Synovial Sarcoma in the Extremity: Diversity of Imaging Features for Diagnosis and Prognosis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Diagnostic Imaging Features of the Extremity Synovial Sarcoma
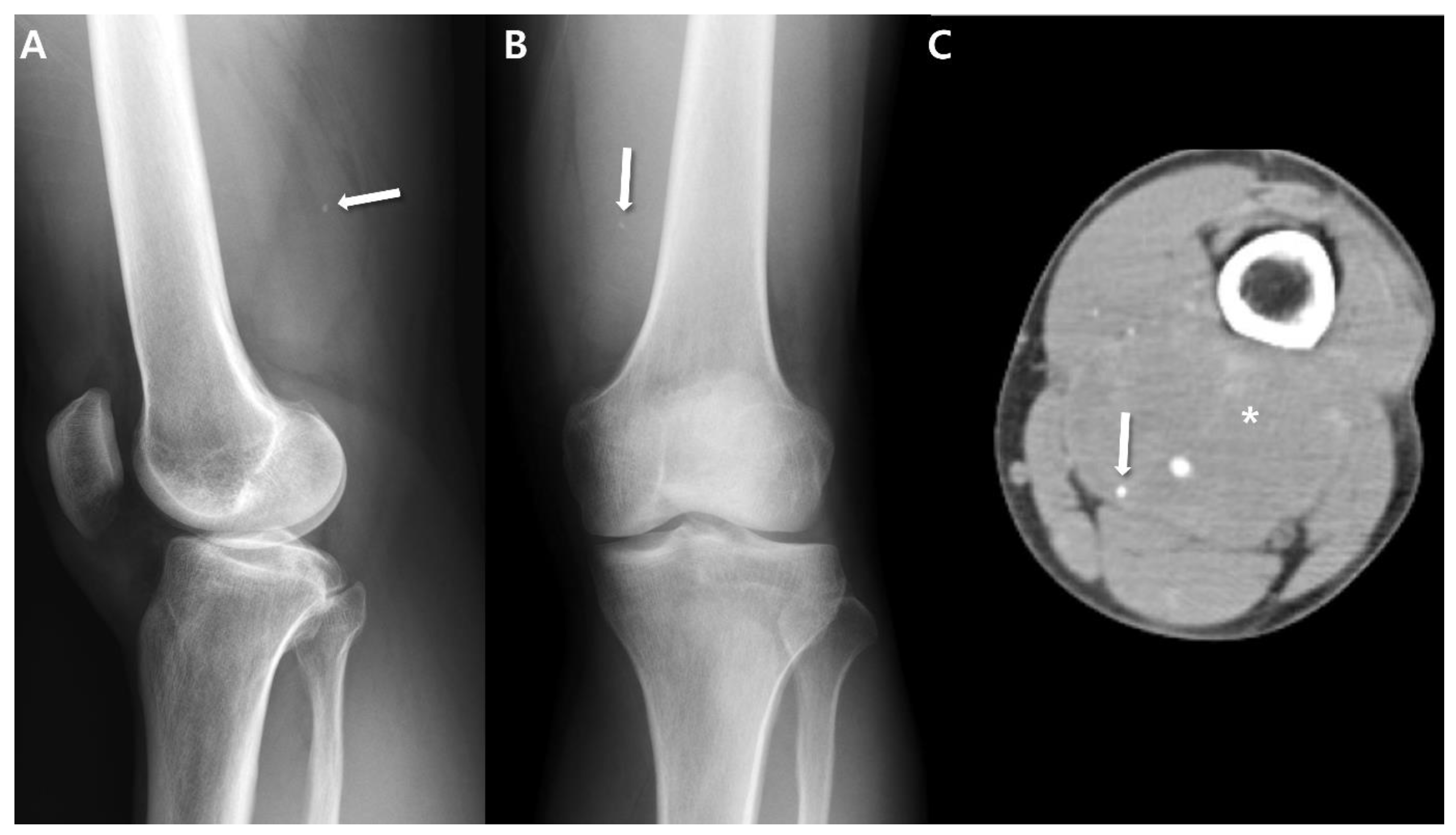
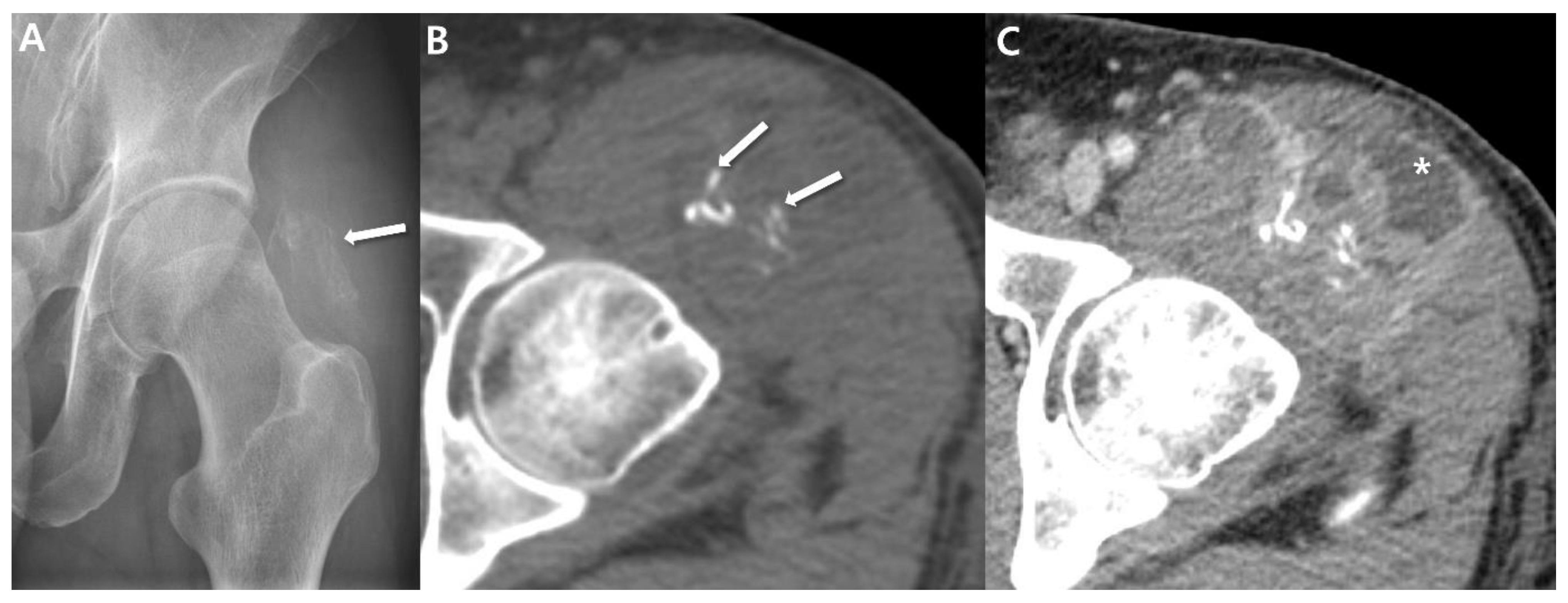
2.1. Radiographs
2.2. Computed Tomography (CT)
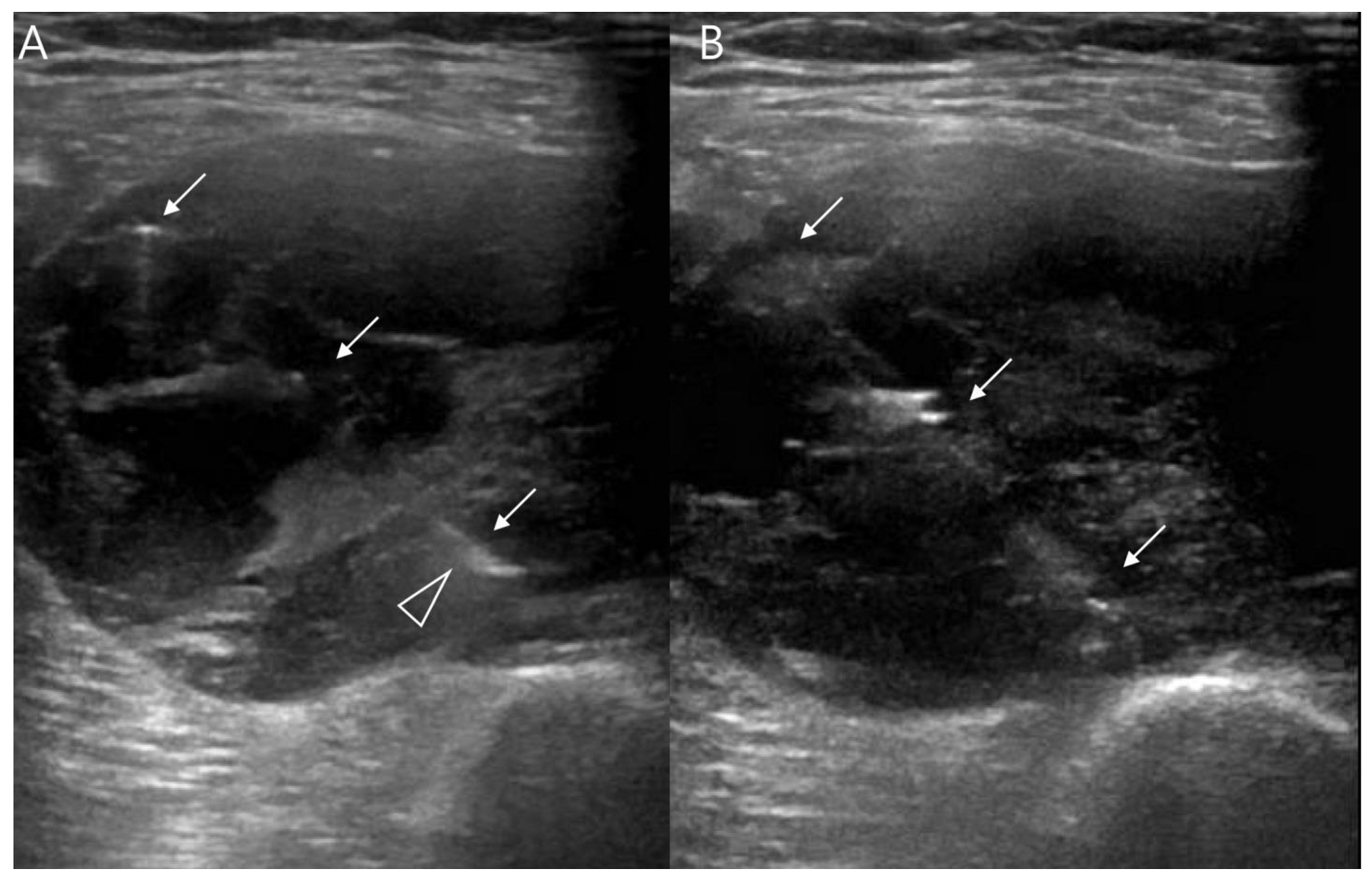
2.3. Ultrasound
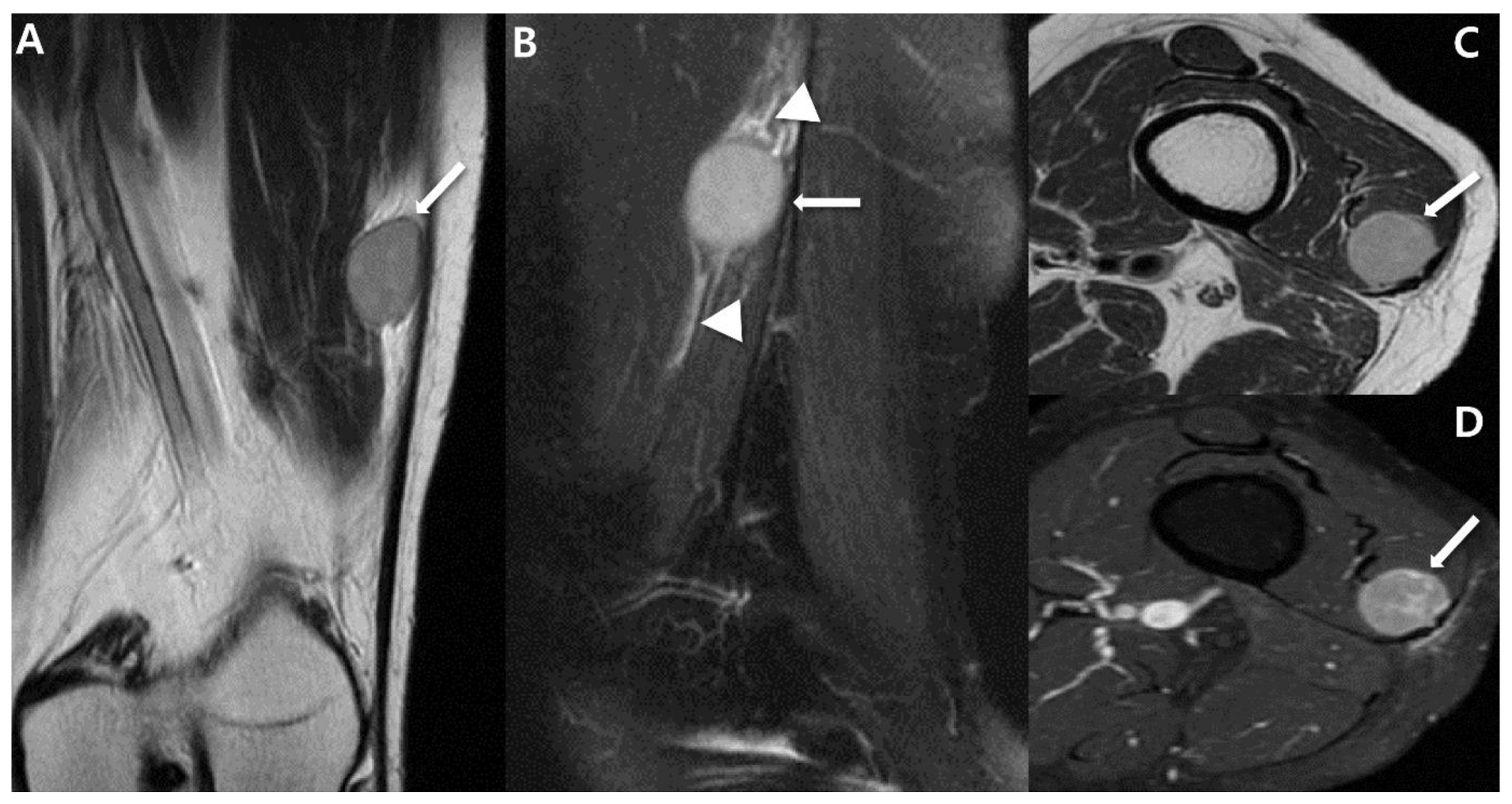
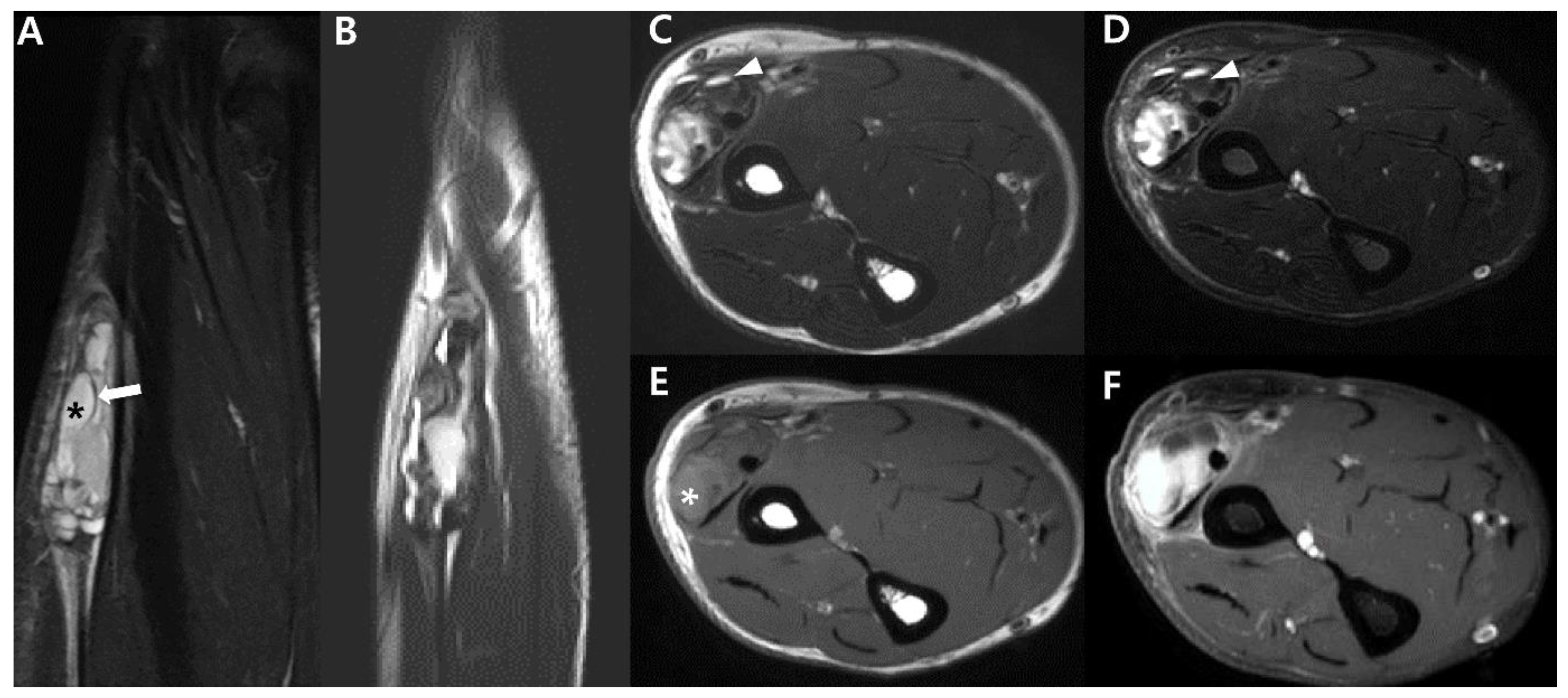
2.4. Magnetic Resonance Imaging (MRI)
2.5. Advanced MRI
3. Uncommon Primary Sites of Synovial Sarcoma in the Extremities
4. Prognostic Imaging Features of Synovial Sarcoma in the Extremity
4.1. Metastatic Pattern
4.2. Prognostic Imaging Features on CT and MRI
5. Treatments and Management
5.1. Surgical Treatment
5.2. Radiation Therapy
5.3. Chemotherapy
5.4. Novel Therapeutic Options
5.5. Role of Radiologist in Treatment and Management
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sultan, I.; Rodriguez-Galindo, C.; Saab, R.; Yasir, S.; Casanova, M.; Ferrari, A. Comparing children and adults with synovial sarcoma in the Surveillance, Epidemiology, and End Results program, 1983 to 2005: An analysis of 1268 patients. Cancer 2009, 115, 3537–3547. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, G.; Coindre, J.M.; Ducimetière, F.; Dei Tos, A.P.; Fadda, E.; Blay, J.Y.; Buja, A.; Fedeli, U.; Cegolon, L.; Frasson, A.; et al. Incidence of soft tissue sarcoma and beyond: A population-based prospective study in 3 European regions. Cancer 2012, 118, 5339–5348. [Google Scholar] [CrossRef]
- Choi, J.H.; Ro, J.Y. The 2020 WHO Classification of Tumors of Soft Tissue: Selected Changes and New Entities. Adv. Anat. Pathol. 2021, 28, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Scalas, G.; Parmeggiani, A.; Martella, C.; Tuzzato, G.; Bianchi, G.; Facchini, G.; Clinca, R.; Spinnato, P. Magnetic resonance imaging of soft tissue sarcoma: Features related to prognosis. Eur. J. Orthop. Surg. Traumatol. Orthop. Traumatol. 2021, 31, 1567–1575. [Google Scholar] [CrossRef]
- Farkas, A.B.; Baghdadi, S.; Arkader, A.; Nguyen, M.K.; Venkatesh, T.P.; Srinivasan, A.S.; Nguyen, J.C. Magnetic resonance imaging findings of synovial sarcoma in children: Location-dependent differences. Pediatr. Radiol. 2021, 51, 2539–2548. [Google Scholar] [CrossRef]
- Aytekin, M.N.; Öztürk, R.; Amer, K.; Yapar, A. Epidemiology, incidence, and survival of synovial sarcoma subtypes: SEER database analysis. J. Orthop. Surg. 2020, 28, 2309499020936009. [Google Scholar] [CrossRef] [PubMed]
- Kransdorf, M.J. Malignant soft-tissue tumors in a large referral population: Distribution of diagnoses by age, sex, and location. AJR Am. J. Roentgenol. 1995, 164, 129–134. [Google Scholar] [CrossRef]
- Ferrari, A.; Sultan, I.; Huang, T.T.; Rodriguez-Galindo, C.; Shehadeh, A.; Meazza, C.; Ness, K.K.; Casanova, M.; Spunt, S.L. Soft tissue sarcoma across the age spectrum: A population-based study from the Surveillance Epidemiology and End Results database. Pediatr. Blood Cancer 2011, 57, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Murphey, M.D.; Gibson, M.S.; Jennings, B.T.; Crespo-Rodríguez, A.M.; Fanburg-Smith, J.; Gajewski, D.A. From the archives of the AFIP: Imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics 2006, 26, 1543–1565. [Google Scholar] [CrossRef]
- Baheti, A.D.; Tirumani, S.H.; Sewatkar, R.; Shinagare, A.B.; Hornick, J.L.; Ramaiya, N.H.; Jagannathan, J.P. Imaging features of primary and metastatic extremity synovial sarcoma: A single institute experience of 78 patients. Br. J. Radiol. 2015, 88, 20140608. [Google Scholar] [CrossRef]
- Al-Daraji, W.; Lasota, J.; Foss, R.; Miettinen, M. Synovial sarcoma involving the head: Analysis of 36 cases with predilection to the parotid and temporal regions. Am. J. Surg. Pathol. 2009, 33, 1494–1503. [Google Scholar] [CrossRef]
- Hu, P.A.; Zhou, Z.R. Clinical, pathological and unusual MRI features of five synovial sarcomas in head and neck. Br. J. Radiol. 2015, 88, 20140843. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Amini, B.; Wagner, M.J.; Nowell, E.N.; Lazar, A.J.; Lin, P.P.; Benjamin, R.S.; Araujo, D.M. Synovial Sarcoma of the Head and Neck: A Single Institution Review. Sarcoma 2017, 2017, 2016752. [Google Scholar] [CrossRef]
- Baheti, A.D.; Sewatkar, R.; Hornick, J.L.; Saboo, S.S.; Jagannathan, J.P.; Ramaiya, N.H.; Tirumani, S.H. Imaging features of primary and recurrent intrathoracic synovial sarcoma: A single-institute experience. Clin. Imaging 2015, 39, 803–808. [Google Scholar] [CrossRef]
- Duran-Mendicuti, A.; Costello, P.; Vargas, S.O. Primary synovial sarcoma of the chest: Radiographic and clinicopathologic correlation. J. Thorac. Imaging 2003, 18, 87–93. [Google Scholar] [CrossRef]
- Skytting, B. Synovial sarcoma. A Scandinavian Sarcoma Group project. Acta Orthop. Scand. Suppl. 2000, 291, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.J.; Alwafi, L.; Pritchett, S.L.; Wehrli, B.M.; Spouge, A.R.I. The Imaging Spectrum of Synovial Sarcomas: A Pictorial Review from a Single-Centre Tertiary Referral Institution. Can. Assoc. Radiol. J. 2021, 72, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Trassard, M.; Le Doussal, V.; Hacène, K.; Terrier, P.; Ranchère, D.; Guillou, L.; Fiche, M.; Collin, F.; Vilain, M.O.; Bertrand, G.; et al. Prognostic factors in localized primary synovial sarcoma: A multicenter study of 128 adult patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2001, 19, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Bakri, A.; Shinagare, A.B.; Krajewski, K.M.; Howard, S.A.; Jagannathan, J.P.; Hornick, J.L.; Ramaiya, N.H. Synovial sarcoma: Imaging features of common and uncommon primary sites, metastatic patterns, and treatment response. AJR Am. J. Roentgenol. 2012, 199, W208–W215. [Google Scholar] [CrossRef]
- Smolle, M.A.; Parry, M.; Jeys, L.; Abudu, S.; Grimer, R. Synovial sarcoma: Do children do better? Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2019, 45, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Vlenterie, M.; Ho, V.K.; Kaal, S.E.; Vlenterie, R.; Haas, R.; van der Graaf, W.T. Age as an independent prognostic factor for survival of localised synovial sarcoma patients. Br. J. Cancer 2015, 113, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, S.; Van Tine, B.A. Synovial Sarcoma: Current Concepts and Future Perspectives. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 180–187. [Google Scholar] [CrossRef]
- Naka, N.; Takenaka, S.; Araki, N.; Miwa, T.; Hashimoto, N.; Yoshioka, K.; Joyama, S.; Hamada, K.; Tsukamoto, Y.; Tomita, Y.; et al. Synovial sarcoma is a stem cell malignancy. Stem Cells 2010, 28, 1119–1131. [Google Scholar] [CrossRef]
- Liang, C.; Mao, H.; Tan, J.; Ji, Y.; Sun, F.; Dou, W.; Wang, H.; Wang, H.; Gao, J. Synovial sarcoma: Magnetic resonance and computed tomography imaging features and differential diagnostic considerations. Oncol. Lett. 2015, 9, 661–666. [Google Scholar] [CrossRef]
- Thway, K.; Fisher, C. Synovial sarcoma: Defining features and diagnostic evolution. Ann. Diagn. Pathol. 2014, 18, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Weissberg, Z.; Sebro, R. Distinguishing synovial sarcoma from benign and malignant mimics: MR Imaging indicators. Appl. Radiol. 2018, 47, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Andrassy, R.J.; Okcu, M.F.; Despa, S.; Raney, R.B. Synovial sarcoma in children: Surgical lessons from a single institution and review of the literature. J. Am. Coll. Surg. 2001, 192, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Bixby, S.D.; Hettmer, S.; Taylor, G.A.; Voss, S.D. Synovial sarcoma in children: Imaging features and common benign mimics. AJR Am. J. Roentgenol. 2010, 195, 1026–1032. [Google Scholar] [CrossRef]
- Crombé, A.; Kind, M.; Fadli, D.; Miceli, M.; Linck, P.A.; Bianchi, G.; Sambri, A.; Spinnato, P. Soft-tissue sarcoma in adults: Imaging appearances, pitfalls and diagnostic algorithms. Diagn. Interv. Imaging 2023, 104, 207–220. [Google Scholar] [CrossRef]
- Wilkerson, B.W.; Crim, J.R.; Hung, M.; Layfield, L.J. Characterization of synovial sarcoma calcification. AJR Am. J. Roentgenol. 2012, 199, W730–W734. [Google Scholar] [CrossRef]
- Tateishi, U.; Hasegawa, T.; Beppu, Y.; Satake, M.; Moriyama, N. Synovial sarcoma of the soft tissues: Prognostic significance of imaging features. J. Comput. Assist. Tomogr. 2004, 28, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Araki, N.; Sawai, Y.; Kudawara, I.; Mano, M.; Ishiguro, S.; Ueda, T.; Yoshikawa, H. Cystic synovial sarcomas: Imaging features with clinical and histopathologic correlation. Skelet. Radiol. 2003, 32, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Marzano, L.; Failoni, S.; Gallazzi, M.; Garbagna, P. The role of diagnostic imaging in synovial sarcoma. Our experience. Radiol. Med. 2004, 107, 533–540. [Google Scholar]
- Zhao, F.; Ahlawat, S.; Farahani, S.J.; Weber, K.L.; Montgomery, E.A.; Carrino, J.A.; Fayad, L.M. Can MR imaging be used to predict tumor grade in soft-tissue sarcoma? Radiology 2014, 272, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Crombé, A.; Marcellin, P.J.; Buy, X.; Stoeckle, E.; Brouste, V.; Italiano, A.; Le Loarer, F.; Kind, M. Soft-Tissue Sarcomas: Assessment of MRI Features Correlating with Histologic Grade and Patient Outcome. Radiology 2019, 291, 710–721. [Google Scholar] [CrossRef]
- Bates, T.; Kao, E.; Alderete, J.; Lybeck, D. Synovial Sarcoma: A Series of Small Tumors in Active Duty Service Members. Mil. Med. 2020, 185, e1864–e1868. [Google Scholar] [CrossRef] [PubMed]
- Blacksin, M.F.; Siegel, J.R.; Benevenia, J.; Aisner, S.C. Synovial sarcoma: Frequency of nonaggressive MR characteristics. J. Comput. Assist. Tomogr. 1997, 21, 785–789. [Google Scholar] [CrossRef]
- O’Sullivan, P.J.; Harris, A.C.; Munk, P.L. Radiological features of synovial cell sarcoma. Br. J. Radiol. 2008, 81, 346–356. [Google Scholar] [CrossRef]
- Jones, B.C.; Sundaram, M.; Kransdorf, M.J. Synovial sarcoma: MR imaging findings in 34 patients. AJR. Am. J. Roentgenol. 1993, 161, 827–830. [Google Scholar] [CrossRef]
- Sedaghat, M.; Sedaghat, S. Primary synovial sarcoma on MRI—A case series and review of the literature. Pol. J. Radiol. 2023, 88, e325–e330. [Google Scholar] [CrossRef]
- Morton, M.J.; Berquist, T.H.; McLeod, R.A.; Unni, K.K.; Sim, F.H. MR imaging of synovial sarcoma. AJR Am. J. Roentgenol. 1991, 156, 337–340. [Google Scholar] [CrossRef]
- Sigal, R.; Chancelier, M.D.; Luboinski, B.; Shapeero, L.G.; Bosq, J.; Vanel, D. Synovial sarcomas of the head and neck: CT and MR findings. AJNR Am. J. Neuroradiol. 1992, 13, 1459–1462. [Google Scholar] [PubMed]
- Ashikyan, O.; Bradshaw, S.B.; Dettori, N.J.; Hwang, H.; Chhabra, A. Conventional and advanced MR imaging insights of synovial sarcoma. Clin. Imaging 2021, 76, 149–155. [Google Scholar] [CrossRef]
- van Rijswijk, C.S.; Geirnaerdt, M.J.; Hogendoorn, P.C.; Taminiau, A.H.; van Coevorden, F.; Zwinderman, A.H.; Pope, T.L.; Bloem, J.L. Soft-tissue tumors: Value of static and dynamic gadopentetate dimeglumine-enhanced MR imaging in prediction of malignancy. Radiology 2004, 233, 493–502. [Google Scholar] [CrossRef]
- Lee, S.K.; Jee, W.H.; Jung, C.K.; Chung, Y.G. Multiparametric quantitative analysis of tumor perfusion and diffusion with 3T MRI: Differentiation between benign and malignant soft tissue tumors. Br. J. Radiol. 2020, 93, 20191035. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Lee, S.K.; Kim, J.Y. Comparison of conventional magnetic resonance imaging and diffusion-weighted imaging in the differentiation of bone plasmacytoma from bone metastasis in the extremities. Diagn. Interv. Imaging 2021, 102, 611–618. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.K.; Kim, J.Y.; Kim, J.H. Pitfalls of Diffusion-Weighted Imaging: Clinical Utility of T2 Shine-through and T2 Black-out for Musculoskeletal Diseases. Diagnostics 2023, 13, 1647. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, D.; Kim, H.S.; Na, K.; Lee, J.Y.; Nam, E.J.; Kim, S.W.; Kim, S.; Kim, Y.T. Integrating a Next Generation Sequencing Panel into Clinical Practice in Ovarian Cancer. Yonsei Med. J. 2019, 60, 914–923. [Google Scholar] [CrossRef]
- Chhabra, A.; Ashikyan, O.; Slepicka, C.; Dettori, N.; Hwang, H.; Callan, A.; Sharma, R.R.; Xi, Y. Conventional MR and diffusion-weighted imaging of musculoskeletal soft tissue malignancy: Correlation with histologic grading. Eur. Radiol. 2019, 29, 4485–4494. [Google Scholar] [CrossRef]
- Hong, J.H.; Jee, W.H.; Jung, C.K.; Jung, J.Y.; Shin, S.H.; Chung, Y.G. Soft tissue sarcoma: Adding diffusion-weighted imaging improves MR imaging evaluation of tumor margin infiltration. Eur. Radiol. 2019, 29, 2589–2597. [Google Scholar] [CrossRef]
- Del Grande, F.; Subhawong, T.; Weber, K.; Aro, M.; Mugera, C.; Fayad, L.M. Detection of soft-tissue sarcoma recurrence: Added value of functional MR imaging techniques at 3.0 T. Radiology 2014, 271, 499–511. [Google Scholar] [CrossRef]
- Yoon, M.A.; Chee, C.G.; Chung, H.W.; Song, J.S.; Lee, J.S.; Kim, W.; Lee, M.H.; Lee, S.H.; Shin, M.J. Added value of diffusion-weighted imaging to conventional MRI for predicting fascial involvement of soft tissue sarcomas. Eur. Radiol. 2019, 29, 1863–1873. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, Y.C.; Seo, S.W.; Choi, Y.L.; Kim, H.S. Soft tissue sarcoma: DWI and DCE-MRI parameters correlate with Ki-67 labeling index. Eur. Radiol. 2020, 30, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Dai, Y.; Liu, Y.; Tao, J.; Pan, Z.; Xie, L.; Wang, S. Soft tissue sarcoma: IVIM and DKI parameters correlate with Ki-67 labeling index on direct comparison of MRI and histopathological slices. Eur. Radiol. 2022, 32, 5659–5668. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Jee, W.H.; Jung, C.K.; Im, S.A.; Chung, N.G.; Chung, Y.G. Prediction of Poor Responders to Neoadjuvant Chemotherapy in Patients with Osteosarcoma: Additive Value of Diffusion-Weighted MRI including Volumetric Analysis to Standard MRI at 3T. PLoS ONE 2020, 15, e0229983. [Google Scholar] [CrossRef] [PubMed]
- Bui-Mansfield, L.T.; O’Brien, S.D. Magnetic resonance appearance of intra-articular synovial sarcoma: Case reports and review of the literature. J. Comput. Assist. Tomogr. 2008, 32, 640–644. [Google Scholar] [CrossRef]
- Ishida, T.; Iijima, T.; Moriyama, S.; Nakamura, C.; Kitagawa, T.; Machinami, R. Intra-articular calcifying synovial sarcoma mimicking synovial chondromatosis. Skelet. Radiol. 1996, 25, 766–769. [Google Scholar] [CrossRef]
- Ayoub, K.S.; Davies, A.M.; Mangham, D.C.; Grimer, R.J.; Twiston Davies, C.W. Synovial sarcoma arising in association with a popliteal cyst. Skelet. Radiol. 2000, 29, 713–716. [Google Scholar] [CrossRef]
- Cao, Q.; Shillingford, N.; Huh, W.; VandenBerg, C.; Raca, G.; Allison, D.C.; Wang, L.; Zhou, S. Primary Knee Intra-articular Synovial Sarcoma in Pediatric and Adolescent Patients. Pediatr. Dev. Pathol. Off. J. Soc. Pediatr. Pathol. Paediatr. Pathol. Soc. 2021, 24, 159–163. [Google Scholar] [CrossRef]
- Van Slyke, M.A.; Moser, R.P., Jr.; Madewell, J.E. MR imaging of periarticular soft-tissue lesions. Magn. Reson. Imaging Clin. N. Am. 1995, 3, 651–667. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, J.Y.; Lee, Y.S.; Jeong, H.S. Intramuscular peripheral nerve sheath tumors: Schwannoma, ancient schwannoma, and neurofibroma. Skelet. Radiol. 2020, 49, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, J.Y.; Jeong, H.S. Benign peripheral nerve sheath tumor of digit versus major-nerve: Comparison of MRI findings. PLoS ONE 2020, 15, e0230816. [Google Scholar] [CrossRef]
- Kim, D.H.; Murovic, J.A.; Tiel, R.L.; Moes, G.; Kline, D.G. A series of 146 peripheral non-neural sheath nerve tumors: 30-year experience at Louisiana State University Health Sciences Center. J. Neurosurg. 2005, 102, 256–266. [Google Scholar] [CrossRef]
- Larque, A.B.; Bredella, M.A.; Nielsen, G.P.; Chebib, I. Synovial sarcoma mimicking benign peripheral nerve sheath tumor. Skelet. Radiol. 2017, 46, 1463–1468. [Google Scholar] [CrossRef]
- Scheithauer, B.W.; Amrami, K.K.; Folpe, A.L.; Silva, A.I.; Edgar, M.A.; Woodruff, J.M.; Levi, A.D.; Spinner, R.J. Synovial sarcoma of nerve. Hum. Pathol. 2011, 42, 568–577. [Google Scholar] [CrossRef]
- Hashimoto, K.; Nishimura, S.; Fujii, K.; Kakinoki, R.; Akagi, M. Intraneural synovial sarcoma of the tibial nerve. Rare Tumors 2018, 10, 2036361318776495. [Google Scholar] [CrossRef] [PubMed]
- Shahid, K.R.; Amrami, K.K.; Spinner, R.J. Primary monophasic synovial sarcoma presenting as a benign neurogenic tumor: Case review and review of the literature. J. Surg. Orthop. Adv. 2010, 19, 129–133. [Google Scholar] [PubMed]
- Kang, S.; Yoo, H.J.; Kim, H.S.; Han, I. Soft tissue sarcoma misdiagnosed as benign peripheral neurogenic tumor. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2015, 20, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.H.; Hefti, F.; Speth, B.M.; Jundt, G.; Guillou, L.; Exner, U.G.; von Hochstetter, A.R.; Cserhati, M.D.; Fuchs, B.; Mouhsine, E.; et al. Synovial sarcomas usually metastasize after >5 years: A multicenter retrospective analysis with minimum follow-up of 10 years for survivors. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22, 458–467. [Google Scholar] [CrossRef]
- Moreau-Bachelard, C.; Campion, L.; Toulmonde, M.; Le Cesne, A.; Brahmi, M.; Italiano, A.; Mir, O.; Piperno-Neumann, S.; Laurence, V.; Firmin, N.; et al. Patterns of care and outcomes of 417 patients with METAstatic SYNovial sarcoma (METASYN): Real-life data from the French Sarcoma Group (FSG). ESMO Open 2022, 7, 100402. [Google Scholar] [CrossRef]
- Farkas, A.; Lirette, S.T.; Al Hmada, Y.; Collier, A.B.; Barr, J.; Vijayakumar, S.; Vijayakumar, V. Single-Institution Experience of Synovial Sarcoma. South. Med. J. 2020, 113, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Venkatramani, R.; Xue, W.; Randall, R.L.; Wolden, S.; Anderson, J.; Lopez-Terrada, D.; Black, J.; Kao, S.C.; Shulkin, B.; Ostrenga, A.; et al. Synovial Sarcoma in Children, Adolescents, and Young Adults: A Report from the Children’s Oncology Group ARST0332 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 3927–3937. [Google Scholar] [CrossRef]
- El Beaino, M.; Rassy, E.; Hadid, B.; Araujo, D.M.; Pavlidis, N.; Lin, P.P. Synovial Sarcoma: A Complex Disease with Multifaceted Signaling and Epigenetic Landscapes. Curr. Oncol. Rep. 2020, 22, 124. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Sambri, A.; Spinnato, P.; Zucchini, R.; Giannini, C.; Caldari, E.; Pirini, M.G.; De Paolis, M. The Biology of Synovial Sarcoma: State-of-the-Art and Future Perspectives. Curr. Treat. Options Oncol. 2021, 22, 109. [Google Scholar] [CrossRef]
- Gazendam, A.M.; Popovic, S.; Munir, S.; Parasu, N.; Wilson, D.; Ghert, M. Synovial Sarcoma: A Clinical Review. Curr. Oncol. 2021, 28, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Hashimoto, H.; Tsuneyoshi, M.; Takeshita, S. Survival in synovial sarcoma. A multivariate study of prognostic factors with special emphasis on the comparison between early death and long-term survival. Am. J. Surg. Pathol. 1993, 17, 35–44. [Google Scholar] [CrossRef]
- Deshmukh, R.; Mankin, H.J.; Singer, S. Synovial sarcoma: The importance of size and location for survival. Clin. Orthop. Relat. Res. 2004, 419, 155–161. [Google Scholar] [CrossRef]
- Crombé, A.; Matcuk, G.R.; Fadli, D.; Sambri, A.; Patel, D.B.; Paioli, A.; Kind, M.; Spinnato, P. Role of Imaging in Initial Prognostication of Locally Advanced Soft Tissue Sarcomas. Acad. Radiol. 2023, 30, 322–340. [Google Scholar] [CrossRef]
- Sánchez Reyes, J.M.; Alcaraz Mexia, M.; Quiñones Tapia, D.; Aramburu, J.A. Extensively calcified synovial sarcoma. Skelet. Radiol. 1997, 26, 671–673. [Google Scholar] [CrossRef]
- Varela-Duran, J.; Enzinger, F.M. Calcifying synovial sarcoma. Cancer 1982, 50, 345–352. [Google Scholar] [CrossRef]
- Tordjman, M.; Honoré, C.; Crombé, A.; Bouhamama, A.; Feydy, A.; Dercle, L.; Haddag, L.; Bouché, P.A.; Ngo, C.; Le Cesne, A.; et al. Prognostic factors of the synovial sarcoma of the extremities: Imaging does matter. Eur. Radiol. 2023, 33, 1162–1173. [Google Scholar] [CrossRef]
- Faur, C.; Pop, D.; Abu-Awwad, A.; Carmen, Z.; Folescu, R.; Gurgus, D.; Motoc, A.; Patrascu, J.; Motoi, S.; Belei, O.; et al. Synovial Sarcoma of the Extremities: A Literature Review. Appl. Sci. 2021, 11, 7407. [Google Scholar] [CrossRef]
- von Mehren, M.; Randall, R.L.; Benjamin, R.S.; Boles, S.; Bui, M.M.; Conrad, E.U., 3rd; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; et al. Soft Tissue Sarcoma, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2016, 14, 758–786. [Google Scholar] [CrossRef]
- Gingrich, A.A.; Marrufo, A.S.; Liu, Y.; Li, C.S.; Darrow, M.A.; Monjazeb, A.M.; Thorpe, S.W.; Canter, R.J. Radiotherapy is Associated with Improved Survival in Patients with Synovial Sarcoma Undergoing Surgery: A National Cancer Database Analysis. J. Surg. Res. 2020, 255, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Park, J.; Kim, H.J.; Kim, I.H.; Han, I.; Kim, H.S.; Kim, S. Effects of Adjuvant Radiotherapy in Patients with Synovial Sarcoma. Am. J. Clin. Oncol. 2017, 40, 306–311. [Google Scholar] [CrossRef]
- Ramu, E.M.; Houdek, M.T.; Isaac, C.E.; Dickie, C.I.; Ferguson, P.C.; Wunder, J.S. Management of soft-tissue sarcomas; treatment strategies, staging, and outcomes. SICOT-J 2017, 3, 20. [Google Scholar] [CrossRef]
- Nakamura, T.; Saito, Y.; Tsuchiya, K.; Miyachi, M.; Iwata, S.; Sudo, A.; Kawai, A. Is perioperative chemotherapy recommended in childhood and adolescent patients with synovial sarcoma? A systematic review. Jpn. J. Clin. Oncol. 2021, 51, 927–931. [Google Scholar] [CrossRef]
- Vlenterie, M.; Litière, S.; Rizzo, E.; Marréaud, S.; Judson, I.; Gelderblom, H.; Le Cesne, A.; Wardelmann, E.; Messiou, C.; Gronchi, A.; et al. Outcome of chemotherapy in advanced synovial sarcoma patients: Review of 15 clinical trials from the European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group; setting a new landmark for studies in this entity. Eur. J. Cancer 2016, 58, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Desar, I.M.E.; Fleuren, E.D.G.; van der Graaf, W.T.A. Systemic Treatment for Adults with Synovial Sarcoma. Curr. Treat. Options Oncol. 2018, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.F.; Morgan, R.A.; Feldman, S.A.; Yang, J.C.; Sherry, R.M.; Dudley, M.E.; Wunderlich, J.R.; Nahvi, A.V.; Helman, L.J.; Mackall, C.L.; et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 2011, 29, 917–924. [Google Scholar] [CrossRef]
- Kasraeian, S.; Allison, D.C.; Ahlmann, E.R.; Fedenko, A.N.; Menendez, L.R. A comparison of fine-needle aspiration, core biopsy, and surgical biopsy in the diagnosis of extremity soft tissue masses. Clin. Orthop. Relat. Res. 2010, 468, 2992–3002. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Damron, T.A. Comparison of needle core biopsy and fine-needle aspiration for diagnostic accuracy in musculoskeletal lesions. Arch. Pathol. Lab. Med. 2004, 128, 759–764. [Google Scholar] [CrossRef]
- Narvani, A.A.; Tsiridis, E.; Saifuddin, A.; Briggs, T.; Cannon, S. Does image guidance improve accuracy of core needle biopsy in diagnosis of soft tissue tumours? Acta Orthop. Belg. 2009, 75, 239–244. [Google Scholar] [PubMed]
- Pang, K.; Guo, X.; Liu, T.; Wang, L.; Chen, R.; Zhang, Z.; Li, L.; He, Y.; Zhang, H.; Fan, S.; et al. The Role of a Multidisciplinary Team in the Diagnosis and Treatment of Bone and Soft Tissue Sarcomas: A Single-Center Experience. J. Pers. Med. 2022, 12, 2079. [Google Scholar] [CrossRef] [PubMed]


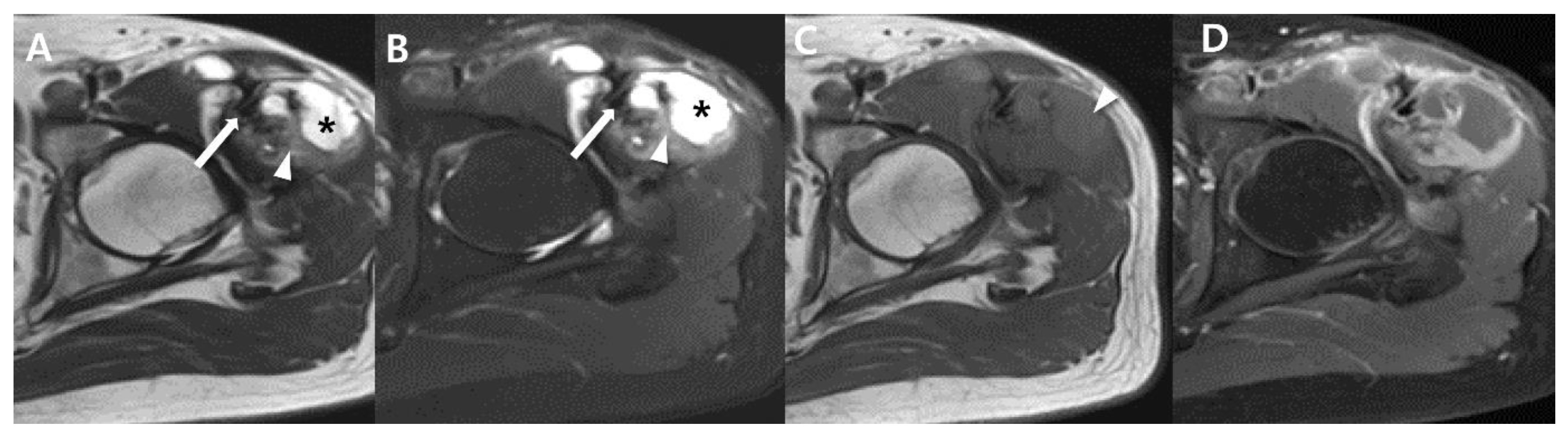
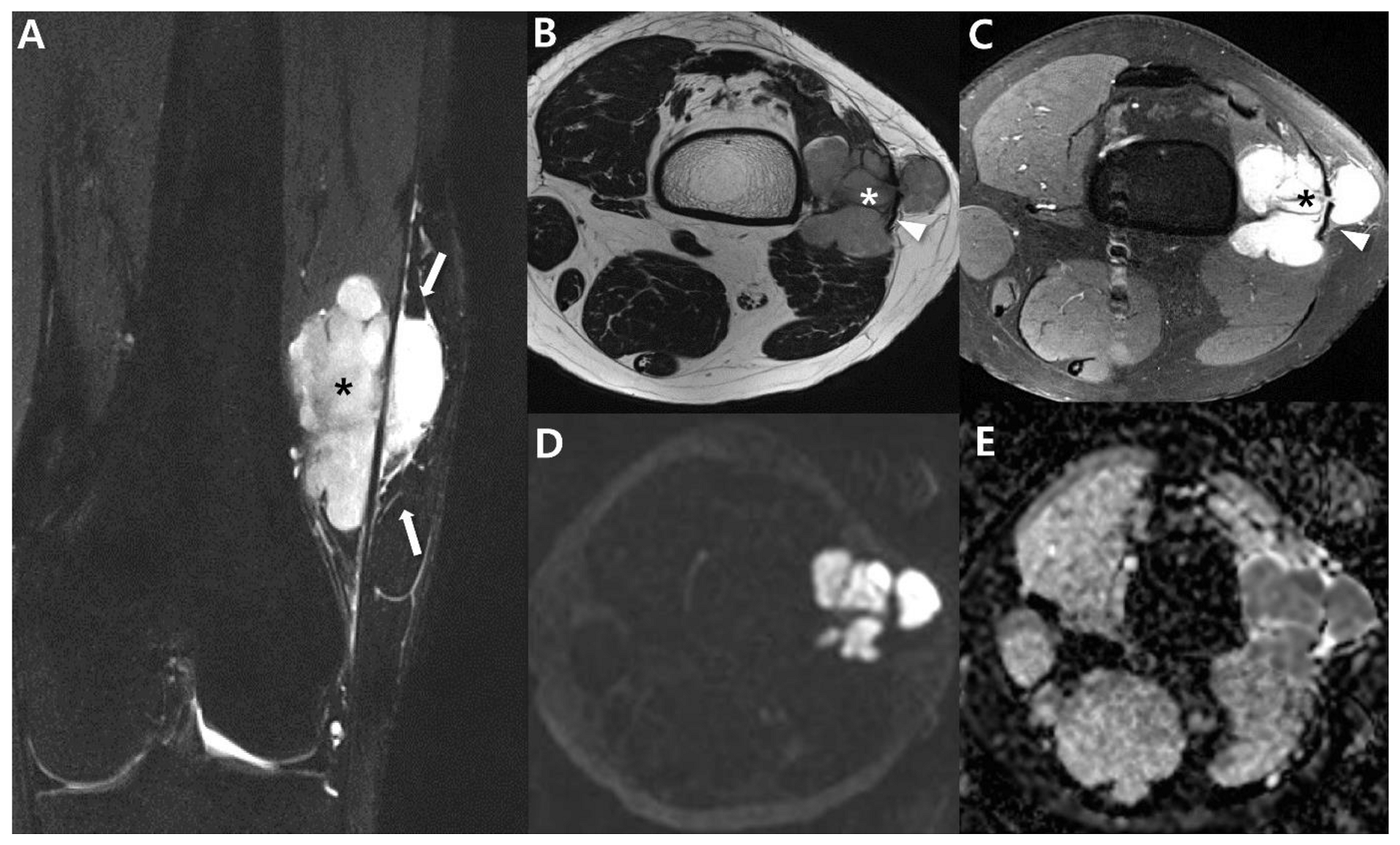

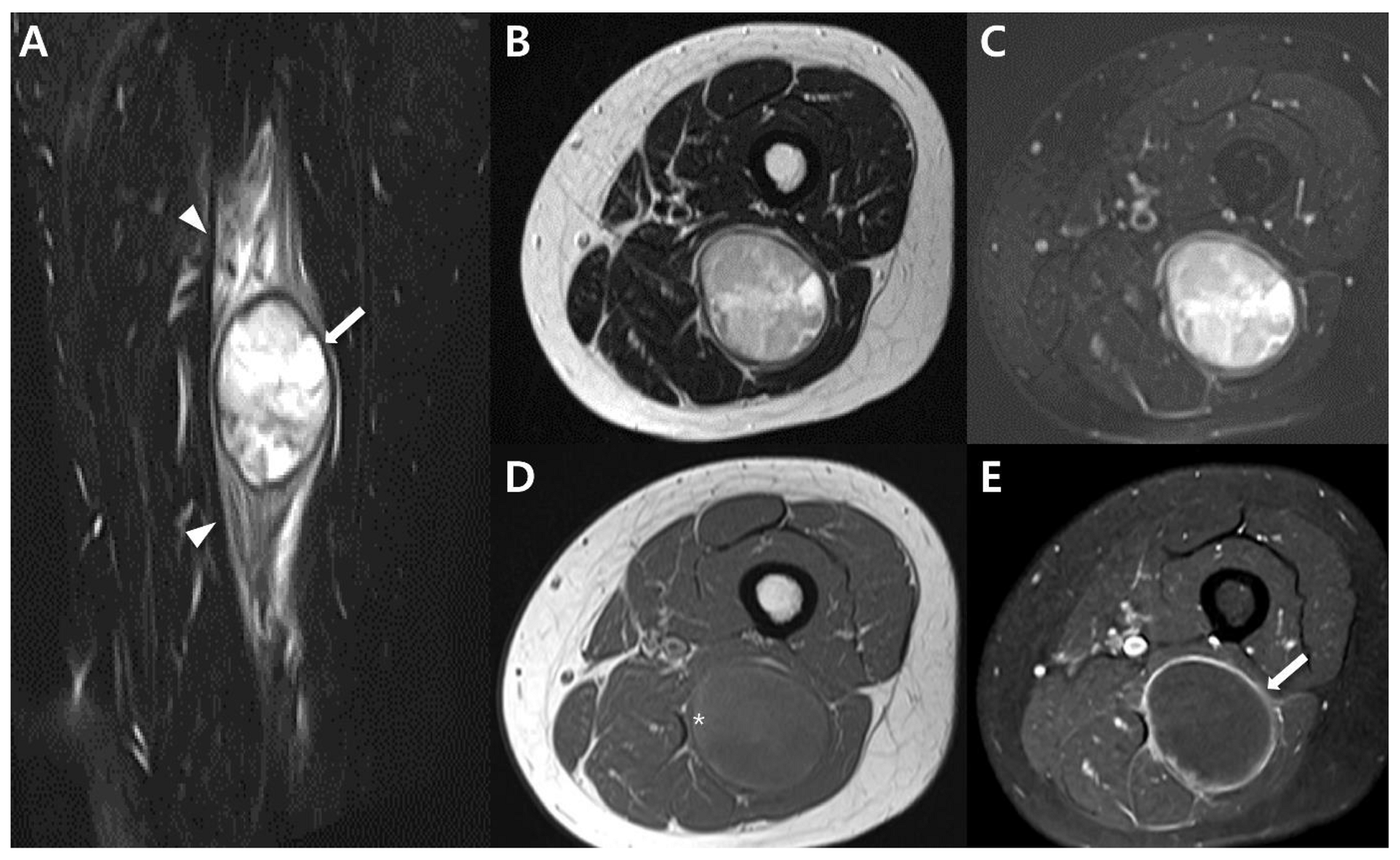
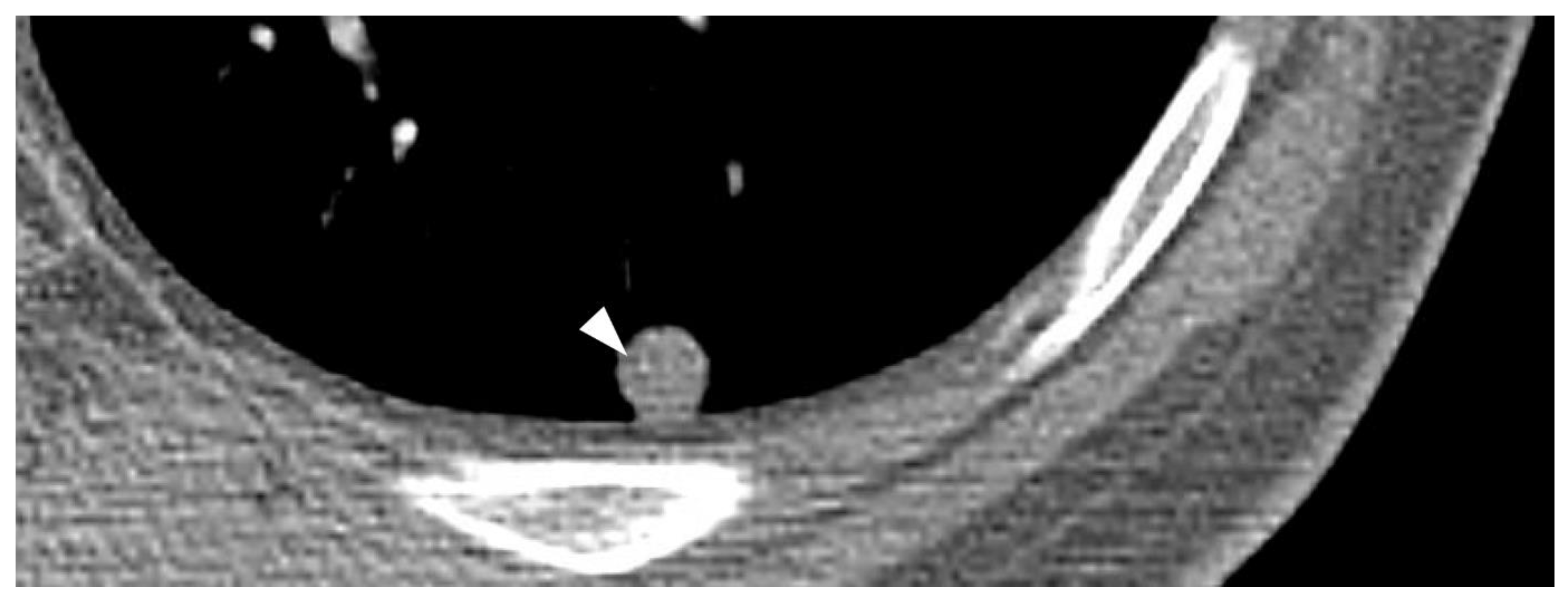
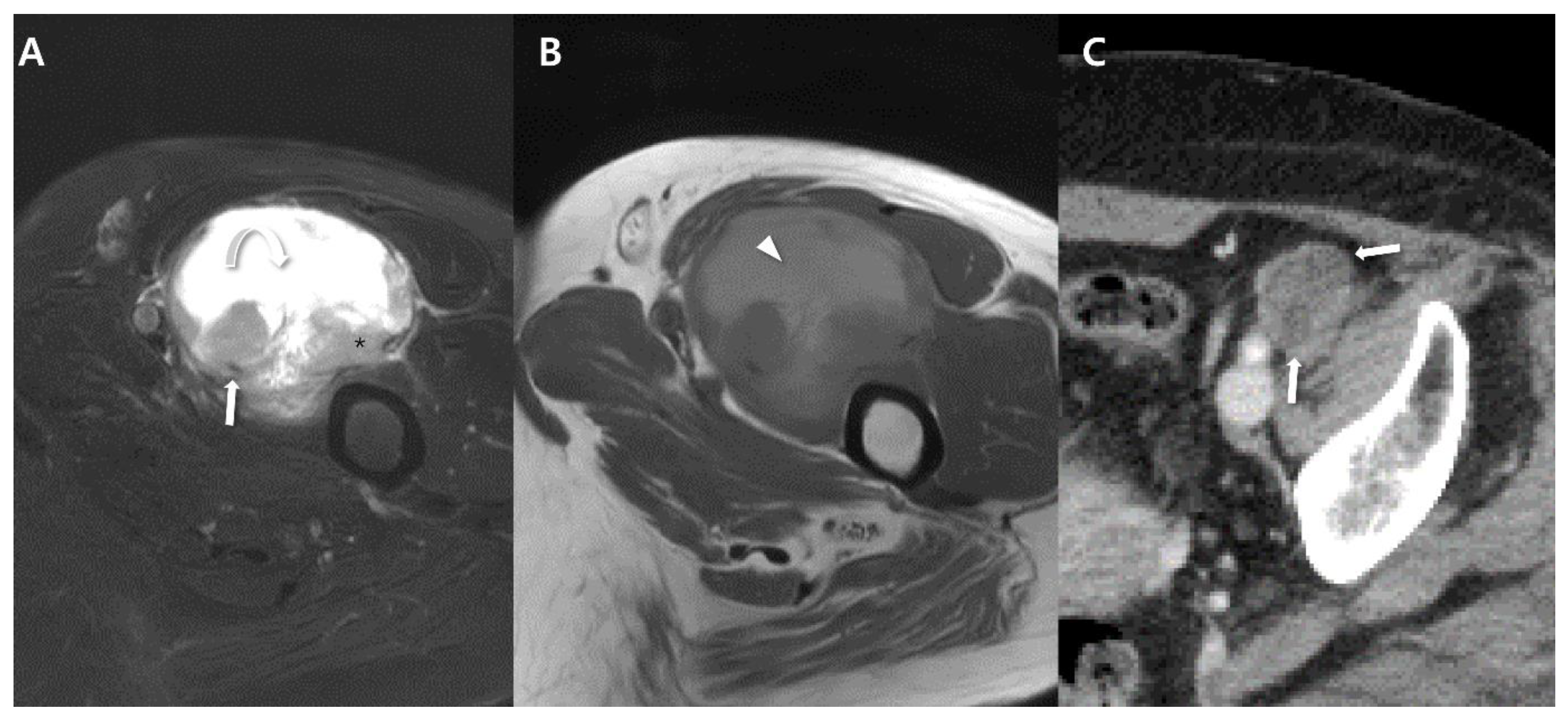
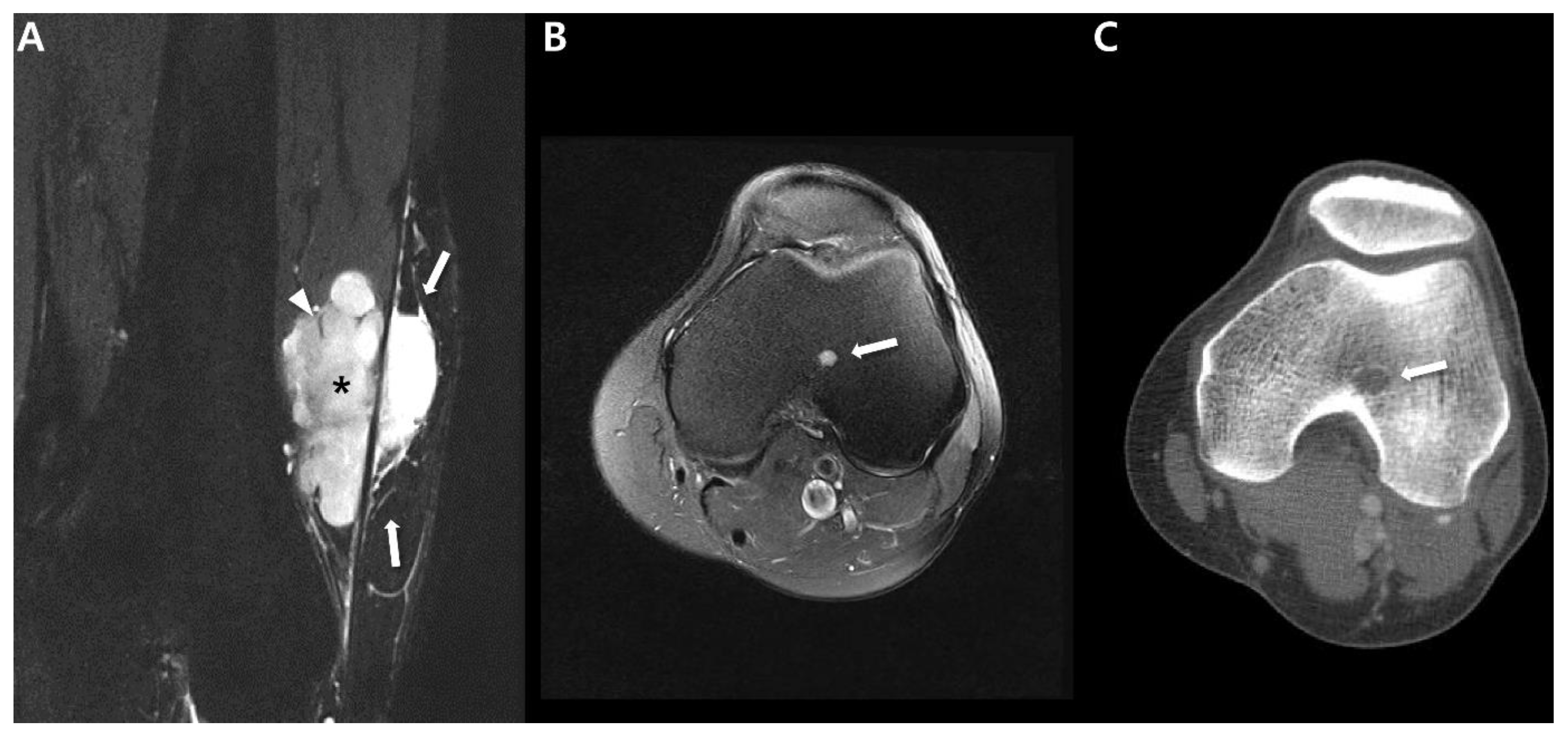

| General imaging features |
|
| Various imaging spectrum depending on tumor size | |
| Small size <5 cm |
|
| Large size >5 cm |
|
| Prognostic Factors | Prognosis | |
|---|---|---|
| Age | Younger than 15–20 years | Favorable |
| Tumor size | Large size >5 cm | Poor |
| Location | Proximal extremity | Poor |
| Inguinal region | ||
| Intrathoracic region | ||
| Trunk | ||
| Imaging features | Intratumoral hemorrhage (fluid-fluid levels) | Poor |
| Triple sign on MRI | ||
| Bowl-of-grapes appearance on MRI | ||
| Intercompartment extension | ||
| Peritumoral enhancement | ||
| Calcification | Favorable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, E.B.; Lee, S.K.; Kim, J.-Y.; Kim, Y. Synovial Sarcoma in the Extremity: Diversity of Imaging Features for Diagnosis and Prognosis. Cancers 2023, 15, 4860. https://doi.org/10.3390/cancers15194860
Cho EB, Lee SK, Kim J-Y, Kim Y. Synovial Sarcoma in the Extremity: Diversity of Imaging Features for Diagnosis and Prognosis. Cancers. 2023; 15(19):4860. https://doi.org/10.3390/cancers15194860
Chicago/Turabian StyleCho, Eun Byul, Seul Ki Lee, Jee-Young Kim, and Yuri Kim. 2023. "Synovial Sarcoma in the Extremity: Diversity of Imaging Features for Diagnosis and Prognosis" Cancers 15, no. 19: 4860. https://doi.org/10.3390/cancers15194860
APA StyleCho, E. B., Lee, S. K., Kim, J.-Y., & Kim, Y. (2023). Synovial Sarcoma in the Extremity: Diversity of Imaging Features for Diagnosis and Prognosis. Cancers, 15(19), 4860. https://doi.org/10.3390/cancers15194860






