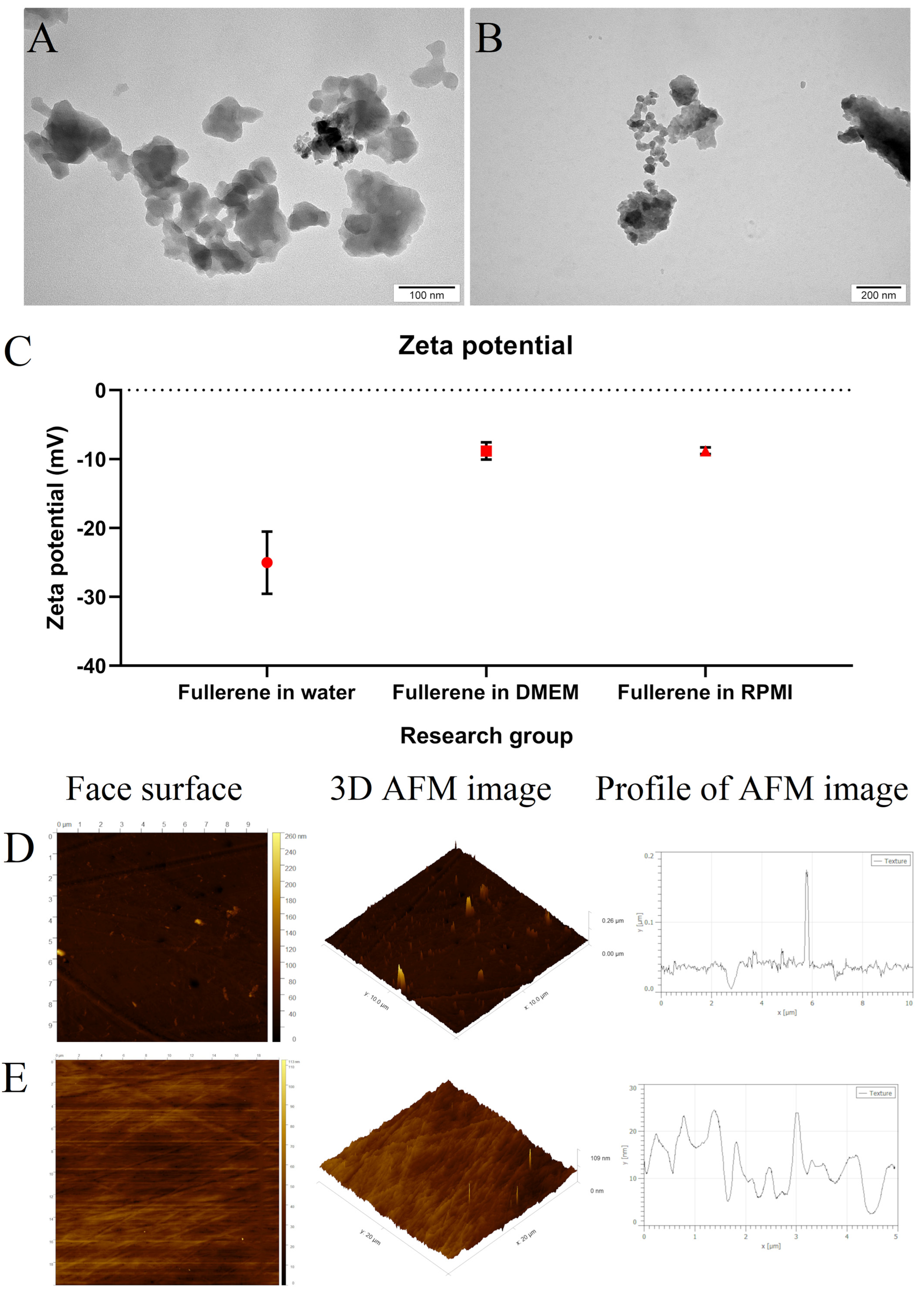
2.1. Characterisation of Fullerene Nanoparticles and Nanofilm
2.2. Cell Culture
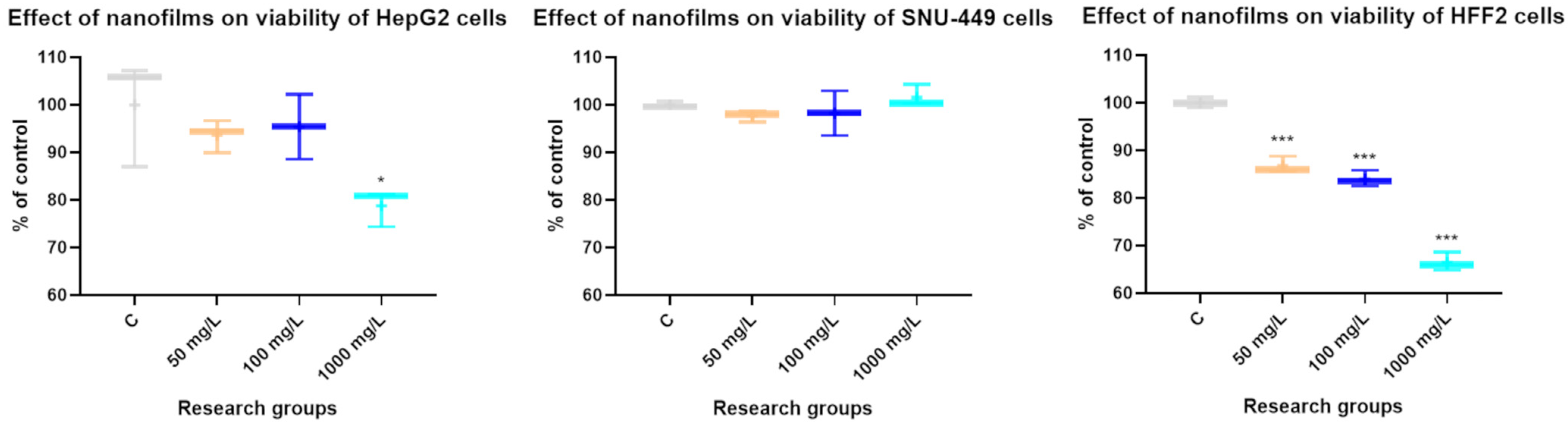
2.3. MTT Assay
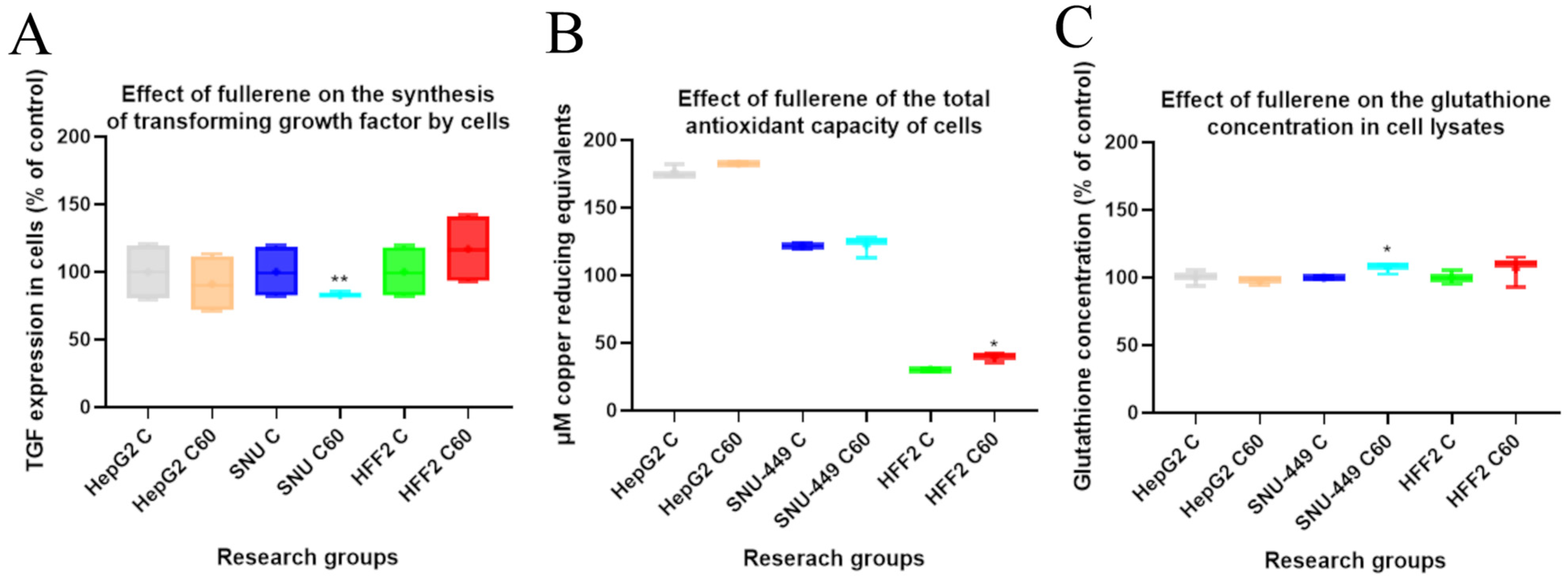
2.4. Anti-Inflammatory and Antioxidative Properties of Fullerene Nanofilm
2.4.1. Quantitative Detection of Transforming Growth Factor Using Enzyme-Linked Immunosorbent Assay
2.4.2. Total Antioxidant Capacity Assay
2.4.3. Quantitative Detection of Glutathione Using Enzyme-Linked Immunosorbent Assay
2.5. Mesenchymal–Epithelial Transition of Cells on Fullerene Nanofilm
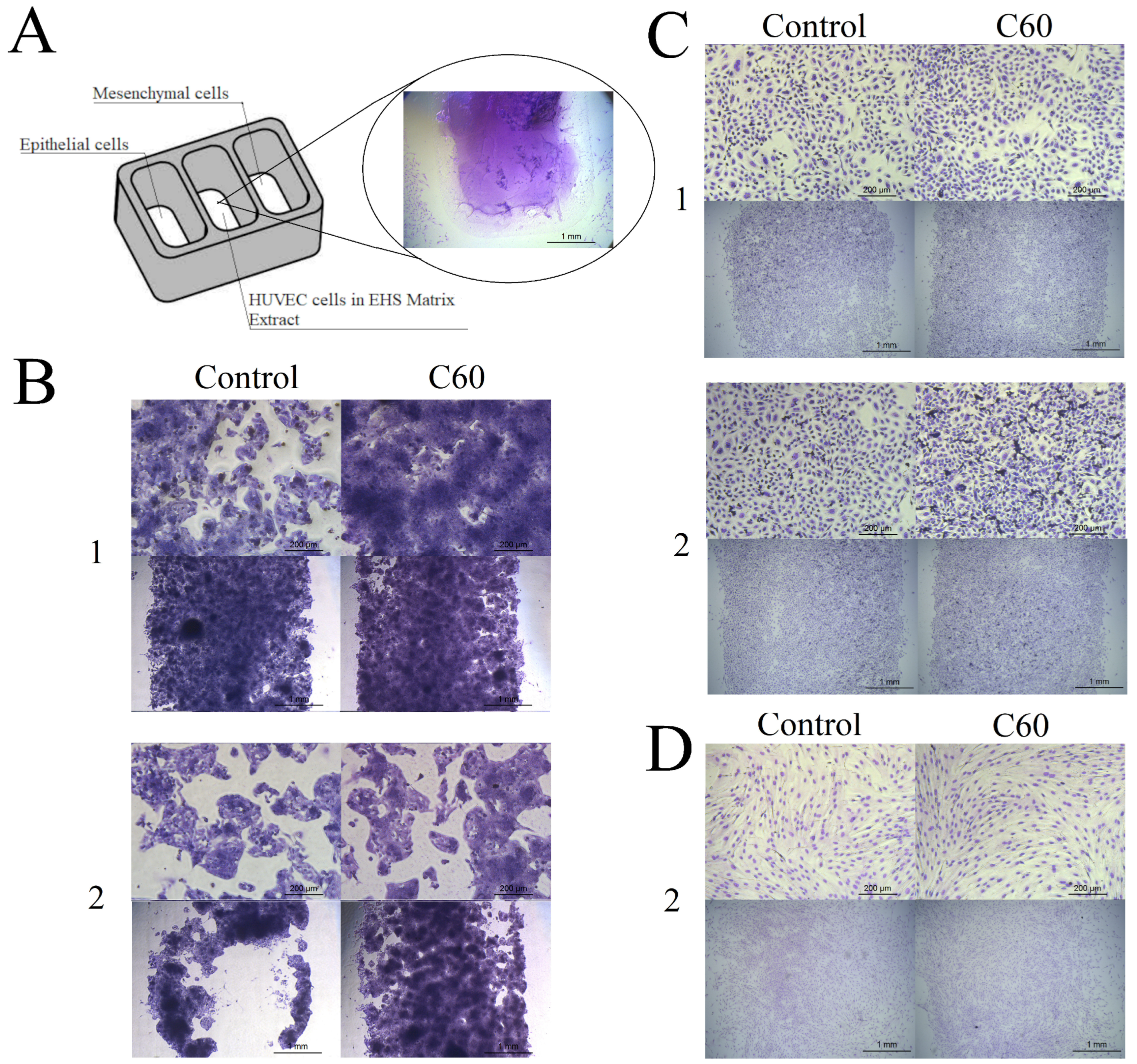
2.5.1. Morphology of Epithelial and Mesenchymal Cells on C60 Nanofilm
2.5.2. Two-Dimensional Invasion Assay
2.5.3. Cell Invasion through the Extracellular Matrix
2.5.4. Metalloproteinase Expression Assay
2.5.5. Expression of Mesenchymal Markers Using Western Blotting
2.5.6. HepG2 Cell Proteome Analysis Using Mass Spectrometry
2.6. Statistical Analysis
3. Results
3.1. Morphology and Stability of Fullerene Nanoparticles
3.2. The Effect of Concentrations of C60 Nanofilm on Cytotoxicity
3.3. The Effect of C60 Nanofilm on Transforming Growth Factor Beta 1 Synthesis and Oxidant Levels
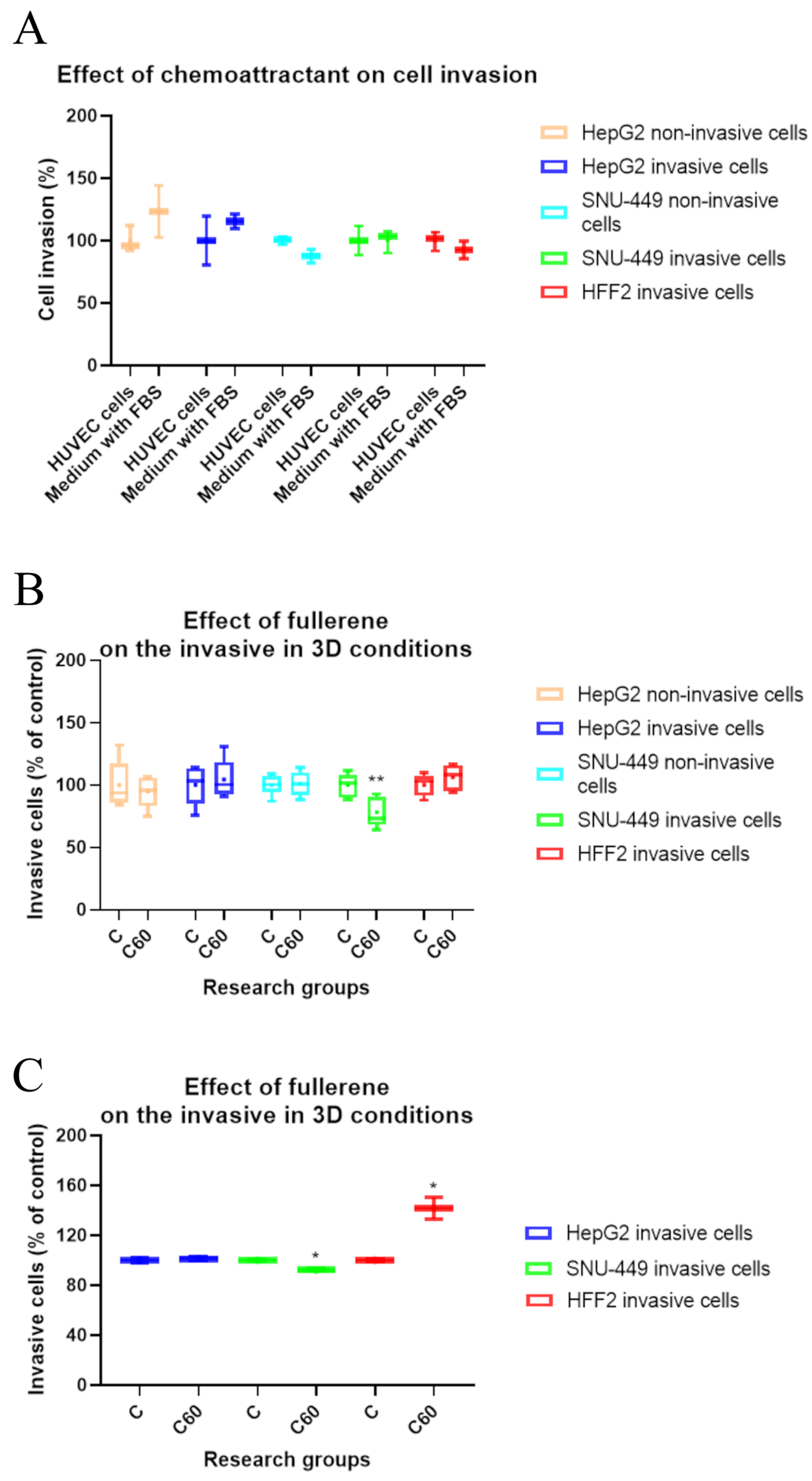
3.4. The Effect of C60 Nanofilm on Cell Morphology and Two-Dimensional Invasion towards Endothelial Cells
3.5. The Effect of C60 Nanofilm on Three-Dimensional Invasion
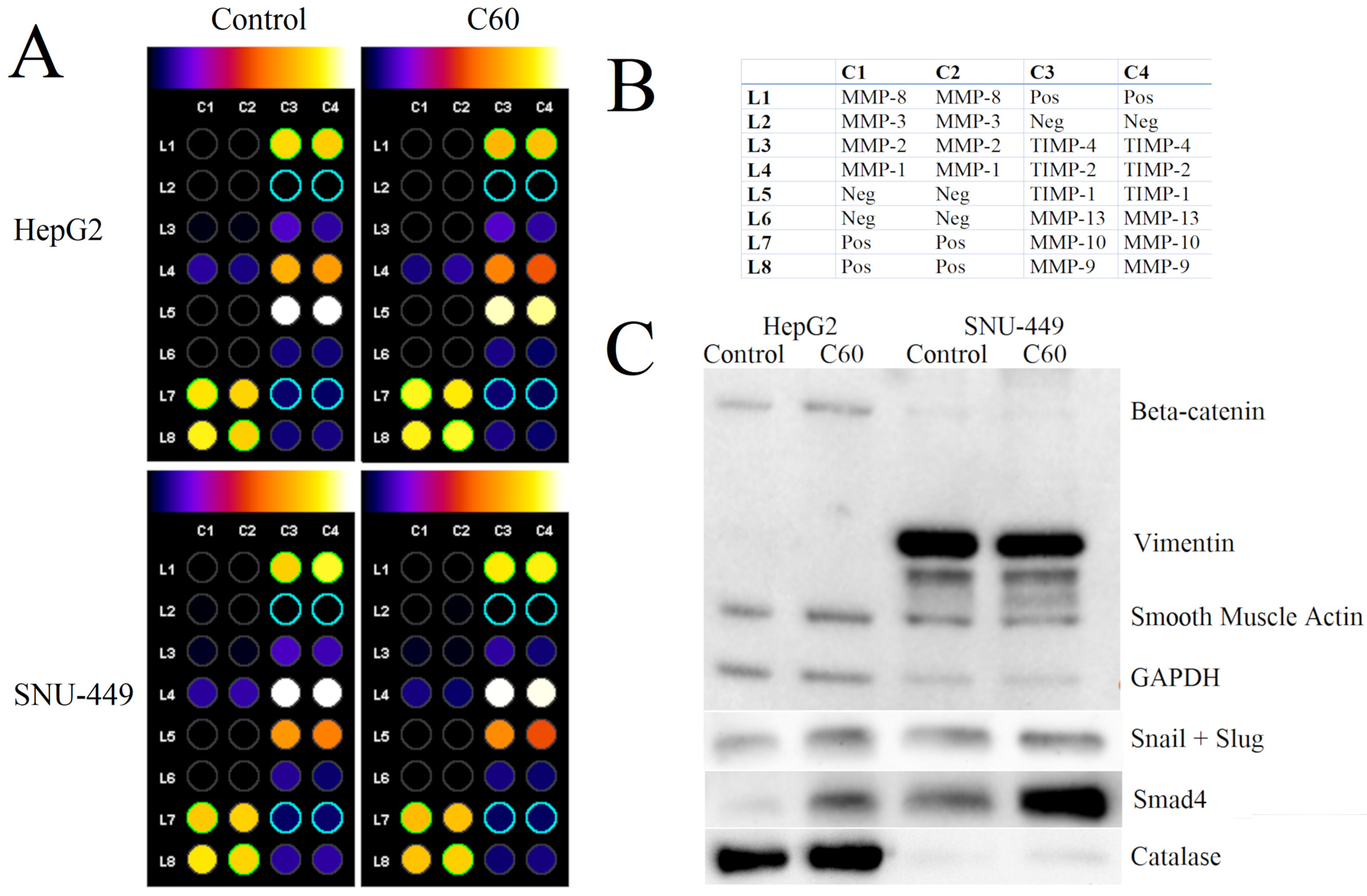
3.6. The Effect of C60 Nanofilm on Degradation of the Extracellular Matrix
3.7. The Effect of C60 Nanofilm on Key Proteins Involved in the Mesenchymal–Epithelial Transition and Oxidative Stress
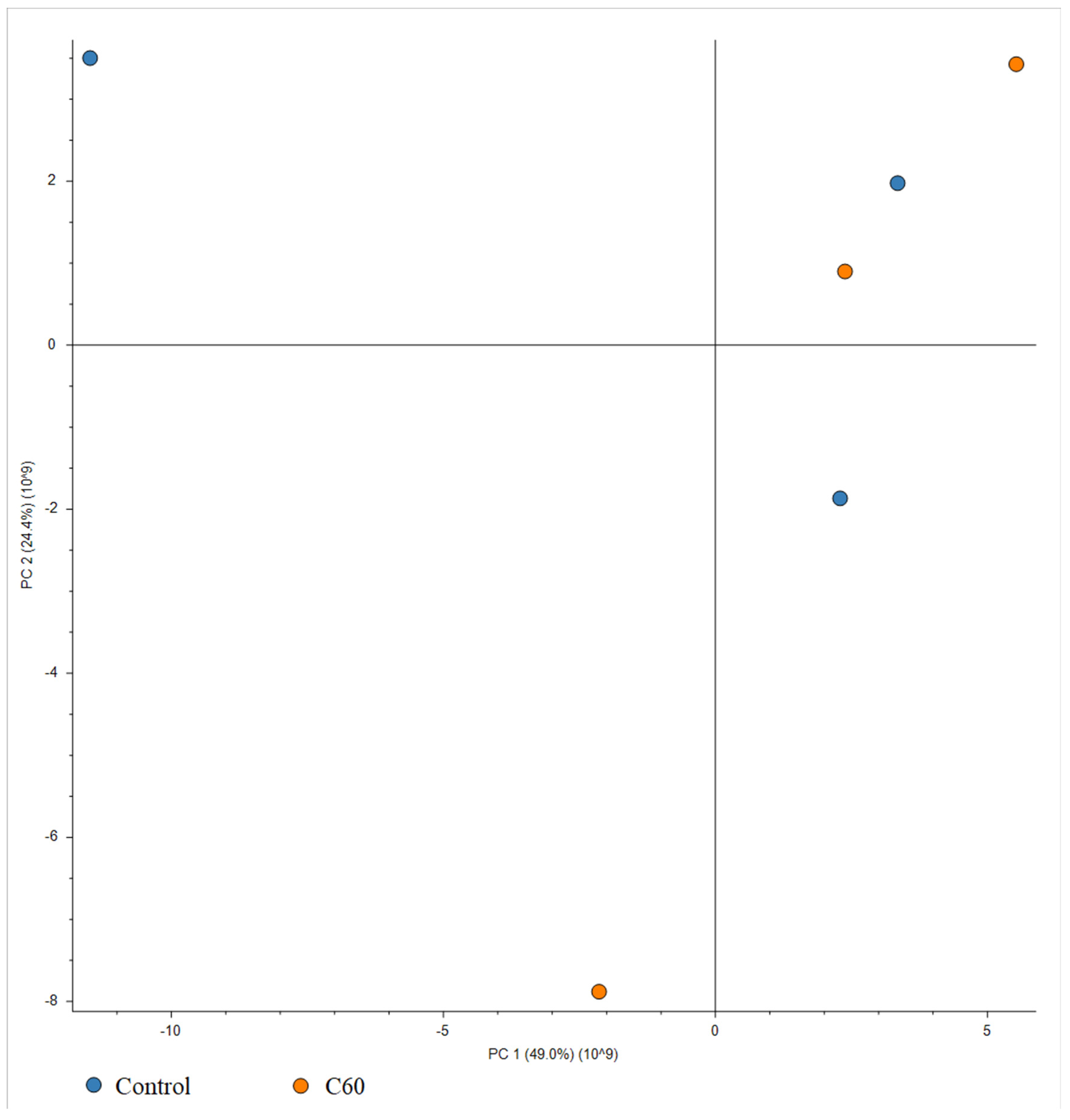
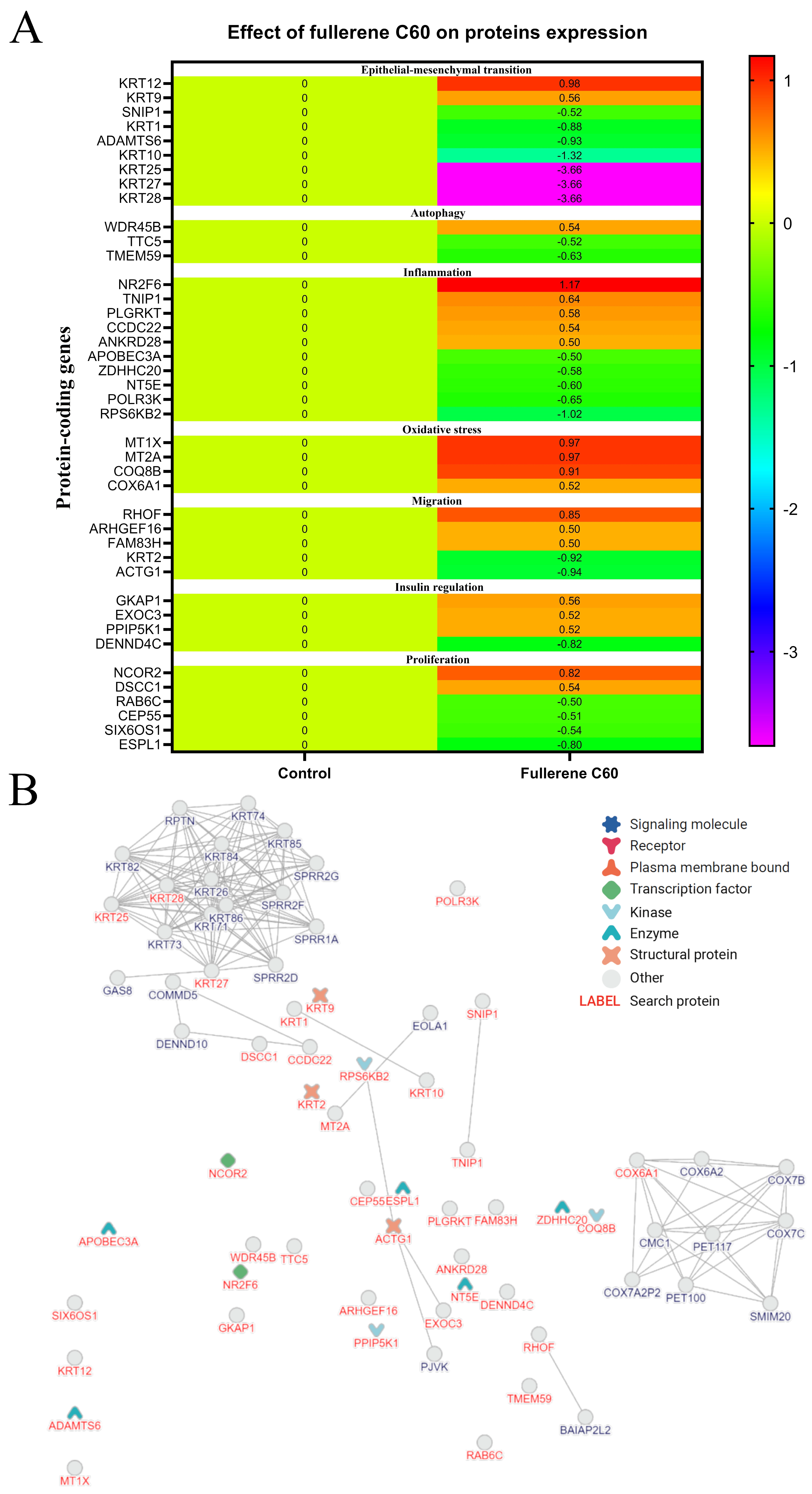
3.8. The Effect of C60 Nanofilm on the Proteomic Profile of HepG2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Shinde, A.; Hardy, S.D.; Kim, D.; Akhand, S.S.; Jolly, M.K.; Wang, W.H.; Anderson, J.C.; Khodadadi, R.B.; Brown, W.S.; George, J.T.; et al. Spleen tyrosine kinase–mediated autophagy is required for epithelial–mesenchymal plasticity and metastasis in breast cancer. Cancer Res. 2019, 79, 1831–1843. [Google Scholar] [CrossRef]
- Asleh, M.A.; Zaher, M.; Asleh, J.; Jadon, J.; Shaulov, L.; Yelin, R.; Schultheiss, T.M. A morphogenetic wave in the chick embryo lateral mesoderm generates mesenchymal-epithelial transition through a 3D-rosette intermediate. Dev. Cell 2023, 58, 951–966. [Google Scholar] [CrossRef]
- Chen, W.; Wu, J.; Shi, W.; Zhang, G.; Chen, X.; Ji, A.; Wang, Z.; Wu, J.; Jiang, C. PRRX1 deficiency induces mesenchymal-epithelial transition through PITX2/miR-200–dependent SLUG/CTNNB1 regulation in hepatocellular carcinoma. Cancer Sci. 2021, 112, 2158–2172. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-mesenchymal transition in cancer: A historical overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.P.; Wrana, J.L. A specialist-generalist framework for epithelial-mesenchymal plasticity in cancer. Trends Cancer 2022, 8, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Scheau, C.; Badarau, I.A.; Costache, R.; Caruntu, C.; Mihai, G.L.; Didilescu, A.C.; Constantin, C.; Neagu, M. The role of matrix metalloproteinases in the epithelial-mesenchymal transition of hepatocellular carcinoma. Anal. Cell. Pathol. 2019, 2019, 9423907. [Google Scholar] [CrossRef] [PubMed]
- White, D.L.; Li, D.; Nurgalieva, Z.; El-Serag, H.B. Genetic variants of glutathione S-transferase as possible risk factors for hepatocellular carcinoma: A HuGE systematic review and meta-analysis. Am. J. Epidemiol. 2008, 167, 377–389. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Allameh, A.; Niayesh-Mehr, R.; Aliarab, A.; Sebastiani, G.; Pantopoulos, K. Oxidative stress in liver pathophysiology and disease. Antioxidants 2023, 12, 1653. [Google Scholar] [CrossRef]
- Sosnowska, M.; Koczoń, P.; Kutwin, M.; Chwalibog, A.; Sawosz, E. Polyhydroxylated fullerene C60(OH)40 nanofilms promote the mesenchymal-epithelial transition of human liver cancer cells via the TGF-β1/Smad pathway. J. Inflamm. Res. 2023, 16, 3739–3761. [Google Scholar] [CrossRef]
- Yazaki, K.; Matsuno, Y.; Yoshida, K.; Sherpa, M.; Nakajima, M.; Matsuyama, M.; Kiwamoto, T.; Morishima, Y.; Ishii, Y.; Hizawa, N. ROS-Nrf2 pathway mediates the development of TGF-β1-induced epithelial-mesenchymal transition through the activation of Notch signaling. Eur. J. Cell Bio. 2021, 100, 151181. [Google Scholar] [CrossRef]
- Muthuramalingam, K.; Cho, M.; Kim, Y. Role of NADPH oxidase and its therapeutic intervention in TGF-β-mediated EMT progression: An in vitro analysis on HeLa cervical cancer cells. Appl. Biol. Chem. 2020, 63, 4. [Google Scholar] [CrossRef]
- Kuznietsova, H.; Dziubenko, N.; Herheliuk, T.; Prylutskyy, Y.; Tauscher, E.; Ritter, U.; Scharff, P. Water-soluble pristine C60 fullerene inhibits liver alterations associated with hepatocellular carcinoma in rat. Pharmaceutics 2020, 12, 794. [Google Scholar] [CrossRef]
- Morry, J.; Ngamcherdtrakul, W.; Yantasee, W. Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol. 2017, 11, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Kollie, L.; Liu, X.; Guo, W.; Ying, X.; Zhu, J.; Yang, S.; Yu, M. Antitumor activity and potential mechanism of novel fullerene derivative nanoparticles. Molecules 2021, 26, 3252. [Google Scholar] [CrossRef]
- Satoh, M.; Takayanagi, I. Pharmacological studies on fullerene (C60), a novel carbon allotrope, and its derivatives. J. Pharmacol. Sci. 2006, 100, 513–518. [Google Scholar] [CrossRef]
- Sayers, B.C.; Germolec, D.R.; Walker, N.J.; Shipkowski, K.A.; Stout, M.D.; Cesta, M.F.; Roycroft, J.H.; White, K.L.; Baker, G.L.; Dill, J.A.; et al. Respiratory toxicity and immunotoxicity evaluations of microparticle and nanoparticle C60 fullerene aggregates in mice and rats following nose-only inhalation for 13 weeks. Nanotoxicology 2016, 10, 1458–1468. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef]
- Saleem, J.; Wang, L.; Chen, C. Carbon-based nanomaterials for cancer therapy via targeting tumor microenvironment. Adv. Healthc. Mater. 2018, 7, 1800525. [Google Scholar] [CrossRef]
- Siringan, M.J.; Dawar, A.; Zhang, J. Interactions between fullerene derivatives and biological systems. Mater. Chem. Front. 2023, 7, 2153–2174. [Google Scholar] [CrossRef]
- Cordani, M.; Strippoli, R.; Somoza, Á. Nanomaterials as inhibitors of epithelial mesenchymal transition in cancer treatment. Cancers 2019, 12, 25. [Google Scholar] [CrossRef]
- Tsubakihara, Y.; Moustakas, A. Epithelial-mesenchymal transition and metastasis under the control of transforming growth factor β. Int. J. Mol. Sci. 2018, 19, 3672. [Google Scholar] [CrossRef]
- Basilico, B.; Palamà, I.E.; D’Amone, S.; Lauro, C.; Rosito, M.; Grieco, M.; Ratano, P.; Cordella, F.; Sanchini, C.; Di Angelantonio, S.; et al. Substrate stiffness effect on molecular crosstalk of epithelial-mesenchymal transition mediators of human glioblastoma cells. Front. Oncol. 2022, 12, 983507. [Google Scholar] [CrossRef]
- Prylutska, S.; Grynyuk, I.; Grebinyk, A.; Hurmach, V.; Shatrava, I.; Sliva, T.; Amirkhanov, V.; Prylutskyy, Y.; Matyshevska, O.; Slobodyanik, M.; et al. Cytotoxic effects of dimorfolido-N-trichloroacetylphosphorylamide and dimorfolido-N-benzoylphosphorylamide in combination with C60 fullerene on leukemic cells and docking study of their interaction with DNA. Nanoscale Res. Lett. 2017, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, Y.; Song, W.; Jiang, X.; Deng, Z.; Xiong, W.; Shen, J. Metabolic reprogramming mediated PD-L1 depression and hypoxia reversion to reactivate tumor therapy. J. Control. Release. 2022, 352, 793–812. [Google Scholar] [CrossRef]
- Kuznietsova, H.; Lynchak, O.; Dziubenko, N.; Herheliuk, T.; Prylutskyy, Y.; Rybalchenko, V.; Ritter, U. Water-soluble pristine C60 fullerene attenuates acetaminophen-induced liver injury. Bioimpacts 2019, 9, 227. [Google Scholar] [CrossRef]
- Isakovic, A.; Markovic, Z.; Todorovic-Markovic, B.; Nikolic, N.; Vranjes-Djuric, S.; Mirkovic, M.; Dramicanin, M.; Harhaji, L.; Raicevic, N.; Nikolic, Z.; et al. Distinct cytotoxic mechanisms of pristine versus hydroxylated fullerene. Toxicol. Sci. 2006, 91, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Athira, S.S.; Biby, E.T.; Mohanan, P.V. Dextran stabilized fullerene soot induced toxicity on alveolar epithelial cells (A549 cells). Environ. Res. 2020, 188, 109716. [Google Scholar] [CrossRef]
- Zogovic, N.S.; Nikolic, N.S.; Vranjes-Djuric, S.D.; Harhaji, L.M.; Vucicevic, L.M.; Janjetovic, K.D.; Misirkic, M.S.; Todorovic-Markovic, B.M.; Markovic, Z.M.; Milonjic, S.K.; et al. Opposite effects of nanocrystalline fullerene (C60) on tumour cell growth in vitro and in vivo and a possible role of immunosupression in the cancer-promoting activity of C60. Biomaterials 2009, 30, 6940–6946. [Google Scholar] [CrossRef]
- Sengez, B.; Carr, B.I.; Alotaibi, H. EMT and inflammation: Crossroads in HCC. J. Gastrointest. Cancer 2023, 54, 204–212. [Google Scholar] [CrossRef]
- Zhu, J.; Li, B.; Xu, M.; Liu, R.; Xia, T.; Zhang, Z.; Xu, J.; Liu, S. Graphene oxide promotes cancer metastasis through associating with plasma membrane to promote TGF-β signaling-dependent epithelial–mesenchymal transition. ACS Nano 2019, 14, 818–827. [Google Scholar] [CrossRef]
- Kuznietsova, H.; Dziubenko, N.; Hurmach, V.; Chereschuk, I.; Motuziuk, O.; Ogloblya, O.; Prylutskyy, Y. Water-soluble pristine C60 fullerenes inhibit liver fibrotic alteration and prevent liver cirrhosis in rats. Oxid. Med. Cell. Longev. 2020, 2020, 8061246. [Google Scholar] [CrossRef] [PubMed]
- Namadr, F.; Bahrami, F.; Bahari, Z.; Ghanbari, B.; Shahyad, S.; Mohammadi, M.T. Fullerene C60 nanoparticles decrease liver oxidative stress through increment of liver antioxidant capacity in streptozotocin-induced diabetes in rats. React. Oxyg. Species 2020, 9, 70–80. [Google Scholar] [CrossRef]
- Vani, J.R.; Mohammadi, M.T.; Foroshani, M.S.; Jafari, M. Polyhydroxylated fullerene nanoparticles attenuate brain infarction and oxidative stress in rat model of ischemic stroke. Excli J. 2016, 15, 378. [Google Scholar] [CrossRef]
- Cheng, S.B.; Liu, H.T.; Chen, S.Y.; Lin, P.T.; Lai, C.Y.; Huang, Y.C. Changes of oxidative stress, glutathione, and its dependent antioxidant enzyme activities in patients with hepatocellular carcinoma before and after tumor resection. PLoS ONE 2017, 12, e0170016. [Google Scholar] [CrossRef]
- Ryan, J.J.; Bateman, H.R.; Stover, A.; Gomez, G.; Norton, S.K.; Zhao, W.; Schwartz, L.B.; Lenk, R.; Kepley, C.L. Fullerene nanomaterials inhibit the allergic response. J. Immunol. 2007, 179, 665–672. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, C.; Qian, P.; Lu, X.; Sun, B.; Zhang, X.; Wang, L.; Gao, X.; Li, H.; Chen, Z.; et al. Gd-metallofullerenol nanomaterial as non-toxic breast cancer stem cell-specific inhibitor. Nat. Commun. 2015, 6, 5988. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, F.; Ding, T.; Liu, X.; Xing, G.; Zhao, Y.; Zhang, N.; Ma, Y. [Gd@C82(OH)22]n nanoparticles inhibit the migration and adhesion of glioblastoma cells. Oncol. Lett. 2010, 1, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Leroy, K.; Silva Costa, C.J.; Pieters, A.; dos Santos Rodrigues, B.; Van Campenhout, R.; Cooreman, A.; Tabernilla, A.; Cogliati, B.; Vinken, M. Expression and functionality of connexin-based channels in human liver cancer cell lines. Int. J. Mol. Sci. 2021, 22, 12187. [Google Scholar] [CrossRef]
- Lucafo, M.; Pelillo, C.; Carini, M.; Da Ros, T.; Prato, M.; Sava, G. A cationic [60] fullerene derivative reduces invasion and migration of HT-29 CRC cells in vitro at dose free of significant effects on cell survival. Nano-Micro Lett. 2014, 6, 163–168. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, F.; Labib, M.; Ahmadi, M.; Chen, H.; Das, J.; Ahmed, S.U.; Angers, S.; Sargent, E.H.; Kelley, S.O. Nanostructured architectures promote the mesenchymal–epithelial transition for invasive cells. ACS Nano 2020, 14, 5324–5336. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, W.; Huang, X.; Sun, Y.; Tian, S.; Cai, P. Reduced graphene oxide triggered epithelial-mesenchymal transition in A549 cells. Sci. Rep. 2018, 8, 15188. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, H.; Yan, M.; Li, W. Matrix metalloproteinase 1 is a poor prognostic biomarker for patients with hepatocellular carcinoma. Clin. Exp. Med. 2022, 23, 2065–2083. [Google Scholar] [CrossRef]
- Peeney, D.; Liu, Y.; Lazaroff, C.; Gurung, S.; Stetler-Stevenson, W.G. Unravelling the distinct biological functions and potential therapeutic applications of TIMP2 in cancer. Carcinogenesis 2022, 43, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Cayetano-Salazar, L.; Nava-Tapia, D.A.; Astudillo-Justo, K.D.; Arizmendi-Izazaga, A.; Sotelo-Leyva, C.; Herrera-Martinez, M.; Villegas-Comonfort, S.; Navarro-Tito, N. Flavonoids as regulators of TIMPs expression in cancer: Consequences, opportunities, and challenges. Life Sci. 2022, 308, 120932. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, B.; Sawosz, E.; Szczepaniak, J.; Strojny, B.; Sosnowska, M.; Daniluk, K.; Zielińska-Górska, M.; Bałaban, J.; Chwalibog, A.; Wierzbicki, M. Effects of metallic and carbon-based nanomaterials on human pancreatic cancer cell lines AsPC-1 and BxPC-3. Int. J. Mol. Sci. 2021, 22, 12100. [Google Scholar] [CrossRef]
- Kai, A.K.L.; Chan, L.K.; Lo, R.C.L.; Lee, J.M.F.; Wong, C.C.L.; Wong, J.C.M.; Ng, I.O.L. Down-regulation of TIMP2 by HIF-1α/miR-210/HIF-3α regulatory feedback circuit enhances cancer metastasis in hepatocellular carcinoma. Hepatology 2016, 64, 473–487. [Google Scholar] [CrossRef]
- Kunte, M.; Desai, K. The inhibitory effect of C-phycocyanin containing protein extract (C-PC Extract) on human matrix metalloproteinases (MMP-2 and MMP-9) in hepatocellular cancer cell line (HepG2). Protein J. 2017, 36, 186–195. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, W.; Xu, Z.; Xing, C.; Xie, H.; Guo, H.; Chen, K.; Song, P.; Gu, Y.; Xiao, F.; et al. Induction of an EMT-like transformation and MET in vitro. J. Transl. Med. 2013, 11, 164. [Google Scholar] [CrossRef][Green Version]
- Yuan, T.; Chen, Z.; Yan, F.; Qian, M.; Luo, H.; Ye, S.; Cao, J.; Ying, M.; Dai, X.; Gai, R.; et al. Deubiquitinating enzyme USP10 promotes hepatocellular carcinoma metastasis through deubiquitinating and stabilizing Smad4 protein. Mol. Oncol. 2020, 14, 197–210. [Google Scholar] [CrossRef]
- Zhang, L.; Niu, H.; Ma, J.; Yuan, B.Y.; Chen, Y.H.; Zhuang, Y.; Chen, G.W.; Zeng, Z.C.; Xiang, Z.L. The molecular mechanism of LncRNA34a-mediated regulation of bone metastasis in hepatocellular carcinoma. Mol. Cancer 2019, 18, 120. [Google Scholar] [CrossRef]
- Chao, D.; Pang, L.; Shi, Y.; Wang, W.; Liu, K. AZD3759 induces apoptosis in hepatoma cells by activating a p53-SMAD4 positive feedback loop. Biochem. Bioph. Res. Commun. 2019, 509, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Grebowski, J.; Kazmierska-Grebowska, P.; Cichon, N.; Piotrowski, P.; Litwinienko, G. The Effect of Fullerenol C60 (OH) 36 on the antioxidant defense system in erythrocytes. Int. J. Mol. Sci. 2021, 23, 119. [Google Scholar] [CrossRef]
- Cho, M.Y.; Cheong, J.Y.; Lim, W.; Jo, S.; Lee, Y.; Wang, H.J.; Han, K.H.; Cho, H. Prognostic significance of catalase expression and its regulatory effects on hepatitis B virus X protein (HBx) in HBV-related advanced hepatocellular carcinomas. Oncotarget 2014, 5, 12233. [Google Scholar] [CrossRef] [PubMed]
- König, K.; Meder, L.; Kröger, C.; Diehl, L.; Florin, A.; Rommerscheidt-Fuss, U.; Kahl, P.; Wardelmann, E.; Magin, T.M.; Buettner, R.; et al. Loss of the keratin cytoskeleton is not sufficient to induce epithelial mesenchymal transition in a novel KRAS driven sporadic lung cancer mouse model. PLoS ONE 2013, 8, e57996. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, W.; Zhang, M.; Wang, X.; Peng, S.; Zhang, R. KRT6A promotes EMT and cancer stem cell transformation in lung adenocarcinoma. Technol. Cancer Res. Treat. 2020, 19, 1533033820921248. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, G.H.; Na, D.C.; Ahn, E.Y.; Kim, G.I.; Lee, J.E.; Cho, J.Y.; Yoo, J.E.; Choi, J.S.; Park, Y.N. Human hepatocellular carcinomas with “Stemness”-related marker expression: Keratin 19 expression and a poor prognosis. Hepatology 2011, 54, 1707–1717. [Google Scholar] [CrossRef]
- Fortier, A.M.; Asselin, E.; Cadrin, M. Keratin 8 and 18 loss in epithelial cancer cells increases collective cell migration and cisplatin sensitivity through claudin1 up-regulation. J. Biol. Chem. 2013, 288, 11555–11571. [Google Scholar] [CrossRef]
- Ten Dijke, P.; Goumans, M.J.; Itoh, F.; Itoh, S. Regulation of cell proliferation by Smad proteins. J. Cell. Physiol. 2002, 191, 1–16. [Google Scholar] [CrossRef]
- Ji, C.; Zhao, H.; Li, D.; Sun, H.; Hao, J.; Chen, R.; Wang, X.; Zhang, H.; Zhao, Y.G. Role of Wdr45b in maintaining neural autophagy and cognitive function. Autophagy 2020, 16, 615–625. [Google Scholar] [CrossRef]
- Liu, Z.; Ning, J.; Zheng, X.; Meng, J.; Han, L.; Zheng, H.; Zhong, L.; Chen, X.F.; Zhang, X.; Luo, H.; et al. TMEM59 interacts with TREM2 and modulates TREM2-dependent microglial activities. Cell Death Dis. 2020, 11, 678. [Google Scholar] [CrossRef]
- Hu, X.; Mullins, R.D. LC3 and STRAP regulate actin filament assembly by JMY during autophagosome formation. J. Cell Biol. 2019, 218, 251–266. [Google Scholar] [CrossRef]
- Chen, H.T.; Liu, H.; Mao, M.J.; Tan, Y.; Mo, X.Q.; Meng, X.J.; Cao, M.T.; Zhong, C.Y.; Liu, Y.; Shan, H.; et al. Crosstalk between autophagy and epithelial-mesenchymal transition and its application in cancer therapy. Mol. Cancer 2019, 18, 101. [Google Scholar] [CrossRef]
- Dash, S.; Sarashetti, P.M.; Rajashekar, B.; Chowdhury, R.; Mukherjee, S. TGF-β2-induced EMT is dampened by inhibition of autophagy and TNF-α treatment. Oncotarget 2018, 9, 6433. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Zhou, L.; Wang, W.; Liu, Z.; Cheng, Y.; Li, J.; Wei, H. Autophagy plays a critical role in insulin resistance-mediated chemoresistance in hepatocellular carcinoma cells by regulating the ER stress. J. Cancer 2018, 9, 4314. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Shinozawa, Y.; Iijima, Y.; Yu, B.C.; Sone, M.; Ooi, Y.; Watanaka, Y.; Chida, K.; Hakuno, F.; Takahashi, S.I. Tumor necrosis factor (TNF)-α-induced repression of GKAP42 protein levels through cGMP-dependent kinase (cGK)-Iα causes insulin resistance in 3T3-L1 adipocytes. J. Biol. Chem. 2015, 290, 5881–5892. [Google Scholar] [CrossRef]
- Fujimoto, B.A.; Young, M.; Nakamura, N.; Ha, H.; Carter, L.; Pitts, M.W.; Torres, D.; Noh, H.L.; Suk, S.; Kim, J.K.; et al. Disrupted glucose homeostasis and skeletal-muscle-specific glucose uptake in an exocyst knockout mouse model. J. Biol. Chem. 2021, 296, 100482. [Google Scholar] [CrossRef]
- Zhou, B.; Jia, L.; Zhang, Z.; Xiang, L.; Yuan, Y.; Zheng, P.; Liu, B.; Ren, X.; Bian, H.; Xie, L.; et al. The nuclear orphan receptor NR2F6 promotes hepatic steatosis through upregulation of fatty acid transporter CD36. Adv. Sci. 2020, 7, 2002273. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Liu, C.; Liu, J.; Yang, L.; Yue, W.; Yang, L.; Xue, R.; Zhang, K.; Zhang, H.; et al. Single-cell RNA seq identifies Plg-RKT-PLG as signals inducing phenotypic transformation of scar-associated macrophage in liver fibrosis. BBA-Mol. Basis Dis. 2023, 1869, 166754. [Google Scholar] [CrossRef]
- Rasmussen, N.L.; Zhou, J.; Olsvik, H.; Kaeser-Pebernard, S.; Lamark, T.; Dengjel, J.; Johansen, T. The inflammation repressor TNIP1/ABIN-1 is degraded by autophagy following TBK1 phosphorylation of its LIR. Autophagy 2023, 19, 2819–2820. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Lundgreen, A.; Herrick, J.S.; Wolff, R.K. Genetic variation in RPS6KA1, RPS6KA2, RPS6KB1, RPS6KB2, and PDK1 and risk of colon or rectal cancer. Mutat. Res. 2011, 706, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Bartuzi, P.; Hofker, M.H.; van de Sluis, B. Tuning NF-κB activity: A touch of COMMD proteins. Biochim. Biophys. Acta 2013, 1832, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Oh, S.; Bournique, E.; Bowen, D.; Jalili, P.; Sanchez, A.; Ward, I.; Dananberg, A.; Manjunath, L.; Tran, G.P.; Semler, B.L.; et al. Genotoxic stress and viral infection induce transient expression of APOBEC3A and pro-inflammatory genes through two distinct pathways. Nat. Commun. 2021, 12, 4917. [Google Scholar] [CrossRef]
- Law, E.K.; Levin-Klein, R.; Jarvis, M.C.; Kim, H.; Argyris, P.P.; Carpenter, M.A.; Starrett, G.J.; Temiz, N.A.; Larson, L.K.; Durfee, C.; et al. APOBEC3A catalyzes mutation and drives carcinogenesis in vivo. J. Exp. Med. 2020, 217, e20200261. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, C.; Xiao, M.; Han, Y.; Zhang, S.; Xu, B. Bioinformatics analysis of the prognostic and biological significance of ZDHHC-protein acyltransferases in kidney renal clear cell carcinoma. Front. Oncol. 2020, 10, 565414. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Liu, Y.Y.; Tao, B.Y.; Xue, X.M.; Zhang, X.X.; Wang, L.L.; Zhong, H.; Zhang, J.; Yang, S.M.; Jiang, Q.Q. NT5E upregulation in head and neck squamous cell carcinoma: A novel biomarker on cancer-associated fibroblasts for predicting immunosuppressive tumor microenvironment. Front. Immunol. 2022, 13, 975847. [Google Scholar] [CrossRef]
- Snider, N.T.; Altshuler, P.J.; Wan, S.; Welling, T.H.; Cavalcoli, J.; Omary, M.B. Alternative splicing of human NT5E in cirrhosis and hepatocellular carcinoma produces a negative regulator of ecto-5′-nucleotidase (CD73). Mol. Biol. Cell 2014, 25, 4024–4033. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ye, Q.; Wu, L.; Gao, F.; Xie, H.; Zhou, L.; Zheng, S.; Xu, X. Metallothionein 1 family profiling identifies MT1X as a tumor suppressor involved in the progression and metastastatic capacity of hepatocellular carcinoma. Mol. Carcinogen. 2018, 57, 1435–1444. [Google Scholar] [CrossRef]
- Shimizu, M.; Koma, Y.I.; Sakamoto, H.; Tsukamoto, S.; Kitamura, Y.; Urakami, S.; Tanigawa, K.; Kodama, T.; Higashino, N.; Nishio, M.; et al. Metallothionein 2A expression in cancer-associated fibroblasts and cancer cells promotes esophageal squamous cell carcinoma progression. Cancers 2021, 13, 4552. [Google Scholar] [CrossRef]
- Shetty, G.A.; Hattiangady, B.; Upadhya, D.; Bates, A.; Attaluri, S.; Shuai, B.; Kodali, M.; Shetty, A.K. Chronic oxidative stress, mitochondrial dysfunction, Nrf2 activation and inflammation in the hippocampus accompany heightened systemic inflammation and oxidative stress in an animal model of gulf war illness. Front. Mol. Neurosci. 2017, 10, 182. [Google Scholar] [CrossRef]
- Luo, P.; Yan, H.; Du, J.; Chen, X.; Shao, J.; Zhang, Y.; Xu, Z.; Jin, Y.; Lin, N.; Yang, B.; et al. PLK1 (polo like kinase 1)-dependent autophagy facilitates gefitinib-induced hepatotoxicity by degrading COX6A1 (cytochrome c oxidase subunit 6A1). Autophagy 2021, 17, 3221–3237. [Google Scholar] [CrossRef] [PubMed]
- Eun, S.Y.; Woo, I.S.; Jang, H.S.; Jin, H.; Kim, M.Y.; Kim, H.J.; Lee, J.H.; Chang, K.C.; Kim, J.H.; Seo, H.G. Identification of cytochrome c oxidase subunit 6A1 as a suppressor of Bax-induced cell death by yeast-based functional screening. Biochem. Bioph. Res. Commun. 2008, 373, 58–63. [Google Scholar] [CrossRef]
- Brea-Calvo, G.; Rodríguez-Hernández, Á.; Fernández-Ayala, D.J.; Navas, P.; Sánchez-Alcázar, J.A. Chemotherapy induces an increase in coenzyme Q10 levels in cancer cell lines. Free Radic. Bio. Med. 2006, 40, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Voon, D.C.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. The EMT spectrum and therapeutic opportunities. Mol. Oncol. 2017, 11, 878–891. [Google Scholar] [CrossRef] [PubMed]

| Primary Antibody | Molecular Mass (kDa) | Catalogue Number | Company |
|---|---|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | 36 | ab157392 | Abcam |
| Smooth muscle actin | 42 | ||
| β-catenin | 92 | ||
| Vimentin | 54 | ||
| Snail and Slug | 29 | ab180714 | |
| Catalase | 60 | ab179843 | |
| Smad4 | 60 | PA5-34806 | Thermo Fisher Scientific |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosnowska, M.; Kutwin, M.; Zawadzka, K.; Pruchniewski, M.; Strojny, B.; Bujalska, Z.; Wierzbicki, M.; Jaworski, S.; Sawosz, E. Influence of C60 Nanofilm on the Expression of Selected Markers of Mesenchymal–Epithelial Transition in Hepatocellular Carcinoma. Cancers 2023, 15, 5553. https://doi.org/10.3390/cancers15235553
Sosnowska M, Kutwin M, Zawadzka K, Pruchniewski M, Strojny B, Bujalska Z, Wierzbicki M, Jaworski S, Sawosz E. Influence of C60 Nanofilm on the Expression of Selected Markers of Mesenchymal–Epithelial Transition in Hepatocellular Carcinoma. Cancers. 2023; 15(23):5553. https://doi.org/10.3390/cancers15235553
Chicago/Turabian StyleSosnowska, Malwina, Marta Kutwin, Katarzyna Zawadzka, Michał Pruchniewski, Barbara Strojny, Zuzanna Bujalska, Mateusz Wierzbicki, Sławomir Jaworski, and Ewa Sawosz. 2023. "Influence of C60 Nanofilm on the Expression of Selected Markers of Mesenchymal–Epithelial Transition in Hepatocellular Carcinoma" Cancers 15, no. 23: 5553. https://doi.org/10.3390/cancers15235553
APA StyleSosnowska, M., Kutwin, M., Zawadzka, K., Pruchniewski, M., Strojny, B., Bujalska, Z., Wierzbicki, M., Jaworski, S., & Sawosz, E. (2023). Influence of C60 Nanofilm on the Expression of Selected Markers of Mesenchymal–Epithelial Transition in Hepatocellular Carcinoma. Cancers, 15(23), 5553. https://doi.org/10.3390/cancers15235553








