The Promise of Semantic Segmentation in Detecting Actinic Keratosis Using Clinical Photography in the Wild
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods
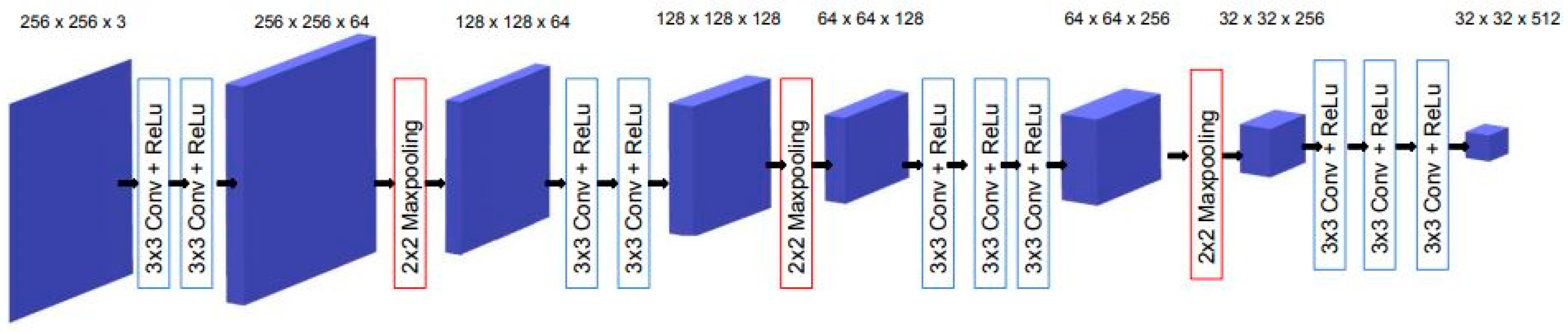
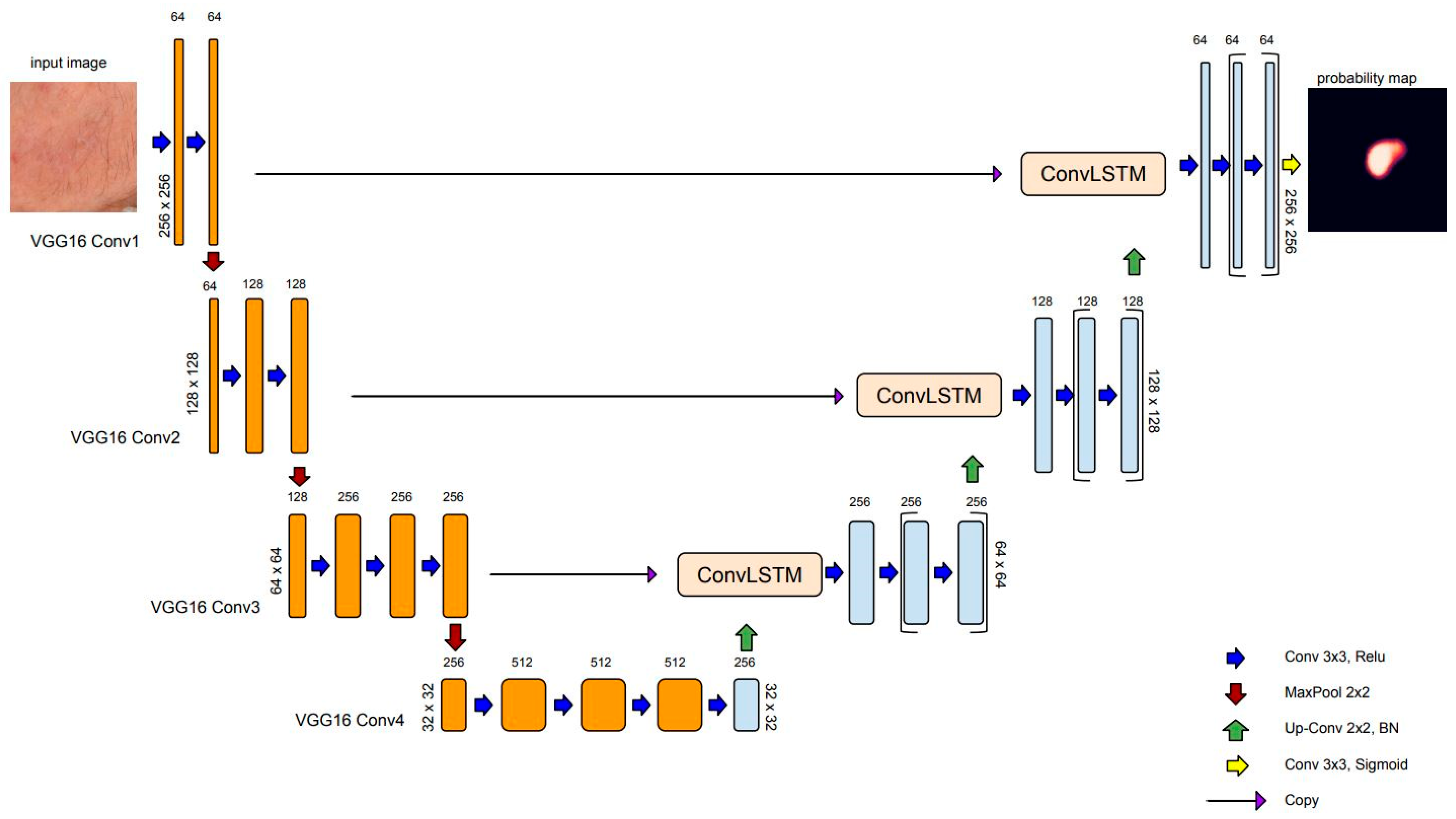
2.1.1. Overview of Semantic Segmentation and U-Net Architecture
2.1.2. Batch Normalization
2.1.3. ConvLSTM: Spatial Recurrent Module in U-Net Architecture
2.1.4. Loss Function
2.1.5. Evaluation
2.1.6. Implementation Details
2.2. Materials
Experimental Settings
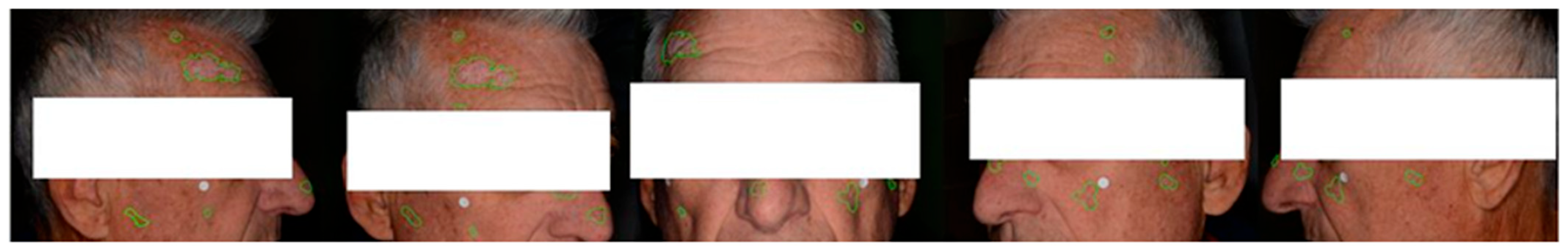
3. Results
- Zero Padding: Appropriate zero-padding was applied to the input image to make it larger and suitable for subsequent cropping.
- Image Cropping: The zero-padded image was divided into crops of 256 × 256 pixels. These crops were used as individual inputs to the AKU-Net model for AK detection.
- Aggregating Results: The obtained segmentation results for each 256 × 256-pixel crop were combined to obtain the overall AK detection for the entire broad skin area.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willenbrink, T.J.; Ruiz, E.S.; Cornejo, C.M.; Schmults, C.D.; Arron, S.T.; Jambusaria-Pahlajani, A. Field cancerization: Definition, epidemiology, risk factors, and outcomes. J. Am. Acad. Dermatol. 2020, 83, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.N.; Sammain, A.; Erdmann, R.; Hartmann, V.; Stockfleth, E.; Nast, A. The natural history of actinic keratosis: A systematic review. Br. J. Dermatol. 2013, 169, 502–518. [Google Scholar] [CrossRef] [PubMed]
- Nart, I.F.; Cerio, R.; Dirschka, T.; Dréno, B.; Lear, J.; Pellacani, G.; Peris, K.; de Casas, A.R.; Progressing Evidence in AK (PEAK) Working Group. Defining the actinic keratosis field: A literature review and discussion. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 544–563. [Google Scholar] [CrossRef] [PubMed]
- Gutzmer, R.; Wiegand, S.; Kölbl, O.; Wermker, K.; Heppt, M.; Berking, C. Actinic Keratosis and Cutaneous Squamous Cell Carcinoma. Dtsch. Arztebl. Int. 2019, 116, 616–626. [Google Scholar] [CrossRef] [PubMed]
- De Berker, D.; McGregor, J.M.; Mustapa, M.F.M.; Exton, L.S.; Hughes, B.R. British Association of Dermatologists’ guidelines for the care of patients with actinic keratosis 2017. Br. J. Dermatol. 2017, 176, 20–43. [Google Scholar] [CrossRef]
- Werner, R.N.; Stockfleth, E.; Connolly, S.; Correia, O.; Erdmann, R.; Foley, P.; Gupta, A.; Jacobs, A.; Kerl, H.; Lim, H.; et al. Evidence- and consensus-based (S3) Guidelines for the Treatment of Actinic Keratosis—International League of Dermatological Societies in cooperation with the European Dermatology Forum—Short version. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2069–2079. [Google Scholar] [CrossRef]
- Eisen, D.B.; Asgari, M.M.; Bennett, D.D.; Connolly, S.M.; Dellavalle, R.P.; Freeman, E.E.; Goldenberg, G.; Leffell, D.J.; Peschin, S.; Sligh, J.E.; et al. Guidelines of care for the management of actinic keratosis. J. Am. Acad. Dermatol. 2021, 85, e209–e233. [Google Scholar] [CrossRef]
- Casari, A.; Chester, J.; Pellacani, G. Actinic Keratosis and Non-Invasive Diagnostic Techniques: An Update. Biomedicines 2018, 6, 8. [Google Scholar] [CrossRef]
- Peris, K.; Micantonio, T.; Piccolo, D.; Concetta, M. Dermoscopic features of actinic keratosis. JDDG J. Dtsch. Dermatol. Gese. 2007, 5, 970–975. [Google Scholar] [CrossRef]
- Schmitz, L.; Gupta, G.; Stücker, M.; Doerler, M.; Gambichler, T.; Welzel, J.; Szeimies, R.; Bierhoff, E.; Stockfleth, E.; Dirschka, T. Evaluation of two histological classifications for actinic keratoses—PRO classification scored highest inter-rater reliability. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1092–1097. [Google Scholar] [CrossRef]
- Daxenberger, F.; Deußing, M.; Eijkenboom, Q.; Gust, C.; Thamm, J.; Hartmann, D.; French, L.E.; Welzel, J.; Schuh, S.; Sattler, E.C. Innovation in Actinic Keratosis Assessment: Artificial Intelligence-Based Approach to LC-OCT PRO Score Evaluation. Cancers 2023, 15, 4457. [Google Scholar] [CrossRef] [PubMed]
- Rigel, D.S.; Gold, L.F.S.; Zografos, P. The importance of early diagnosis and treatment of actinic keratosis. J. Am. Acad. Dermatol. 2013, 68 (Suppl. S1), S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Dirschka, T.; Pellacani, G.; Micali, G.; Malvehy, J.; Stratigos, A.J.; Casari, A.; Schmitz, L.; Gupta, G.; Athens AK Study Group. A proposed scoring system for assessing the severity of actinic keratosis on the head: Actinic keratosis area and severity index. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1295–1302. [Google Scholar] [CrossRef]
- Dreno, B.; Cerio, R.; Dirschka, T.; Nart, I.F.; Lear, J.T.; Peris, K.; de Casas, A.R.; Kaleci, S.; Pellacani, G. A novel actinic keratosis field assessment scale for grading actinic keratosis disease severity. Acta Derm. Venereol. 2017, 97, 1108–1113. [Google Scholar] [CrossRef]
- Schmitz, L.; Broganelli, P.; Boada, A. Classifying Actinic Keratosis: What the Reality of Everyday Clinical Practice Shows Us. J. Drugs Dermatol. 2022, 21, 845–849. [Google Scholar] [CrossRef]
- Epstein, E. Quantifying actinic keratosis: Assessing the evidence. Am. J. Clin. Dermatol. 2004, 5, 141–144. [Google Scholar] [CrossRef]
- Steeb, T.; Wessely, A.; Petzold, A.; Schmitz, L.; Dirschka, T.; Berking, C.; Heppt, M.V. How to Assess the Efficacy of Interventions for Actinic Keratosis? A Review with a Focus on Long-Term Results. J. Clin. Med. 2021, 10, 4736. [Google Scholar] [CrossRef] [PubMed]
- Criscione, V.D.; Weinstock, M.A.; Naylor, M.F.; Luque, C.; Eide, M.J.; Bingham, S.F. Actinic keratoses: Natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer 2009, 115, 2523–2530. [Google Scholar] [CrossRef]
- Adegun, A.; Viriri, S. Deep learning techniques for skin lesion analysis and melanoma cancer detection: A survey of state-of-the-art. Artif. Intell. Rev. 2020, 54, 811–841. [Google Scholar] [CrossRef]
- Jeong, H.K.; Park, C.; Henao, R.; Kheterpal, M. Deep Learning in Dermatology: A Systematic Review of Current Approaches, Outcomes, and Limitations. JID Innov. 2023, 3, 100150. [Google Scholar] [CrossRef]
- Li, L.-F.; Wang, X.; Hu, W.-J.; Xiong, N.N.; Du, Y.-X.; Li, B.-S. Deep Learning in Skin Disease Image Recognition: A Review. IEEE Access 2020, 8, 208264–208280. [Google Scholar] [CrossRef]
- Kassem, M.A.; Hosny, K.M.; Damaševičius, R.; Eltoukhy, M.M. Machine Learning and Deep Learning Methods for Skin Lesion Classification and Diagnosis: A Systematic Review. Diagnostics 2021, 11, 1390. [Google Scholar] [CrossRef]
- Wang, L.; Chen, A.; Zhang, Y.; Wang, X.; Zhang, Y.; Shen, Q.; Xue, Y. AK-DL: A shallow neural network model for diagnosing actinic keratosis with better performance than deep neural networks. Diagnostics 2020, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Maron, R.C.; Weichenthal, M.; Utikal, J.S.; Hekler, A.; Berking, C.; Hauschild, A.; Enk, A.H.; Haferkamp, S.; Klode, J.; Schadendorf, D.; et al. Systematic outperformance of 112 dermatologists in multiclass skin cancer image classification by convolutional neural networks. Eur. J. Cancer 2019, 119, 57–65. [Google Scholar] [CrossRef]
- Tschandl, P.; Rosendahl, C.; Akay, B.N.; Argenziano, G.; Blum, A.; Braun, R.P.; Cabo, H.; Gourhant, J.-Y.; Kreusch, J.; Lallas, A.; et al. Expert-Level Diagnosis of Nonpigmented Skin Cancer by Combined Convolutional Neural Networks. JAMA Dermatol. 2019, 155, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.G.C.; Krohling, R.A. The impact of patient clinical information on automated skin cancer detection. Comput. Biol. Med. 2020, 116, 103545. [Google Scholar] [CrossRef]
- Liu, Y.; Jain, A.; Eng, C.; Way, D.H.; Lee, K.; Bui, P.; Kanada, K.; de Oliveira Marinho, G.; Gallegos, J.; Gabriele, S.; et al. A deep learning system for differential diagnosis of skin diseases. Nat. Med. 2020, 26, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Karthik, R.; Vaichole, T.S.; Kulkarni, S.K.; Yadav, O.; Khan, F. Eff2Net: An efficient channel attention-based convolutional neural network for skin disease classification. Biomed. Signal Process. Control 2021, 73, 103406. [Google Scholar] [CrossRef]
- Han, S.S.; Kim, M.S.; Lim, W.; Park, G.H.; Park, I.; Chang, S.E. Classification of the Clinical Images for Benign and Malignant Cutaneous Tumors Using a Deep Learning Algorithm. J. Investig. Dermatol. 2018, 138, 1529–1538. [Google Scholar] [CrossRef]
- Fujisawa, Y.; Otomo, Y.; Ogata, Y.; Nakamura, Y.; Fujita, R.; Ishitsuka, Y.; Watanabe, R.; Okiyama, N.; Ohara, K.; Fujimoto, M. Deep-learning-based, computer-aided classifier developed with a small dataset of clinical images surpasses board-certified dermatologists in skin tumour diagnosis. Br. J. Dermatol. 2019, 180, 373–381. [Google Scholar] [CrossRef]
- Han, S.S.; Moon, I.J.; Lim, W.; Suh, I.S.; Lee, S.Y.; Na, J.-I.; Kim, S.H.; Chang, S.E. Keratinocytic Skin Cancer Detection on the Face Using Region-Based Convolutional Neural Network. JAMA Dermatol. 2020, 156, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Lippman, S.M.; Flaherty, K.T.; Kurzrock, R. The conundrum of genetic ‘Drivers’ in benign conditions. J. Natl. Cancer Inst. 2016, 108, djw036. [Google Scholar] [CrossRef] [PubMed]
- Hames, S.C.; Sinnya, S.; Tan, J.-M.; Morze, C.; Sahebian, A.; Soyer, H.P.; Prow, T.W. Automated detection of actinic keratoses in clinical photographs. PLoS ONE 2015, 10, e0112447. [Google Scholar] [CrossRef] [PubMed]
- Spyridonos, P.; Gaitanis, G.; Likas, A.; Bassukas, I.D. Automatic discrimination of actinic keratoses from clinical photographs. Comput. Biol. Med. 2017, 88, 50–59. [Google Scholar] [CrossRef]
- South, A.P.; Purdie, K.J.; Watt, S.A.; Haldenby, S.; Breems, N.Y.D.; Dimon, M.; Arron, S.T.; Kluk, M.J.; Aster, J.C.; McHugh, A.; et al. NOTCH1 mutations occur early during cutaneous squamous cell carcinogenesis. J. Investig. Dermatol. 2014, 134, 2630–2638. [Google Scholar] [CrossRef]
- Durinck, S.; Ho, C.; Wang, N.J.; Liao, W.; Jakkula, L.R.; Collisson, E.A.; Pons, J.; Chan, S.-W.; Lam, E.T.; Chu, C.; et al. Temporal dissection of tumorigenesis in primary cancers. Cancer Discov. 2011, 1, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Spyridonos, P.; Gaitanis, G.; Likas, A.; Bassukas, I.D. A convolutional neural network based system for detection of actinic keratosis in clinical images of cutaneous field cancerization. Biomed. Signal Process. Control 2023, 79, 104059. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-net: Convolutional networks for biomedical image segmentation. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2015, Proocedings of the 18th International Conference, Munich, Germany, 5–9 October 2015; Part of the Lecture Notes in Computer Science book series; Springer: Berlin/Heidelberg, Germany, 2015; Volume 9351, pp. 234–241. [Google Scholar] [CrossRef]
- Mirikharaji, Z.; Abhishek, K.; Bissoto, A.; Barata, C.; Avila, S.; Valle, E.; Celebi, M.E.; Hamarneh, G. A survey on deep learning for skin lesion segmentation. Med. Image Anal. 2023, 88, 102863. [Google Scholar] [CrossRef]
- Hasan, M.K.; Ahamad, M.A.; Yap, C.H.; Yang, G. A survey, review, and future trends of skin lesion segmentation and classification. Comput. Biol. Med. 2023, 155, 106624. [Google Scholar] [CrossRef]
- Aljabri, M.; AlGhamdi, M. A review on the use of deep learning for medical images segmentation. Neurocomputing 2022, 506, 311–335. [Google Scholar] [CrossRef]
- Siddique, N.; Paheding, S.; Elkin, C.P.; Devabhaktuni, V. U-net and its variants for medical image segmentation: A review of theory and applications. IEEE Access 2021, 9, 82031–82057. [Google Scholar] [CrossRef]
- Azad, R.; Aghdam, E.K.; Rauland, A.; Jia, Y.; Avval, A.H.; Bozorgpour, A.; Karimijafarbigloo, S.; Cohen, J.P.; Adeli, E.; Merhof, D. Medical Image Segmentation Review: The success of U-Net. arXiv 2022, arXiv:2211.14830. [Google Scholar] [CrossRef]
- Ghafoorian, M.; Mehrtash, A.; Kapur, T.; Karssemeijer, N.; Marchiori, E.; Pesteie, M.; Guttmann, C.R.G.; de Leeuw, F.-E.; Tempany, C.M.; van Ginneken, B.; et al. Transfer Learning for Domain Adaptation in MRI: Application in Brain Lesion Segmentation. In Medical Image Computing and Computer Assisted Intervention—MICCAI 2017, Proceedings of the 20th International Conference, Quabec City, QC, Canada, 11–13 September 2017; Springer: Berlin/Heidelberg, Germany, 2017; pp. 516–524. [Google Scholar]
- Feng, R.; Liu, X.; Chen, J.; Chen, D.Z.; Gao, H.; Wu, J. A Deep Learning Approach for Colonoscopy Pathology WSI Analysis: Accurate Segmentation and Classification. IEEE J. Biomed. Health Inform. 2021, 25, 3700–3708. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Jiang, L.; Zhang, J.; Wang, Q. Attention-VGG16-UNet: A novel deep learning approach for automatic segmentation of the median nerve in ultrasound images. Quant. Imaging Med. Surg. 2022, 12, 3138–3150. [Google Scholar] [CrossRef]
- Sharma, N.; Gupta, S.; Koundal, D.; Alyami, S.; Alshahrani, H.; Asiri, Y.; Shaikh, A. U-Net Model with Transfer Learning Model as a Backbone for Segmentation of Gastrointestinal Tract. Bioengineering 2023, 10, 119. [Google Scholar] [CrossRef]
- Simonyan, K.; Zisserman, A. Very Deep Convolutional Networks for Large-Scale Image Recognition. In Proceedings of the 3rd International Conference on Learning Representations, San Diego, CA, USA, 7–9 May 2015; pp. 1–14. [Google Scholar]
- Deng, J.; Dong, W.; Socher, R.; Li, L.-J.; Li, K.; Fei-Fei, L. ImageNet: A large-scale hierarchical image database. In Proceedings of the 2009 IEEE Conference on Computer Vision and Pattern Recognition, Miami, FL, USA, 20–25 June 2009; pp. 248–255. [Google Scholar] [CrossRef]
- Ioffe, S.; Szegedy, C. Batch Normalization: Accelerating Deep Network Training by Reducing Internal Covariate Shift. In Proceedings of the 32nd International Conference on International Conference on Machine Learning (ICML’15), Lille, France, 6–11 July 2015; Volume 1, pp. 448–456. Available online: https://arxiv.org/abs/1502.03167v3 (accessed on 10 July 2023).
- Yu, Y.; Si, X.; Hu, C.; Zhang, J. A review of recurrent neural networks: Lstm cells and network architectures. Neural Comput. 2019, 31, 1235–1270. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Z.; Wang, H.; Yeung, D.Y.; Wong, W.K.; Woo, W.C. Convolutional LSTM Network: A Machine Learning Approach for Precipitation Nowcasting. In Proceedings of the Advances in Neural Information Processing Systems 28 (NIPS 2015), Montreal, QC, Canada, 7–12 December 2015; Volume 2015, pp. 802–810. Available online: https://arxiv.org/abs/1506.04214v2 (accessed on 4 July 2023).
- Alom, M.Z.; Yakopcic, C.; Hasan, M.; Taha, T.M.; Asari, V.K. Recurrent residual U-Net for medical image segmentation. J. Med. Imaging 2019, 6, 1. [Google Scholar] [CrossRef]
- Arbelle, A.; Cohen, S.; Raviv, T.R. Dual-Task ConvLSTM-UNet for Instance Segmentation of Weakly Annotated Microscopy Videos. IEEE Trans. Med. Imaging 2022, 41, 1948–1960. [Google Scholar] [CrossRef]
- Attia, M.; Hossny, M.; Nahavandi, S.; Yazdabadi, A. Skin melanoma segmentation using recurrent and convolutional neural networks. In Proceedings of the 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017), Melbourne, VIC, Australia, 18–21 April 2017; pp. 292–296. [Google Scholar] [CrossRef]
- Azad, R.; Asadi-Aghbolaghi, M.; Fathy, M.; Escalera, S. Bi-Directional ConvLSTM U-Net with Densley Connected Convolutions. In Proceedings of the 2019 IEEE/CVF International Conference on Computer Vision Workshop (ICCVW), Seoul, Republic of Korea, 27–28 October 2019; pp. 406–415. [Google Scholar] [CrossRef]
- Jiang, X.; Jiang, J.; Wang, B.; Yu, J.; Wang, J. SEACU-Net: Attentive ConvLSTM U-Net with squeeze-and-excitation layer for skin lesion segmentation. Comput. Methods Programs Biomed. 2022, 225, 107076. [Google Scholar] [CrossRef]
- Zhou, Z.; Siddiquee, M.M.R.; Tajbakhsh, N.; Liang, J. UNet++: A Nested U-Net Architecture for Medical Image Segmentation. In Deep Learning in Medical Image Analysis and Multimodal Learning for Clinical Decision Support, Proceedings of the 4th International Workshop, DLMIA 2018 and 8th International Workshop, ML-CDS 2018 Held in Conjunction with MICCAI 2018, Granada, Spain, 20 September 2018; Springer: Berlin/Heidelberg, Germany, 2018; Volume 11045, pp. 3–11. [Google Scholar] [CrossRef]
- Olsen, E.A.; Abernethy, M.L.; Kulp-Shorten, C.; Callen, J.P.; Glazer, S.D.; Huntley, A.; McCray, M.; Monroe, A.B.; Tschen, E.; Wolf, J.E. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J. Am. Acad. Dermatol. 1991, 24 Pt 1, 738–743. [Google Scholar] [CrossRef]






| Patients | Images | Crops | Augmentation | |
|---|---|---|---|---|
| Train | 93 | 410 | 13,190 | Yes |
| Validation | 5 | 100 | 3298 | Yes |
| Test | 17 | 59 | 403 | None |
| Total | 115 | 569 | 16,891 |
| Architecture | Dice (Mean) | IoU (Mean) |
|---|---|---|
| U-Net | 0.14 | 0.48 |
| U-Net++ | 0.39 | 0.55 |
| AKU-Net | 0.50 | 0.63 |
| AKCNN | AKU-Net | |||||
|---|---|---|---|---|---|---|
| Frame | ||||||
| 1 | 0.96 | 0.67 | 0.79 | 0.81 | 0.67 | 0.73 |
| 2 | 0.77 | 0.6 | 0.67 | 0.56 | 0.50 | 0.53 |
| 3 | 0.77 | 0.56 | 0.65 | 0.94 | 0.56 | 0.70 |
| 4 | 0.5 | 1 | 0.67 | 0.88 | 0.80 | 0.84 |
| 5 | 1 | 0.25 | 0.4 | 1.00 | 0.50 | 0.67 |
| 6 | 0.26 | 1 | 0.41 | 0.69 | 1.00 | 0.81 |
| 7 | 0.7 | 0.67 | 0.68 | 0.33 | 0.67 | 0.44 |
| 8 | 0.86 | 1 | 0.93 | 0.73 | 1.00 | 0.84 |
| 9 | 0.99 | 0.27 | 0.43 | 0.74 | 0.45 | 0.56 |
| 10 | 0.96 | 0.6 | 0.74 | 0.60 | 0.60 | 0.60 |
| Median | 0.82 | 0.64 | 0.67 | 0.73 | 0.63 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derekas, P.; Spyridonos, P.; Likas, A.; Zampeta, A.; Gaitanis, G.; Bassukas, I. The Promise of Semantic Segmentation in Detecting Actinic Keratosis Using Clinical Photography in the Wild. Cancers 2023, 15, 4861. https://doi.org/10.3390/cancers15194861
Derekas P, Spyridonos P, Likas A, Zampeta A, Gaitanis G, Bassukas I. The Promise of Semantic Segmentation in Detecting Actinic Keratosis Using Clinical Photography in the Wild. Cancers. 2023; 15(19):4861. https://doi.org/10.3390/cancers15194861
Chicago/Turabian StyleDerekas, Panagiotis, Panagiota Spyridonos, Aristidis Likas, Athanasia Zampeta, Georgios Gaitanis, and Ioannis Bassukas. 2023. "The Promise of Semantic Segmentation in Detecting Actinic Keratosis Using Clinical Photography in the Wild" Cancers 15, no. 19: 4861. https://doi.org/10.3390/cancers15194861
APA StyleDerekas, P., Spyridonos, P., Likas, A., Zampeta, A., Gaitanis, G., & Bassukas, I. (2023). The Promise of Semantic Segmentation in Detecting Actinic Keratosis Using Clinical Photography in the Wild. Cancers, 15(19), 4861. https://doi.org/10.3390/cancers15194861







