Alzheimer’s Disease and Different Types of Cancer Likelihood: Unveiling Disparities and Potential Protective Effects in a Korean Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Definition of Alzheimer’s Disease
2.2. Definition of Cancers
2.3. Participant Selection
2.4. Covariates
2.5. Statistical Analyses
3. Results
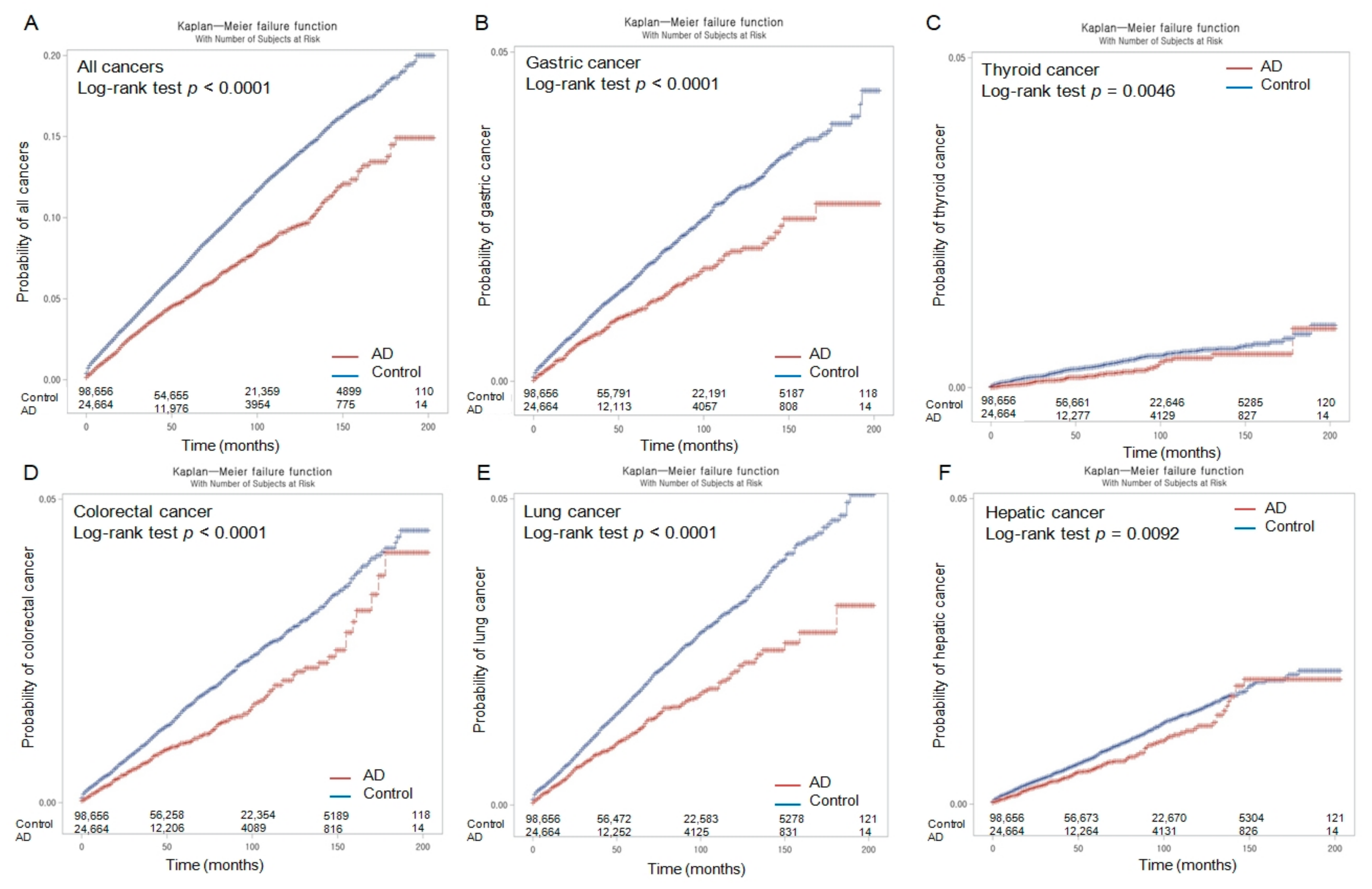
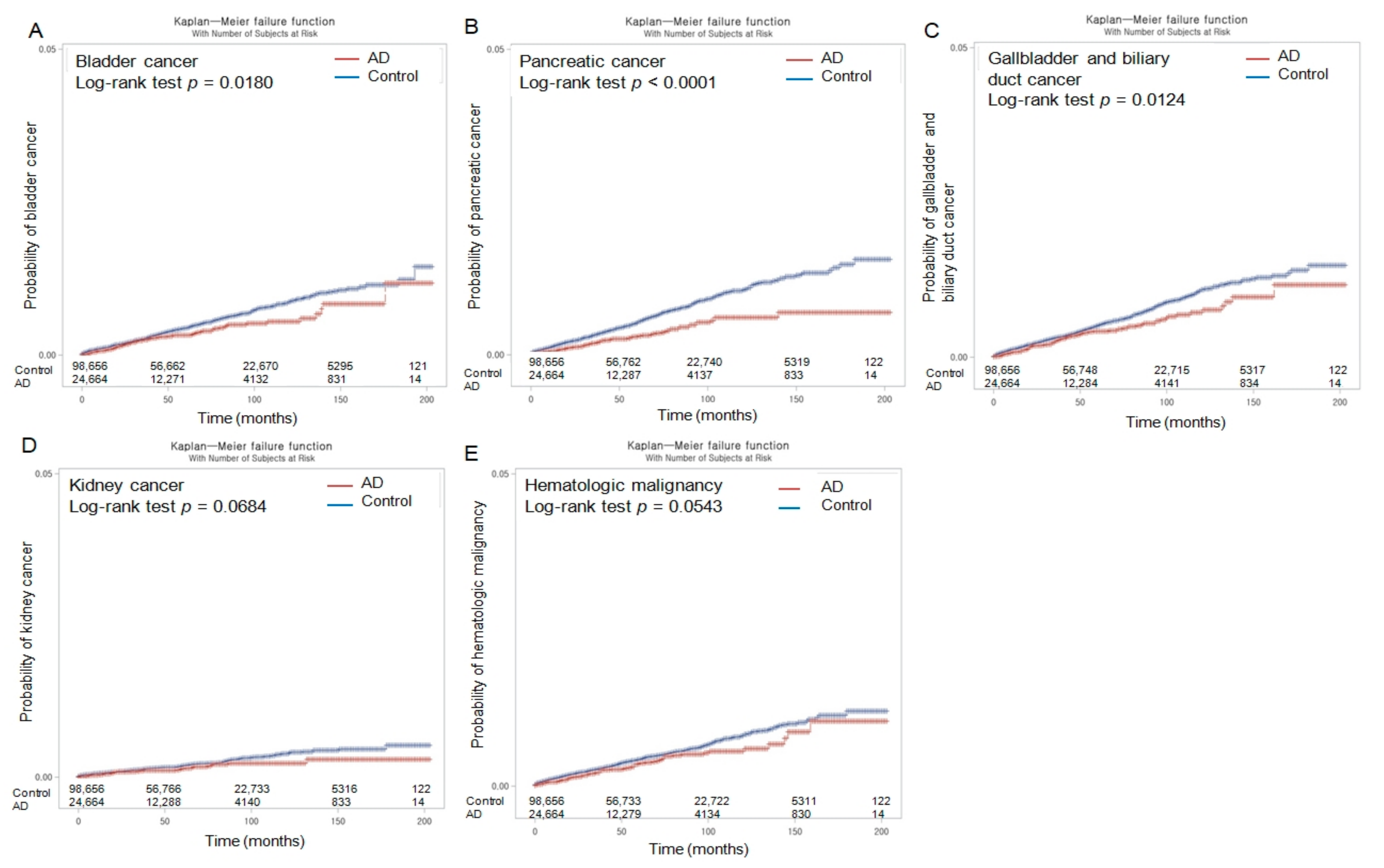
3.1. Association of Occurrence of Malignancy between the Group with AD and the Controls
3.2. Subgroup Analysis According to Sex
3.3. Subgroup Analysis According to Age
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, H. Cancer Management Among Older Adults Living with Dementia: A Call to Action from Asian Perspectives. Asia-Pac. J. Oncol. Nurs. 2022, 9, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Hebert, L.E.; Scherr, P.A.; Bienias, J.L.; Bennett, D.A.; Evans, D.A. Alzheimer Disease in the US Population: Prevalence Estimates Using the 2000 Census. Arch. Neurol. 2003, 60, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the Global Prevalence of Dementia in 2019 and Forecasted Prevalence in 2050: An Analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Pilleron, S.; Sarfati, D.; Janssen-Heijnen, M.; Vignat, J.; Ferlay, J.; Bray, F.; Soerjomataram, I. Global Cancer Incidence in Older Adults, 2012 and 2035: A Population-Based Study. Int. J. Cancer 2019, 144, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, A.; Driver, J.A.; Schernhammer, E.S. Parkinson’s Disease and Cancer Risk: A Systematic Review and Meta-analysis. Cancer Causes Control 2010, 21, 697–707. [Google Scholar] [CrossRef]
- Ording, A.G.; Veres, K.; Horváth-Puhó, E.; Glymour, M.M.; Rørth, M.; Henderson, V.W.; Sørensen, H.T. Alzheimer’s and Parkinson’s Diseases and the Risk of Cancer: A Cohort Study. J. Alzheimers Dis. 2019, 72, 1269–1277. [Google Scholar] [CrossRef]
- Scheuner, D.; Eckman, C.; Jensen, M.; Song, X.; Citron, M.; Suzuki, N.; Bird, T.D.; Hardy, J.; Hutton, M.; Kukull, W.; et al. Secreted Amyloid Beta-Protein Similar to That in the Senile Plaques of Alzheimer’s Disease Is Increased In Vivo by the Presenilin 1 and 2 and APP Mutations Linked to Familial Alzheimer’s Disease. Nat. Med. 1996, 2, 864–870. [Google Scholar] [CrossRef]
- Gómez-Isla, T.; Hollister, R.; West, H.; Mui, S.; Growdon, J.H.; Petersen, R.C.; Parisi, J.E.; Hyman, B.T. Neuronal Loss Correlates with but Exceeds Neurofibrillary Tangles in Alzheimer’s Disease. Ann. Neurol. 1997, 41, 17–24. [Google Scholar] [CrossRef]
- Behrens, M.I.; Lendon, C.; Roe, C.M. A Common Biological Mechanism in Cancer and Alzheimer’s Disease? Curr. Alzheimer Res. 2009, 6, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Townsley, C.A.; Selby, R.; Siu, L.L. Systematic Review of Barriers to the Recruitment of Older Patients with Cancer onto Clinical Trials. J. Clin. Oncol. 2005, 23, 3112–3124. [Google Scholar] [CrossRef]
- D’Amelio, M.; Ragonese, P.; Sconzo, G.; Aridon, P.; Savettieri, G. Parkinson’s Disease and Cancer: Insights for Pathogenesis from Epidemiology. Ann. N. Y. Acad. Sci. 2009, 1155, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Inzelberg, R.; Jankovic, J. Are Parkinson Disease Patients Protected from Some but Not All Cancers? Neurology 2007, 69, 1542–1550. [Google Scholar] [CrossRef]
- Roe, C.M.; Fitzpatrick, A.L.; Xiong, C.; Sieh, W.; Kuller, L.; Miller, J.P.; Williams, M.M.; Kopan, R.; Behrens, M.I.; Morris, J.C. Cancer Linked to Alzheimer Disease but Not Vascular Dementia. Neurology 2010, 74, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Realmuto, S.; Cinturino, A.; Arnao, V.; Mazzola, M.A.; Cupidi, C.; Aridon, P.; Ragonese, P.; Savettieri, G.; D’Amelio, M. Tumor Diagnosis Preceding Alzheimer’s Disease Onset: Is There a Link Between Cancer and Alzheimer’s Disease? J. Alzheimers Dis. 2012, 31, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Driver, J.A.; Beiser, A.; Au, R.; Kreger, B.E.; Splansky, G.L.; Kurth, T.; Kiel, D.P.; Lu, K.P.; Seshadri, S.; Wolf, P.A. Inverse Association Between Cancer and Alzheimer’s Disease: Results from the Framingham Heart Study. BMJ 2012, 344, e1442. [Google Scholar] [CrossRef] [PubMed]
- Musicco, M.; Adorni, F.; Di Santo, S.; Prinelli, F.; Pettenati, C.; Caltagirone, C.; Palmer, K.; Russo, A. Inverse Occurrence of Cancer and Alzheimer Disease: A Population-Based Incidence Study. Neurology 2013, 81, 322–328. [Google Scholar] [CrossRef]
- Ou, S.M.; Lee, Y.J.; Hu, Y.W.; Liu, C.J.; Chen, T.J.; Fuh, J.L.; Wang, S.J. Does Alzheimer’s Disease Protect Against Cancers? A Nationwide Population-Based Study. Neuroepidemiology 2013, 40, 42–49. [Google Scholar] [CrossRef]
- Lin, H.L.; Lin, H.C.; Tseng, Y.F.; Chen, S.C.; Hsu, C.Y. Inverse Association Between Cancer and Dementia: A Population-Based Registry Study in Taiwan. Alzheimer Dis. Assoc. Disord. 2016, 30, 118–122. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, D.; Lee, J.H. Association Between Alzheimer’s Disease and Cancer Risk in South Korea: An 11-Year Nationwide Population-Based Study. Dement. Neurocogn. Disord. 2018, 17, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.J.; Huang, Q.; Xu, G.; Gu, K.; Dammer, E.B.; Wang, C.F.; Xie, X.Y.; Chen, W.; Shao, Z.Y.; Chen, S.D.; et al. Association Between Alzheimer’s Disease and Risk of Cancer: A Retrospective Cohort Study in Shanghai, China. Alzheimers Dement. 2022, 18, 924–933. [Google Scholar] [CrossRef]
- Kendall, B.E.; Fox, G.A.; Fujiwara, M.; Nogeire, T.M. Demographic Heterogeneity, Cohort Selection, and Population Growth. Ecology 2011, 92, 1985–1993. [Google Scholar] [CrossRef]
- Papageorgakopoulos, T.N.; Moraitou, D.; Papanikolaou, M.; Tsolaki, M. The Association Between Alzheimer’s Disease and Cancer: Systematic Review—Meta-analysis. Hell. J. Nucl. Med. 2017, 20, 45–57. [Google Scholar] [PubMed]
- Ospina-Romero, M.; Glymour, M.M.; Hayes-Larson, E.; Mayeda, E.R.; Graff, R.E.; Brenowitz, W.D.; Ackley, S.F.; Witte, J.S.; Kobayashi, L.C. Association Between Alzheimer Disease and Cancer with Evaluation of Study Biases: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2025515. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Liyanage, S.I.; Weaver, D.F. Cancer and Alzheimer’s Inverse Correlation: An Immunogenetic Analysis. Mol. Neurobiol. 2023, 60, 3086–3099. [Google Scholar] [CrossRef]
- Seong, S.C.; Kim, Y.Y.; Park, S.K.; Khang, Y.H.; Kim, H.C.; Park, J.H.; Kang, H.J.; Do, C.H.; Song, J.S.; Lee, E.J.; et al. Cohort Profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 2017, 7, e016640. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Tobacco Smoking and Alcohol Consumption Are Related to Benign Parotid Tumor: A Nested Case-Control Study Using a National Health Screening Cohort. Clin. Exp. Otorhinolaryngol. 2019, 12, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.G.; Kim, J.H.; Kim, J.H.; Kim, E.S.; Park, H.Y.; Min, K.W.; Kwon, M.J. Associations between Proton Pump Inhibitors and Alzheimer’s Disease: A Nested Case-Control Study Using a Korean Nationwide Health Screening Cohort. Alzheimers Res. Ther. 2022, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.G.; Lee, H.K.; Kang, H.S.; Lim, H.; Kim, J.H.; Kim, J.H.; Kim, N.Y.; Cho, S.J.; Nam, E.S.; Min, K.W.; et al. Possible Association Between the Use of Proton Pump Inhibitors and H(2) Receptor Antagonists, and Esophageal Cancer: A Nested Case-Control Study Using a Korean National Health Screening Cohort. Pharmaceuticals 2022, 15, 517. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kang, H.S.; Kim, J.H.; Kim, J.H.; Kim, S.H.; Kim, N.Y.; Nam, E.S.; Min, K.W.; Choi, H.G. Association Between Statin Use and Gastric Cancer: A Nested Case-Control Study Using a National Health Screening Cohort in Korea. Pharmaceuticals 2021, 14, 1283. [Google Scholar] [CrossRef]
- Choi, H.G.; Kang, H.S.; Lim, H.; Kim, J.H.; Kim, J.H.; Cho, S.J.; Nam, E.S.; Min, K.W.; Park, H.Y.; Kim, N.Y.; et al. Potential Cancer Risk in Patients with Rheumatoid Arthritis: A Longitudinal Korean Population-Based Analysis. J. Pers. Med. 2022, 12, 965. [Google Scholar] [CrossRef]
- Kwon, M.J.; Byun, S.H.; Kim, J.H.; Kim, J.H.; Kim, S.H.; Kim, N.Y.; Park, H.R.; Choi, H.G. Longitudinal Follow-Up Study of the Association Between Statin Use and Chronic Periodontitis Using National Health Screening Cohort of Korean Population. Sci. Rep. 2022, 12, 5504. [Google Scholar] [CrossRef] [PubMed]
- WHO; IASO; IOTF. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; Health Communications Australia Pty Limited: Sydney, NSW, Australia, 2000. [Google Scholar]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data from 6 Countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Balance Diagnostics for Comparing the Distribution of Baseline Covariates Between Treatment Groups in Propensity-Score Matched Samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Catalá-López, F.; Driver, J.A.; Page, M.J.; Hutton, B.; Ridao, M.; Berrozpe-Villabona, C.; Alonso-Arroyo, A.; Fraga-Medín, C.A.; Bernal-Delgado, E.; Valencia, A.; et al. Design and Methodological Characteristics of Studies Using Observational Routinely Collected Health Data for Investigating the Link Between Cancer and Neurodegenerative Diseases: Protocol for a Meta Research Study. BMJ Open 2022, 12, e058738. [Google Scholar] [CrossRef]
- Attner, B.; Lithman, T.; Noreen, D.; Olsson, H. Low Cancer Rates Among Patients with Dementia in a Population-Based Register Study in Sweden. Dement. Geriatr. Cogn. Disord. 2010, 30, 39–42. [Google Scholar] [CrossRef]
- Zhang, D.D.; Ou, Y.N.; Yang, L.; Ma, Y.H.; Tan, L.; Feng, J.F.; Cheng, W.; Yu, J.T. Investigating the Association Between Cancer and Dementia Risk: A Longitudinal Cohort Study. Alzheimers Res. Ther. 2022, 14, 146. [Google Scholar] [CrossRef]
- Du, X.L.; Song, L.; Schulz, P.E.; Xu, H.; Chan, W. Associations Between Vascular Diseases and Alzheimer’s Disease or Related Dementias in a Large Cohort of Men and Women with Colorectal Cancer. J. Alzheimers Dis. 2022, 90, 211–231. [Google Scholar] [CrossRef]
- Kent, D.M.; Rothwell, P.M.; Ioannidis, J.P.; Altman, D.G.; Hayward, R.A. Assessing and Reporting Heterogeneity in Treatment Effects in Clinical Trials: A Proposal. Trials 2010, 11, 85. [Google Scholar] [CrossRef]
- Kang, M.J.; Won, Y.J.; Lee, J.J.; Jung, K.W.; Kim, H.J.; Kong, H.J.; Im, J.S.; Seo, H.G.; Community of Population-Based Regional Cancer Registries. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2019. Cancer Res. Treat. 2022, 54, 330–344. [Google Scholar] [CrossRef]
- Jung, K.W.; Won, Y.J.; Hong, S.; Kong, H.J.; Im, J.S.; Seo, H.G. Prediction of Cancer Incidence and Mortality in Korea, 2021. Cancer Res. Treat. 2021, 53, 316–322. [Google Scholar] [CrossRef]
- Zabłocka, A.; Kazana, W.; Sochocka, M.; Stańczykiewicz, B.; Janusz, M.; Leszek, J.; Orzechowska, B. Inverse Correlation Between Alzheimer’s Disease and Cancer: Short Overview. Mol. Neurobiol. 2021, 58, 6335–6349. [Google Scholar] [CrossRef] [PubMed]
- Forés-Martos, J.; Boullosa, C.; Rodrigo-Domínguez, D.; Sánchez-Valle, J.; Suay-García, B.; Climent, J.; Falcó, A.; Valencia, A.; Puig-Butillé, J.A.; Puig, S.; et al. Transcriptomic and Genetic Associations Between Alzheimer’s Disease, Parkinson’s Disease, and Cancer. Cancers 2021, 13, 2990. [Google Scholar] [CrossRef] [PubMed]
- Remiro-Azócar, A.; Heath, A.; Baio, G. Methods for Population Adjustment with Limited Access to Individual Patient Data: A Review and Simulation Study. Res. Synth. Methods 2021, 12, 750–775. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A Common Classification Framework for Neuroendocrine Neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) Expert Consensus Proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef]
- Xia, S.; Yu, X.; Chen, G. Pain as a Protective Factor for Alzheimer Disease in Patients with Cancer. Cancers 2022, 15, 248. [Google Scholar] [CrossRef]
- Dong, Z.; Xu, M.; Sun, X.; Wang, X. Mendelian Randomization and Transcriptomic Analysis Reveal an Inverse Causal Relationship Between Alzheimer’s Disease and Cancer. J. Transl. Med. 2023, 21, 527. [Google Scholar] [CrossRef]
- Seddighi, S.; Houck, A.L.; Rowe, J.B.; Pharoah, P.D.P. Evidence of a Causal Association Between Cancer and Alzheimer’s Disease: A Mendelian Randomization Analysis. Sci. Rep. 2019, 9, 13548. [Google Scholar] [CrossRef]
- Silva, M.V.F.; Loures, C.M.G.; Alves, L.C.V.; de Souza, L.C.; Borges, K.B.G.; Carvalho, M.D.G. Alzheimer’s Disease: Risk Factors and Potentially Protective Measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Before Overlap Weighting Adjustment | After Overlap Weighting Adjustment | |||||
|---|---|---|---|---|---|---|---|
| Dementia (n = 24,664) | Control (n = 98,656) | Standardized Difference | Dementia (n = 24,664) | Control (n = 98,656) | Standardized Difference | ||
| Age (y), % | 0.00 | 0.00 | |||||
| 40–44 | 0.01 | 0.01 | 0.01 | 0.01 | |||
| 45–49 | 0.21 | 0.21 | 0.20 | 0.20 | |||
| 50–54 | 0.79 | 0.79 | 0.77 | 0.77 | |||
| 55–59 | 2.57 | 2.57 | 2.53 | 2.53 | |||
| 60–64 | 5.83 | 5.83 | 5.75 | 5.75 | |||
| 65–69 | 13.36 | 13.36 | 13.22 | 13.22 | |||
| 70–74 | 25.25 | 25.25 | 25.14 | 25.14 | |||
| 75–79 | 35.62 | 35.62 | 35.78 | 35.78 | |||
| 80–84 | 15.30 | 15.30 | 15.51 | 15.51 | |||
| 85+ | 1.06 | 1.06 | 1.09 | 1.09 | |||
| Sex, % | 0.00 | 0.00 | |||||
| Male | 42.60 | 42.60 | 42.11 | 42.11 | |||
| Female | 57.40 | 57.40 | 57.89 | 57.89 | |||
| Income, % | 0.00 | 0.00 | |||||
| 1 (lowest) | 19.94 | 19.94 | 19.62 | 19.62 | |||
| 2 | 10.72 | 10.72 | 10.64 | 10.64 | |||
| 3 | 13.19 | 13.19 | 13.24 | 13.24 | |||
| 4 | 19.01 | 19.01 | 19.04 | 19.04 | |||
| 5 (highest) | 37.15 | 37.15 | 37.45 | 37.45 | |||
| Region of residence, % | 0.00 | 0.00 | |||||
| Urban | 35.91 | 35.91 | 35.82 | 35.82 | |||
| Rural | 64.09 | 64.09 | 64.18 | 64.18 | |||
| Obesity, % | 0.12 | 0.00 | |||||
| Underweight | 4.69 | 3.41 | 4.38 | 4.38 | |||
| Normal | 38.49 | 34.46 | 37.64 | 37.64 | |||
| Overweight | 24.34 | 26.35 | 24.89 | 24.89 | |||
| Obese I | 29.22 | 32.26 | 29.80 | 29.80 | |||
| Obese II | 3.26 | 3.51 | 3.29 | 3.29 | |||
| Smoking status, % | 0.06 | 0.00 | |||||
| Nonsmoker | 77.21 | 76.97 | 77.50 | 77.50 | |||
| Past smoker | 11.87 | 13.46 | 12.18 | 12.18 | |||
| Current smoker | 10.93 | 9.57 | 10.31 | 10.31 | |||
| Alcohol consumption, % | 0.07 | 0.00 | |||||
| <1 time a week | 71.34 | 68.02 | 70.72 | 70.72 | |||
| ≥1 time a week | 28.66 | 31.98 | 29.28 | 29.28 | |||
| Systolic blood pressure (mean, SD) | 130.41 (17.56) | 130.50 (16.60) | 0.01 | 130.34 (14.77) | 130.34 (7.18) | 0.00 | |
| Diastolic blood pressure (mean, SD) | 78.28 (10.94) | 78.08 (10.37) | 0.02 | 78.16 (9.20) | 78.16 (4.50) | 0.00 | |
| Fasting blood glucose (mean, SD) | 107.85 (37.71) | 103.82 (29.07) | 0.12 | 106.22 (29.41) | 106.22 (14.41) | 0.00 | |
| Total cholesterol (mean, SD) | 195.41 (42.06) | 195.30 (40.26) | 0.00 | 195.40 (35.03) | 195.40 (17.77) | 0.00 | |
| CCI score (mean, SD) | 1.84 (1.76) | 0.76 (1.19) | 0.72 | 1.43 (1.22) | 1.43 (0.71) | 0.00 | |
| All cancer, % | 4.90 | 7.99 | 0.13 | 4.84 | 8.73 | 0.15 | |
| Gastric cancer, % | 0.99 | 1.66 | 0.06 | 0.98 | 1.76 | 0.07 | |
| Thyroid cancer, % | 0.19 | 0.33 | 0.03 | 0.20 | 0.34 | 0.03 | |
| Colorectal cancer, % | 0.97 | 1.62 | 0.06 | 0.99 | 1.71 | 0.06 | |
| Lung cancer, % | 1.11 | 1.89 | 0.06 | 1.12 | 2.07 | 0.08 | |
| Hepatic cancer, % | 0.61 | 0.87 | 0.03 | 0.54 | 1.09 | 0.06 | |
| Bladder cancer, % | 0.32 | 0.48 | 0.03 | 0.32 | 0.51 | 0.03 | |
| Pancreatic cancer, % | 0.29 | 0.59 | 0.05 | 0.30 | 0.67 | 0.05 | |
| Gallbladder and biliary duct, % | 0.38 | 0.57 | 0.03 | 0.38 | 0.61 | 0.03 | |
| Kidney cancer, % | 0.13 | 0.21 | 0.02 | 0.13 | 0.23 | 0.02 | |
| Hematologic malignancy, % | 0.32 | 0.46 | 0.02 | 0.32 | 0.50 | 0.03 | |
| Dependent Variable | IR per 1000 PY | IRD per 1000 PY (95% CI) | Hazard Ratios for Cancers (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| AD (n = 3070) | Control (n = 12,280) | Crude † | p | Model 1 †,‡ | p | ||
| All cancer (n = 9087) | 11.63 | 16.48 | −4.85 (−5.69 to −4.02) | 0.70 (0.66–0.75) | <0.001 * | 0.63 (0.59–0.68) | <0.001 * |
| Gastric cancer (n = 1883) | 2.33 | 3.35 | −1.02 (−1.40 to −0.65) | 0.68 (0.60–0.78) | <0.001 * | 0.63 (0.55–0.73) | <0.001 * |
| Thyroid cancer (n = 374) | 0.44 | 0.66 | −0.22 (−0.38 to −0.05) | 0.63 (0.46–0.85) | 0.003 * | 0.65 (0.47–0.90) | 0.009 * |
| Colorectal cancer (n = 1838) | 2.27 | 3.25 | −0.98 (−1.35 to −0.61) | 0.69 (0.61–0.80) | <0.001 * | 0.67 (0.58–0.77) | <0.001 * |
| Lung cancer (n = 2133) | 2.57 | 3.76 | −1.19 (−1.59 to −0.80) | 0.69 (0.61–0.79) | <0.001 * | 0.64 (0.56–0.73) | <0.001 * |
| Hepatic cancer (n = 1009) | 1.41 | 1.73 | −0.32 (−0.59 to −0.05) | 0.81 (0.68–0.97) | 0.020 * | 0.60 (0.50–0.72) | <0.001 * |
| Bladder cancer (n = 557) | 0.74 | 0.96 | −0.22 (−0.42 to −0.02) | 0.79 (0.62–1.00) | 0.051 | 0.76 (0.59–0.98) | 0.031 * |
| Pancreatic cancer (n = 658) | 0.68 | 1.18 | −0.50 (−0.72 to −0.29) | 0.57 (0.45–0.73) | <0.001 * | 0.50 (0.39–0.65) | <0.001 * |
| Gallbladder and BD (n = 654) | 0.87 | 1.13 | −0.26 (−0.48 to −0.04) | 0.78 (0.63–0.98) | 0.029 * | 0.73 (0.58–0.92) | 0.007 * |
| Kidney cancer (n = 242) | 0.31 | 0.42 | −0.11 (−0.24 to 0.02) | 0.72 (0.50–1.05) | 0.084 | 0.63 (0.43–0.93) | 0.019 * |
| Hematologic malignancy (n = 537) | 0.75 | 0.92 | −0.17 (−0.37 to 0.03) | 0.81 (0.64–1.02) | 0.078 | 0.73 (0.57–0.94) | 0.014 * |
| Dependent Variable | IR per 1000 PY | IRD per 1000 PY (95% CI) | Hazard Ratios for Cancers (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| AD | Control | Crude † | p | Model 1 †,‡ | p | ||
| Men (n = AD: 10,506, control: 42,024) | |||||||
| All cancer (n = 5172) | 18.43 | 25.09 | −6.66 (−8.40 to −4.92) | 0.71 (0.66–0.77) | <0.001 * | 0.64 (0.59–0.69) | <0.001 * |
| Gastric cancer (n = 1119) | 3.72 | 5.32 | −1.60 (−2.39 to −0.81) | 0.67 (0.56–0.80) | <0.001 * | 0.62 (0.52–0.75) | <0.001 * |
| Thyroid cancer (n = 69) | 0.32 | 0.3 | 0.01 (−0.18 to 0.21) | 1.04 (0.56–1.94) | 0.906 | 1.02 (0.52–1.98) | 0.966 |
| Colorectal cancer (n = 981) | 2.99 | 4.66 | −1.67 (−2.40 to −0.93) | 0.63 (0.51–0.76) | <0.001 * | 0.60 (0.49–0.73) | <0.001 * |
| Lung cancer (n = 1439) | 4.99 | 6.66 | −1.68 (−2.56 to −0.79) | 0.74 (0.63–0.86) | <0.001 * | 0.67 (0.57–0.79) | <0.001 * |
| Hepatic cancer (n = 595) | 2.45 | 2.66 | −0.21 (−0.78 to 0.35) | 0.89 (0.71–1.11) | 0.298 | 0.63 (0.50–0.80) | <0.001 * |
| Bladder cancer (n = 412) | 1.55 | 1.87 | −0.32 (−0.79 to 0.15) | 0.81 (0.62–1.07) | 0.145 | 0.77 (0.58–1.02) | 0.072 |
| Pancreatic cancer (n = 293) | 0.71 | 1.41 | −0.70 (−1.09 to −0.30) | 0.49 (0.33–0.73) | <0.001 * | 0.44 (0.29–0.66) | <0.001 * |
| Gallbladder and BD (n = 335) | 1.23 | 1.52 | −0.29 (−0.71 to 0.13) | 0.81 (0.60–1.10) | 0.182 | 0.76 (0.55–1.05) | 0.095 |
| Kidney cancer (n = 151) | 0.55 | 0.69 | 0.11 (−0.71 to 0.93) | 0.76 (0.48–1.21) | 0.243 | 0.68 (0.42–1.10) | 0.118 |
| Hematologic malignancy (n = 266) | 1.1 | 1.19 | −0.08 (−0.46 to 0.30) | 0.91 (0.65–1.26) | 0.556 | 0.84 (0.59–1.19) | 0.316 |
| Women (n = AD: 14,158, control: 56,632) | |||||||
| All cancer (n = 3915) | 7.9 | 11.32 | −3.43 (−4.29 to −2.56) | 0.69 (0.63–0.75) | <0.001 * | 0.63 (0.57–0.69) | <0.001 * |
| Gastric cancer (n = 764) | 1.56 | 2.16 | −0.60 (−0.98 to −0.22) | 0.71 (0.58–0.87) | <0.001 * | 0.65 (0.53–0.81) | <0.001 * |
| Thyroid cancer (n = 305) | 0.51 | 0.88 | −0.37 (−0.61 to −0.13) | 0.55 (0.39–0.78) | 0.001 * | 0.59 (0.41–0.84) | 0.004 * |
| Colorectal cancer (n = 857) | 1.87 | 2.39 | −0.52 (−0.92 to −0.13) | 0.77 (0.64–0.93) | 0.007 * | 0.75 (0.61–0.91) | 0.003 * |
| Lung cancer (n = 694) | 1.23 | 1.99 | −0.76 (−1.12 to −0.40) | 0.61 (0.49–0.77) | <0.001 * | 0.58 (0.45–0.73) | <0.001 * |
| Hepatic cancer (n = 414) | 0.83 | 1.16 | −0.33 (−0.60 to −0.05) | 0.71 (0.54–0.95) | 0.018 * | 0.56 (0.41–0.74) | <0.001 * |
| Bladder cancer (n = 145) | 0.29 | 0.41 | −0.11 (−0.28 to 0.05) | 0.72 (0.45–1.16) | 0.174 | 0.73 (0.45–1.20) | 0.213 |
| Pancreatic cancer (n = 365) | 0.66 | 1.04 | −0.38 (−0.64 to −0.12) | 0.63 (0.46–0.86) | 0.003 * | 0.55 (0.40–0.76) | 0.003 * |
| Gallbladder and BD (n = 319) | 0.67 | 0.89 | −0.22 (−0.46 to 0.03) | 0.76 (0.55–1.03) | 0.080 | 0.70 (0.51–0.97) | 0.033 * |
| Kidney cancer (n = 91) | 0.18 | 0.26 | −0.08 (−0.21 to 0.05) | 0.67 (0.36–1.23) | 0.192 | 0.57 (0.30–1.07) | 0.081 |
| Hematologic malignancy (n = 271) | 0.56 | 0.76 | −0.20 (−0.42 to 0.02) | 0.72 (0.51–1.02) | 0.061 | 0.64 (0.45–0.92) | 0.014 * |
| Dependent Variable | IR per 1000 PY | IRD per 1000 PY (95% CI) | Hazard Ratios for Cancers (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| AD | Control | Crude † | p | Model 1 †,‡ | p | ||
| Age < 60 (n = AD: 250, control: 1000) | |||||||
| All cancer (n = 72) | 5.75 | 6.53 | −0.78 (−4.58 to 3.02) | 0.88 (0.47–1.64) | 0.695 | 0.68 (0.32–1.48) | 0.332 |
| Gastric cancer (n = 16) | 0.47 | 1.60 | −1.13 (−2.89 to 0.63) | 0.31 (0.04–2.34) | 0.254 | 0.20 (0.02–2.16) | 0.185 |
| Thyroid cancer (n = 9) | 0.94 | 0.74 | 0.19 (−1.12 to 1.51) | 1.25 (0.26–6.01) | 0.785 | 1.33 (0.23–7.72) | 0.753 |
| Colorectal cancer (n = 12) | 0.94 | 1.06 | −0.13 (−1.64 to 1.39) | 0.87 (0.19–3.99) | 0.859 | 2.03 (0.28–14.80) | 0.486 |
| Lung cancer (n = 8) | 0.00 | 0.85 | −0.85 (−2.08 to 0.38) | N/A | 0.995 | N/A | 0.994 |
| Hepatic cancer (n = 13) | 2.36 | 0.85 | 1.51 (−0.07 to 3.09) | 2.78 (0.91–8.52) | 0.074 | 1.92 (0.30–12.24) | 0.490 |
| Bladder cancer (n = 3) | 0.00 | 0.32 | −0.32 (−1.07 to 0.44) | N/A | 0.997 | N/A | 0.998 |
| Pancreatic cancer (n = 3) | 0.00 | 0.32 | −0.32 (−1.07 to 0.44) | N/A | 0.997 | N/A | 0.999 |
| Gallbladder and BD (n = 1) | 0.00 | 0.11 | −0.11 (−0.54 to 0.33) | N/A | 0.999 | N/A | 1.000 |
| Kidney cancer (n = 4) | 0.00 | 0.42 | −0.42 (−1.30 to 0.45) | N/A | 0.997 | N/A | 1.000 |
| Hematologic malignancy (n = 6) | 1.40 | 0.32 | 1.09 (0.02 to 2.16) | 4.27 (0.86–21.17) | 0.076 | 2.87 (0.24–33.99) | 0.403 |
| Age ≥ 60 (n = AD: 24,414, control: 97,656) | |||||||
| All cancer (n = 9015) | 11.75 | 16.68 | −4.93 (−5.78 to −4.08) | 0.70 (0.66–0.74) | <0.001 * | 0.63 (0.59–0.67) | <0.001 * |
| Gastric cancer (n = 1867) | 2.37 | 3.39 | −1.02 (−1.40 to −0.64) | 0.69 (0.60–0.79) | <0.001 * | 0.64 (0.55–0.73) | <0.001 * |
| Thyroid cancer (n = 365) | 0.43 | 0.66 | −0.23 (−0.39 to −0.06) | 0.61 (0.45–0.84) | 0.002 * | 0.64 (0.46–0.88) | 0.007 * |
| Colorectal cancer (n = 1826) | 2.29 | 3.29 | −1.00 (−1.37 to −0.62) | 0.69 (0.61–0.80) | <0.001 * | 0.67 (0.58–0.77) | <0.001 * |
| Lung cancer (n = 2125) | 2.62 | 3.82 | −1.20 (−1.60 to −0.80) | 0.70 (0.61–0.79) | <0.001 * | 0.64 (0.56–0.73) | <0.001 * |
| Hepatic cancer (n = 996) | 1.39 | 1.75 | −0.36 (−0.63 to −0.08) | 0.79 (0.67–0.95) | <0.001 * | 0.59 (0.49–0.71) | <0.001 * |
| Bladder cancer (n = 554) | 0.76 | 0.98 | −0.22 (−0.42 to −0.01) | 0.79 (0.63–1.01) | 0.058 | 0.77 (0.60–0.98) | 0.036 * |
| Pancreatic cancer (n = 655) | 0.69 | 1.20 | −0.51 (−0.73 to −0.28) | 0.57 (0.45–0.73) | <0.001 * | 0.51 (0.39–0.65) | <0.001 * |
| Gallbladder and BD (n = 653) | 0.89 | 1.15 | −0.26 (−0.48 to −0.04) | 0.78 (0.63–0.98) | 0.030 * | 0.73 (0.58–0.92) | 0.008 * |
| Kidney cancer (n = 238) | 0.32 | 0.42 | −0.10 (−0.24 to 0.03) | 0.74 (0.51–1.07) | 0.105 | 0.65 (0.44–0.96) | 0.028 * |
| Hematologic malignancy (n = 531) | 0.74 | 0.93 | −0.19 (−0.39 to 0.01) | 0.78 (0.62–1.00) | 0.048 * | 0.71 (0.55–0.91) | 0.008 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.S.; Kim, J.H.; Lim, H.; Kim, J.-H.; Noh, H.-M.; Choi, H.G.; Min, K.-W.; Kim, N.Y.; Kwon, M.J. Alzheimer’s Disease and Different Types of Cancer Likelihood: Unveiling Disparities and Potential Protective Effects in a Korean Cohort Study. Cancers 2023, 15, 4615. https://doi.org/10.3390/cancers15184615
Kang HS, Kim JH, Lim H, Kim J-H, Noh H-M, Choi HG, Min K-W, Kim NY, Kwon MJ. Alzheimer’s Disease and Different Types of Cancer Likelihood: Unveiling Disparities and Potential Protective Effects in a Korean Cohort Study. Cancers. 2023; 15(18):4615. https://doi.org/10.3390/cancers15184615
Chicago/Turabian StyleKang, Ho Suk, Ji Hee Kim, Hyun Lim, Joo-Hee Kim, Hye-Mi Noh, Hyo Geun Choi, Kyueng-Whan Min, Nan Young Kim, and Mi Jung Kwon. 2023. "Alzheimer’s Disease and Different Types of Cancer Likelihood: Unveiling Disparities and Potential Protective Effects in a Korean Cohort Study" Cancers 15, no. 18: 4615. https://doi.org/10.3390/cancers15184615
APA StyleKang, H. S., Kim, J. H., Lim, H., Kim, J.-H., Noh, H.-M., Choi, H. G., Min, K.-W., Kim, N. Y., & Kwon, M. J. (2023). Alzheimer’s Disease and Different Types of Cancer Likelihood: Unveiling Disparities and Potential Protective Effects in a Korean Cohort Study. Cancers, 15(18), 4615. https://doi.org/10.3390/cancers15184615





