Differential Expression and Diagnostic Value of MUC5AC Glycoforms in Pancreatic Ductal Adenocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen
2.2. Staining
2.3. Statistics
3. Results
3.1. Baseline Characteristics
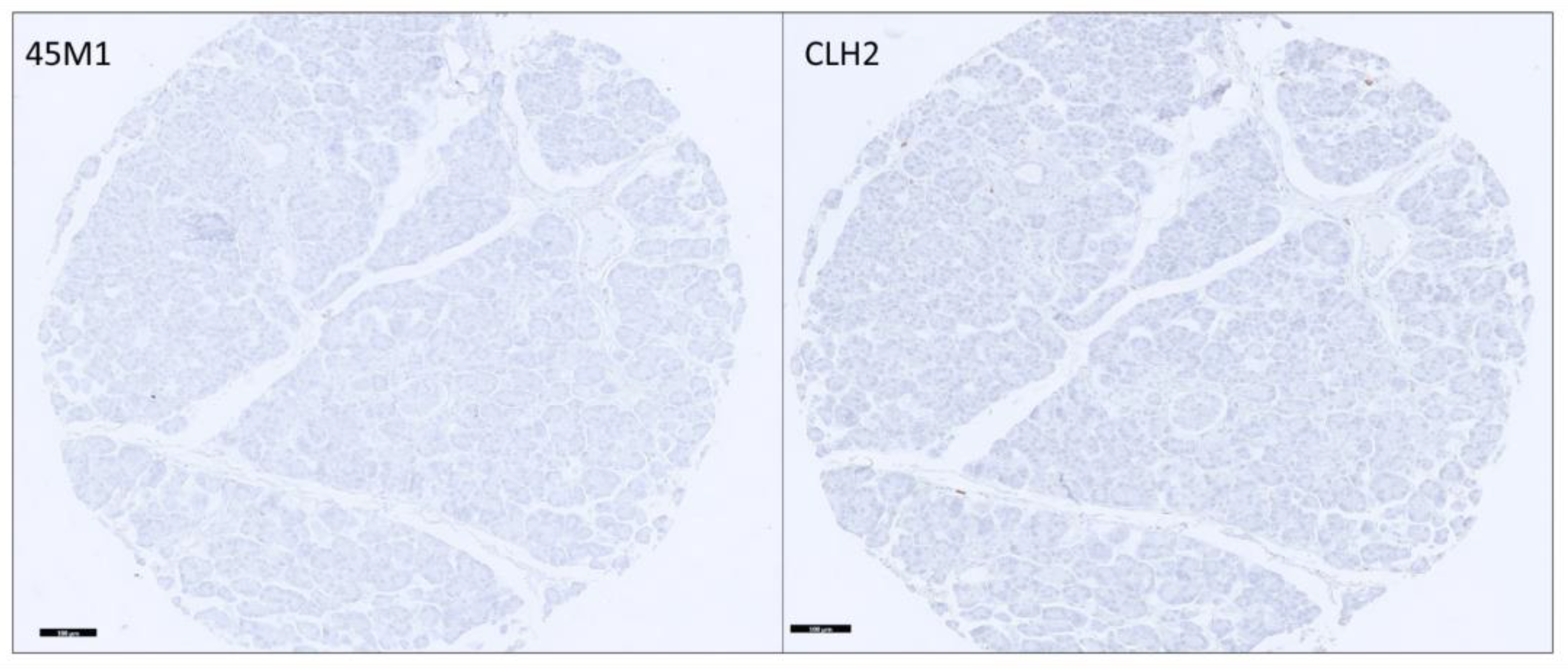
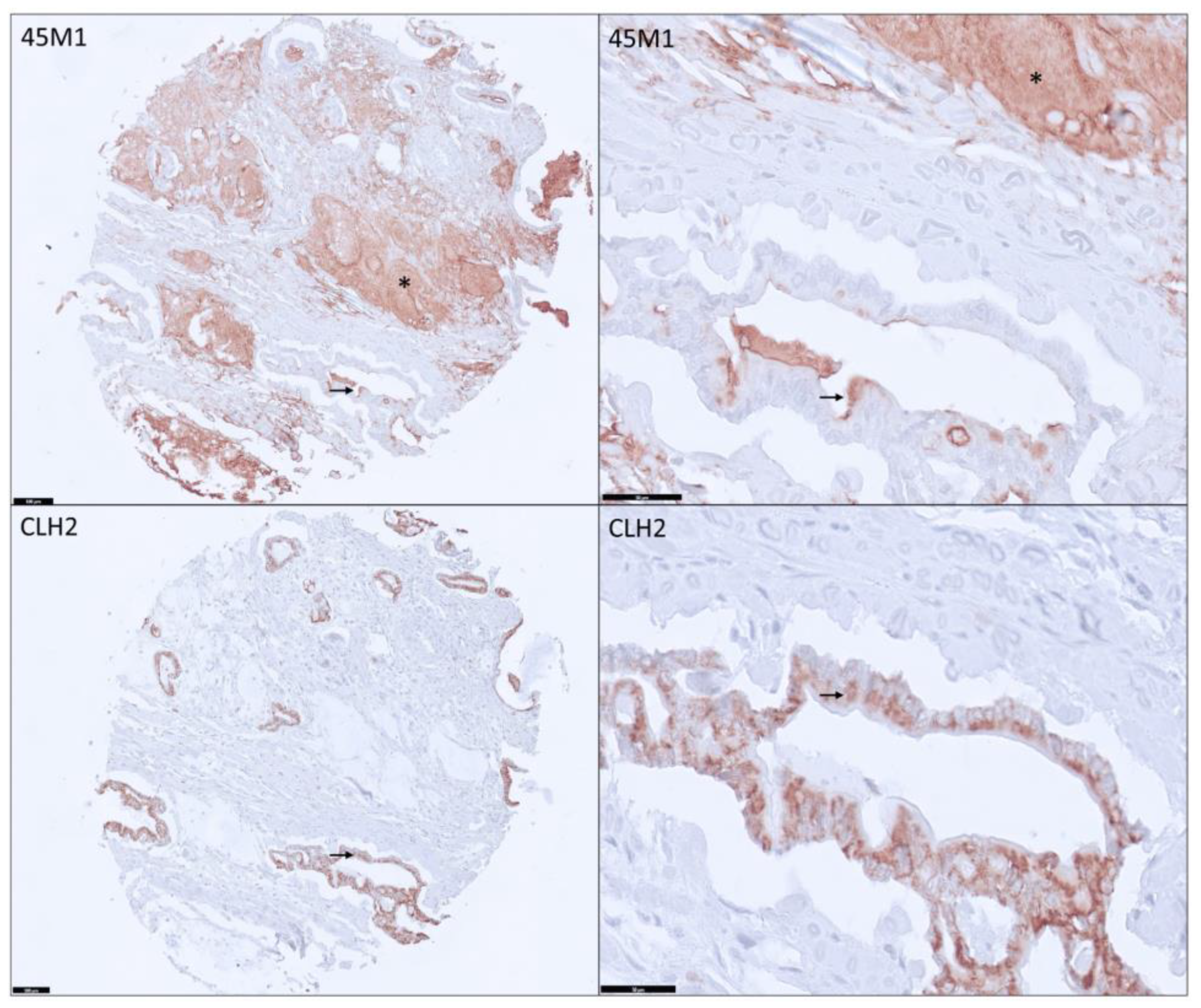
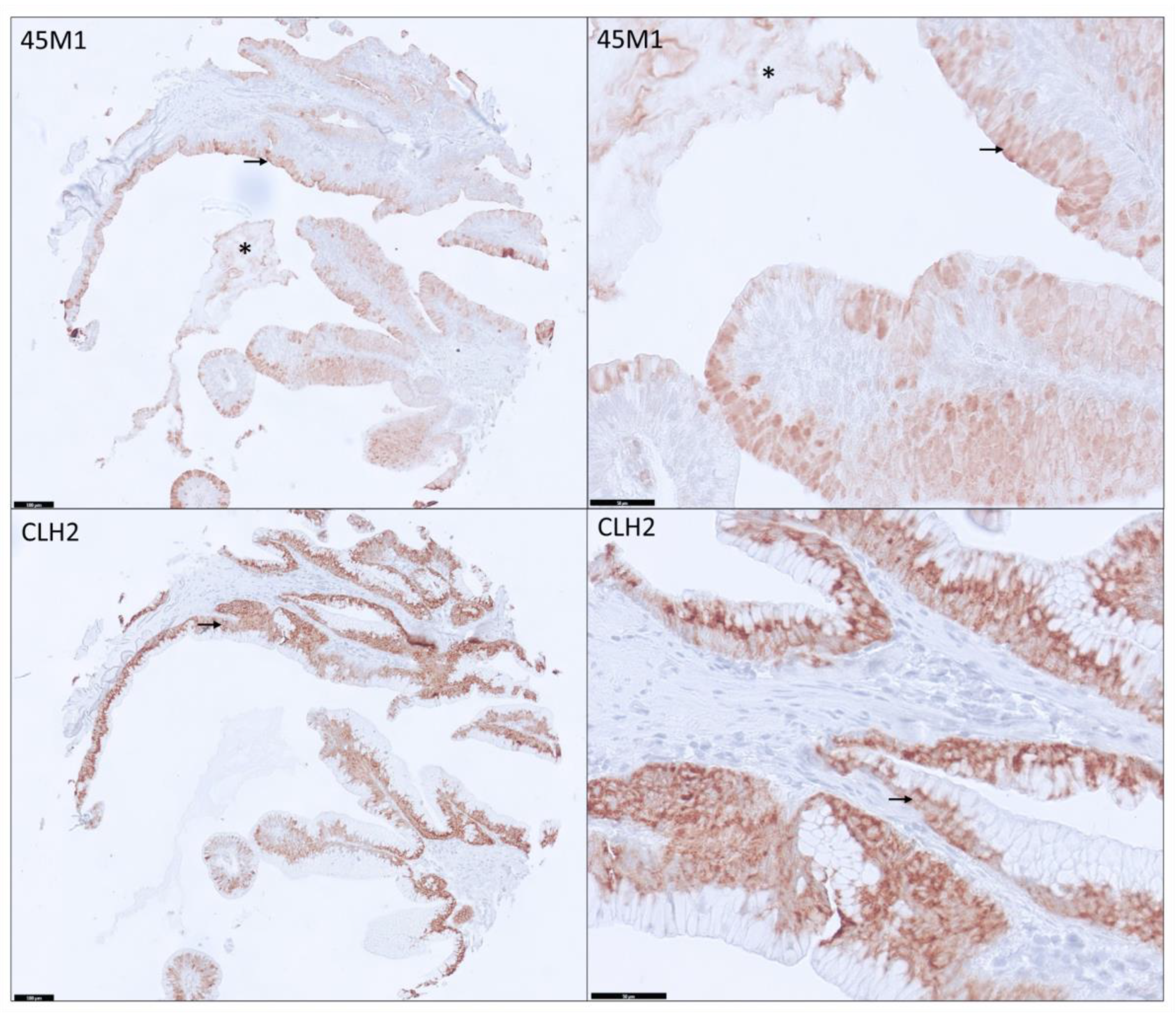
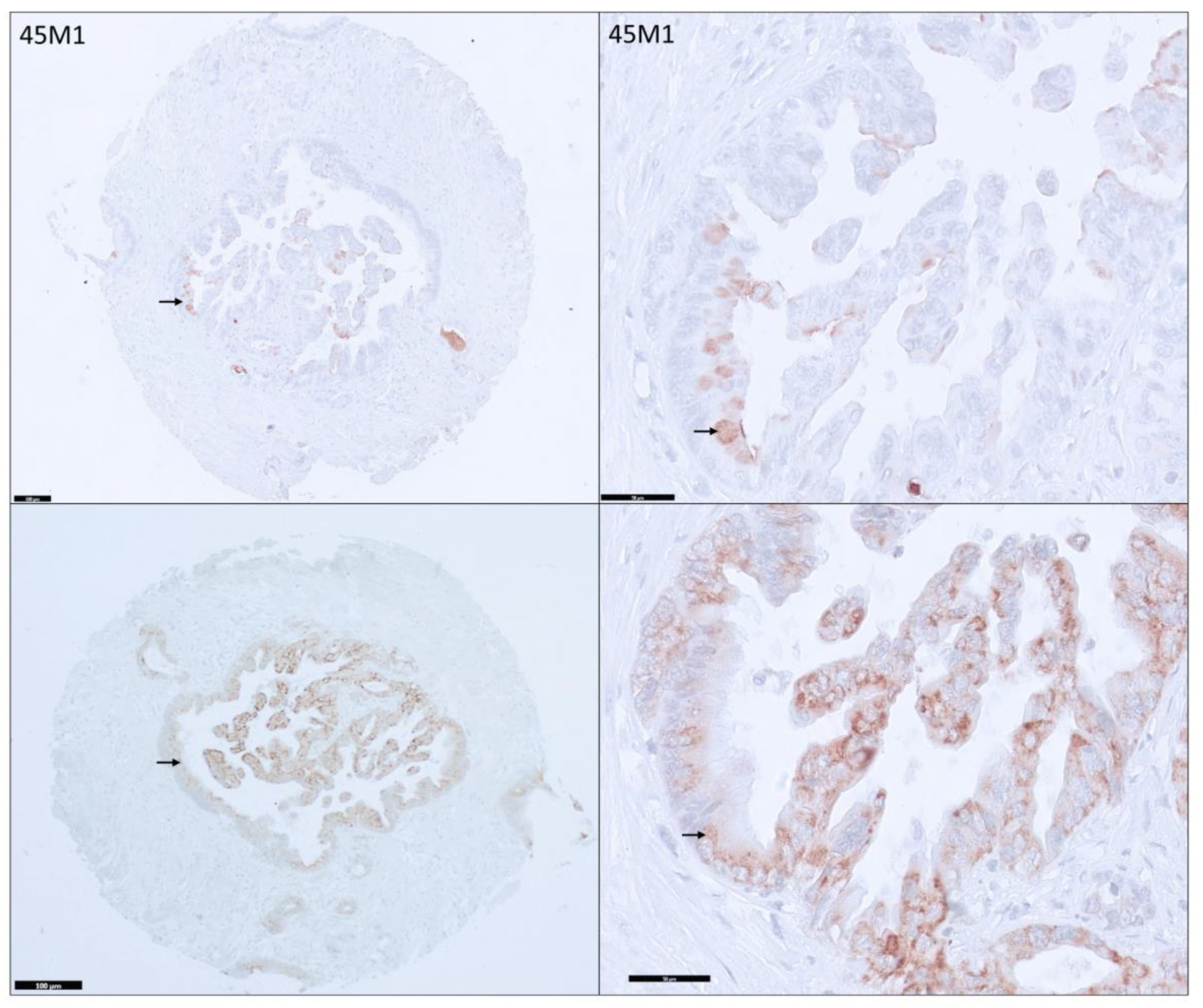
3.2. Distribution and Differential Expression of MUC5AC Glycoforms in Pancreatic Tissues
3.3. Distribution among PDAs
3.4. Differential Expression of MUC5AC Glycoforms in PDA
3.5. MUC5AC Expression and Pathological Characteristics in PDA
3.6. Diagnostic Value of MUC5AC
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER Research Data, 9 Registries, November 2019 Sub (1975–2017). Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 6 September 2023).
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Curry, S.J.; Doubeni, C.A.; Epling, J.W., Jr.; Kubik, M.; et al. Screening for Pancreatic Cancer: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA 2019, 322, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Sheel, A.; Addison, S.; Nuguru, S.P.; Manne, A. Is Cell-Free DNA Testing in Pancreatic Ductal Adenocarcinoma Ready for Prime Time? Cancers 2022, 14, 3453. [Google Scholar] [CrossRef] [PubMed]
- Manne, A.; Esnakula, A.; Abushahin, L.; Tsung, A. Understanding the Clinical Impact of MUC5AC Expression on Pancreatic Ductal Adenocarcinoma. Cancers 2021, 13, 3059. [Google Scholar] [CrossRef] [PubMed]
- Manne, A.; Kasi, A.; Esnakula, A.K.; Paluri, R.K. Predictive Value of MUC5AC Signature in Pancreatic Ductal Adenocarcinoma: A Hypothesis Based on Preclinical Evidence. Int. J. Mol. Sci. 2023, 24, 8087. [Google Scholar] [CrossRef] [PubMed]
- Manne, A.; Cheng, X.; Tsung, A.; Esnakula, A.K. Expression profiles of MUC5AC glycoforms in neoplastic and non-neoplastic pancreatic tissue. J. Clin. Oncol. 2023, 41, e16008. [Google Scholar] [CrossRef]
- Manne, A.; Mneimneh, W.; Elkadi, O.; Escobar, D.E.; Coley, J.; Guzman, G.B.; Fnu, S.M.d.; Alkharabsheh, O.; Khushman, M.d.M. The pattern of mucin 5AC (MUC5AC) expression using immunohistochemistry and its prognostic significance in patients with pancreatic ductal adenocarcinoma. J. Clin. Oncol. 2020, 38, e16756. [Google Scholar] [CrossRef]
- Lüttges, J.; Feyerabend, B.; Buchelt, T.; Pacena, M.; Klöppel, G. The mucin profile of noninvasive and invasive mucinous cystic neoplasms of the pancreas. Am. J. Surg. Pathol. 2002, 26, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Dwertmann Rico, S.; Büscheck, F.; Dum, D.; Luebke, A.M.; Kluth, M.; Hube-Magg, C.; Hinsch, A.; Höflmayer, D.; Perez, D.; Izbicki, J.R.; et al. Mucin 5AC expression is common but unrelated to tumor progression in pancreatic adenocarcinoma. Int. J. Immunopathol. Pharmacol. 2022, 36, 039463202211065. [Google Scholar] [CrossRef] [PubMed]
- Krishn, S.R. Secretory Mucin MUC5AC in Gastrointestinal Malignancies. Ph.D. Thesis, University of Nebraska Medical Center, Omaha, NE, USA, 2016. [Google Scholar]
- Kaur, S.; Smith, L.M.; Patel, A.; Menning, M.; Watley, D.C.; Malik, S.S.; Krishn, S.R.; Mallya, K.; Aithal, A.; Sasson, A.R.; et al. A Combination of MUC5AC and CA19-9 Improves the Diagnosis of Pancreatic Cancer: A Multicenter Study. Am. J. Gastroenterol. 2017, 112, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Meyerholz, D.K.; Beck, A.P. Principles and approaches for reproducible scoring of tissue stains in research. Lab. Invest. 2018, 98, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Horinouchi, M.; Saitou, M.; Higashi, M.; Nomoto, M.; Goto, M.; Yonezawa, S. Mucin expression profile in pancreatic cancer and the precursor lesions. J. Hepatobiliary Pancreat. Surg. 2007, 14, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Rico, S.D.; Mahnken, M.; Büscheck, F.; Dum, D.; Luebke, A.M.; Kluth, M.; Hube-Magg, C.; Hinsch, A.; Höflmayer, D.; Möller-Koop, C.; et al. MUC5AC Expression in Various Tumor Types and Nonneoplastic Tissue: A Tissue Microarray Study on 10 399 Tissue Samples. Technol. Cancer Res. Treat. 2021, 20, 15330338211043328. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.E.; Bae, H.I.; Park, H.U.; Kuan, S.F.; Crawley, S.C.; Ho, J.J.L.; Kim, Y.S. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology 2002, 123, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, M.; Nagata, K.; Nakamura, A.; Goto, M.; Takao, S.; Sakamoto, M.; Fukushima, N.; Miwa, A.; Irimura, T.; Imai, K.; et al. Expression of Different Glycoforms of Membrane Mucin(MUC1) and Secretory Mucin (MUC2, MUC5AC and MUC6) in Pancreatic Neoplasms. Acta Histochem. Et Cytochem. 2003, 36, 443–453. [Google Scholar] [CrossRef]
- Krishn, S.R.; Ganguly, K.; Kaur, S.; Batra, S.K. Ramifications of secreted mucin MUC5AC in malignant journey: A holistic view. Carcinogenesis 2018, 39, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Nishida, Y.; Yokoyama, S.; Tsutsumida, H.; Houjou, I.; Kitamoto, S.; Goto, M.; Higashi, M.; Yonezawa, S. Expression of MUC5AC, an early marker of pancreatobiliary cancer, is regulated by DNA methylation in the distal promoter region in cancer cells. J. Hepato Biliary Pancreat. Sci. 2010, 17, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Perrais, M.; Desseyn, J.L.; Aubert, J.P.; Pigny, P.; Van Seuningen, I. Epigenetic regulation (DNA methylation, histone modifications) of the 11p15 mucin genes (MUC2, MUC5AC, MUC5B, MUC6) in epithelial cancer cells. Oncogene 2007, 26, 6566–6576. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.J.; Han, S.W.; Pan, P.L.; Deng, G.; Kuan, S.F.; Kim, Y.S. Methylation status of promoters and expression of MUC2 and MUC5AC mucins in pancreatic cancer cells. Int. J. Oncol. 2003, 22, 273–279. [Google Scholar] [CrossRef] [PubMed]
| N | MM ± IM | MM-Positive | MM-Only | Ap/Cy/Ec | IM-Only | MM + IM | All Negative | |
|---|---|---|---|---|---|---|---|---|
| Neoplastic | 54 | 30 | 19 | 2 | 12/16/9 | 11 | 17 | 24 |
| • PDA | 43 | 28 | 19 | 2 | 12/16/9 | 9 | 17 | 15 |
| 38 5 | 27 1 | 18 1 | 2 0 | 11/15/9 1/1/0 | 9 0 | 16 1 | 11 4 |
| • NET | 11 | 2 | 0 | 0 | 0 | 2 | 0 | 9 |
| Non-neoplastic | 42 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| • Normal adjacent | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| • Normal | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| • Pancreatitis | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MUC5AC Glycoform | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95% CI) | p Value |
|---|---|---|---|---|---|---|
| MM or IM | 71.05% (54.1%–84.58%) | 96.23% (87.02%–99.54%) | 93.10% (77.23%–99.15%) | 82.23% (70.47%–90.8%) | 85.71% (76.81%–92.17%) | <0.0001 |
| MM + IM | 42.11% (26.31%–59.18%) | 100% (93.28%–100.00%) | 100% (79.41%–100.00%) | 70.67% (59.02%–80.62%) | 75.82% (65.72%–84.19%) | <0.0001 |
| MM positive | 47.37% (30.98%–64.18%) | 100% (93.28%–100%) | 100% (81.47%–100%) | 72.60% (60.91%–82.39%) | 78.02% (68.12%–86.03%) | <0.0001 |
| IM positive | 65.79% (48.65%–80.37%) | 96.23% (87.02%–99.54%) | 92.59% (75.71%–99.09%) | 79.69% (67.77%–88.72%) | 83.52% (74.27%–90.47%) | <0.0001 |
| MM Ap | 28.95% (15.42%–45.90%) | 100% (93.28%–100%) | 100% (71.51%–100%) | 66.25% (54.81%–76.45%) | 70.33% (59.84%–79.45%) | <0.0001 |
| MM Cy | 39.47% (24.04%–56.61%) | 100.00% (93.28% to 100%) | 100% (78.20%–100.00%) | 69.74% (58.13%–79.75%) | 74.73% (64.53%–83.25%) | <0.0001 |
| MM Ec | 23.68% (11.44%–40.24%) | 100 (93.28%–100%) | 100% (66.37%–100%) | 64.63% (53.30%–74.88%) | 68.13% (57.53%–77.51%) | 0.0002 |
| MUC5AC Glycoform | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95% CI) | p Value |
|---|---|---|---|---|---|---|
| MM or IM | 59.2% (44.21%—73%) | 100% (91.59%–100%) | 100% (88.06%-100%) | 67.7% (54.66%–79.06%) | 78.1% (68.12%–86.03%) | <0.0001 |
| MM+ IM | 32.65% (19.95%–47.54%) | 100% (91.59%–100%) | 100% (79.41%–100%) | 56% (44.06%–64.36%) | 63.74% (52.99%–73.56%) | <0.0001 |
| MM positive | 36.73% (23.42%–51.71%) | 100% (91.59%–100%) | 100% (81.47%–100%) | 57.53% (45.41%–69.03%) | 65.93% (55.25%–75.55%) | <0.0001 |
| IM positive | 55.1% (40.23%–69.33%) | 100% (91.59%–100%) | 100% (87.23–100% | 65.625% (52.7%–77.05%) | 75.82% (65.72%–84.19%) | <0.0001 |
| MM Ap | 22.45% (11.77%–36.62%) | 100% (91.59%–100%) | 100% (71.51%–100%) | 52.5 (41.02%–63.79%) | 58.2% (47.43%–68.5%) | 0.0011 |
| MM Cy | 30.61% (18.25%–45.42%) | 100.00% (91.59%–100%) | 100% (78.20%–100%) | 55.26% (43.41%–66.69%) | 62.64% (51.87%–72.56%) | <0.0001 |
| MM Ec | 18.36% (8.76%–32.02%) | 100% (91.59%–100%) | 100% (66.37%–100%) | 51.22% (39.92%–62.42%) | 56.04% (45.25%–66.44%) | 0.0034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manne, A.; Yu, L.; Hart, P.A.; Tsung, A.; Esnakula, A. Differential Expression and Diagnostic Value of MUC5AC Glycoforms in Pancreatic Ductal Adenocarcinoma. Cancers 2023, 15, 4832. https://doi.org/10.3390/cancers15194832
Manne A, Yu L, Hart PA, Tsung A, Esnakula A. Differential Expression and Diagnostic Value of MUC5AC Glycoforms in Pancreatic Ductal Adenocarcinoma. Cancers. 2023; 15(19):4832. https://doi.org/10.3390/cancers15194832
Chicago/Turabian StyleManne, Ashish, Lianbo Yu, Phil A Hart, Allan Tsung, and Ashwini Esnakula. 2023. "Differential Expression and Diagnostic Value of MUC5AC Glycoforms in Pancreatic Ductal Adenocarcinoma" Cancers 15, no. 19: 4832. https://doi.org/10.3390/cancers15194832
APA StyleManne, A., Yu, L., Hart, P. A., Tsung, A., & Esnakula, A. (2023). Differential Expression and Diagnostic Value of MUC5AC Glycoforms in Pancreatic Ductal Adenocarcinoma. Cancers, 15(19), 4832. https://doi.org/10.3390/cancers15194832







