Disparities Contributing to Late-Stage Diagnosis of Lung, Colorectal, Breast, and Cervical Cancers: Rural and Urban Poverty in Florida
Abstract
Simple Summary
Abstract
1. Introduction
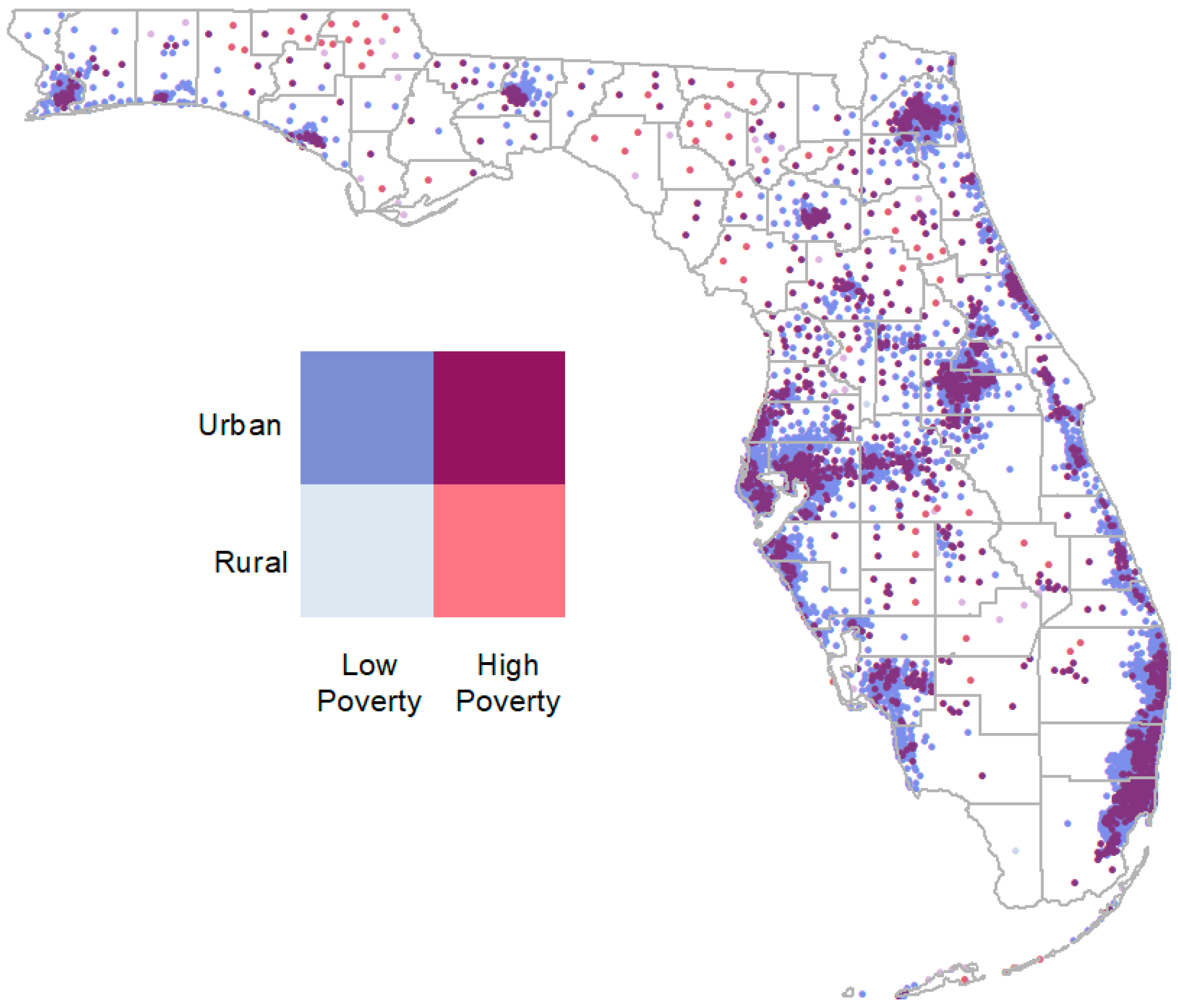
2. Materials and Methods
3. Results
3.1. Summary Statistics
3.2. Multi-Variable Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Cancer Facts & Figures 2022|American Cancer Society. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html (accessed on 28 March 2023).
- Leading Health Indicators—Healthy People 2030|health.gov. Available online: https://health.gov/healthypeople/objectives-and-data/leading-health-indicators (accessed on 1 February 2023).
- Ahmad, A. (Ed.) Breast Cancer Statistics: Recent Trends. In Breast Cancer Metastasis and Drug Resistance: Challenges and Progress; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–7. [Google Scholar] [CrossRef]
- Siegel, R.L.; Jemal, A.; Wender, R.C.; Gansler, T.; Ma, J.; Brawley, O.W.; Dvm, A.J.; Gansler, T. An assessment of progress in cancer control. CA A Cancer J. Clin. 2018, 68, 329–339. [Google Scholar] [CrossRef]
- Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement|Cancer Screening, Prevention, Control|JAMA|JAMA Network. Available online: https://jamanetwork.com/journals/jama/article-abstract/2529486 (accessed on 20 December 2022).
- Cervical Cancer Elimination Initiative. Available online: https://www.who.int/initiatives/cervical-cancer-elimination-initiative (accessed on 23 March 2023).
- Rivera-Franco, M.M.; Leon-Rodriguez, E. Delays in Breast Cancer Detection and Treatment in Developing Countries. Breast Cancer 2018, 12, 1178223417752677. [Google Scholar] [CrossRef]
- Ladabaum, U.; Dominitz, J.A.; Kahi, C.; Schoen, R.E. Strategies for Colorectal Cancer Screening. Gastroenterology 2020, 158, 418–432. [Google Scholar] [CrossRef]
- Cancer Screening Prevalence and Associated Factors Among US Adults. Available online: https://www.cdc.gov/pcd/collections/Cancer_Screening_Collection.htm (accessed on 21 June 2023).
- Benavidez, G.A. Disparities in Meeting USPSTF Breast, Cervical, and Colorectal Cancer Screening Guidelines among Women in the United States. Prev. Chronic Dis. 2021, 18, 200315. [Google Scholar] [CrossRef]
- Lewis-Thames, M.W.; Langston, M.E.; Khan, S.; Han, Y.; Fuzzell, L.; Xu, S.; Moore, J.X. Racial and Ethnic Differences in Rural-Urban Trends in 5-Year Survival of Patients with Lung, Prostate, Breast, and Colorectal Cancers: 1975–2011 Surveillance, Epidemiology, and End Results (SEER). JAMA Netw. Open 2022, 5, e2212246. [Google Scholar] [CrossRef]
- Hall, J.M.; Szurek, S.M.; Cho, H.; Guo, Y.; Gutter, M.S.; Khalil, G.E.; Licht, J.D.; Shenkman, E.A. Cancer disparities related to poverty and rurality for 22 top cancers in Florida. Prev. Med. Rep. 2022, 29, 101922. [Google Scholar] [CrossRef]
- Wang, H.; Roy, S.; Kim, J.; Farazi, P.A.; Siahpush, M.; Su, D. Barriers of colorectal cancer screening in rural USA: A systematic review. Rural. Remote Health 2019, 19, 5181. [Google Scholar] [CrossRef]
- Massarweh, N.N.; Chiang, Y.-J.; Xing, Y.; Chang, G.J.; Haynes, A.B.; You, Y.N.; Feig, B.W.; Cormier, J.N. Association Between Travel Distance and Metastatic Disease at Diagnosis among Patients with Colon Cancer. J. Clin. Oncol. 2014, 32, 942–948. [Google Scholar] [CrossRef]
- Yu, L.; Sabatino, S.A.; White, M.C. Rural–Urban and Racial/Ethnic Disparities in Invasive Cervical Cancer Incidence in the United States, 2010–2014. Prev. Chronic Dis. 2019, 16, 180447. [Google Scholar] [CrossRef]
- Lung Cancer Statistics|How Common Is Lung Cancer? Available online: https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html (accessed on 8 January 2023).
- Home Page|United States Preventive Services Taskforce. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/ (accessed on 2 January 2023).
- Association, A.L. State of Lung Cancer|Racial and Ethnic Disparities. Available online: https://www.lung.org/research/state-of-lung-cancer/racial-and-ethnic-disparities (accessed on 29 March 2023).
- Cavers, D.; Nelson, M.; Rostron, J.; Robb, K.A.; Brown, L.R.; Campbell, C.; Akram, A.R.; Dickie, G.; Mackean, M.; van Beek, E.J.R.; et al. Understanding patient barriers and facilitators to uptake of lung screening using low dose computed tomography: A mixed methods scoping review of the current literature. Respir. Res. 2022, 23, 374. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Lamanna, A.; Sheaffer, H.; Guerra, C.; Kochman, M. Colorectal Cancer Screening Navigation for the Underserved: Experience of an Urban Program. Gastroenterol. Hepatol. 2016, 12, 547–551. [Google Scholar]
- Muller, C.; Ihionkhan, E.; Stoffel, E.M.; Kupfer, S.S. Disparities in Early-Onset Colorectal Cancer. Cells 2021, 10, 1018. [Google Scholar] [CrossRef]
- Global Burden of Disease 2019 Cancer Collaboration; Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life Years for 29 Cancer Groups from 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [CrossRef]
- Bleyer, A.; Welch, H.G. Effect of Three Decades of Screening Mammography on Breast-Cancer Incidence. N. Engl. J. Med. 2012, 367, 1998–2005. [Google Scholar] [CrossRef]
- Taplin, S.H.; Ichikawa, L.; Yood, M.U.; Manos, M.M.; Geiger, A.M.; Weinmann, S.; Gilbert, J.; Mouchawar, J.; Leyden, W.A.; Altaras, R.; et al. Reason for Late-Stage Breast Cancer: Absence of Screening or Detection, or Breakdown in Follow-up? JNCI J. Natl. Cancer Inst. 2004, 96, 1518–1527. [Google Scholar] [CrossRef]
- Jemal, A.; Simard, E.P.; Dorell, C.; Noone, A.-M.; Markowitz, L.E.; Kohler, B.; Eheman, C.; Saraiya, M.; Bandi, P.; Saslow, D.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, Featuring the Burden and Trends in Human Papillomavirus (HPV)–Associated Cancers and HPV Vaccination Coverage Levels. J. Natl. Cancer Inst. 2013, 105, 175–201. [Google Scholar] [CrossRef]
- Gultekin, M.; Ramirez, P.T.; Broutet, N.; Hutubessy, R. World Health Organization call for action to eliminate cervical cancer globally. Int. J. Gynecol. Cancer 2020, 30, 426–427. [Google Scholar] [CrossRef]
- Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; Kubik, M.; et al. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 320, 674. [Google Scholar] [CrossRef]
- Fontham, E.T.H.; Wolf, A.M.D.; Church, T.R.; Etzioni, R.; Flowers, C.R.; Herzig, A.; Guerra, C.E.; Oeffinger, K.C.; Shih, Y.T.; Walter, L.C.; et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA A Cancer J. Clin. 2020, 70, 321–346. [Google Scholar] [CrossRef]
- Buskwofie, A.; David-West, G.; Clare, C.A. A Review of Cervical Cancer: Incidence and Disparities. J. Natl. Med. Assoc. 2020, 112, 229–232. [Google Scholar] [CrossRef]
- CDC. CDC Vitalsign: Cervical Cancer Is Preventable. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/vitalsigns/cervical-cancer/index.html (accessed on 29 March 2023).
- The Florida Cancer Data System Home Page. Available online: https://fcds.med.miami.edu/inc/welcome.shtml (accessed on 2 January 2023).
- Cancer Staging—NCI. Available online: https://www.cancer.gov/about-cancer/diagnosis-staging/staging (accessed on 13 December 2022).
- USDA ERS—Rural-Urban Commuting Area Codes. Available online: https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/ (accessed on 2 January 2023).
- U.C. Bureau, “Census.gov”, Census.gov. Available online: https://www.census.gov/en.html (accessed on 2 January 2023).
- SAS Help Center: PROC LOGISTIC Statement. Available online: https://documentation.sas.com/doc/en/pgmsascdc/9.4_3.4/statug/statug_logistic_syntax01.htm (accessed on 18 September 2023).
- Patel, A.; Gantz, O.; Zagadailov, P.; Merchant, A.M. The role of socioeconomic disparity in colorectal cancer stage at presentation. Updates Surg. 2019, 71, 523–531. [Google Scholar] [CrossRef]
- Hoffman, C.; Paradise, J. Health Insurance and Access to Health Care in the United States. Ann. N. Y. Acad. Sci. 2008, 1136, 149–160. [Google Scholar] [CrossRef]
- Orwat, J.; Caputo, N.; Key, W.; De Sa, J. Comparing Rural and Urban Cervical and Breast Cancer Screening Rates in a Privately Insured Population. Soc. Work. Public Health 2017, 32, 311–323. [Google Scholar] [CrossRef]
- Elovainio, M.; Lumme, S.; Arffman, M.; Manderbacka, K.; Pukkala, E.; Hakulinen, C. Living alone as a risk factor for cancer incidence, case-fatality and all-cause mortality: A nationwide registry study. SSM Popul. Health 2021, 15, 100826. [Google Scholar] [CrossRef]
- Tung, E.L.; Hawkley, L.C.; Cagney, K.A.; Peek, M.E. Social Isolation, Loneliness, and Violence Exposure in Urban Adults. Health Aff. 2019, 38, 1670–1678. [Google Scholar] [CrossRef]
- Laconi, E.; Marongiu, F.; DeGregori, J. Cancer as a disease of old age: Changing mutational and microenvironmental landscapes. Br. J. Cancer 2020, 122, 943–952. [Google Scholar] [CrossRef]
- Liu, B.; Quan, X.; Xu, C.; Lv, J.; Li, C.; Dong, L.; Liu, M. Lung cancer in young adults aged 35 years or younger: A full-scale analysis and review. J. Cancer 2019, 10, 3553–3559. [Google Scholar] [CrossRef]
- Steele, S.R.; Park, G.E.; Johnson, E.K.; Martin, M.J.; Stojadinovic, A.; Maykel, J.A.; Causey, M.W. The Impact of Age on Colorectal Cancer Incidence, Treatment, and Outcomes in an Equal-Access Health Care System. Dis. Colon Rectum 2014, 57, 303. [Google Scholar] [CrossRef]
- Chelmow, D.; Pearlman, M.D.; Young, A.; Bozzuto, L.; Dayaratna, S.; Jeudy, M.; Kremer, M.E.; Scott, D.M.; O’Hara, J.S. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet. Gynecol. 2020, 135, 1457–1478. [Google Scholar] [CrossRef] [PubMed]
- Cooley, J.J.P.; Maguire, F.B.; Morris, C.R.; Parikh-Patel, A.; Abrahão, R.; Chen, H.A.; Keegan, T.H. Cervical Cancer Stage at Diagnosis and Survival among Women ≥65 Years in California. Cancer Epidemiol. Biomark. Prev. 2023, 32, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.J.; Tangka, F.K.L.; Sabatino, S.A.; Thompson, T.D.; Graubard, B.I.; Breen, N. Patterns and Trends in Cancer Screening in the United States. Prev. Chronic Dis. 2018, 15, 170465. [Google Scholar] [CrossRef]
- Sabik, L.M.; Adunlin, G. The ACA and Cancer Screening and Diagnosis. Cancer J. 2017, 23, 151–162. [Google Scholar] [CrossRef]
- Lin, L.; Soni, A.; Sabik, L.M.; Drake, C. Early- and Late-Stage Cancer Diagnosis Under 3 Years of Medicaid Expansion. Am. J. Prev. Med. 2021, 60, 104–109. [Google Scholar] [CrossRef]
- Jemal, A.; Lin, C.C.; Davidoff, A.J.; Han, X. Changes in Insurance Coverage and Stage at Diagnosis Among Nonelderly Patients with Cancer after the Affordable Care Act. J. Clin. Oncol. 2017, 35, 3906–3915. [Google Scholar] [CrossRef]
- Sabik, L.M.; Tarazi, W.W.; Bradley, C.J. State Medicaid Expansion Decisions and Disparities in Women’s Cancer Screening. Am. J. Prev. Med. 2015, 48, 98–103. [Google Scholar] [CrossRef]
- Davidoff, A.J.; Guy, G.P.J.; Hu, X.M.; Gonzales, F.; Han, X.; Zheng, Z.; Parsons, H.; Ekwueme, D.U.; Jemal, A.D. Changes in Health Insurance Coverage Associated with the Affordable Care Act Among Adults with and Without a Cancer History. Med. Care 2018, 56, 220–227. [Google Scholar] [CrossRef]
- Robbins, A.S.; Han, X.; Ward, E.M.; Simard, E.P.; Zheng, Z.; Jemal, A. Association Between the Affordable Care Act Dependent Coverage Expansion and Cervical Cancer Stage and Treatment in Young Women. JAMA 2015, 314, 2189–2191. [Google Scholar] [CrossRef][Green Version]
- About the National Breast and Cervical Cancer Early Detection Program|CDC. Available online: https://www.cdc.gov/cancer/nbccedp/about.htm (accessed on 27 March 2023).
- Ladapo, J. The Florida Breast Cancer Early Detection and Treatment Referral Program Report. Florida Breast and Cervical Cancer Early Detection Program. 2020. Available online: https://www.floridahealth.gov/diseases-and-conditions/cancer/breast-cancer/_documents/2020FloridaBreastCancerEarlyDetectionandTreatmentReferralProgram.pdf (accessed on 22 September 2023).
- National Breast and Cervical Cancer Early Detection Program|CDC. Available online: https://www.cdc.gov/cancer/nbccedp/index.htm (accessed on 1 February 2023).
- Tejeda, S.; Darnell, J.S.; Cho, Y.I.; Stolley, M.R.; Markossian, T.W.; Calhoun, E.A. Patient Barriers to Follow-Up Care for Breast and Cervical Cancer Abnormalities. J. Womens Health 2013, 22, 507–517. [Google Scholar] [CrossRef]
- Parsons, J.; Bryce, C.; Atherton, H. Which patients miss appointments with general practice and the reasons why: A systematic review. Br. J. Gen. Pract. 2021, 71, e406–e412. [Google Scholar] [CrossRef]
- Wang, G.X.; Baggett, T.P.; Pandharipande, P.V.; Park, E.R.; Percac-Lima, S.; Shepard, J.-A.O.; Fintelmann, F.J.; Flores, E.J. Barriers to Lung Cancer Screening Engagement from the Patient and Provider Perspective. Radiology 2019, 290, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Mohamoud, Y.A.; Kirby, R.S.; Ehrenthal, D.B. Poverty, urban-rural classification and term infant mortality: A population-based multilevel analysis. BMC Pregnancy Childbirth 2019, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Silva-Laya, M.; D’Angelo, N.; García, E.; Zúñiga, L.; Fernández, T. Urban poverty and education. A systematic literature review. Educ. Res. Rev. 2020, 29, 100280. [Google Scholar] [CrossRef]
- Nardone, A.; Chiang, J.; Corburn, J. Historic Redlining and Urban Health Today in U.S. Cities. Environ. Justice 2020, 13, 109–119. [Google Scholar] [CrossRef]
- Adie, Y.; Kats, D.J.; Tlimat, A.; Perzynski, A.; Dalton, J.; Gunzler, D.; Tarabichi, Y. Neighborhood Disadvantage and Lung Cancer Incidence in Ever-Smokers at a Safety Net Health-Care System: A Retrospective Study. Chest 2020, 157, 1021–1029. [Google Scholar] [CrossRef]
- Freeman, H.P.; Rodriguez, R.L. History and principles of patient navigation. Cancer 2011, 117, 3537–3540. [Google Scholar] [CrossRef]
- Charlot, M.; Stein, J.N.; Damone, E.; Wood, I.; Forster, M.; Baker, S.; Emerson, M.; Samuel-Ryals, C.; Yongue, C.; Eng, E.; et al. Effect of an Antiracism Intervention on Racial Disparities in Time to Lung Cancer Surgery. J. Clin. Oncol. 2022, 40, 1755–1762. [Google Scholar] [CrossRef]
| Lung | Colorectal | Breast | Cervical | |
|---|---|---|---|---|
| (n = 84,175) | (n = 51,700) | (n = 85,371) | (n = 5232) | |
| Age | ||||
| 20–49 | 1898 (2.3%) | 5040 (9.7%) | 13,230 (15.3%) | 2296 (43.9%) |
| 50–64 | 20,698 (24.6%) | 15,510 (30.0%) | 28,404 (32.9%) | 1683 (32.2%) |
| 65–75 | 32,913 (39.1%) | 15,326 (29.6%) | 27,791 (32.2%) | 810 (15.5%) |
| >75 | 28,646 (34.0%) | 15,725 (30.4%) | 16,799 (19.5%) | 438 (8.4%) |
| Sex | ||||
| Women | 40,689 (48.3%) | 24,106 (46.6%) | 85,371 (100%) | 5232 (100%) |
| Men | 43,464 (51.6%) | 27,579 (53.3%) | ||
| Race–Ethnicity | ||||
| Non-Hispanic White | 67,308 (80.0%) | 35,814 (69.3%) | 60,057 (69.6%) | 2864 (54.7%) |
| Non-Hispanic Black | 6621 (7.9%) | 6094 (11.8%) | 9886 (11.5%) | 945 (18.1%) |
| Non-Hispanic Other | 1422 (1.7%) | 1252 (2.4%) | 2337 (2.7%) | 173 (3.3%) |
| Hispanic | 8241 (9.8%) | 8173 (15.8%) | 13,299 (15.4%) | 1209 (23.1%) |
| Payer | ||||
| Private | 13,892 (16.5%) | 14,870 (28.8%) | 32,982 (38.2%) | 2158 (41.2%) |
| No insurance | 2321 (2.8%) | 1983 (3.8%) | 2240 (2.6%) | 513 (9.8%) |
| Medicaid | 4218 (5.0%) | 2856 (5.5%) | 3610 (4.2%) | 818 (15.6%) |
| Medicare | 53,979 (64.1%) | 27,024 (52.3%) | 40,551 (47.0%) | 1172 (22.4%) |
| Other Governmental | 2558 (3.0%) | 1003 (1.9%) | 1507 (1.7%) | 83 (1.6%) |
| Unknown | 7207 (8.6%) | 3964 (7.7%) | 5343 (6.2%) | 488 (9.3%) |
| Poverty–Rurality | ||||
| Low poverty, urban | 49,505 (58.8%) | 30,401 (58.8%) | 55,453 (64.3%) | 2591 (49.5%) |
| Low poverty, rural | 1078 (1.3%) | 612 (1.2%) | 848 (1.0%) | 51 (1.0%) |
| High poverty, urban | 29,896 (35.5%) | 18,563 (35.9%) | 26,982 (31.3%) | 2405 (46.0%) |
| High poverty, rural | 2161 (2.6%) | 1154 (2.2%) | 1420 (1.6%) | 98 (1.9%) |
| Lung | Colorectal | Breast | Cervical | |
|---|---|---|---|---|
| 65.3 | 57.7 | 32.4 | 51.1 | |
| Age | ||||
| 20–49 | 77.7 | 67.2 | 45.7 | 43.2 |
| 50–64 | 73.2 | 60.6 | 35.2 | 56.4 |
| 65–75 | 64.6 | 54.9 | 26 | 58.1 |
| >75 | 59.6 | 54.6 | 27.6 | 59.4 |
| Sex | ||||
| Women | 63.5 | 57.2 | 32.4 | 51.1 |
| Men | 66.9 | 58.1 | ||
| Race–Ethnicity | ||||
| Non-Hispanic White | 64.9 | 57.2 | 29.6 | 52.6 |
| Non-Hispanic Black | 69.4 | 59.9 | 43.4 | 53.3 |
| Non-Hispanic Other | 69.8 | 59.5 | 35.6 | 45.1 |
| Hispanic | 65.2 | 58.4 | 36.6 | 47.1 |
| Payer | ||||
| Private | 71.1 | 60.2 | 35.2 | 44.6 |
| No insurance | 81.3 | 68 | 51.6 | 61.8 |
| Medicaid | 79.3 | 67.1 | 51.3 | 57.3 |
| Medicare | 66.1 | 56.8 | 27.6 | 60.1 |
| Other Governmental | 61.6 | 59.5 | 33.0 | 44.6 |
| Unknown | 36.0 | 41.5 | 30.9 | 38.1 |
| Poverty–Rurality | ||||
| Low poverty, urban | 64.6 | 57.9 | 30.7 | 50.3 |
| Low poverty, rural | 66.0 | 58.5 | 34.6 | 56.9 |
| High poverty, urban | 66.6 | 57.8 | 35.9 | 52.5 |
| High poverty, rural | 66.4 | 56.3 | 36.7 | 52.0 |
| Lung | Colorectal | Breast | Cervical | |
|---|---|---|---|---|
| (n = 84,175) | (n = 51,700) | (n = 85,371) | (n = 5232) | |
| Age | ||||
| 20–49 | Reference | Reference | Reference | Reference |
| 50–64 | 0.82 (0.72, 0.94) | 0.76 (0.71, 0.82) | 0.67 (0.64, 0.70) | 1.97 (1.71, 2.27) |
| 65–75 | 0.59 (0.51, 0.67) | 0.61 (0.57, 0.67) | 0.45 (0.43, 0.48) | 2.06 (1.64, 2.60) |
| >75 | 0.52 (0.46, 0.60) | 0.69 (0.63, 0.75) | 0.52 (0.49, 0.56) | 2.94 (2.17, 3.98) |
| Sex | ||||
| Women | Reference | Reference | ||
| Men | 1.33 (1.29, 1.38) | 1.05 (1.01, 1.09) | ||
| Race–Ethnicity | ||||
| Non-Hispanic White | Reference | Reference | Reference | Reference |
| Non-Hispanic Black | 1.12 (1.05, 1.20) | 1.14 (1.07, 1.21) | 1.57 (1.50, 1.65) | 1.02 (0.85, 1.21) |
| Non-Hispanic Other | 1.18 (1.04, 1.34) | 1.06 (0.94, 1.20) | 1.15 (1.05, 1.25) | 0.88 (0.63, 1.23) |
| Hispanic | 1.00 (0.95, 1.06) | 1.08 (1.03, 1.15) | 1.18 (1.13, 1.23) | 0.78 (0.67, 0.91) |
| Payer | ||||
| Private | Reference | Reference | Reference | Reference |
| No insurance | 1.92 (1.69, 2.18) | 1.54 (1.38, 1.72) | 1.98 (1.80, 2.16) | 2.38 (1.91, 2.97) |
| Medicaid | 1.64 (1.50, 1.81) | 1.51 (1.37, 1.66) | 1.83 (1.70, 1.97) | 2.11 (1.76, 2.53) |
| Medicare | 1.04 (0.98, 1.09) | 1.09 (1.03, 1.16) | 1.07 (1.02, 1.12) | 1.50 (1.21, 1.87) |
| Other Governmental | 0.76 (0.69, 0.84) | 1.24 (1.07, 1.43) | 0.97 (0.87, 1.09) | 0.99 (0.63, 1.58) |
| Unknown | 1.27 (1.16, 1.40) | 1.17 (1.07, 1.28) | 1.26 (1.18, 1.35) | 1.17 (0.93, 1.48) |
| Poverty–Rurality | ||||
| Low poverty, urban | Reference | Reference | Reference | |
| Low poverty, rural | 1.16 (1.00, 1.35) | 1.24 (1.07, 1.44) | 1.10 (0.61, 2.00) | |
| High poverty, urban | 1.08 (1.04, 1.12) | 1.14 (1.10, 1.18) | 1.16 (1.02, 1.32) | |
| High poverty, rural | 1.23 (1.10, 1.37) | 1.31 (1.17, 1.47) | 1.03 (0.66, 1.62) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, J.M.; Mkuu, R.S.; Cho, H.D.; Woodard, J.N.; Kaye, F.J.; Bian, J.; Shenkman, E.A.; Guo, Y. Disparities Contributing to Late-Stage Diagnosis of Lung, Colorectal, Breast, and Cervical Cancers: Rural and Urban Poverty in Florida. Cancers 2023, 15, 5226. https://doi.org/10.3390/cancers15215226
Hall JM, Mkuu RS, Cho HD, Woodard JN, Kaye FJ, Bian J, Shenkman EA, Guo Y. Disparities Contributing to Late-Stage Diagnosis of Lung, Colorectal, Breast, and Cervical Cancers: Rural and Urban Poverty in Florida. Cancers. 2023; 15(21):5226. https://doi.org/10.3390/cancers15215226
Chicago/Turabian StyleHall, Jaclyn M., Rahma S. Mkuu, Hee Deok Cho, Jennifer N. Woodard, Frederic J. Kaye, Jiang Bian, Elizabeth A. Shenkman, and Yi Guo. 2023. "Disparities Contributing to Late-Stage Diagnosis of Lung, Colorectal, Breast, and Cervical Cancers: Rural and Urban Poverty in Florida" Cancers 15, no. 21: 5226. https://doi.org/10.3390/cancers15215226
APA StyleHall, J. M., Mkuu, R. S., Cho, H. D., Woodard, J. N., Kaye, F. J., Bian, J., Shenkman, E. A., & Guo, Y. (2023). Disparities Contributing to Late-Stage Diagnosis of Lung, Colorectal, Breast, and Cervical Cancers: Rural and Urban Poverty in Florida. Cancers, 15(21), 5226. https://doi.org/10.3390/cancers15215226








