Impact of Autoimmune Gastritis on Occurrence of Metachronous Gastric Neoplasms after Endoscopic Resection for Gastric Neoplasms
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
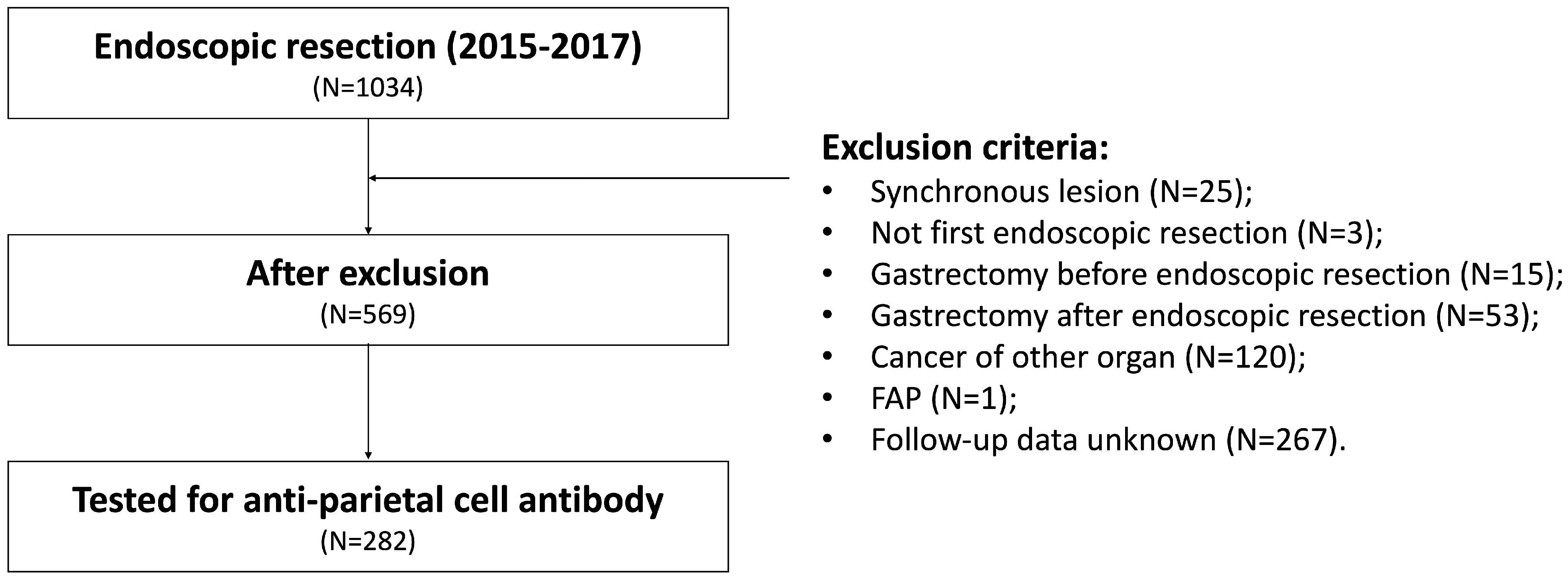
2.1. Patients
2.2. Endoscopic Procedures and Follow-Up
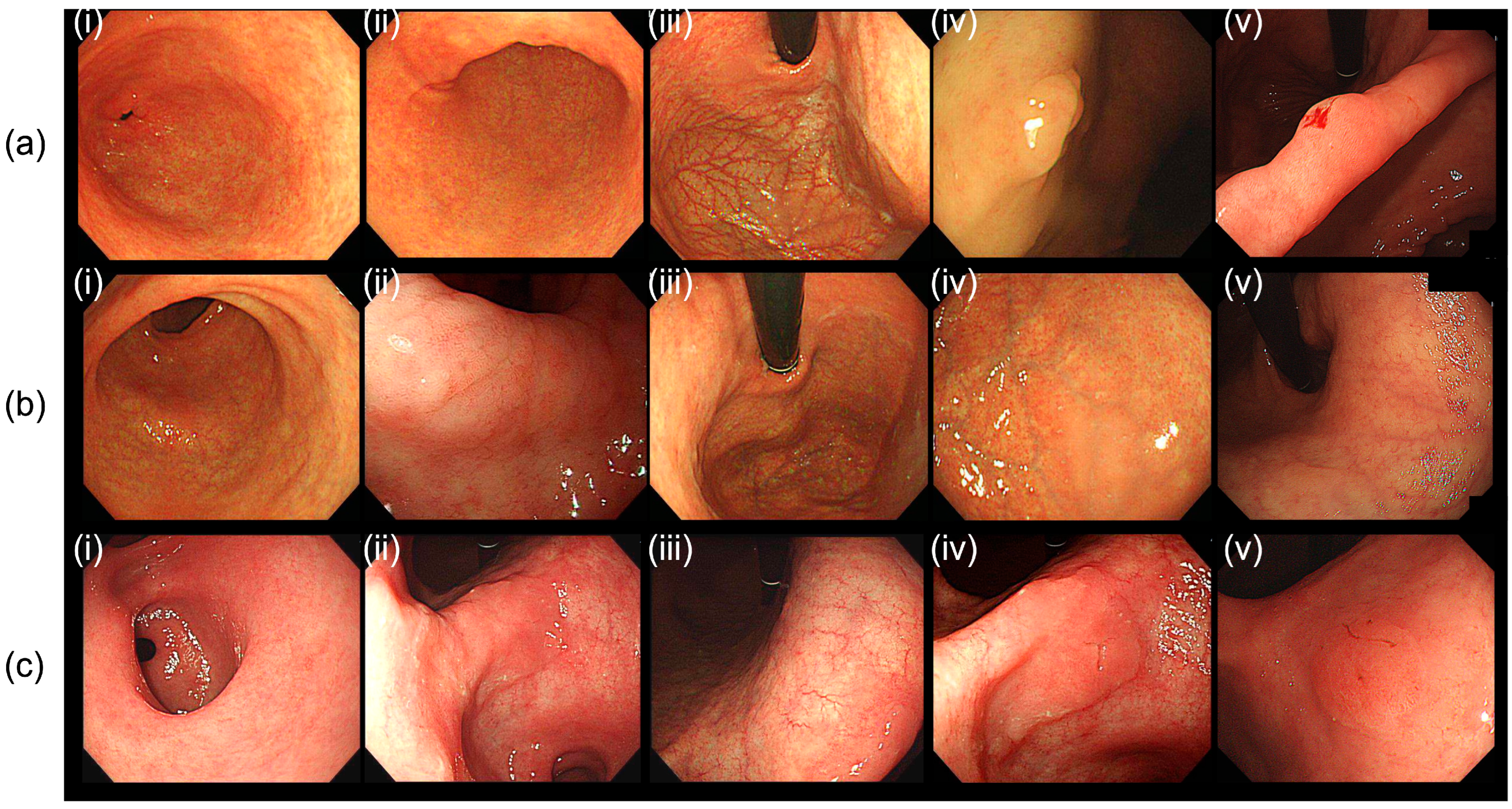
2.3. Clinicopathological Evaluations
2.4. Outcome Measures
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
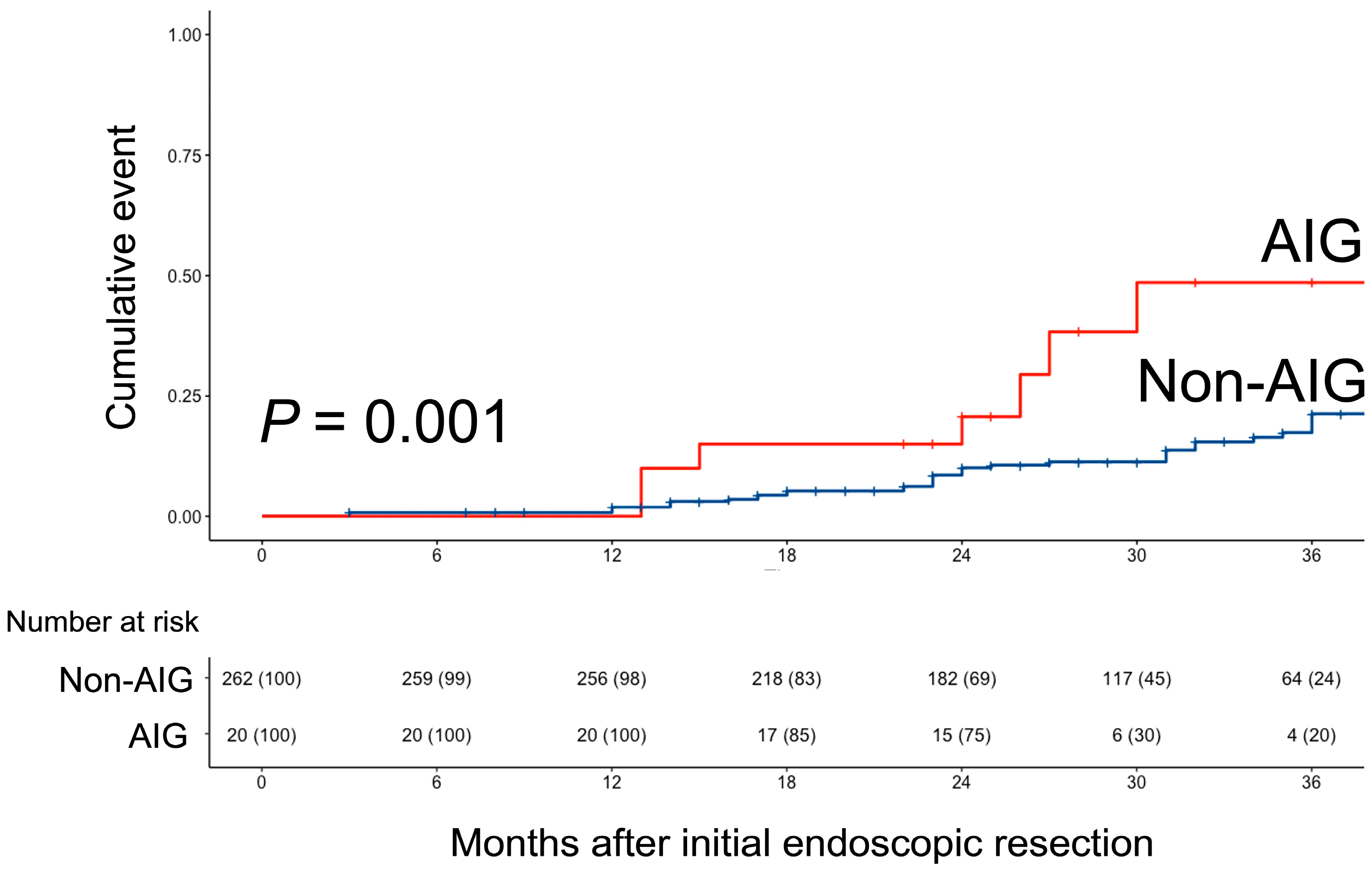
3.2. Metachronous Tumor Occurrence Patterns in Both Groups
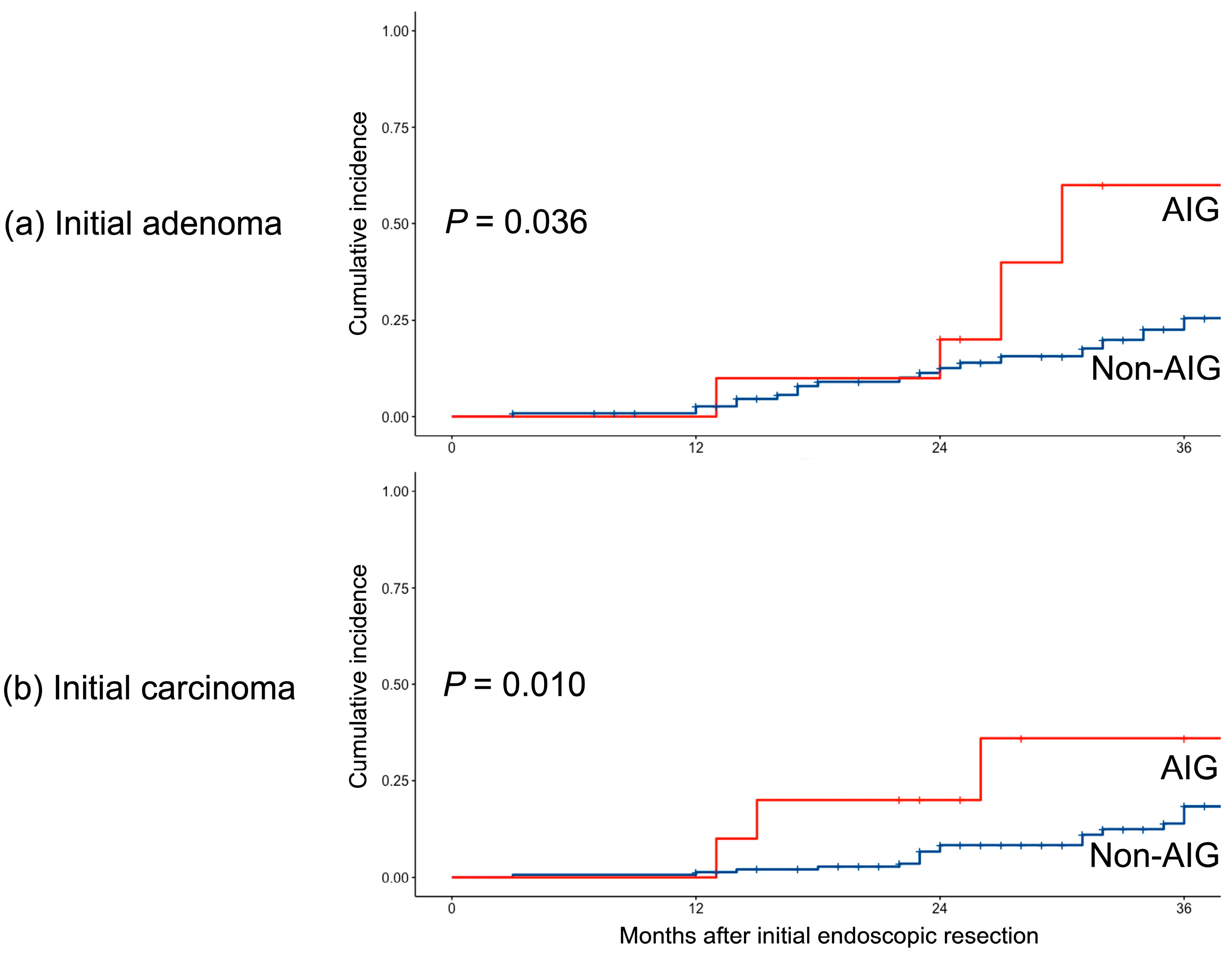
3.3. Subgroup Analysis Based on the Initial Pathology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zheng, Y.; Wang, H.L.; Wu, J. Global Patterns and Trends in Gastric Cancer Incidence Rates (1988–2012) and Predictions to 2030. Gastroenterology 2021, 161, 116–127.e118. [Google Scholar] [CrossRef] [PubMed]
- Neumann, W.L.; Coss, E.; Rugge, M.; Genta, R.M. Autoimmune atrophic gastritis—Pathogenesis, pathology and management. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Minalyan, A.; Benhammou, J.N.; Artashesyan, A.; Lewis, M.S.; Pisegna, J.R. Autoimmune atrophic gastritis: Current perspectives. Clin. Exp. Gastroenterol. 2017, 10, 19–27. [Google Scholar] [CrossRef]
- Annibale, B.; Esposito, G.; Lahner, E. A current clinical overview of atrophic gastritis. Expert. Rev. Gastroenterol. Hepatol. 2020, 14, 93–102. [Google Scholar] [CrossRef]
- Waldum, H.L.; Fossmark, R. Role of Autoimmune Gastritis in Gastric Cancer. Clin. Transl. Gastroenterol. 2019, 10, e00080. [Google Scholar] [CrossRef]
- Elsborg, L.; Mosbech, J. Pernicious anaemia as a risk factor in gastric cancer. Acta Medica Scand. 1979, 206, 315–318. [Google Scholar] [CrossRef]
- Correa, P. A human model of gastric carcinogenesis. Cancer Res. 1988, 48, 3554–3560. [Google Scholar]
- Hoft, S.G.; Noto, C.N.; DiPaolo, R.J. Two Distinct Etiologies of Gastric Cancer: Infection and Autoimmunity. Front. Cell Dev. Biol. 2021, 9, 752346. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Dinis-Ribeiro, M.; Ponchon, T.; Repici, A.; Vieth, M.; De Ceglie, A.; Amato, A.; Berr, F.; Bhandari, P.; Bialek, A.; et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015, 47, 829–854. [Google Scholar] [CrossRef]
- ASGE Standards of Practice Committee; Evans, J.A.; Chandrasekhara, V.; Chathadi, K.V.; Decker, G.A.; Early, D.S.; Fisher, D.A.; Foley, K.; Hwang, J.H.; Jue, T.L.; et al. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest. Endosc. 2015, 82, 1–8. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017, 20, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Takemoto, T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969, 1, 87–97. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, N.; Shin, C.M.; Lee, H.S.; Kim, B.K.; Kang, G.H.; Kim, J.M.; Kim, J.S.; Lee, D.H.; Jung, H.C. Risk Factors for Metachronous Gastric Neoplasms in Patients Who Underwent Endoscopic Resection of a Gastric Neoplasm. Gut Liver 2016, 10, 228–236. [Google Scholar] [CrossRef]
- Kim, Y.I.; Park, J.Y.; Kim, B.J.; Hwang, H.W.; Hong, S.A.; Kim, J.G. Risk of metachronous gastric neoplasm occurrence during intermediate-term follow-up period after endoscopic submucosal dissection for gastric dysplasia. Sci. Rep. 2020, 10, 6747. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, S.G.; Kim, B.; Kim, J.L.; Kim, J.; Chung, H.; Cho, S.J. Metachronous gastric neoplasm beyond 5 years after endoscopic resection for early gastric cancer. Surg. Endosc. 2023, 37, 3901–3910. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.J.; Kook, M.C.; Kim, Y.I.; Cho, S.J.; Lee, J.Y.; Kim, C.G.; Park, B.; Nam, B.H. Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer. N. Engl. J. Med. 2018, 378, 1085–1095. [Google Scholar] [CrossRef]
- Yamanaka, K.; Miyatani, H.; Yoshida, Y.; Ishii, T.; Asabe, S.; Takada, O.; Nokubi, M.; Mashima, H. Malignant transformation of a gastric hyperplastic polyp in a context of Helicobacter pylori-negative autoimmune gastritis: A case report. BMC Gastroenterol. 2016, 16, 130. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, X.; Song, Z.; Cui, R.; Jin, Z. Hyperplastic polyps arising in autoimmune metaplastic atrophic gastritis patients: Is this a distinct clinicopathological entity? Scand. J. Gastroenterol. 2018, 53, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Rugge, M.; Bricca, L.; Guzzinati, S.; Sacchi, D.; Pizzi, M.; Savarino, E.; Farinati, F.; Zorzi, M.; Fassan, M.; Tos, A.P.D.; et al. Autoimmune gastritis: Long-term natural history in naïve Helicobacter pylori-negative patients. Gut 2023, 72, 30–38. [Google Scholar] [CrossRef] [PubMed]
| Total (N = 282) | Non-AIG (N = 262) | AIG (N = 20) | p Value | |
|---|---|---|---|---|
| Age (mean ± SD) | 65.1 ± 9.4 | 65.1 ± 9.3 | 65.0 ± 11.8 | 0.965 |
| Male, n (%) | 201 (71.3%) | 187 (71.4%) | 14 (70.0%) | 1.000 |
| BMI, kg/m2, (mean ± SD) | 24.2 ± 3.1 | 24.2 ± 2.9 | 22.9 ± 5.4 | 0.296 |
| Cardiovascular disease, n (%) | 5 (1.8%) | 5 (2.0%) | 0 (0.0%) | 1.000 |
| Cerebrovascular disease, n (%) | 2 (0.7%) | 2 (0.8%) | 0 (0.0%) | 1.000 |
| Diabetes mellitus, n (%) | 48 (17.5%) | 44 (17.3%) | 4 (21.1%) | 0.915 |
| Hypertension, n (%) | 104 (38.0%) | 99 (38.8%) | 5 (26.3%) | 0.402 |
| Initial carcinoma, n (%) | 156 (55.3%) | 146 (55.7%) | 10 (50.0%) | 0.792 |
| Pathologic diagnosis, n (%) | 0.670 | |||
| Tubular adenocarcinoma, WD | 116 (41.2%) | 110 (42.0%) | 6 (30.0%) | |
| Tubular adenocarcinoma, MD | 32 (11.3%) | 30 (11.5%) | 2 (10.0%) | |
| Poorly differentiated adenocarcinoma | 7 (2.5%) | 6 (2.3%) | 1 (5.0%) | |
| Adenoma | 127 (45.0%) | 116 (44.3%) | 11 (55.0%) | |
| H. pylori status, n (%) | ||||
| Negative | 136 (48.2%) | 124 (47.3%) | 12 (60.0%) | 0.750 |
| Eradicated | 111 (39.4%) | 105 (40.1%) | 6 (30.0%) | |
| Persistent | 16 (5.7%) | 15 (5.7%) | 1 (5.0%) | |
| Unknown | 19 (6.7%) | 18 (6.9%) | 1 (5.0%) | |
| Lesion size, mm (mean ± SD) | 15.3 ± 11.7 | 15.3 ± 11.7 | 16.1 ± 12.0 | 0.749 |
| Location, n (%) | ||||
| Lower | 162 (57.4%) | 150 (57.3%) | 12 (60.0%) | 0.830 |
| Middle | 99 (35.1%) | 93 (35.5%) | 6 (30.0%) | |
| Upper | 21 (7.4%) | 19 (7.3%) | 2 (10.0%) | |
| Mucosal atrophy | ||||
| No atrophy | 2 (0.7%) | 2 (0.8%) | 0 (0.0%) | 0.379 |
| Closed type | 43 (15.2%) | 42 (16.0%) | 1 (5.0%) | |
| Open type | 237 (84.0%) | 218 (83.2%) | 19 (95.0%) | |
| MGN occurrence, n (%) | 57 (20.2%) | 48 (18.3%) | 9 (45.0%) | 0.010 |
| MGN pattern, n (%) | ||||
| No MGN | 225 (79.8%) | 214 (81.7%) | 11 (55.0%) | 0.048 |
| Adenoma → Adenoma | 23 (8.2%) | 19 (7.3%) | 4 (20.0%) | |
| Adenoma → Carcinoma | 8 (2.8%) | 7 (2.7%) | 1 (5.0%) | |
| Carcinoma → Adenoma | 15 (5.3%) | 12 (4.6%) | 3 (15.0%) | |
| Carcinoma → Carcinoma | 11 (3.95) | 10 (3.8%) | 1 (5.0%) |
| N = 57 | Non-AIG (N = 48) | AIG (N = 9) | p Value |
|---|---|---|---|
| Adenoma → Adenoma | 19 (39.6%) | 4 (44.4%) | 0.878 |
| Adenoma → Carcinoma | 7 (14.6%) | 1 (11.1%) | |
| Carcinoma → Adenoma | 12 (25.0%) | 3 (33.3%) | |
| Carcinoma → Carcinoma | 10 (20.8%) | 1 (11.1%) |
| N = 282 | Overall Survival | |||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age | 1.03 (1.00–1.06) | 0.043 | 1.03 (1.00–1.06) | 0.070 |
| Male sex | 1.21 (0.66–2.21) | 0.500 | 0.97 (0.51–1.87) | >0.900 |
| BMI | 1.16 (1.06–1.28) | 0.001 | 1.16 (1.06–1.27) | 0.002 |
| Primarily cancer | 0.57 (0.34–0.97) | 0.038 | ||
| AIG | 3.16 (1.53–6.52) | 0.002 | 3.32 (1.55–7.10) | 0.002 |
| H. pylori status | ||||
| HPE (vs. HPN) | 0.70 (0.38–1.27) | 0.200 | 0.85 (0.45–1.60) | 0.600 |
| HPP (vs. HPN) | 0.94 (0.29–3.09) | >0.900 | 0.85 (0.25–2.90) | 0.800 |
| HPU (vs. HPN) | 0.45 (0.11–1.89) | 0.300 | 0.43 (0.10–1.85) | 0.300 |
| Mucosal atrophy (closed vs. open) | 1.03 (0.52–2.04) | >0.900 | 1.01 (0.49–2.07) | >0.900 |
| Initial lesion location | ||||
| Middle (vs. Lower) | 0.92 (0.52–1.61) | 0.800 | ||
| Upper (vs. Lower) | 0.98 (0.35–2.79) | >0.900 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, D.; Lim, C.-H.; Kim, J.S.; Cho, Y.K.; Park, J.M.; Choi, M.-G. Impact of Autoimmune Gastritis on Occurrence of Metachronous Gastric Neoplasms after Endoscopic Resection for Gastric Neoplasms. Cancers 2023, 15, 4859. https://doi.org/10.3390/cancers15194859
Kang D, Lim C-H, Kim JS, Cho YK, Park JM, Choi M-G. Impact of Autoimmune Gastritis on Occurrence of Metachronous Gastric Neoplasms after Endoscopic Resection for Gastric Neoplasms. Cancers. 2023; 15(19):4859. https://doi.org/10.3390/cancers15194859
Chicago/Turabian StyleKang, Donghoon, Chul-Hyun Lim, Jin Su Kim, Yu Kyung Cho, Jae Myung Park, and Myung-Gyu Choi. 2023. "Impact of Autoimmune Gastritis on Occurrence of Metachronous Gastric Neoplasms after Endoscopic Resection for Gastric Neoplasms" Cancers 15, no. 19: 4859. https://doi.org/10.3390/cancers15194859
APA StyleKang, D., Lim, C.-H., Kim, J. S., Cho, Y. K., Park, J. M., & Choi, M.-G. (2023). Impact of Autoimmune Gastritis on Occurrence of Metachronous Gastric Neoplasms after Endoscopic Resection for Gastric Neoplasms. Cancers, 15(19), 4859. https://doi.org/10.3390/cancers15194859







