Diabetes Mellitus Is a Strong Independent Negative Prognostic Factor in Patients with Brain Metastases Treated with Radiotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Age, KPS and Tumor Histology Influenced Survival Outcomes
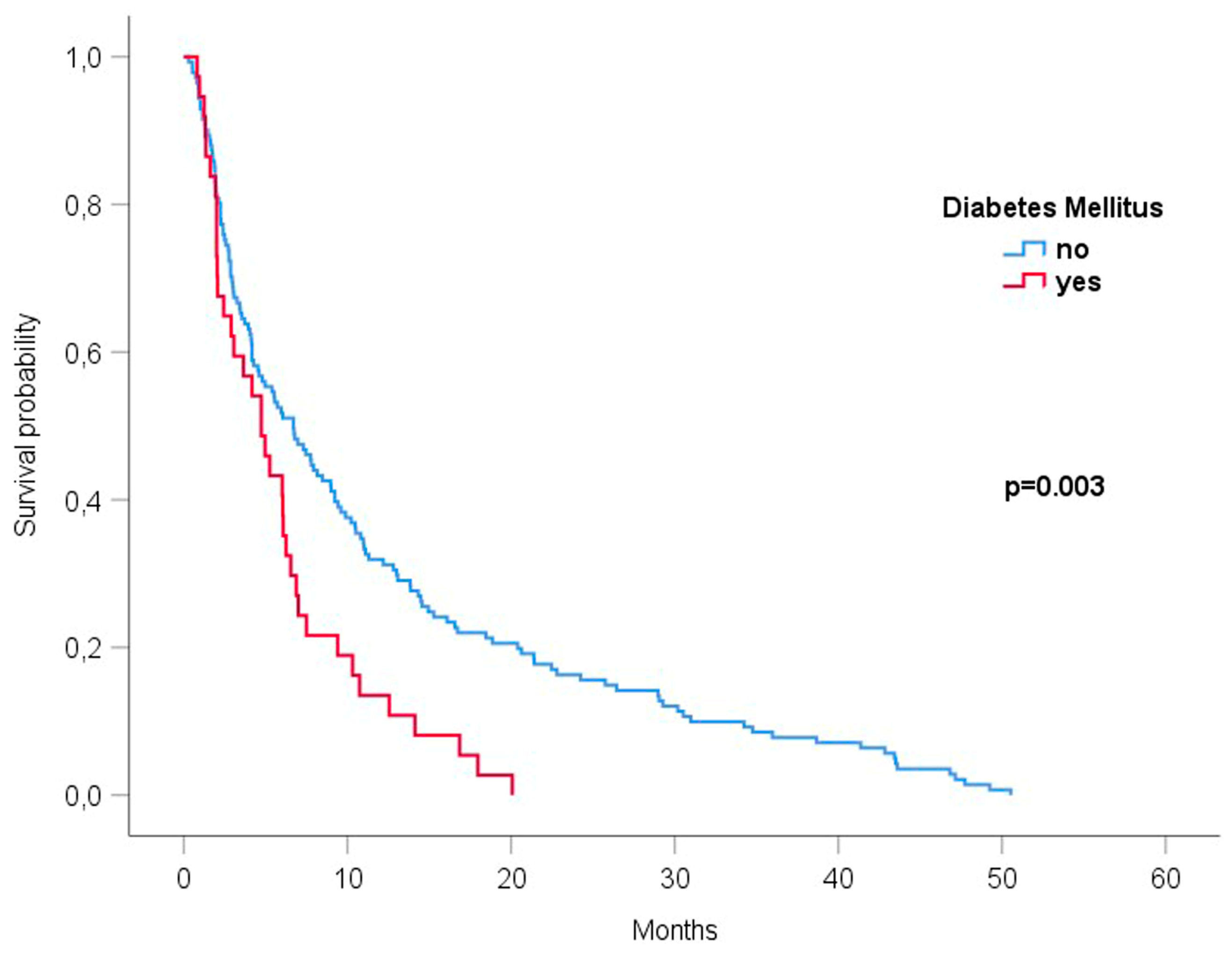
3.3. Diabetes Mellitus Was Associated with the Reduced Survival of Patients with Brain Metastases Undergoing Radiotherapy
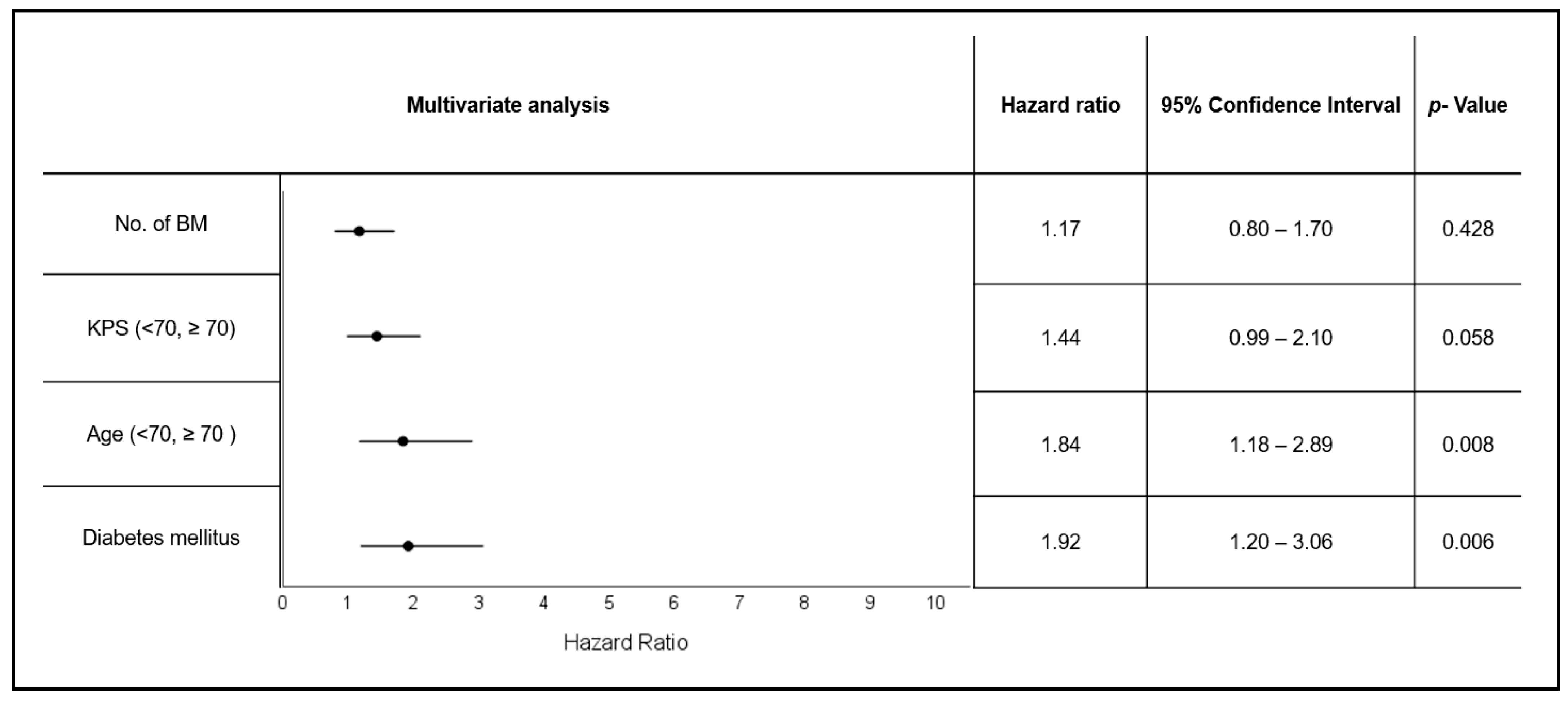
3.4. Diabetes and Patient Age Are Independent Negative Prognostic Factors in Patients with Brain Metastases Undergoing Radiotherapy
3.5. Diabetes Mellitus Is a Negative Prognostic Factor for Brain Metastases of Distinct Histologies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cagney, D.N.; Martin, A.M.; Catalano, P.J.; Redig, A.J.; Lin, N.U.; Lee, E.Q.; Wen, P.Y.; Dunn, I.F.; Bi, W.L.; Weiss, S.E.; et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: A population-based study. Neuro-Oncol. 2017, 19, 1511–1521. [Google Scholar] [CrossRef]
- Li, A.Y.; Gaebe, K.; Jerzak, K.J.; Cheema, P.K.; Sahgal, A.; Das, S. Intracranial Metastatic Disease: Present Challenges, Future Opportunities. Front. Oncol. 2022, 12, 855182. [Google Scholar] [CrossRef] [PubMed]
- Habbous, S.; Forster, K.; Darling, G.; Jerzak, K.; Holloway, C.M.B.; Sahgal, A.; Das, S. Incidence and real-world burden of brain metastases from solid tumors and hematologic malignancies in Ontario: A population-based study. Neuro-Oncol. Adv. 2021, 3, vdaa178. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Handa, P.; Sahgal, A.; Lo, S.; Vellayappan, B. Risk-reduction strategies for late complications arising from brain metastases treated with radiotherapy: A narrative review. Chin. Clin. Oncol. 2022, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Nieder, C.; Spanne, O.; Mehta, M.P.; Grosu, A.L.; Geinitz, H. Presentation, patterns of care, and survival in patients with brain metastases: What has changed in the last 20 years? Cancer 2011, 117, 2505–2512. [Google Scholar] [CrossRef]
- Soffietti, R.; Rudā, R.; Mutani, R. Management of brain metastases. J. Neurol. 2002, 249, 1357–1369. [Google Scholar] [CrossRef]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G.; Deming, R.; Burri, S.H.; et al. Effect of Radiosurgery Alone vs Radiosurgery with Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016, 316, 401–409. [Google Scholar] [CrossRef]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef]
- Yamamoto, M.; Serizawa, T.; Shuto, T.; Akabane, A.; Higuchi, Y.; Kawagishi, J.; Yamanaka, K.; Sato, Y.; Jokura, H.; Yomo, S.; et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014, 15, 387–395. [Google Scholar] [CrossRef]
- Yamamoto, M.; Serizawa, T.; Higuchi, Y.; Sato, Y.; Kawagishi, J.; Yamanaka, K.; Shuto, T.; Akabane, A.; Jokura, H.; Yomo, S.; et al. A Multi-institutional Prospective Observational Study of Stereotactic Radiosurgery for Patients with Multiple Brain Metastases (JLGK0901 Study Update): Irradiation-related Complications and Long-term Maintenance of Mini-Mental State Examination Scores. Int. J. Radiat. Oncol. 2017, 99, 31–40. [Google Scholar] [CrossRef]
- Gondi, V.; Pugh, S.L.; Tome, W.A.; Caine, C.; Corn, B.; Kanner, A.; Rowley, H.; Kundapur, V.; DeNittis, A.; Greenspoon, J.N.; et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): A phase II multi-institutional trial. J. Clin. Oncol. 2014, 32, 3810–3816. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Gondi, V.; Pugh, S.; Tome, W.A.; Wefel, J.S.; Armstrong, T.S.; Bovi, J.A.; Robinson, C.; Konski, A.; Khuntia, D.; et al. Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients with Brain Metastases: Phase III Trial NRG Oncology CC001. J. Clin. Oncol. 2020, 38, 1019–1029. [Google Scholar] [CrossRef]
- Brown, P.D.; Pugh, S.; Laack, N.N.; Wefel, J.S.; Khuntia, D.; Meyers, C.; Choucair, A.; Fox, S.; Suh, J.H.; Roberge, D.; et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro-Oncology 2013, 15, 1429–1437. [Google Scholar] [CrossRef]
- Gaspar, L.; Scott, C.; Rotman, M.; Asbell, S.; Phillips, T.; Wasserman, T.; McKenna, W.G.; Byhardt, R. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Kased, N.; Roberge, D.; Xu, Z.; Shanley, R.; Luo, X.; Sneed, P.K.; Chao, S.T.; Weil, R.J.; Suh, J.; et al. Summary Report on the Graded Prognostic Assessment: An Accurate and Facile Diagnosis-Specific Tool to Estimate Survival for Patients with Brain Metastases. J. Clin. Oncol. 2012, 30, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; De, B.; Li, J.; Carpenter, D.; Kwithatrick, J.; Milligan, M.; Shih, H.A.; Kutuk, T.; Kotecha, R.; Higaki, H.; et al. Graded Prognostic Assessment (GPA) for Patients with Lung Cancer and Brain Metastases: Initial Report of the Small Cell Lung Cancer GPA and Update of the Non-Small Cell Lung Cancer GPA Including the Effect of Programmed Death Ligand 1 and Other Prognostic Factors. Int. J. Radiat. Oncol. 2022, 114, 60–74. [Google Scholar] [CrossRef]
- Bellera, C.A.; Rainfray, M.; Mathoulin-Pélissier, S.; Mertens, C.; Delva, F.; Fonck, M.; Soubeyran, P.L. Screening older cancer patients: First evaluation of the G-8 geriatric screening tool. Ann. Oncol. 2012, 23, 2166–2172. [Google Scholar] [CrossRef]
- Extermann, M.; Hurria, A. Comprehensive Geriatric Assessment for Older Patients with Cancer. J. Clin. Oncol. 2007, 25, 1824–1831. [Google Scholar] [CrossRef]
- Boakye, D.; Rillmann, B.; Walter, V.; Jansen, L.; Hoffmeister, M.; Brenner, H. Impact of comorbidity and frailty on prognosis in colorectal cancer patients: A systematic review and meta-analysis. Cancer Treat. Rev. 2018, 64, 30–39. [Google Scholar] [CrossRef]
- Land, L.H.; Dalton, S.O.; Jørgensen, T.L.; Ewertz, M. Comorbidity and survival after early breast cancer. A review. Crit. Rev. Oncol. 2011, 81, 196–205. [Google Scholar] [CrossRef]
- Firat, S.; Bousamra, M.; Gore, E.; Byhardt, R.W. Comorbidity and KPS are independent prognostic factors in stage I non-small-cell lung cancer. Int. J. Radiat. Oncol. 2002, 52, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, F.; Saito, E.; Lin, Y.; Song, M.; Luu, H.N.; Gupta, P.C.; Sawada, N.; Tamakoshi, A.; Shu, X.-O.; et al. Association between type 2 diabetes and risk of cancer mortality: A pooled analysis of over 771,000 individuals in the Asia Cohort Consortium. Diabetologia 2017, 60, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, B.S.; Horsman, M.R. Tumor Hypoxia: Impact on Radiation Therapy and Molecular Pathways. Front. Oncol. 2020, 10, 562. [Google Scholar] [CrossRef] [PubMed]
- Corroyer-Dulmont, A.; Valable, S.; Fantin, J.; Chatre, L.; Toutain, J.; Teulier, S.; Bazille, C.; Letissier, E.; Levallet, J.; Divoux, D.; et al. Multimodal evaluation of hypoxia in brain metastases of lung cancer and interest of hypoxia image-guided radiotherapy. Sci. Rep. 2021, 11, 11239. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Oeffinger, K.C.; Chen, Y.; Kawashima, T.; Yasui, Y.; Leisenring, W.; Stovall, M.; Chow, E.J.; Sklar, C.A.; Mulrooney, D.A.; et al. Modifiable Risk Factors and Major Cardiac Events Among Adult Survivors of Childhood Cancer. J. Clin. Oncol. 2013, 31, 3673–3680. [Google Scholar] [CrossRef]
- Cohen, J.B.; Geara, A.S.; Hogan, J.J.; Townsend, R.R. Hypertension in Cancer Patients and Survivors. JACC CardioOncol. 2019, 1, 238–251. [Google Scholar] [CrossRef]
- Huang, L.; Shi, Y. Prognostic value of pretreatment smoking status for small cell lung cancer: A meta-analysis. Thorac. Cancer 2020, 11, 3252–3259. [Google Scholar] [CrossRef]
- Ban, W.H.; Yeo, C.D.; Han, S.; Kang, H.S.; Park, C.K.; Kim, J.S.; Kim, J.W.; Kim, S.J.; Lee, S.H.; Kim, S.K. Impact of smoking amount on clinicopathological features and survival in non-small cell lung cancer. BMC Cancer 2020, 20, 848. [Google Scholar] [CrossRef]
- Passarelli, M.N.; Newcomb, P.A.; Hampton, J.M.; Trentham-Dietz, A.; Titus, L.J.; Egan, K.M.; Baron, J.A.; Willett, W.C. Cigarette Smoking Before and After Breast Cancer Diagnosis: Mortality from Breast Cancer and Smoking-Related Diseases. J. Clin. Oncol. 2016, 34, 1315–1322. [Google Scholar] [CrossRef]
- Jaiswal, V.; Agrawal, V.; Ang, S.P.; Saleeb, M.; Ishak, A.; Hameed, M.; Rajak, K.; Kalra, K.; Jaiswal, A. Post-diagnostic statin use and its association with cancer recurrence and mortality in breast cancer patients: A systematic review and meta-analysis. Eur. Hear. J. Cardiovasc. Pharmacother. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Barone, B.B.; Yeh, H.-C.; Snyder, C.F.; Peairs, K.S.; Stein, K.B.; Derr, R.L.; Wolff, A.C.; Brancati, F.L. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. JAMA 2008, 300, 2754–2764. [Google Scholar] [CrossRef] [PubMed]
- Lipscombe, L.L.; Goodwin, P.J.; Zinman, B.; McLaughlin, J.R.; Hux, J.E. The impact of diabetes on survival following breast cancer. Breast Cancer Res. Treat. 2008, 109, 389–395. [Google Scholar] [CrossRef]
- Lorger, M.; Felding-Habermann, B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am. J. Pathol. 2010, 176, 2958–2971. [Google Scholar] [CrossRef]
- Kienast, Y.; Baumgarten, L.v.; Fuhrmann, M.; Klinkert, W.E.F.; Goldbrunner, R.; Herms, J.; Winkler, F. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 2010, 16, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Łazarczyk, M.; Mickael, M.E.; Skiba, D.; Kurzejamska, E.; Ławiński, M.; Horbańczuk, J.O.; Radziszewski, J.; Fraczek, K.; Wolinska, R.; Paszkiewicz, J.; et al. The Journey of Cancer Cells to the Brain: Challenges and Opportunities. Int. J. Mol. Sci. 2023, 24, 3854. [Google Scholar] [CrossRef] [PubMed]
- Hartford, A.C.; Gill, G.S.; Ravi, D.; Tosteson, T.D.; Li, Z.; Russo, G.; Eskey, C.J.; Jarvis, L.A.; Simmons, N.E.; Evans, L.T.; et al. Sensitizing brain metastases to stereotactic radiosurgery using hyperbaric oxygen: A proof-of-principle study. Radiother. Oncol. 2022, 177, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, Y.; Fei, S.; Wei, C.; Tong, F.; Wu, G.; Ma, H.; Dong, X. The effect of combining Endostar with radiotherapy on blood vessels, tumor-associated macrophages, and T cells in brain metastases of Lewis lung cancer. Transl. Lung Cancer Res. 2020, 9, 745–760. [Google Scholar] [CrossRef]
- Corroyer-Dulmont, A.; Valable, S.; Falzone, N.; Frelin-Labalme, A.-M.; Tietz, O.; Toutain, J.; Soto, M.S.; Divoux, D.; Chazalviel, L.; Pérès, E.A.; et al. VCAM-1 targeted alpha-particle therapy for early brain metastases. Neuro-Oncol. 2020, 22, 357–368. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Preusser, M. Anti-angiogenic therapies in brain metastases. Memo 2018, 11, 14–17. [Google Scholar] [CrossRef]
- Sigurdsson, S.; Aspelund, T.; Kjartansson, O.; Gudmundsson, E.; Jonsson, P.V.; van Buchem, M.A.; Gudnason, V.; Launer, L.J. Cerebrovascular Risk-Factors of Prevalent and Incident Brain Infarcts in the General Population: The AGES-Reykjavik Study. Stroke 2022, 53, 1199–1206. [Google Scholar] [CrossRef]
- Knopman, D.S.; Penman, A.D.; Catellier, D.J.; Coker, L.H.; Shibata, D.K.; Sharrett, A.R.; Mosley, T.H. Vascular risk factors and longitudinal changes on brain MRI: The ARIC study. Neurology 2011, 76, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. The Lancet. Neurology 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Chen, R.; Ovbiagele, B.; Feng, W. Diabetes and Stroke: Epidemiology, Pathophysiology, Pharmaceuticals and Outcomes. Am. J. Med. Sci. 2016, 351, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Shenker, R.F.; McTyre, E.R.; Ruiz, J.; Weaver, K.E.; Cramer, C.; Alphonse-Sullivan, N.K.; Farris, M.; Petty, W.J.; Bonomi, M.R.; Watabe, K.; et al. The Effects of smoking status and smoking history on patients with brain metastases from lung cancer. Cancer Med. 2017, 6, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Berk, B.-A.; Nagel, S.; Hering, K.; Paschke, S.; Hoffmann, K.-T.; Kortmann, R.-D.; Gaudino, C.; Seidel, C. White matter lesions reduce number of brain metastases in different cancers: A high-resolution MRI study. J. Neuro-Oncol. 2016, 130, 203–209. [Google Scholar] [CrossRef]
- Mazzone, P.J.; Marchi, N.; Fazio, V.; Taylor, J.M.; Masaryk, T.; Bury, L.; Mekhail, T.; Janigro, D. Small vessel ischemic disease of the brain and brain metastases in lung cancer patients. PLoS ONE 2009, 4, e7242. [Google Scholar] [CrossRef]
- Berk, B.-A.; Hering, K.; Kortmann, R.-D.; Hoffmann, K.-T.; Ziemer, M.; Seidel, C. Vascular white matter lesions negatively correlate with brain metastases in malignant melanoma-Results from a retrospective comparative analysis. Clin. Neurol. Neurosurg. 2019, 180, 117–121. [Google Scholar] [CrossRef] [PubMed]
- McCall, N.S.; Simone, B.A.; Mehta, M.; Zhan, T.; Ko, K.; Nowak-Choi, K.; Rese, A.; Venkataraman, C.; Andrews, D.W.; Anne’, P.R.; et al. Onco-metabolism: Defining the prognostic significance of obesity and diabetes in women with brain metastases from breast cancer. Breast Cancer Res. Treat. 2018, 172, 221–230. [Google Scholar] [CrossRef]
- LeCompte, M.C.; McTyre, E.R.; Strowd, R.E.; Lanier, C.; Soike, M.H.; Hughes, R.T.; Masters, A.H.; Cramer, C.K.; Farris, M.; Ruiz, J.; et al. Impact of diabetes mellitus on outcomes in patients with brain metastasis treated with stereotactic radiosurgery. J. Radiosurgery SBRT 2018, 5, 285–291. [Google Scholar] [CrossRef]
- Shahid, R.K.; Ahmed, S.; Le, D.; Yadav, S. Diabetes and Cancer: Risk, Challenges, Management and Outcomes. Cancers 2021, 13, 5735. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, K.; Ji, W.; Zhang, W.; Li, Y.; Liu, M.; Cui, J.; Li, W. Effects of Diabetes on Inflammatory Status and Prognosis in Cancer Patients. Front. Nutr. 2022, 9, 792577. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Liao, Z. Diabetes and cancer: Epidemiological and biological links. World J. Diabetes 2020, 11, 227–238. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Kajio, H.; Sugiyama, T. Association between hyperinsulinemia and increased risk of cancer death in nonobese and obese people: A population-based observational study. Int. J. Cancer 2017, 141, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, R.D.; Gallagher, E.J.; Cohen, D.; Tobin-Hess, A.; Alikhani, N.; Novosyadlyy, R.; Haddad, N.; Yakar, S.; LeRoith, D. Hyperinsulinemia promotes metastasis to the lung in a mouse model of Her2-mediated breast cancer. Endocr. Relat. Cancer 2013, 20, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Gallo, M.; Muscogiuri, G.; Felicetti, F.; Faggiano, A.; Trimarchi, F.; Arvat, E.; Vigneri, R.; Colao, A. Adverse glycaemic effects of cancer therapy: Indications for a rational approach to cancer patients with diabetes. Metab. Clin. Exp. 2018, 78, 141–154. [Google Scholar] [CrossRef]
- Adekola, K.; Rosen, S.T.; Shanmugam, M. Glucose transporters in cancer metabolism. Curr. Opin. Oncol. 2012, 24, 650–654. [Google Scholar] [CrossRef]
- Ramteke, P.; Deb, A.; Shepal, V.; Bhat, M.K. Hyperglycemia Associated Metabolic and Molecular Alterations in Cancer Risk, Progression, Treatment, and Mortality. Cancers 2019, 11, 1402. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, H.; Qian, W.; Cheng, L.; Yan, B.; Han, L.; Xu, Q.; Ma, Q.; Ma, J. Hyperglycemia aggravates microenvironment hypoxia and promotes the metastatic ability of pancreatic cancer. Comput. Struct. Biotechnol. J. 2018, 16, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Masur, K.; Vetter, C.; Hinz, A.; Tomas, N.; Henrich, H.; Niggemann, B.; Zänker, K.S. Diabetogenic glucose and insulin concentrations modulate transcriptome and protein levels involved in tumour cell migration, adhesion and proliferation. Br. J. Cancer 2011, 104, 345–352. [Google Scholar] [CrossRef] [PubMed]
- García-Jiménez, C.; García-Martínez, J.M.; Chocarro-Calvo, A.; La Vieja, A.d. A new link between diabetes and cancer: Enhanced WNT/β-catenin signaling by high glucose. J. Mol. Endocrinol. 2014, 52, R51-66. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [CrossRef]
- Leshem, Y.; Dolev, Y.; Siegelmann-Danieli, N.; Sharman Moser, S.; Apter, L.; Chodick, G.; Nikolaevski-Berlin, A.; Shamai, S.; Merimsky, O.; Wolf, I. Association between diabetes mellitus and reduced efficacy of pembrolizumab in non-small cell lung cancer. Cancer 2023, 129, 2789–2797. [Google Scholar] [CrossRef]
- Duan, Q.; Li, H.; Gao, C.; Zhao, H.; Wu, S.; Wu, H.; Wang, C.; Shen, Q.; Yin, T. High glucose promotes pancreatic cancer cells to escape from immune surveillance via AMPK-Bmi1-GATA2-MICA/B pathway. J. Exp. Clin. Cancer Res. CR 2019, 38, 192. [Google Scholar] [CrossRef]
- Kislinger, T.; Fu, C.; Huber, B.; Qu, W.; Taguchi, A.; Du Yan, S.; Hofmann, M.; Yan, S.F.; Pischetsrieder, M.; Stern, D.; et al. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J. Biol. Chem. 1999, 274, 31740–31749. [Google Scholar] [CrossRef]
- Rojas, A.; González, I.; Morales, E.; Pérez-Castro, R.; Romero, J.; Figueroa, H. Diabetes and cancer: Looking at the multiligand/RAGE axis. World J. Diabetes 2011, 2, 108–113. [Google Scholar] [CrossRef]
- Rojas, A.; Figueroa, H.; Morales, E. Fueling inflammation at tumor microenvironment: The role of multiligand/RAGE axis. Carcinogenesis 2010, 31, 334–341. [Google Scholar] [CrossRef]
- Monteiro, C.; Miarka, L.; Perea-García, M.; Priego, N.; García-Gómez, P.; Álvaro-Espinosa, L.; Pablos-Aragoneses, A.d.; Yebra, N.; Retana, D.; Baena, P.; et al. Stratification of radiosensitive brain metastases based on an actionable S100A9/RAGE resistance mechanism. Nat. Med. 2022, 28, 752–765. [Google Scholar] [CrossRef]
- Valiente, M.; Sepúlveda, J.M.; Pérez, A. Emerging targets for cancer treatment: S100A9/RAGE. ESMO Open 2023, 8, 100751. [Google Scholar] [CrossRef]
- Mayer, A.; Haist, M.; Loquai, C.; Grabbe, S.; Rapp, M.; Roth, W.; Vaupel, P.; Schmidberger, H. Role of Hypoxia and the Adenosine System in Immune Evasion and Prognosis of Patients with Brain Metastases of Melanoma: A Multiplex Whole Slide Immunofluorescence Study. Cancers 2020, 12, 3753. [Google Scholar] [CrossRef]
- Pitter, K.L.; Tamagno, I.; Alikhanyan, K.; Hosni-Ahmed, A.; Pattwell, S.S.; Donnola, S.; Dai, C.; Ozawa, T.; Chang, M.; Chan, T.A.; et al. Corticosteroids compromise survival in glioblastoma. Brain 2016, 139 Pt 5, 1458–1471. [Google Scholar] [CrossRef] [PubMed]
- Churilla, T.M.; Handorf, E.; Collette, S.; Collette, L.; Dong, Y.; Aizer, A.A.; Kocher, M.; Soffietti, R.; Alexander, B.M.; Weiss, S.E. Whole brain radiotherapy after stereotactic radiosurgery or surgical resection among patients with one to three brain metastases and favorable prognoses: A secondary analysis of EORTC 22952–26001. Ann. Oncol. 2017, 28, 2588–2594. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, L.; Zhao, M.; Cao, K.; Li, Y.; Liu, X.; Hou, Y.; Li, L.; Wang, L.; Chang, L.; et al. Real-world analysis of different intracranial radiation therapies in non-small cell lung cancer patients with 1–4 brain metastases. BMC Cancer 2022, 22, 1010. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Group | Patients n = 203 (%) |
|---|---|---|
| Age | <70 years | 154 (76) |
| ≥70 years | 49 (24) | |
| KPS | ≥70 | 104 (51.2) |
| <70 | 52 (25.6) | |
| Missing data | 47 (23.2) | |
| No. of brain metastases | >3 | 90 (44.3) |
| ≤3 | 80 (39.4) | |
| Missing data | 33 (16.3) | |
| Tumor type | NSCLC | 84 (41.4) |
| SCLC | 20 (9.9) | |
| Breast cancer | 21 (10.3) | |
| Melanoma | 28 (13.8) | |
| RCC | 20 (9.9) | |
| Other | 30 (14.7) | |
| Stable systemic disease | Yes | 27 (13.3) |
| No | 71 (34.9) | |
| Synchronous BM | 66 (32.5) | |
| Missing data | 39 (19.2) | |
| Radiotherapy technique | SRT | 40 (19.7) |
| WBRT ± SRT | 152 (74.8) | |
| Missing data | 11 (5.5) | |
| Diabetes mellitus | Yes | 39 (19.2) |
| No | 164 (80.3) | |
| Missing data | 0 (0) | |
| Arterial hypertension | Yes | 100 (49.3) |
| No | 94 (46.3) | |
| Missing data | 9 (4.4) | |
| Smoking | Yes | 68 (33.5) |
| No | 128 (63.1) | |
| Missing data | 7 (3.4) | |
| Peripheral arterial occlusive disease | Yes | 23 (11.3) |
| No | 176 (86.7) | |
| Missing data | 4 (2) | |
| Hypercholesterolemia | Yes | 7 (3.5) |
| No | 189 (93) | |
| Missing data | 7 (3.5) |
| Characteristic | Median OS | p-Value (Log Rank) |
|---|---|---|
| Age | 0.001 | |
| <70 years | 7.29 (5.34–9.24) | |
| ≥70 years | 4.14 (2.43–5.85) | |
| KPS | 0.03 | |
| ≥70 | 6.70 (2.99–10.42) | |
| <70 | 3.06 (1.16–4.95) | |
| No. of brain metastases | 0.443 | |
| >3 | 5.55 (4.03–7.07) | |
| ≤3 | 6.74 (4.63–8.84) | |
| Tumor type | 0.02 | |
| NSCLC | 6.01 (3.93–8.09) | |
| SCLC | 5.52 (3.2–7.84) | |
| Breast cancer | 7.46 (1.89–13.03) | |
| Melanoma | 9.53 (3.73–15.32) | |
| RCC | 2.96 (1.73–4.18) | |
| other | 4.17 (2.84–5.5) | |
| Diabetes mellitus | 0.003 | |
| Yes | 4.73 (2.81–6.65) | |
| No | 6.7 (4.5–8.89) | |
| Arterial hypertension | 0.443 | |
| Yes | 5.95 (4.29–7.61) | |
| No | 6.05 (3.51–8.58) | |
| Smoking history | 0.307 | |
| Yes | 6.08 (3.96–8.20) | |
| No | 5.72 (3.67–7.77) | |
| PAOD | 0.266 | |
| Yes | 4.17 (0–9.3) | |
| No | 5.95 (4.54–7.36) | |
| Hypercholesterolemia | 0.157 | |
| Yes | 4.11 (0–8.62) | |
| No | 0.71 (4.65–7.45) |
| Characteristic | n | With DM | Without DM | p-Value |
|---|---|---|---|---|
| Age (years) | 0.027 | |||
| <70 | 149 | 24 (61.5%) | 125 (78.6%) | |
| ≥70 | 49 | 15 (38.5%) | 34 (21.4%) | |
| KPS | 0.841 | |||
| <70 | 51 | 10 (34.5%) | 41 (32.5%) | |
| ≥70 | 104 | 19 (65.5%) | 85 (67.5%) | |
| Histology | 0.606 | |||
| NSCLC | 84 | 20 (51,3%) | 64 (40.3%) | |
| SCLC | 20 | 4 (10%) | 16 (10.1%) | |
| Melanoma | 28 | 2 (5.1%) | 26 (16.4%) | |
| Breast cancer | 20 | 3 (7.7%) | 17 (10.7%) | |
| Colorectal cancer | 5 | 1 (2.6%) | 4 (2.5%) | |
| RCC | 19 | 5 (12.8%) | 14 (8.8%) | |
| Other | 22 | 4 (10.3%) | 18 (11.3%) | |
| No. BM | 0.792 | |||
| ≤3 | 77 | 16 (44.4%) | 61 (46.9%) | |
| >3 | 69 | 20 (55.6%) | 69 (53.1%) | |
| Stable systemic disease | 0.154 | |||
| Yes | 27 | 2 (5.7%) | 25 (19.4%) | |
| No | 71 | 17 (48.6%) | 54 (41.9%) | |
| Synchronous BM | 66 | 16 (45.7%) | 50 (38.8%) | |
| Radiation modality | 0.441 | |||
| SRT alone | 40 | 6 (16.2%) | 34 (21.9%) | |
| WBRT ± SRT | 152 | 31 (83.8%) | 121 (78.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.; Poudyal, S.; Klagges, S.; Kuhnt, T.; Papsdorf, K.; Hambsch, P.; Wach, J.; Güresir, E.; Nägler, F.; Rühle, A.; et al. Diabetes Mellitus Is a Strong Independent Negative Prognostic Factor in Patients with Brain Metastases Treated with Radiotherapy. Cancers 2023, 15, 4845. https://doi.org/10.3390/cancers15194845
Jeong S, Poudyal S, Klagges S, Kuhnt T, Papsdorf K, Hambsch P, Wach J, Güresir E, Nägler F, Rühle A, et al. Diabetes Mellitus Is a Strong Independent Negative Prognostic Factor in Patients with Brain Metastases Treated with Radiotherapy. Cancers. 2023; 15(19):4845. https://doi.org/10.3390/cancers15194845
Chicago/Turabian StyleJeong, Seong, Soniya Poudyal, Sabine Klagges, Thomas Kuhnt, Kirsten Papsdorf, Peter Hambsch, Johannes Wach, Erdem Güresir, Franziska Nägler, Alexander Rühle, and et al. 2023. "Diabetes Mellitus Is a Strong Independent Negative Prognostic Factor in Patients with Brain Metastases Treated with Radiotherapy" Cancers 15, no. 19: 4845. https://doi.org/10.3390/cancers15194845
APA StyleJeong, S., Poudyal, S., Klagges, S., Kuhnt, T., Papsdorf, K., Hambsch, P., Wach, J., Güresir, E., Nägler, F., Rühle, A., Nicolay, N. H., & Seidel, C. (2023). Diabetes Mellitus Is a Strong Independent Negative Prognostic Factor in Patients with Brain Metastases Treated with Radiotherapy. Cancers, 15(19), 4845. https://doi.org/10.3390/cancers15194845








