Clinical Effectiveness of Fluorescence Lymph Node Mapping Using ICG for Laparoscopic Right Hemicolectomy: A Prospective Case–Control Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Fluorescence Lymph Nodes Mapping
2.3. FLNM-Guided D3 Lymph Node Dissection
2.4. Pathologic Evaluation
2.5. Clinical Outcomes
2.6. Statistical Analysis
3. Results
3.1. Patients
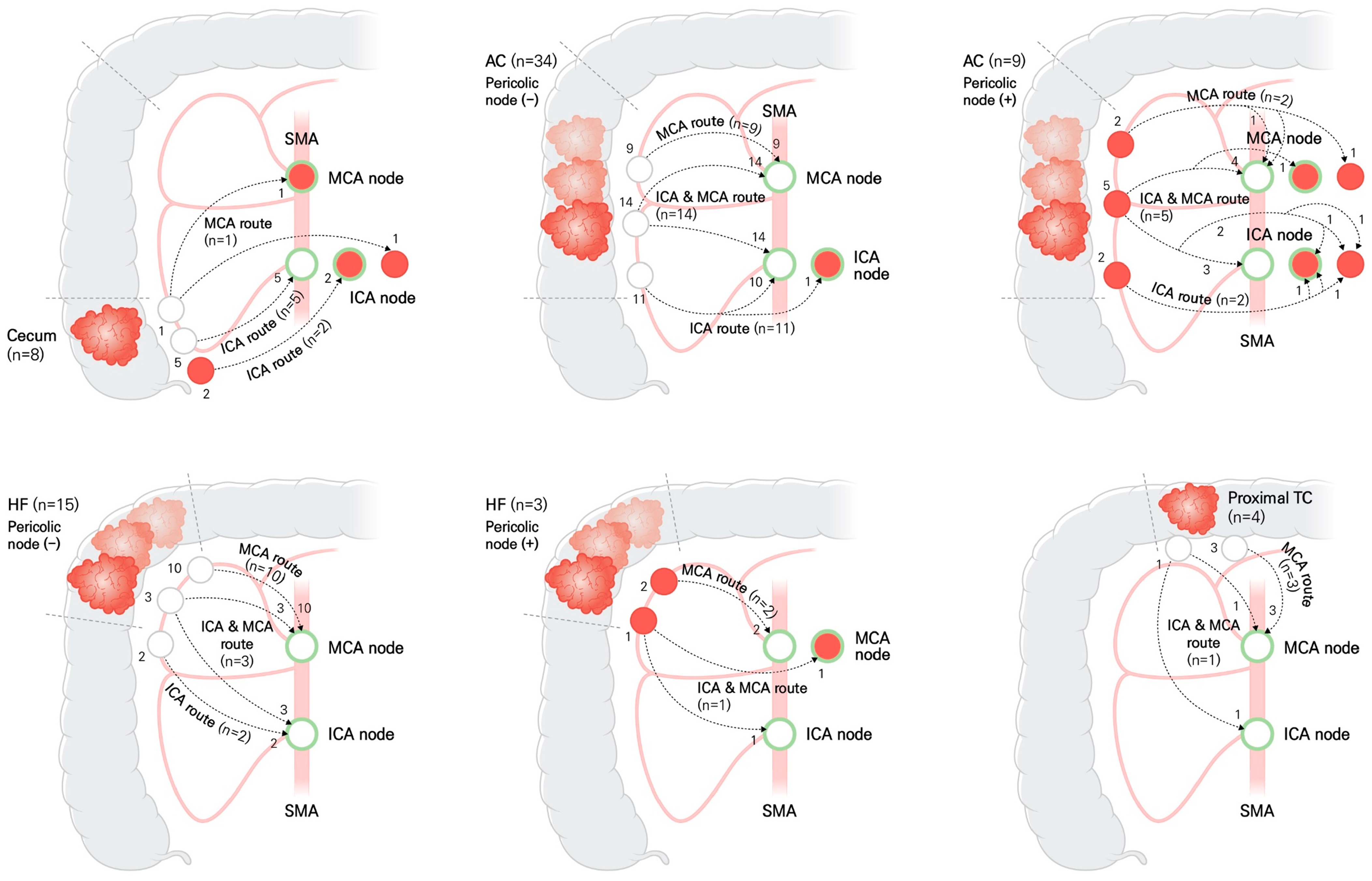
3.2. Fluorescent Lymphatic Drainage Pathway
3.3. Fluorescence Lymph Node Mapping and Metastatic Lymph Nodes
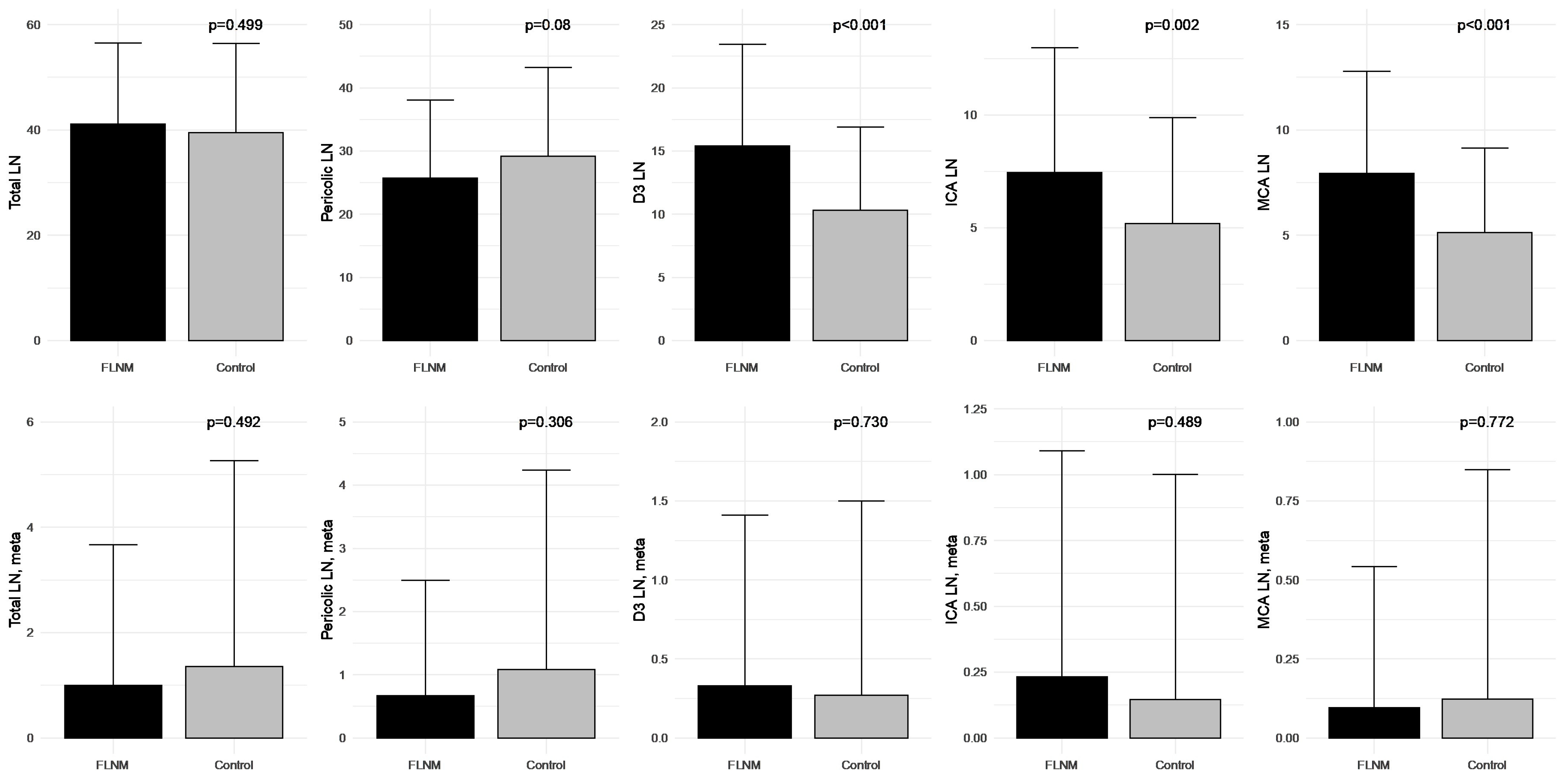
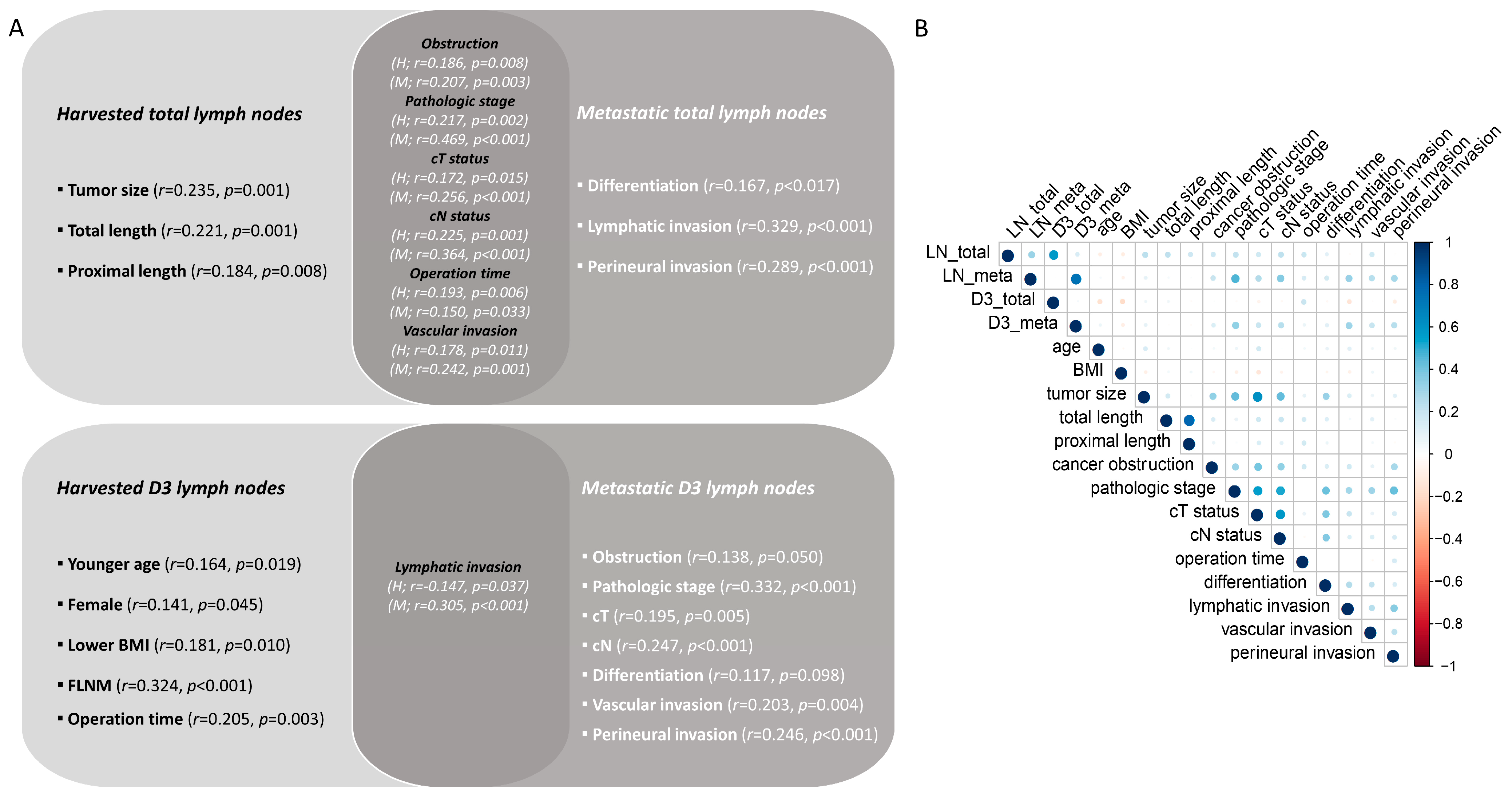
3.4. Correlation of Harvested and Metastatic Lymph Nodes
3.5. Regression Analysis for FLNM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lykke, J.; Roikjaer, O.; Jess, P.; Rosenberg, J.; Danish Colorectal Cancer Group. Identification of Risk Factors Associated with Stage III Disease in Nonmetastatic Colon Cancer: Results from a Prospective National Cohort Study. Ann. Coloproctol. 2020, 36, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Kim, J.S.; Kim, J.Y.; Lee, K.H. Prognostic Factor and Survival Benefit of Adjuvant Chemotherapy in Stage IIA Colon Cancer. Ann. Coloproctol. 2021, 37, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.F.; Naeem, A.; Haq, I.U.; Riaz, S.; Shakeel, O.; Panteleimonitis, S.; Khattak, S.; Syed, A.A.; Parvaiz, A. Laparoscopy offers better clinical outcomes and long-term survival in patients with right colon cancer: Experience from national cancer center. Ann. Coloproctol. 2022, 38, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef]
- Seow-En, I.; Chen, W.T. Complete mesocolic excision with central venous ligation/D3 lymphadenectomy for colon cancer—A comprehensive review of the evidence. Surg. Oncol. 2022, 42, 101755. [Google Scholar] [CrossRef]
- Bertelsen, C.A.; Kirkegaard-Klitbo, A.; Nielsen, M.; Leotta, S.M.; Daisuke, F.; Gögenur, I. Pattern of Colon Cancer Lymph Node Metastases in Patients Undergoing Central Mesocolic Lymph Node Excision: A Systematic Review. Dis. Colon. Rectum 2016, 59, 1209–1221. [Google Scholar] [CrossRef]
- Lal, N.; Chan, D.K.H.; Ng, M.E.; Vermeulen, L.; Buczacki, S.J.A. Primary tumour immune response and lymph node yields in colon cancer. Br. J. Cancer 2022, 126, 1178–1185. [Google Scholar] [CrossRef]
- Son, G.M.; Lee, I.Y.; Lee, Y.S.; Kye, B.H.; Cho, H.M.; Jang, J.H.; Kim, C.N.; Lee, K.Y.; Lee, S.H.; Kim, J.G.; et al. Is Laparoscopic Complete Mesocolic Excision and Central Vascular Ligation Really Necessary for All Patients with Right-Sided Colon Cancer? Ann. Coloproctol. 2021, 37, 434–444. [Google Scholar] [CrossRef]
- Ahn, H.M.; Son, G.M.; Lee, I.Y.; Shin, D.H.; Kim, T.K.; Park, S.B.; Kim, H.W. Optimal ICG dosage of preoperative colonoscopic tattooing for fluorescence-guided laparoscopic colorectal surgery. Surg. Endosc. 2022, 36, 1152–1163. [Google Scholar] [CrossRef]
- Watanabe, J.; Kanemitsu, Y.; Suwa, H.; Kakeji, Y.; Ishihara, S.; Shinto, E.; Ozawa, H.; Suto, T.; Kawamura, J.; Fujita, F.; et al. A multicenter cohort study on mapping of lymph node metastasis for splenic flexural colon cancer. Ann. Gastroenterol. Surg. 2022, 7, 265–271. [Google Scholar] [CrossRef]
- Fukuoka, H.; Fukunaga, Y.; Nagasaki, T.; Akiyoshi, T.; Konishi, T.; Nagayama, S.; Ueno, M. Lymph Node Mapping in Transverse Colon Cancer Treated Using Laparoscopic Colectomy with D3 Lymph Node Dissection. Dis. Colon. Rectum 2022, 65, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, J.S.; Kim, H.J.; Woo, I.T.; Park, I.K.; Choi, G.S. Indocyanine Green Fluorescence Imaging-Guided Laparoscopic Surgery Could Achieve Radical D3 Dissection in Patients with Advanced Right-Sided Colon Cancer. Dis. Colon. Rectum 2020, 63, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Chand, M.; Keller, D.S.; Joshi, H.M.; Devoto, L.; Rodriguez-Justo, M.; Cohen, R. Feasibility of fluorescence lymph node imaging in colon cancer: FLICC. Tech. Coloproctol. 2018, 22, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, H.; Lu, X.; Yi, X.; Wan, J.; Liao, W.; Wang, J.; Ke, Y.; Tan, P.; Chen, J.; et al. Regional lymph nodes distribution pattern in central area of right-sided colon cancer: In-vivo detection and the update on the clinical exploration. Am. J. Cancer Res. 2021, 11, 2095–2105. [Google Scholar]
- Wexner, S.; Abu-Gazala, M.; Boni, L.; Buxey, K.; Cahill, R.; Carus, T.; Chadi, S.; Chand, M.; Cunningham, C.; Emile, S.H.; et al. Use of fluorescence imaging and indocyanine green during colorectal surgery: Results of an intercontinental Delphi survey. Surgery 2022, 172, S38–S45. [Google Scholar] [CrossRef]
- Son, G.M.; Ahn, H.M.; Lee, I.Y.; Ha, G.W. Multifunctional Indocyanine Green Applications for Fluorescence-Guided Laparoscopic Colorectal Surgery. Ann. Coloproctol. 2021, 37, 133–140. [Google Scholar] [CrossRef]
- Kim, H.; Seo, A.N.; Park, S.Y. Laparoscopic right hemicolectomy with aortocaval lymphadenectomy, and pelvic peritoneum partial resection for ascending colon cancer. Ann. Coloproctol. 2023, 39, 283–286. [Google Scholar] [CrossRef]
- Jung, W.B. To what extent does endoscopic tattooing marking boost lymph node retrieval? Ann. Coloproctol. 2023, 39, 95–96. [Google Scholar] [CrossRef]
- Park, K.J. Against all odds: Why surgeons need to be more aggressive in the era of the multidisciplinary team approach to colorectal cancer. Ann. Coloproctol. 2022, 38, 393–397. [Google Scholar] [CrossRef]
- Hacım, N.A.; Akbaş, A.; Ulgen, Y.; Aktokmakyan, T.V.; Meric, S.; Tokocin, M.; Karabay, O.; Altinel, Y. Influence of colonic mesenteric area on the number of lymph node retrieval for colon cancer: A prospective cohort study. Ann. Coloproctol. 2023, 39, 77–84. [Google Scholar] [CrossRef]
- Lucas, K.; Melling, N.; Giannou, A.D.; Reeh, M.; Mann, O.; Hackert, T.; Izbicki, J.R.; Perez, D.; Grass, J.K. Lymphatic Mapping in Colon Cancer Depending on Injection Time and Tracing Agent: A Systematic Review and Meta-Analysis of Prospective Designed Studies. Cancers 2023, 15, 3196. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.N.; Ricciardi, R.; Simunovic, M.; Urbach, D.R.; Virnig, B.A. An evaluation of the relationship between lymph node number and staging in pT3 colon cancer using population-based data. Dis. Colon. Rectum 2010, 53, 65–70. [Google Scholar] [CrossRef]
- Imaoka, K.; Yano, T.; Yoshimitsu, M.; Fukuhara, S.; Oshita, K.; Nakano, K.; Kunihiro, M.; Idani, H.; Okajima, M. Preoperative endoscopic tattoo marking improves lymph node retrieval in laparoscopic rectal resection: A retrospective cohort study. Ann. Coloproctol. 2023, 39, 115–122. [Google Scholar] [CrossRef]
- Kakizoe, M.; Watanabe, J.; Suwa, Y.; Nakagawa, K.; Suwa, H.; Ozawa, M.; Ishibe, A.; Masui, H.; Nagahori, K. The histopathological evaluation based on the indocyanine green fluorescence imaging of regional lymph node metastasis of splenic flexural colon cancer by near-infrared observation. Int. J. Colorectal Dis. 2021, 36, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Watanabe, T.; Fujita, Y.; Matsumura, S.; Ueda, K.; Nagano, S.; Kinoshita, I.; Murakami, D.; Tabata, H.; Tsuji, T.; et al. Histology of metastatic colorectal cancer in a lymph node. PLoS ONE 2023, 18, e0284536. [Google Scholar] [CrossRef]
- Carrara, A.; Motter, M.; Amabile, D.; Pellecchia, L.; Moscatelli, P.; Pertile, R.; Barbareschi, M.; Decarli, N.L.; Ferrari, M.; Tirone, G. Predictive value of the sentinel lymph node procedure in the staging of non-metastatic colorectal cancer. Int. J. Color. Dis. 2020, 35, 1921–1928. [Google Scholar] [CrossRef]
- Saha, S.; Philimon, B.; Efeson, M.; Helina, A.; Elgamal, M.; Kiya, G.; Hilkiah, S.; Arora, M.; Wiese, D.; Kitagawa, Y. The role of sentinel lymph node mapping in colon cancer: Detection of micro-metastasis, effect on survival, and driver of a paradigm shift in extent of colon resection. Clin. Exp. Metastasis 2022, 39, 109–115. [Google Scholar] [CrossRef]
- Villegas-Tovar, E.; Jimenez-Lillo, J.; Jimenez-Valerio, V.; Diaz-Giron-Gidi, A.; Faes-Petersen, R.; Otero-Piñeiro, A.; De Lacy, F.B.; Martinez-Portilla, R.J.; Lacy, A.M. Performance of Indocyanine green for sentinel lymph node mapping and lymph node metastasis in colorectal cancer: A diagnostic test accuracy meta-analysis. Surg. Endosc. 2020, 34, 1035–1047. [Google Scholar] [CrossRef]
- Liang, Y.; Guo, W.; Li, C.; Shen, G.; Tan, H.; Sun, P.; Chen, Z.; Huang, H.; Li, Z.; Li, Z.; et al. Tumor-Targeted Polydopamine-Based Nanoparticles for Multimodal Mapping Following Photothermal Therapy of Metastatic Lymph Nodes. Int. J. Nanomed. 2022, 17, 4659–4675. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Xu, T.; Xue, R.; Yu, L.; Zhu, Y.; Wu, Y.; Zhang, Q.; Li, D.; Shen, S.; et al. Mapping the spreading routes of lymphatic metastases in human colorectal cancer. Nat. Commun. 2020, 11, 1993. [Google Scholar] [CrossRef]
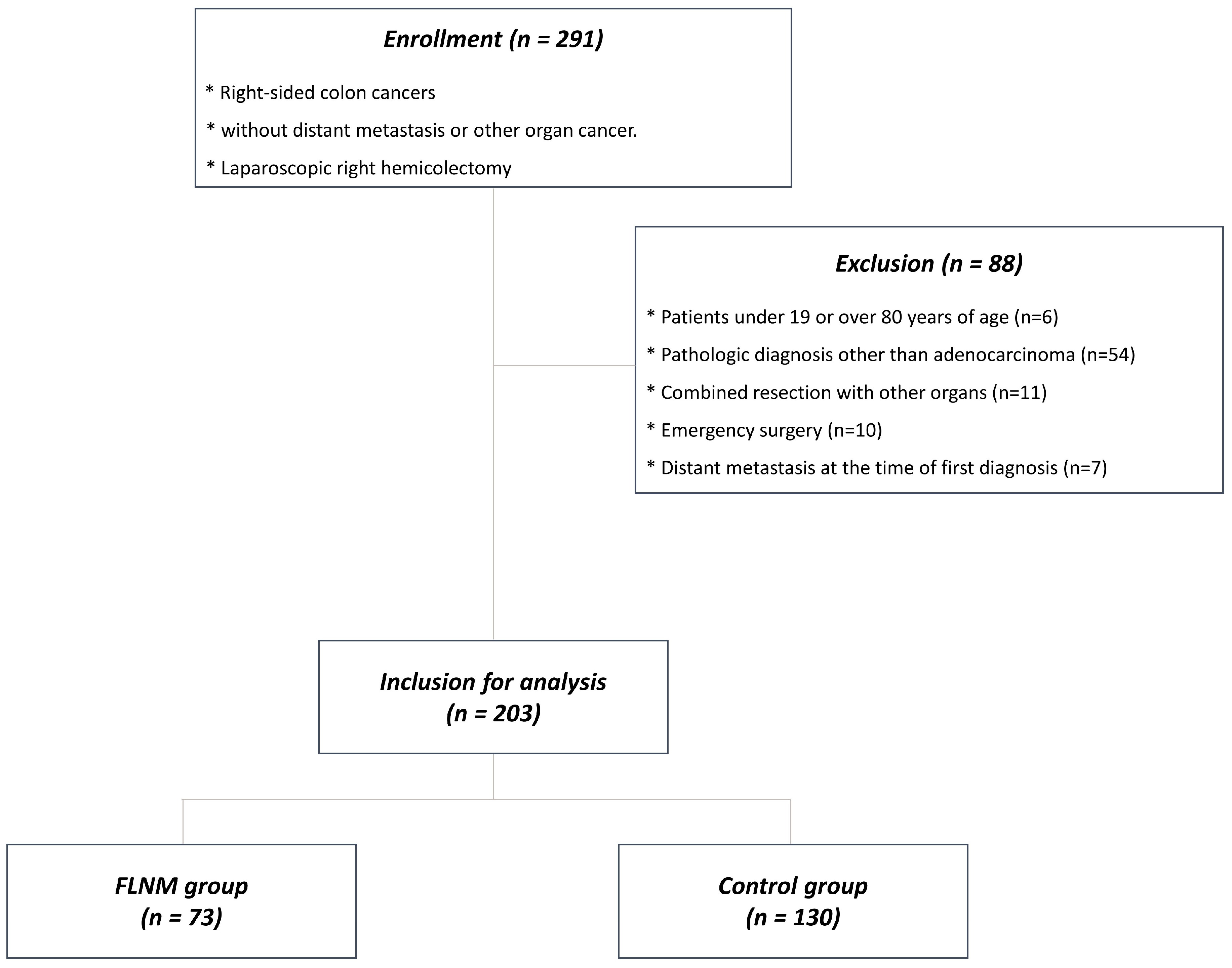
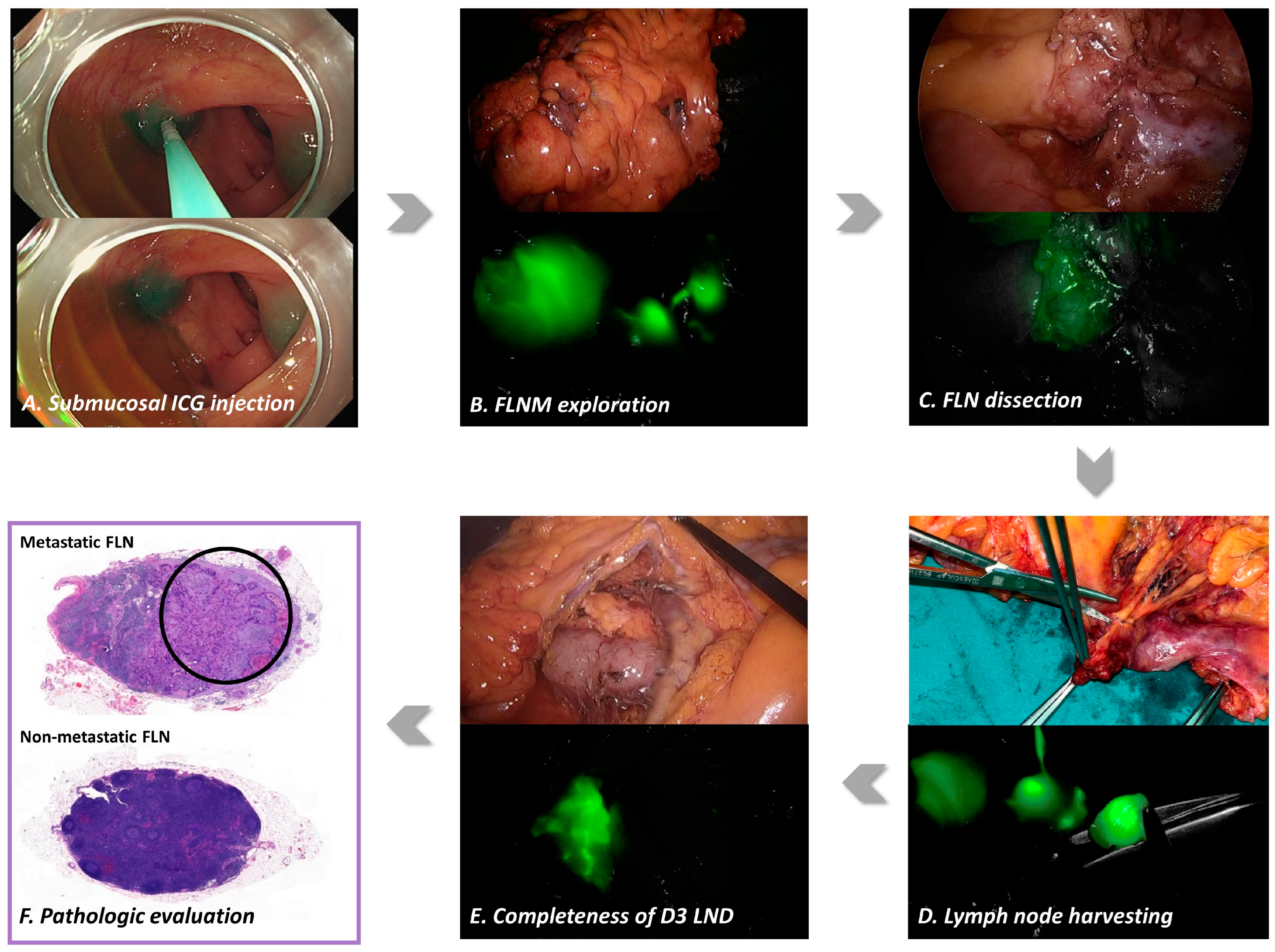

| Clinical Variables | FLNM (n = 73) | Control (n = 130) | |
|---|---|---|---|
| n (%) | n (%) | ||
| Age, yr | mean ± SD | 67.7 ± 11.8 | 67.6 ± 10.5 |
| Sex | male | 36 (49.3) | 61 (46.9) |
| female | 37 (50.7) | 69 (53.1) | |
| BMI (kg/m2) | mean ± SD | 23.5 ± 3.1 | 23.9 ± 2.8 |
| Cancer location | cecum | 8 (11.0) | 19 (14.6) |
| ascending colon | 43 (58.9) | 80 (61.5) | |
| hepatic flexure | 18 (24.7) | 19 (14.6) | |
| proximal transverse colon | 4 (5.5) | 12 (9.2) | |
| cT status | cT 1–2 | 42 (57.5) | 58 (44.6) |
| cT 3–4 | 31 (42.5) | 72 (55.4) | |
| cN status | cN 0 | 48 (65.8) | 51 (39.2) |
| cN 1–2 | 25 (34.2) | 79 (60.8) | |
| pT status | pT 1–2 | 30 (41.1) | 39 (30.0) |
| pT 3–4 | 43 (58.9) | 91 (70.0) | |
| pN status | pN 0 | 57 (78.1) | 89 (68.5) |
| pN 1–2 | 16 (21.9) | 41 (31.5) | |
| Pathologic stage | I | 29 (39.7) | 34 (26.2) |
| II | 28 (38.4) | 55 (42.3) | |
| III | 16 (21.9) | 41 (31.5) | |
| Tumor size (cm) | mean ± SD | 3.9 ± 2.2 | 4.4 ± 2.4 |
| Differentiation | well | 34 (46.6) | 27 (20.8) |
| moderate | 37 (50.7) | 95 (73.1) | |
| poorly | 2 (2.7) | 8 (6.2) | |
| Cancer obstruction | positive | 15 (20.5) | 28 (21.5) |
| Lymphatic invasion | positive | 13 (17.8) | 26 (20.0) |
| Vascular invasion | positive | 4 (5.5) | 14 (10.8) |
| Perineural invasion | positive | 14 (19.2) | 26 (20.0) |
| Cancer Location, n (%) | p Value | |||||
|---|---|---|---|---|---|---|
| Cecum | Ascending Colon | Hepatic Flexure | Proximal Transverse Colon | |||
| FLNM group | ||||||
| Lymphatic drainage pattern | ||||||
| ICA route | 7 (87.5) | 13 (30.2) | 2 (11.1) | 0 | <0.001 † | |
| MCA route | 1 (12.5) | 11 (25.6) | 12 (66.7) | 3 (75.0) | ||
| Dual route | 0 | 19 (44.2) | 4 (22.2) | 1 (25.0) | ||
| D3 LN metastasis | ||||||
| Negative | 5 (62.5) | 37 (86.0) | 17 (94.4) | 4 (100) | 0.311 † | |
| ICA LNs | 2 (25.0) | 4 (9.3) | 0 | 0 | ||
| MCA LNs | 1 (12.5) | 1 (2.3) | 1 (5.6) | 0 | ||
| Both LNs | 0 | 1 (2.3) | 0 | 0 | ||
| Control group | ||||||
| D3 LN metastasis | ||||||
| Negative | 17 (89.5) | 75 (93.8) | 15 (78.9) | 12 (100) | 0.090 † | |
| ICA LNs | 1 (5.3) | 3 (3.8) | 0 | 0 | ||
| MCA LNs | 0 | 2 (2.5) | 3 (15.8) | 0 | ||
| Both LNs | 1 (5.3) | 0 | 1 (5.3) | 0 | ||
| Total | ||||||
| D3 LN metastasis | ||||||
| Negative | 22 (81.5) | 112 (91.1) | 32 (86.5) | 16 (100) | 0.125 † | |
| ICA LNs | 3 (11.1) | 7 (5.7) | 0 | 0 | ||
| MCA LNs | 1 (3.7) | 3 (2.4) | 4 (10.8) | 0 | ||
| Both LNs | 1 (3.7) | 1 (0.8) | 1 (2.7) | 0 | ||
| Stage III | FLNM Group, n (%) | Control Group, n (%) | p Value | |
|---|---|---|---|---|
| Percolic LNs metastasis | negative | 2 (12.5) | 0 | 0.075 |
| positive | 14 (87.5) | 41 (100) | ||
| ICA LNs metastasis | negative | 8 (50.0) | 35 (85.4) | 0.005 |
| positive | 8 (50.0) | 6 (14.6) | ||
| MCA LNs metastasis | negative | 12 (75.0) | 34 (82.9) | 0.496 |
| positive | 4 (25.0) | 7 (17.1) | ||
| Total D3 LNs metastasis | negative | 6 (37.5) | 30 (73.2) | 0.012 |
| positive | 10 (62.5) | 11 (26.8) |
| D3 LN Metastasis | ||||||
|---|---|---|---|---|---|---|
| Negative | ICA LNs | MCA LNs | Both LNs | p Value | ||
| Pericolic LN metastasis; negative (n = 59) | ||||||
| ICA route | 17 (29.8) | 1 (100) | 0 | 0 | 0.852 † | |
| MCA route | 22 (38.6) | 0 | 0 | 1 (100) | ||
| Dual route | 18 (31.6) | 0 | 0 | 0 | ||
| Pericolic LN metastasis; positive (n = 14) | ||||||
| ICA route | 0 | 4 (80.0) | 0 | 0 | 0.039 † | |
| MCA route | 3 (50.0) | 0 | 1 (50.0) | 0 | ||
| Dual route | 3 (50.0) | 1 (20.0) | 1 (50.0) | 1 (100) | ||
| Total (n = 73) | ||||||
| ICA route | 17 (27.0) | 5 (83.3) | 0 | 0 | 0.041 † | |
| MCA route | 25 (39.7) | 0 | 1 (50.0) | 1 (50.0) | ||
| Dual route | 21 (33.3) | 1 (16.7) | 1 (50.0) | 1 (50.0) | ||
| Stage III of FLNM Group | Fluorescent D3 LNs Metastasis, n (%) | |||||
|---|---|---|---|---|---|---|
| Negative | ICA LNs | MCA LNs | Both LNs | Total | ||
| Non-fluorescent D3 LNs metastasis | Negative | 6 (37.5) * | 4 (25.0) | 1 (6.3) | 0 | 11 (68.8) |
| ICA LNs | 1 (6.3) | 1 (6.3) | 1 (6.3) | 1 (6.3) | 4 (25.0) | |
| MCA LNs | 1 (6.3) | 0 | 0 | 0 | 1 (6.3) | |
| Total | 8 (50.0) | 5 (31.3) | 2 (12.5) | 1 (6.3) | 16 (100) | |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Harvested D3 LNs | ||||||
| Variable | Beta | 95% CI | p Value | Beta | 95% CI | p Value |
| age | −0.113 | −0.205, −0.017 | 0.020 | −0.112 | −0.199, −0.026 | 0.011 |
| sex (male) | −2.129 | −4.161, −0.019 | 0.048 | −2.645 | −4.590, −0.699 | 0.008 |
| BMI | −0.465 | −0.818, −0.113 | 0.010 | −0.451 | −0.775, −0.126 | 0.007 |
| operation time (min) | 0.042 | 0.014, 0.069 | 0.003 | 0.031 | 0.003, 0.060 | 0.030 |
| lymphatic invasion | −2.802 | −5.431, −0.173 | 0.038 | −2.359 | −4.774, 0.057 | 0.056 |
| FLNM | 5.072 | 3.005, 7.138 | <0.001 | 3.942 | 1.806, 6.078 | <0.001 |
| Metastatic D3 LNs | ||||||
| Variable | Beta | 95% CI | p Value | Beta | 95% CI | p Value |
| cancer obstruction | 0.398 | 0.003, 0.794 | 0.049 | |||
| pathologic stage | 0.507 | 0.306, 0.707 | <0.001 | 0.321 | 0.082, 0.559 | 0.009 |
| cT status | 0.212 | 0.064, 0.359 | 0.005 | |||
| cN status | 0.365 | 0.163, 0.566 | <0.001 | 0.200 | −0.031, 0.432 | 0.089 |
| differentiation | 0.255 | −0048, 0.558 | 0.099 | |||
| lymphatic invasion | 0.910 | 0.515, 1.305 | <0.001 | 0.682 | 0.284, 1.080 | 0.001 |
| vascular invasion | 0.839 | 0.277, 1.402 | 0.012 | |||
| perineural invasion | 0.728 | 0.330, 0.324 | <0.001 | |||
| FLNM | 0.06 | −0.281, 0.040 | 0.730 | 0.237 | −0.088, 0.561 | 0.152 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Harvested D3 LNs | ||||||
| Variable | Beta | 95% CI | p Value | Beta | 95% CI | p Value |
| age | −0.108 | −0.281, 0.065 | 0.216 | −0.121 | −0.210, −0.032 | 0.008 |
| FLNM | 7.567 | 3.706, 11.428 | <0.001 | −5.243 | −7.278, −3.208 | <0.001 |
| length (total) | 0.048 | −0.083, 0.180 | 0.465 | 0.122 | 0.021, 0.222 | 0.018 |
| length (proximal) | −0.013 | −0.163, 0.136 | 0.858 | −0.103 | −0.218, 0.011 | 0.076 |
| Metastatic D3 LNs | ||||||
| Variable | Beta | 95% CI | p Value | Beta | 95% CI | p Value |
| sex (male) | −0.28 | −1.377, 0.818 | 0.612 | −0.969 | −2.076, 0.138 | 0.085 |
| operation time (min) | 0.009 | −0.006, 0.024 | 0.224 | 0.013 | −0.002, 0.028 | 0.085 |
| lymphatic invasion | 1.486 | 0.407, 2.566 | 0.008 | 1.664 | 0.576, 2.752 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, G.M.; Yun, M.S.; Lee, I.Y.; Im, S.B.; Kim, K.H.; Park, S.B.; Kim, T.U.; Shin, D.-H.; Nazir, A.M.; Ha, G.W. Clinical Effectiveness of Fluorescence Lymph Node Mapping Using ICG for Laparoscopic Right Hemicolectomy: A Prospective Case–Control Study. Cancers 2023, 15, 4927. https://doi.org/10.3390/cancers15204927
Son GM, Yun MS, Lee IY, Im SB, Kim KH, Park SB, Kim TU, Shin D-H, Nazir AM, Ha GW. Clinical Effectiveness of Fluorescence Lymph Node Mapping Using ICG for Laparoscopic Right Hemicolectomy: A Prospective Case–Control Study. Cancers. 2023; 15(20):4927. https://doi.org/10.3390/cancers15204927
Chicago/Turabian StyleSon, Gyung Mo, Mi Sook Yun, In Young Lee, Sun Bin Im, Kyung Hee Kim, Su Bum Park, Tae Un Kim, Dong-Hoon Shin, Armaan M. Nazir, and Gi Won Ha. 2023. "Clinical Effectiveness of Fluorescence Lymph Node Mapping Using ICG for Laparoscopic Right Hemicolectomy: A Prospective Case–Control Study" Cancers 15, no. 20: 4927. https://doi.org/10.3390/cancers15204927
APA StyleSon, G. M., Yun, M. S., Lee, I. Y., Im, S. B., Kim, K. H., Park, S. B., Kim, T. U., Shin, D.-H., Nazir, A. M., & Ha, G. W. (2023). Clinical Effectiveness of Fluorescence Lymph Node Mapping Using ICG for Laparoscopic Right Hemicolectomy: A Prospective Case–Control Study. Cancers, 15(20), 4927. https://doi.org/10.3390/cancers15204927





