Integrating [18F]-Fluorodeoxyglucose Positron Emission Tomography with Computed Tomography with Radiation Therapy and Immunomodulation in Precision Therapy for Solid Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Imaging Biomarkers Derived from Baseline PET
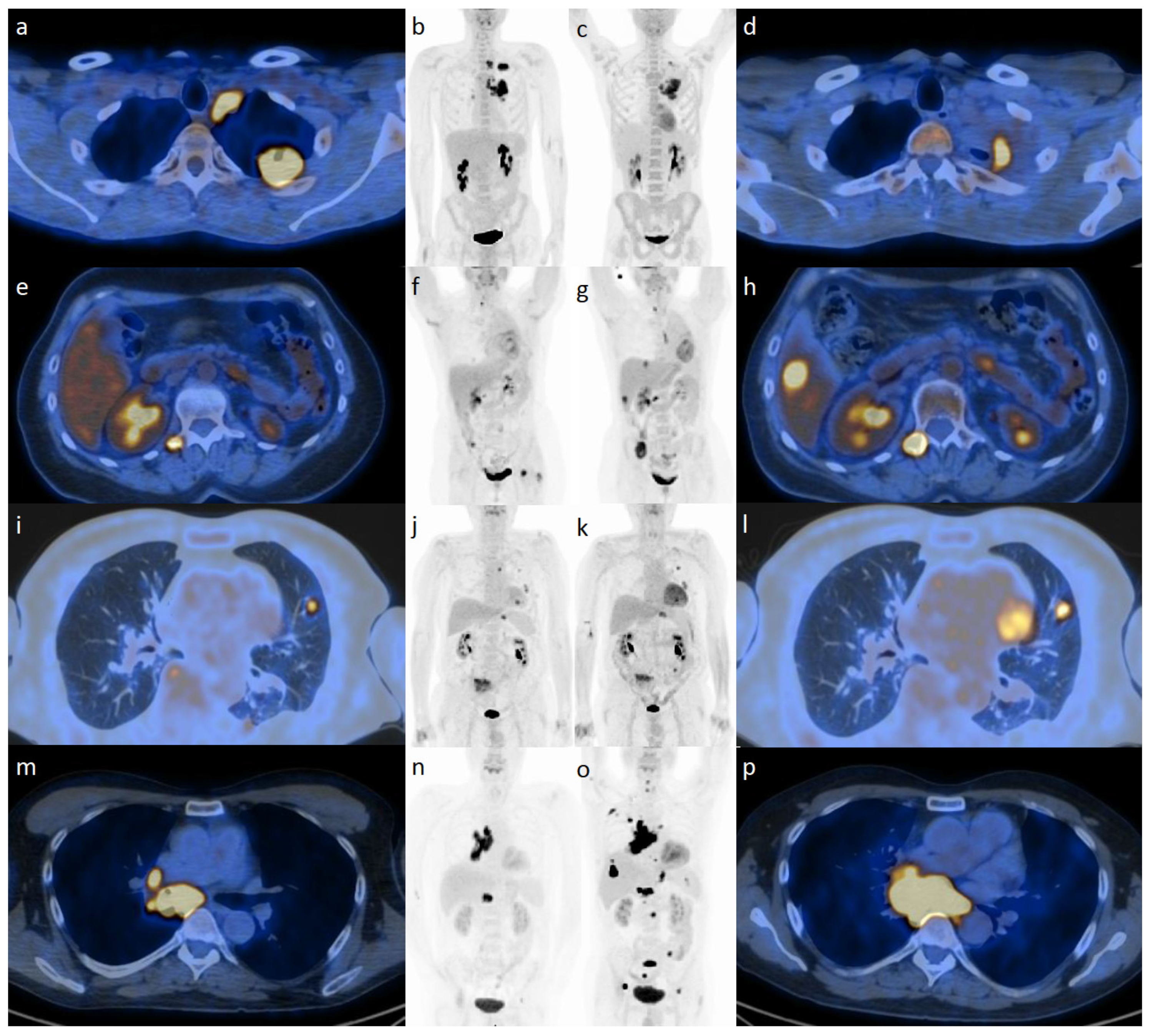
2.1. [18F]-FDG PET
2.2. Immuno-PET
3. The New Role of PET for Defining the Effect of IO/RT
Understanding the Abscopal Effect
4. The Role of PET for Refining Patterns of Response to IO/RT
4.1. Role of PET for Hyperprogression
4.2. Role of PET for Oligoprogression
4.3. Role of PET for Pseudoprogression
4.4. Role of PET for the Diagnosis of Immune-Related Adverse Events
5. Documentation and Reporting: Adaptation of the Joint Guidelines
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| [18F]-FDG-PET | [18]Fluorodeoxyglucose Positron Emission Tomography |
| CT | Computed Tomography |
| ANZSNM | Australian and New Zealand Society of Nuclear Medicine |
| CMR | Complete Metabolic Response |
| EANM | European Association of Nuclear Medicine |
| IO | Immuno-oncology |
| irAE | Immune-related Adverse Events |
| MATV | Metabolic Activity Tumor Volume |
| PD-1 | Programmed Death Protein/Ligand |
| PERCMIT | PET Response Evaluation Criteria for Immunotherapy |
| RT | Radiation Therapy |
| SNMMI | Society of Nuclear Medicine and Molecular Imaging |
| SUV | Standardized Uptake Value |
| TLG | Total Lesion Glycolysis |
References
- Lopci, E.; Hicks, R.J.; Dimitrakopoulou-Strauss, A.; Dercle, L.; Iravani, A.; Seban, R.D.; Sachpekidis, C.; Humbert, O.; Gheysens, O.; Glaudemans, A.W.J.M.; et al. Joint EANM/SNMMI/ANZSNM practice guidelines/procedure standards on recommended use of [18F]FDG PET/CT imaging during immunomodulatory treatments in patients with solid tumors version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2323–2341. [Google Scholar] [CrossRef]
- Dercle, L.; Sun, S.; Seban, R.D.; Mekki, A.; Sun, R.; Tselikas, L.; Hans, S.; Bernard-Tessier, A.; Bouvier, F.M.; Aide, N.; et al. Emerging and Evolving Concepts in Cancer Immunotherapy Imaging. Radiology 2023, 306, e239003, Erratum in Radiology 2023, 306, 32–46. [Google Scholar] [CrossRef]
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Mekki, A.; Dercle, L.; Lichtenstein, P.; Marabelle, A.; Michot, J.M.; Lambotte, O.; Le Pavec, J.; De Martin, E.; Balleyguier, C.; Champiat, S.; et al. Detection of immune-related adverse events by medical imaging in patients treated with anti-programmed cell death 1. Eur. J. Cancer 2018, 96, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Rosenkrans, Z.T.; Liu, J.; Huang, G.; Luo, Q.-Y.; Cai, W. Immunopet: Concept, design, and applications. Chem. Rev. 2020, 120, 3787–3851. [Google Scholar] [CrossRef] [PubMed]
- Lopci, E. Immunotherapy Monitoring with Immune Checkpoint Inhibitors Based on [18F]FDG PET/CT in Metastatic Melanomas and Lung Cancer. J. Clin. Med. 2021, 10, 5160. [Google Scholar] [CrossRef]
- Kok, I.C.; Hooiveld, J.S.; van de Donk, P.P.; Giesen, D.; van der Veen, E.L.; Lub-de Hooge, M.N.; Brouwers, A.H.; Hiltermann, T.J.N.; van der Wekken, A.J.; Hijmering-Kappelle, L.B.M.; et al. 89Zr-pembrolizumab imaging as a non-invasive approach to assess clinical response to PD-1 blockade in cancer. Ann. Oncol. 2022, 33, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Niemeijer, A.N.; Leung, D.; Huisman, M.C.; Bahce, I.; Hoekstra, O.S.; van Dongen, G.A.M.S.; Boellaard, R.; Du, S.; Hayes, W.; Smith, R.; et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat. Commun. 2018, 9, 4664. [Google Scholar] [CrossRef] [PubMed]
- Bensch, F.; van der Veen, E.L.; Lub-de Hooge, M.N.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schröder, C.P.; Hiltermann, T.J.N.; van der Wekken, A.J.; et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 2018, 24, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Young, K.H.; Baird, J.R.; Savage, T.; Cottam, B.; Friedman, D.; Bambina, S.; Messenheimer, D.J.; Fox, B.; Newell, P.; Bahjat, K.S.; et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS ONE 2016, 11, e0157164. [Google Scholar] [CrossRef]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, Y.; Kong, L.; Shi, F.; Zhu, H.; Yu, J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Janopaul-Naylor, J.R.; Shen, Y.; Qian, D.C.; Buchwald, Z.S. The abscopal effect: A review of pre-clinical and clinical advances. Int. J. Mol. Sci. 2021, 22, 11061. [Google Scholar] [CrossRef] [PubMed]
- Aide, N.; Hicks, R.J.; Le Tourneau, C.; Lheureux, S.; Fanti, S.; Lopci, E. FDG PET/CT for assessing tumour response to immunotherapy: Report on the EANM symposium on immune modulation and recent review of the literature. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Seban, R.D.; Schwartz, L.H.; Bonardel, G.; Dercle, L. Diagnosis of Hyperprogressive Disease in Patients Treated with Checkpoint Inhibitors Using 18F-FDG PET/CT. J. Nucl. Med. 2020, 61, 1404–1405. [Google Scholar] [CrossRef] [PubMed]
- Laurie, S.A.; Banerji, S.; Blais, N.; Brule, S.; Cheema, P.K.; Cheung, P.; Daaboul, N.; Hao, D.; Hirsh, V.; Juergens, R.; et al. Canadian consensus: Oligoprogressive, pseudoprogressive, and oligometastatic non-small-cell lung cancer. Curr. Oncol. 2019, 26, e81–e93. [Google Scholar] [CrossRef] [PubMed]
- Cherk, M.H.; Nadebaum, D.P.; Barber, T.W.; Beech, P.; Haydon, A.; Yap, K.S. 18F-FDG PET features of immune-related adverse events and pitfalls following immunotherapy. J. Med. Imaging Radiat. Oncol. 2022, 66, 483–494. [Google Scholar] [CrossRef]
- Costa, L.B.; Queiroz, M.A.; Barbosa, F.G.; Nunes, R.F.; Zaniboni, E.C.; Ruiz, M.M.; Jardim, D.; Gomes Marin, J.F.; Cerri, G.G.; Buchpiguel, C.A. Reassessing Patterns of Response to Immunotherapy with PET: From Morphology to Metabolism. Radiographics 2021, 41, 120–143. [Google Scholar] [CrossRef]
- Lopci, E.; Aide, N.; Dimitrakopoulou-Strauss, A.; Dercle, L.; Iravani, A.; Seban, R.D.; Sachpekidis, C.; Humbert, O.; Gheysens, O.; Glaudemans, A.W.J.M.; et al. Perspectives on joint EANM/SNMMI/ANZSNM practice guidelines/procedure standards for [18F]FDG PET/CT imaging during immunomodulatory treatments in patients with solid tumors. Cancer Imaging 2022, 22, 73. [Google Scholar] [CrossRef]
- Lopci, E.; Castello, A.; Filippi, L. Novelties from the Joint EANM/SNMMI/ANZSNM Guidelines on Immunotherapy. Cancer Biother. Radiopharm. 2023. epub ahead of print. [Google Scholar] [CrossRef]
- Young, H.; Baum, R.; Cremerius, U.; Herholz, K.; Hoekstra, O.; Lammertsma, A.A.; Pruim, J.; Price, P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur. J. Cancer 1999, 35, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50 (Suppl. S1), S122–S150. [Google Scholar] [CrossRef]
- Cheson, B.D.; Ansell, S.; Schwartz, L.; Gordon, L.I.; Advani, R.; Jacene, H.A.; Hoos, A.; Barrington, S.F.; Armand, P. Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood 2016, 128, 2489–2496. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Lipson, E.J.; Im, H.-J.; Rowe, S.P.; Gonzalez, E.M.; Blackford, A.; Chirindel, A.; Pardoll, D.M.; Topalian, S.L.; Wahl, R.L. Prediction of response to immune checkpoint inhibitor therapy using early-time-point (18)F-FDG PET/CT imaging in patients with advanced melanoma. J. Nucl. Med. 2017, 58, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Anwar, H.; Sachpekidis, C.; Winkler, J.; Kopp-Schneider, A.; Haberkorn, U.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. Absolute number of new lesions on (18)F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Teng, R.; Schöder, H.; Humm, J.L.; Ni, A.; Michaud, L.; Nakajima, R.; Yamashita, R.; Wolchok, J.D.; Weber, W.A. (18)F-FDG PET/CT for monitoring of ipilimumab therapy in patients with metastatic melanoma. J. Nucl. Med. 2019, 60, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, L.; Duchemann, B.; Chouahnia, K.; Zelek, L.; Soussan, M. Monitoring anti-PD-1-based immunotherapy in non-small cell lung cancer with FDG PET: Introduction of iPERCIST. EJNMMI Res. 2019, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Dercle, L.; Henry, T.; Carré, A.; Paragios, N.; Deutsch, E.; Robert, C. Reinventing radiation therapy with machine learning and imaging bio-markers (radiomics): State-of-the-art, challenges and perspectives. Methods 2021, 188, 44–60. [Google Scholar] [CrossRef]
- Michot, J.-M.; Mazeron, R.; Dercle, L.; Ammari, S.; Canova, C.; Marabelle, A.; Rose, S.; Rubin, E.; Deutsch, E.; Soria, J.-C.; et al. Abscopal effect in a Hodgkin lymphoma patient treated by an anti-programmed death 1 antibody. Eur. J. Cancer 2016, 66, 91–94. [Google Scholar] [CrossRef]
- Dercle, L.; McGale, J.; Sun, S.; Marabelle, A.; Yeh, R.; Deutsch, E.; Mokrane, F.-Z.; Farwell, M.; Ammari, S.; Schoder, H.; et al. Artificial intelligence and radiomics: Fundamentals, applications, and challenges in immunotherapy. J. Immunother. Cancer 2022, 10, e005292. [Google Scholar] [CrossRef]

| PET Parameters | Point of Interest |
|---|---|
| Standardized uptake value (SUV) | Dimensionless value of tumor’s’ uptake of the radiotracer, quantified as SUVmax, SUVmean, SUVpeak; helps distinguish between metabolically normal and abnormal tissues |
| Metabolically active tumor volume (MATV) | Volume of [18F]-FDG-avid tumor delineated on PET images; utilized for prognosis and quantifying response to treatment |
| Total lesion glycolysis (TLG) | Product of the mean standardized uptake value and metabolic activity tumor volume; used to quantify metabolic tumor burden |
| Bone marrow avidity | Metric of [18F]-FDG uptake in bone marrow; often higher than other organs such as the liver and could be a biomarker for detecting immunosuppressive cancers or infiltration by cancers such as lymphomas |
| Bone marrow to liver ratio/spleen to liver ratio | Ratio between the [18F]-FDG uptake in bone marrow or in the spleen compared to the liver uptake; used to assess the immune system activation in the context of IO |
| EORTC | PERCIST 1.0 | LYRIC | PECRIT | PERCIMT | imPERCIST | iPERCIST | |
|---|---|---|---|---|---|---|---|
| Authors | Young et al. [21] | Wahl et al. [22] | Cheson et al. [23] | Cho et al. [24] | Anwar et al. [25] | Ito et al. [26] | Goldfarb et al. [27] |
| Tumor type/Modality | Solid tumor/[18F]-FDG PET | Solid tumor/[18F]-FDG PET | Lymphoma/CT and [18F]-FDG PET | Melanoma/CT and [18F]-FDG PET/CT | CT and [18F]-FDG PET/CT | [18F]-FDG PET/CT | [18F]-FDG PET/CT |
| Year | 1999 | 2009 | 2016 | 2017 | 2017 | 2019 | 2019 |
| Lesion measurement | - | - | Bidimensional | Unidimensional | - | - | - |
| Baseline size | - | - | >15 mm | >10 mm | - | - | - |
| Baseline lesion number | - | 5 lesions total, 2 per organ | 6 lesions total (nodes and extranodal sites) | 5 lesions (2 per organ) | According to RECIST 1.1 and PERCIST 1 | 5 lesions total, 2 per organ | 5 lesions total, 2 per organ |
| New lesion | Results in PD | Results in PMD | Considered as IR2a | Results in PD | PD depends on number and functional size of new lesion(s) | SULpeak of new lesion(s) included in the sum of SULpeak | To be confirmed by a new imaging evaluation at least 4 weeks later |
| Non-index lesion | - | - | Considered as IR2b | Same as RECIST 1.1 | - | - | - |
| Complete resolution | Complete resolution of [18F]-FDG PET uptake within the tumor volume so that it is indistinguishable from surrounding normal tissue | Disappearance of all metabolically active lesions | [18F]-FDG PET-uptake < liver (score 1, 2, 3) without a residual mass OR on CT, target nodes/nodal masses must regress to <15 mm in longest diameter | See RECIST 1.1 Results in clinical benefit | No new lesions | Disappearance of all lesions | Disappearance of all lesions |
| Partial reduction | A reduction of a minimum of 15–25% in tumor SUV after one cycle of chemotherapy, and >25% after more than one treatment cycle | Reduction in SULpeak in target lesions of > 30% and absolute drop in SUL > 0.8 SUL units | [18F]-FDG PET-uptake > liver (score 4 or 5) with reduced uptake compared with baseline and residual masse(es) of any size OR on CT > 50% decrease in SPDof up to 6 measurable nodes and extranodal sites | See RECIST 1.1 Results in clinical benefit | No new lesions | ≥30% decrease in sum of SULpeak of target lesions and decrease of ≥0.8 SUL units | ≥30% decrease in sum of SULpeak |
| Stable disease | An increase in SUV < 25% or a decrease < 15% and no visible increase in extent of [18F]-FDG PET tumor uptake (>20% in the longest dimension) | Neither CR/PR nor PD can be established | Neither CR/PR nor PD can be established | See RECIST 1.1 If SULpeak decreases by more than 15.5%, clinical benefit If SULpeak decreases by less than 15.5%, no clinical benefit | Neither CR/PR nor PD can be established | Neither CR/PR nor PD can be established | Neither CR/PR nor PD can be established |
| Progressive disease | An increase in SUV > 25% within the tumor region defined on the baseline scan, visible increase in the extent [18F]-FDG PET tumor uptake (>20% in the longest dimension) or the appearance of new [18F]-FDG PET uptake in metastatic lesions | Increase in SULpeak of > 30% or the appearance of a new lesion | First PD is IR (indeterminate response) Increase > 5 mm (if <2 cm) or 10 mm (if >2 cm) of at least one lesion Criteria for IR IR1: >50% increase in SPD in first 12 weeks IR2a: <50% increase in SPD with new lesion IR2b: <50% increase in SPD with >50% increase in product of the perpendicular diameters of a lesion or set of lesions IR3: increase in [18F]-FDG PET uptake without a concomitant increase in lesion size meeting criteria for IR1 or IR2 | See RECIST 1.1 Results in no clinical benefit | Four or more new lesions of less than 1.0 cm in functional diameter, or three or more new lesions of more than 1.0 cm in functional diameter, or two or more new lesions of more than 1.5 cm in functional diameter Predicts clinical PD, and no clinical benefit | >30% increase in sum of SULpeak | >30% increase in sum of SULpeak or new lesions Results in UPMD Clinical stability is considered to decide if treatment should be continued after UPMD |
| Confirmation PD | No | No | Yes, wait up to 12 weeks | No | No | No | Yes, 4–8 weeks later for CPMD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prendergast, C.M.; Lopci, E.; Seban, R.-D.; De Jong, D.; Ammari, S.; Aneja, S.; Lévy, A.; Sajan, A.; Salvatore, M.M.; Cappacione, K.M.; et al. Integrating [18F]-Fluorodeoxyglucose Positron Emission Tomography with Computed Tomography with Radiation Therapy and Immunomodulation in Precision Therapy for Solid Tumors. Cancers 2023, 15, 5179. https://doi.org/10.3390/cancers15215179
Prendergast CM, Lopci E, Seban R-D, De Jong D, Ammari S, Aneja S, Lévy A, Sajan A, Salvatore MM, Cappacione KM, et al. Integrating [18F]-Fluorodeoxyglucose Positron Emission Tomography with Computed Tomography with Radiation Therapy and Immunomodulation in Precision Therapy for Solid Tumors. Cancers. 2023; 15(21):5179. https://doi.org/10.3390/cancers15215179
Chicago/Turabian StylePrendergast, Conor M., Egesta Lopci, Romain-David Seban, Dorine De Jong, Samy Ammari, Sanjay Aneja, Antonin Lévy, Abin Sajan, Mary M. Salvatore, Kathleen M. Cappacione, and et al. 2023. "Integrating [18F]-Fluorodeoxyglucose Positron Emission Tomography with Computed Tomography with Radiation Therapy and Immunomodulation in Precision Therapy for Solid Tumors" Cancers 15, no. 21: 5179. https://doi.org/10.3390/cancers15215179
APA StylePrendergast, C. M., Lopci, E., Seban, R.-D., De Jong, D., Ammari, S., Aneja, S., Lévy, A., Sajan, A., Salvatore, M. M., Cappacione, K. M., Schwartz, L. H., Deutsch, E., & Dercle, L. (2023). Integrating [18F]-Fluorodeoxyglucose Positron Emission Tomography with Computed Tomography with Radiation Therapy and Immunomodulation in Precision Therapy for Solid Tumors. Cancers, 15(21), 5179. https://doi.org/10.3390/cancers15215179









