Impact of Radiotherapy on Malfunctions and Battery Life of Cardiac Implantable Electronic Devices in Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
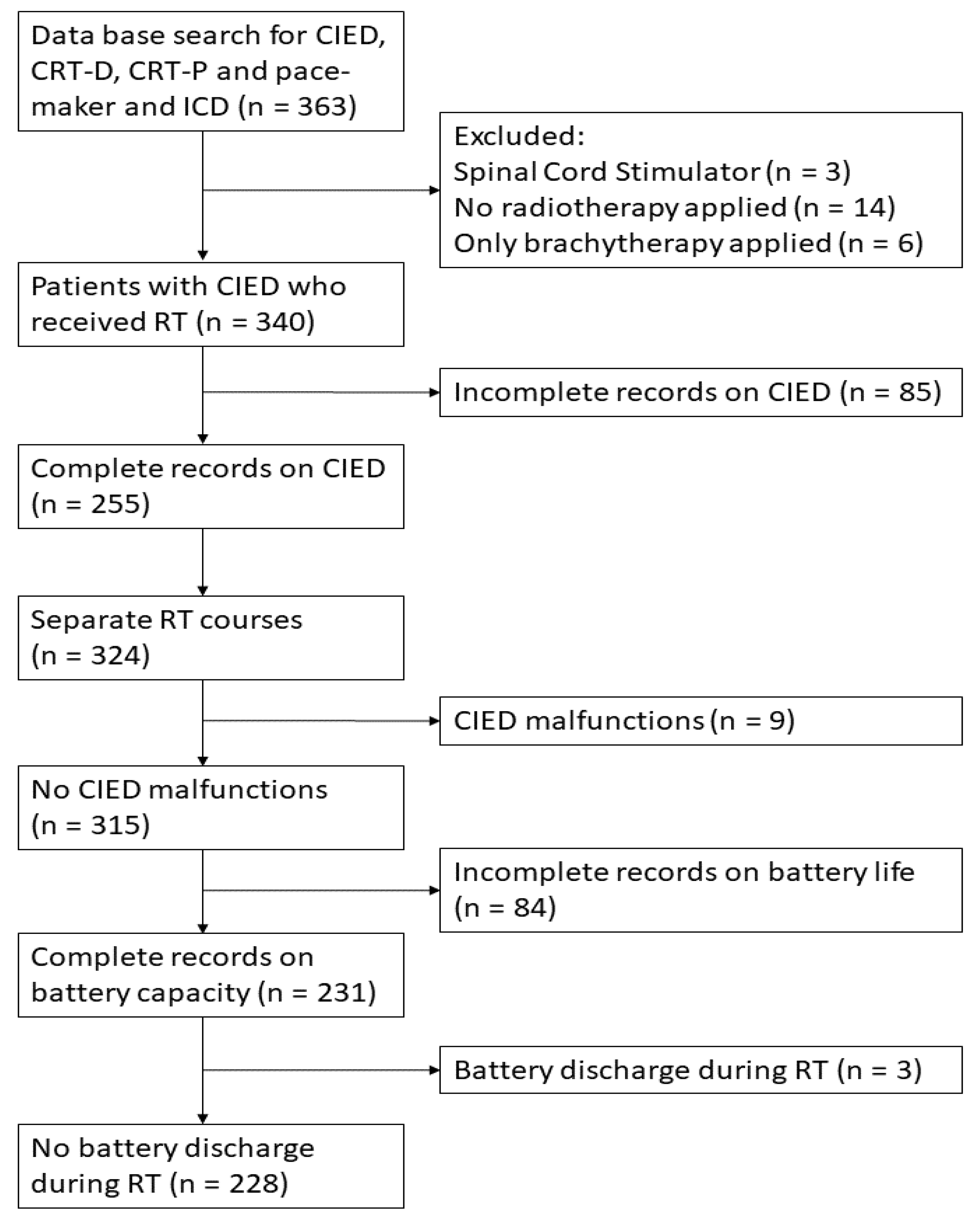
2. Materials and Methods
3. Results
3.1. Results on Patient and Treatment Characteristics
3.2. CIED Malfunctions
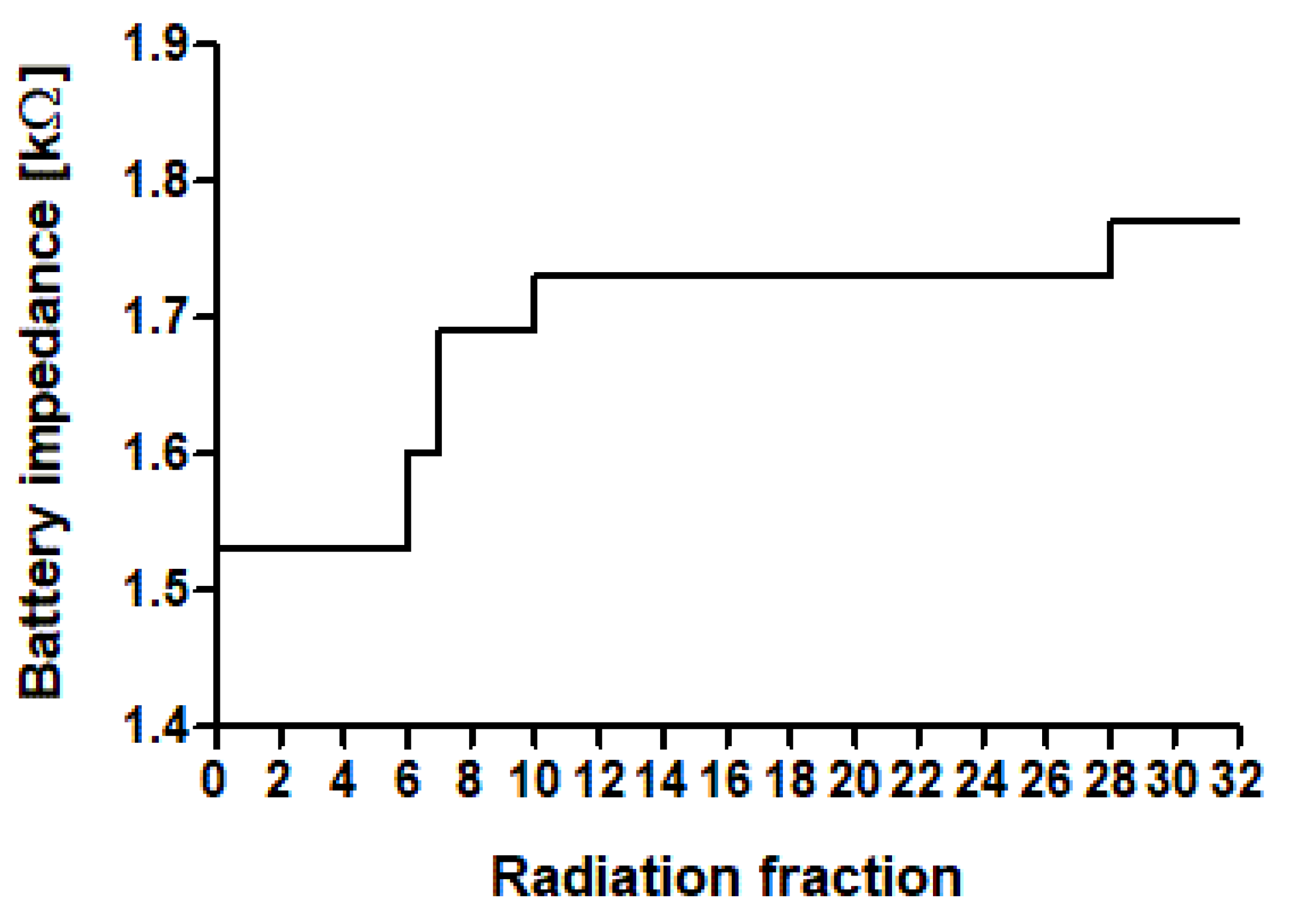
3.3. Premature Battery Depletion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valzania, C.; Torbica, A.; Tarricone, R.; Leyva, F.; Boriani, G. Implant Rates of Cardiac Implantable Electrical Devices in Europe: A Systematic Literature Review. Health Policy 2016, 120, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zecchin, M.; Torre, M.; Carrani, E.; Sampaolo, L.; Ciminello, E.; Ortis, B.; Ricci, R.; Proclemer, A.; Sinagra, G.; Boriani, G. Seventeen-Year Trend (2001–2017) in Pacemaker and Implantable Cardioverter-Defibrillator Utilization Based on Hospital Discharge Database Data: An Analysis by Age Groups. Eur. J. Intern. Med. 2021, 84, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef]
- Miften, M.; Mihailidis, D.; Kry, S.F.; Reft, C.; Esquivel, C.; Farr, J.; Followill, D.; Hurkmans, C.; Liu, A.; Gayou, O.; et al. Management of Radiotherapy Patients with Implanted Cardiac Pacemakers and Defibrillators: A Report of the AAPM TG-203. Med. Phys. 2019, 46, e757–e788. [Google Scholar] [CrossRef] [PubMed]
- Gauter-Fleckenstein, B.; Israel, C.W.; Dorenkamp, M.; Dunst, J.; Roser, M.; Schimpf, R.; Steil, V.; Schäfer, J.; Höller, U.; Wenz, F.; et al. DEGRO/DGK Guideline for Radiotherapy in Patients with Cardiac Implantable Electronic Devices. Strahlenther. Onkol. 2015, 191, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Zecchin, M.; Severgnini, M.; Fiorentino, A.; Malavasi, V.L.; Menegotti, L.; Alongi, F.; Catanzariti, D.; Jereczek-Fossa, B.A.; Stasi, M.; Russi, E.; et al. Management of Patients with Cardiac Implantable Electronic Devices (CIED) Undergoing Radiotherapy: A Consensus Document from Associazione Italiana Aritmologia E Cardiostimolazione (AIAC), Associazione Italiana Radioterapia Oncologica (AIRO), Associazione Italiana Fisica Medica (AIFM). Int. J. Cardiol. 2018, 255, 175–183. [Google Scholar]
- Escande, A.; Frey, P.; Lacornerie, T.; Mervoyer, E.; Chargari, C.; Laurans, M.; Mornex, F.; Marijon, É.; Giraud, P. Radiotherapy for Patient with Cardiac Implantable Electronic Device, Consensus from French Radiation Oncology Society. Cancer Radiother. 2022, 26, 404–410. [Google Scholar] [CrossRef]
- Indik, J.H.; Gimbel, J.R.; Abe, H.; Alkmim-Teixeira, R.; Birgersdotter-Green, U.; Clarke, G.D.; Dickfeld, T.-M.L.; Froelich, J.W.; Grant, J.; Hayes, D.L.; et al. 2017 HRS Expert Consensus Statement on Magnetic Resonance Imaging and Radiation Exposure in Patients with Cardiovascular Implantable Electronic Devices. Heart Rhythm 2017, 14, e97–e153. [Google Scholar] [CrossRef]
- Hurkmans, C.W.; Knegjens, J.L.; Oei, B.S.; Maas, A.J.J.; Uiterwaal, G.J.; van der Borden, A.J.; Ploegmakers, M.M.J.; van Erven, L. Dutch Society of Radiotherapy and Oncology (NVRO) Management of Radiation Oncology Patients with a Pacemaker or ICD: A New Comprehensive Practical Guideline in The Netherlands. Radiat. Oncol. 2012, 7, 198. [Google Scholar] [CrossRef]
- Ohno, T.; Soejima, T.; Sekiguchi, Y.; Hashimoto, T.; Koike, I.; Matsubara, H.; Nakamura, K.; Nitta, K.; Takahashi, S.; Tsujino, K.; et al. JASTRO/JCS Guidelines for Radiotherapy in Patients with Cardiac Implantable Electronic Devices. J. Radiat. Res. 2021, 62, 172–184. [Google Scholar] [CrossRef]
- Tajstra, M.; Blamek, S.; Niedziela, J.T.; Gadula-Gacek, E.; Przybylski, A.; Blicharz, J.; Oręziak, A.; Miszczyk, L.; Gepner, K.; Fijuth, J.; et al. Patients with Cardiac Implantable Electronic Devices Undergoing Radiotherapy in Poland. Expert Opinion of the Heart Rhythm Section of the Polish Cardiac Society and the Polish Society of Radiation Oncology. Kardiol. Pol. 2019, 77, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Bagur, R.; Chamula, M.; Brouillard, É.; Lavoie, C.; Nombela-Franco, L.; Julien, A.-S.; Archambault, L.; Varfalvy, N.; Gaudreault, V.; Joncas, S.X.; et al. Radiotherapy-Induced Cardiac Implantable Electronic Device Dysfunction in Patients With Cancer. Am. J. Cardiol. 2017, 119, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Frey, P.; Irles, D.; Dompnier, A.; Akret, C.; Hosu, I.C.; Narayanan, K.; Mazoyer, F.; Yayehd, K.; Guillon, B.; Marijon, E. Cardiac Implantable Electronic Device Dysfunctions in Patients Undergoing Radiotherapy: A Prospective Cohort Study. J. Cardiovasc. Electrophysiol. 2022, 33, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.D.; Jensen, G.L.; Tang, C.; Pollard, J.M.; Kry, S.F.; Krishnan, S.; Dougherty, A.H.; Gomez, D.R.; Rozner, M.A. Radiotherapy-Induced Malfunction in Contemporary Cardiovascular Implantable Electronic Devices: Clinical Incidence and Predictors. JAMA Oncol. 2015, 1, 624–632. [Google Scholar] [CrossRef]
- Malavasi, V.L.; De Marco, G.; Imberti, J.F.; Placentino, F.; Vitolo, M.; Mazzeo, E.; Cicoria, G.; Casali, E.; Turco, V.; Lohr, F.; et al. Radiotherapy-Induced Malfunctions of Cardiac Implantable Electronic Devices in Cancer Patients. Intern. Emerg. Med. 2020, 15, 967–973. [Google Scholar] [CrossRef]
- Niedziela, J.T.; Blamek, S.; Gadula-Gacek, E.; Gorol, J.; Kurek, A.; Witek, M.; Wojtaszczyk, A.; Plaza, P.; Miszczyk, L.; Gąsior, M.; et al. Radiation Therapy in Patients with Cardiac Implantable Electronic Devices. Kardiol. Pol. 2021, 79, 156–160. [Google Scholar] [CrossRef]
- Sharifzadehgan, A.; Laurans, M.; Thuillot, M.; Huertas, A.; Baudinaud, P.; Narayanan, K.; Mirabel, M.; Bibault, J.-E.; Frey, P.; Waldmann, V.; et al. Radiotherapy in Patients With a Cardiac Implantable Electronic Device. Am. J. Cardiol. 2020, 128, 196–201. [Google Scholar] [CrossRef]
- Zaremba, T.; Jakobsen, A.R.; Søgaard, M.; Thøgersen, A.M.; Johansen, M.B.; Madsen, L.B.; Riahi, S. Risk of Device Malfunction in Cancer Patients with Implantable Cardiac Device Undergoing Radiotherapy: A Population-Based Cohort Study. Pacing Clin. Electrophysiol. 2015, 38, 343–356. [Google Scholar] [CrossRef]
- Brambatti, M.; Mathew, R.; Strang, B.; Dean, J.; Goyal, A.; Hayward, J.E.; Long, L.; DeMeis, P.; Smoke, M.; Connolly, S.J.; et al. Management of Patients with Implantable Cardioverter-Defibrillators and Pacemakers Who Require Radiation Therapy. Heart Rhythm 2015, 12, 2148–2154. [Google Scholar] [CrossRef]
- Kapa, S.; Fong, L.; Blackwell, C.R.; Herman, M.G.; Schomberg, P.J.; Hayes, D.L. Effects of Scatter Radiation on ICD and CRT Function. Pacing Clin. Electrophysiol. 2008, 31, 727–732. [Google Scholar] [CrossRef]
- Ferrara, T.; Baiotto, B.; Malinverni, G.; Caria, N.; Garibaldi, E.; Barboni, G.; Stasi, M.; Gabriele, P. Irradiation of Pacemakers and Cardio-Defibrillators in Patients Submitted to Radiotherapy: A Clinical Experience. Tumori 2010, 96, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Levis, M.; Andreis, A.; Badellino, S.; Budano, C.; Caivano, D.; Cerrato, M.; Orlandi, E.; Bissolino, A.; Angelico, G.; Cavallin, C.; et al. Safety of Lung Stereotactic Ablative Radiotherapy for the Functioning of Cardiac Implantable Electronic Devices. Radiother. Oncol. 2021, 156, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Brouillard, É.; Chamula, M.; Lavoie, C.; Varfalvy, N.; Archambault, L. Radiation Therapy-Induced Dysfunction in Cardiovascular Implantable Electronic Devices. Pract. Radiat. Oncol. 2019, 9, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.; Rice, S.; Lamichhane, N.; Chen, S.; Mohindra, P. Conformal Radiation Therapy in Patients With Cardiovascular Implantable Electronic Devices: Proposed Practical Implementation of the 2019 American Association of Physicists in Medicine Task Group No. 203 Risk-Stratified Interrogation Schedule. Pract. Radiat. Oncol. 2021, 11, e402–e414. [Google Scholar] [CrossRef]
- Gauter-Fleckenstein, B.; Barthel, C.; Büttner, S.; Wenz, F.; Borggrefe, M.; Tülümen, E. Effectivity and Applicability of the German DEGRO/DGK-Guideline for Radiotherapy in CIED-Bearing Patients. Radiother. Oncol. 2020, 152, 208–215. [Google Scholar] [CrossRef]
- Bravo-Jaimes, K.; Samala, V.; Fernandez, G.; Moravan, M.J.; Dhakal, S.; Shah, A.H.; Messing, S.; Singh, K.; Aktas, M.K. CIED Malfunction in Patients Receiving Radiation Is a Rare Event That Could Be Detected by Remote Monitoring. J. Cardiovasc. Electrophysiol. 2018, 29, 1268–1275. [Google Scholar] [CrossRef]
- Yeung, C.; Hazim, B.; Campbell, D.; Gooding, J.; Li, S.X.; Tam, H.K.; Hopman, W.M.; Chacko, S.; Redfearn, D.P.; Simpson, C.; et al. Radiotherapy for Patients with Cardiovascular Implantable Electronic Devices: An 11-Year Experience. J. Interv. Card. Electrophysiol. 2019, 55, 333–341. [Google Scholar] [CrossRef]
- Maisel, W.H.; Moynahan, M.; Zuckerman, B.D.; Gross, T.P.; Tovar, O.H.; Tillman, D.-B.; Schultz, D.B. Pacemaker and ICD Generator Malfunctions: Analysis of Food and Drug Administration Annual Reports. JAMA 2006, 295, 1901–1906. [Google Scholar] [CrossRef]
- Malavasi, V.L.; Imberti, J.F.; Tosetti, A.; Romiti, G.F.; Vitolo, M.; Zecchin, M.; Mazzeo, E.; Giuseppina, D.M.; Lohr, F.; Lopez-Fernandez, T.; et al. A Systematic Review and Meta-analysis on Oncological Radiotherapy in Patients with a Cardiac Implantable Electronic Device: Prevalence and Predictors of Device Malfunction in 3121 Patients. Eur. J. Clin. Investig. 2023, 53, e13862. [Google Scholar] [CrossRef]
- Elders, J.; Kunze-Busch, M.; Smeenk, R.J.; Smeets, J.L.R.M. High Incidence of Implantable Cardioverter Defibrillator Malfunctions during Radiation Therapy: Neutrons as a Probable Cause of Soft Errors. Europace 2013, 15, 60–65. [Google Scholar] [CrossRef]
- Wilkinson, J.D.; Bounds, C.; Brown, T.; Gerbi, B.J.; Peltier, J. Cancer-Radiotherapy Equipment as a Cause of Soft Errors in Electronic Equipment. IEEE Trans. Device Mater. Reliab. 2005, 5, 449–451. [Google Scholar] [CrossRef]
- Gauter-Fleckenstein, B.; Tülümen, E.; Jahnke, L.; Nguyen, J.; Wenz, F. Investigation of Mechanisms of Radiation-Induced CIED Failures with Flattening Filter-Free-VMAT. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, E661. [Google Scholar] [CrossRef][Green Version]
- Barmore, W.; Patel, H.; Voong, C.; Tarallo, C.; Calkins, J.B., Jr. Effects of Medically Generated Electromagnetic Interference from Medical Devices on Cardiac Implantable Electronic Devices: A Review. World J. Cardiol. 2022, 14, 446–453. [Google Scholar] [CrossRef]
- Burke, B.; Lamey, M.; Rathee, S.; Murray, B.; Fallone, B.G. Radio Frequency Noise from Clinical Linear Accelerators. Phys. Med. Biol. 2009, 54, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Prisciandaro, J.I.; Makkar, A.; Fox, C.J.; Hayman, J.A.; Horwood, L.; Pelosi, F.; Moran, J.M. Dosimetric Review of Cardiac Implantable Electronic Device Patients Receiving Radiotherapy. J. Appl. Clin. Med. Phys. 2015, 16, 5189. [Google Scholar] [CrossRef] [PubMed]
- Azraai, M.; D’Souza, D.; Lin, Y.-H.; Nadurata, V. Current Clinical Practice in Patients with Cardiac Implantable Electronic Devices Undergoing Radiotherapy: A Literature Review. Europace 2022, 24, 362–374. [Google Scholar] [CrossRef] [PubMed]
- López-Honrubia, V.; Hidalgo-Olivares, V.M.; Dobón-Roux, M.; Martí-Laosa, M.M.; Castro-Larefors, S.; Fernandez-Lopez, J.; Andres, I.; Roche, O.; Rovirosa, A.; Arenas, M.; et al. Radiotherapy Is Safe in Patients with Implantable Cardiac Devices. Analysis of a Systematic Interrogation Follow-Up. Clin. Transl. Oncol. 2020, 22, 2286–2292. [Google Scholar] [CrossRef]
- Gomez, D.R.; Poenisch, F.; Pinnix, C.C.; Sheu, T.; Chang, J.Y.; Memon, N.; Mohan, R.; Rozner, M.A.; Dougherty, A.H. Malfunctions of Implantable Cardiac Devices in Patients Receiving Proton Beam Therapy: Incidence and Predictors. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 570–575. [Google Scholar] [CrossRef]
- Zagzoog, A.; Wronski, M.; Birnie, D.H.; Yeung, C.; Baranchuk, A.; Healey, J.S.; Golian, M.; Boles, U.; Carrizo, A.G.; Turner, S.; et al. Assessment of Radiation-Induced Malfunction in Cardiac Implantable Electronic Devices. CJC Open 2021, 3, 1438–1443. [Google Scholar] [CrossRef]
- Lau, E.W. Technologies for Prolonging Cardiac Implantable Electronic Device Longevity. Pacing Clin. Electrophysiol. 2017, 40, 75–96. [Google Scholar] [CrossRef]
- Guédon-Moreau, L.; Finat, L.; Klein, C.; Kouakam, C.; Marquié, C.; Klug, D.; Potelle, C.; Ninni, S.; Brigadeau, F.; Mirabel, X.; et al. Usefulness of Remote Monitoring for the Early Detection of Back-up Mode in Implantable Cardioverter Defibrillators. Arch. Cardiovasc. Dis. 2021, 114, 287–292. [Google Scholar] [CrossRef] [PubMed]
| Variable | Number | (%) | |
|---|---|---|---|
| Localization | |||
| Head and neck | 73 | 22.5% | |
| Thorax | 119 | 36.7% | |
| Abdomen | 25 | 7.7% | |
| Pelvis | 89 | 27.5% | |
| Upper extremities | 7 | 2.2% | |
| Lower extremities | 11 | 3.4% | |
| Radiation therapy dose in Gy | |||
| Median dose (IQR) | 40.05 | (30.0–59.3) | |
| Boost dose (IQR) | 10.0 | (6.9–14.0) | |
| Total median dose (IQR) | 47.5 | (30.0–60.4) | |
| Total median dose per fraction (IQR) | 2.67 | (2.1–4.0) | |
| Calculated dose of CIED in Gy (n = 122) | |||
| Median dose (IQR) | 0.9 | (0.3–2.0) | |
| Median maximum dose (IQR) | 2.4 | (0.6–6.0) | |
| Target volume in cm3 | |||
| Mean planning target volume (SD) | 714.8 | (662.7) | |
| Mean boost target volume (SD) | 220.1 | (246.4) | |
| Beam modality | |||
| Max. photon beam energy of 18 MV | 55 | 17.0% | |
| Max. photon beam energy of 10 MV | 56 | 17.3% | |
| Max. photon beam energy of 6 MV | 202 | 62.3% | |
| Electron therapy only | 11 | 3.4% | |
| Radiation technique (n = 391) | |||
| 3D-CRT | 140 | 35.8% | |
| IMRT | 46 | 11.8% | |
| VMAT | 121 | 31.0% | |
| Radiotherapy without simulation CT | 60 | 15.3% | |
| FFF-RT | 24 | 6.1% | |
| No. | Device Type | Manufacturer | Model | Anatomic Area Irradiated | Total Dose/Number of Fractions Planned | Dose until Malfunction | RT Sessions Applied until Malfunction | Max. Dose at CIED at Time of Malfunction | Beam Type | Maximum Beam Energy | Description |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (1.) | PM | Biotronik | Entovis DR-T | Lung, left upper lobe | 45 Gy/18 fx | 42.5 Gy | 17 | 2.7 Gy | Photons | 10 MV | Device in back-up mode |
| (2.) | ICD | Biotronik | Lumax DR-T | Mediastinum, interpleural space | 9 Gy/3 fx | 9 Gy + 15.4 Gy = 24.4 Gy | 10 | 1.25 Gy | Photons | 18 MV | Software error with multiple mode switches |
| 61.6 Gy/28 fx | |||||||||||
| (3.) | PM | Biotronik | Cylos DR | Left groin | 30 Gy/15 fx | 30 Gy | 15 | 0 Gy | Photons | 18 MV | Device in safety mode, reprogramming unsuccessful, device exchanged |
| (4.) | CRT-P | Boston Scientific | Contak Renewal TR | Left breast | 50 Gy/25 fx | 36 Gy | 18 | 2.1 Gy | Photons | 6 MV | Failure of automatic read-out |
| (5.) | CRT-D | Biotronik | Itrevia 7 HF-T/QP | Lower abdomen | 36 Gy/18 fx | (errors several times during RT) | (errors several times during RT) | 0 Gy | Photons | 18 MV | Functional software errors occurring several times during RT |
| (6.) | CRT-D | Biotronik | Rivacor 5 HF-T/QP | Prostate | 76.23/33 fx | 43.89 Gy | 19 | 0 Gy | Photons | 10 MV | Device in safety mode |
| (7.) | ICD | Biotronik | Lumax 340 VR-T | Lower Esophagus | 64 Gy/32 fx | 26 Gy | 13 | 0 Gy | Photons | 18 MV | Device in safety mode |
| (8.) | ICD | Biotronik | Lumax 740 VR-T DX | Thoracic spine (6–10) | 30 Gy/10 fx | 15 Gy | 5 | 0.36 Gy | Photons | 6 MV | Antitachycardia functions were deactivated |
| ICD | Biotronik | Lumax 740 VR-T DX | Left ribs (6–10) | 30 Gy/10 fx | 15 Gy | 5 | 0.25 Gy | Photons | 6 MV | Antitachycardia functions were deactivated |
| DEGRO/DKG Guideline | Dutch Guideline | AIAC/AIRO/AIFM Consensus | SFRO Consensus | PTK/PTRO Opinion | HRS Consensus | AAPM TG-34 Guideline | ||
|---|---|---|---|---|---|---|---|---|
| Risk stratification | ||||||||
| Low risk | - CIED dose < 2 Gy without pacemaker dependency or history of prior ventricular fibrillation | - CIED dose < 2 Gy without pacemaker dependency | - CIED dose ≤ 2 Gy without pacemaker dependency or frequent ICD intervention | - CIED dose ≤ 5 Gy without pacemaker dependency and without production of secondary neutrons | - PM dose < 5 Gy without pacemaker dependency | - CIED dose ≤ 5 Gy without pacemaker dependency and without production of secondary neutrons | - CIED dose < 2 Gy without pacemaker dependency | |
| Intermediate risk | - CIED dose 2–10 Gy without pacemaker dependency or history of prior ventricular fibrillation - CIED dose < 2 Gy with pacemaker dependency or history of prior ventricular fibrillation | - CIED dose 2–10 Gy without pacemaker dependency or history of prior ventricular fibrillation - CIED dose < 2 Gy with pacemaker dependency or history of prior ventricular fibrillation | - CIED dose 2–10 Gy without pacemaker dependency or frequent ICD intervention - CIED dose ≤ 10 Gy without pacemaker dependency but with frequent ICD intervention or RT with protons or photons > 6 MV - CIED dose ≤ 2 Gy with pacemaker dependency | - CIED dose ≤ 5 Gy with pacemaker dependency and/or production of secondary neutrons | - ICD dose < 5 Gy - PM dose < 5 Gy with pacemaker dependency | - CIED dose ≤ 5 Gy with pacemaker dependency | - CIED dose 2– 5 Gy without pacemaker dependency - CIED dose ≤ 5 Gy with pacemaker dependency | |
| High risk | - CIED dose > 10 Gy - CIED dose > 2 Gy with pacemaker dependency or history of prior ventricular fibrillation | - CIED dose > 10 Gy - CIED dose > 2 Gy with pacemaker dependency or history of prior ventricular fibrillation | - CIED dose > 10 Gy - CIED dose > 2 Gy with pacemaker dependency - CIED any dose with pacemaker dependency/frequent ICD intervention and RT with protons or photons > 6 MV | - CIED dose > 5 Gy | - CIED dose ≥ 5 Gy - RT with ≥10 MV | - CIED dose > 5 Gy - RT with production of secondary neutrons | - CIED dose > 5 Gy - RT with production of secondary neutrons | |
| PM-dependency as independent risk factor | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Secondary neutron production as independent risk factor | No * | No * | Yes | Yes | Yes | Yes | Yes | |
| Emergency protocol required | Yes | Yes † | Yes | No | No | No | Yes | |
| Frequency of interrogations, monitoring and precaution stratified by risk group | ||||||||
| Low risk | - interrogation after every RT session - personnel qualified for specific procedures in respect to CIED patients | - weekly interrogations - audiovisual contact - deactivation of ATP therapy of ICDs through reprogramming or magnet placement during RT | - first and mid-treatment interrogations - audiovisual contact | - interrogation only at the end of RT | - interrogations every 2 weeks - disabling (temporarily) of the “R” function, automatic measurement and setting safe stimulation impulses (with a margin of at least 1 V above the stimulation threshold) | - interrogation after the last RT session | - interrogation before first and after last RT session | |
| Intermediate risk | - interrogation before and after every RT session - ECG and SpO2 monitoring - external defibrillator and external pacemaker available, programming device available | - weekly interrogations - medical emergency cart available - external pacemaker available - trained staff with cardiology expertise can be present within 10 min | - first and mid-treatment interrogations - ECG and SpO2 monitoring | - weekly interrogations | - weekly interrogations - setting stimulation to a frequency other than the default frequency of a reset device - presence of a cardiologist experienced in the use of CIED during the first RT session | - consider weekly interrogations | - additional interrogation at mid-treatment - formal consultation with cardiology/electrophysiology - pacing-dependent: consultation with cardiology/electrophysiology on the use of magnet and SpO2 | |
| High risk | - interrogation before and immediately after every RT session - device relocation or replanning of RT with dose reduction at CIED - if reduction of CIED dose is impossible then consider RT on individual basis - cardiologist or anesthesiologist present | - in exceptional cases a decision to start RT can be made - interrogations within 24 h after every RT session - ECG-monitoring during RT | - weekly interrogations in addition to the first and mid-treatment interrogations | - weekly interrogations - telemetry monitoring - presence of cardiologist/intensivist - magnet placement depending at the discretion of the cardiologist | - interrogation immediately before and after completing every RT session - presence of cardiologist during RT | - weekly interrogations | - weekly interrogations once the device receives >5 Gy - weekly ECG monitoring - cardiologist/pacemaker technologist should be available, if needed | |
| Interrogations, monitoring and precaution independent of risk group | ||||||||
| During RT | - RT with photon beam energy ≤ 10 MV - evaluation of RT dose at CIED during first RT session and comparison with calculated CIED dose - deactivation of ATP therapy of ICDs through reprogramming or magnet placement during RT - audiovisual contact - continuous ECG and SpO2 monitoring in patients with suspended ATP therapy - availability of cardiologist and programming device | - RT with photon beam energy ≤ 10 MV | - interrogation in office/remote after the first RT session - interrogation at mid-treatment | - medical emergency cart available with physician on-site - audiovisual contact during RT - telemetric surveillance if available | - RT with photon beam energy < 10 MV - access to external defibrillator with external stimulation option - audiovisual contact, ECG, BP and SpO2 monitoring in high-risk patients, readiness for resuscitation - maintaining contact with the programmer - deactivation of ATP therapy of ICDs through reprogramming or magnet placement during RT - evaluation of the RT dose during the first RT sessions | - audiovisual contact - CIED relocation if it interferes with adequate tumor treatment - CIED relocation not recommended for CIED with dose <5 Gy - non–neutron-producing treatment preferred over neutron-producing treatment | - PM magnet, SpO2 and AED available - audiovisual contact - communication with cardiology/electrophysiology | |
| After RT | - interrogation of CIED after the last RT session - checkups of CIED at 1, 3 and 6 months after RT - asynchronous stimulation not longer than necessary - analysis of any CIED irregularities in connection to RT and forwarding of data to manufacturer - exchange of CIEDs with significant defects even if the malfunction is temporary and full device recovery is observed - telemetric surveillance if available | - interrogation of CIED after the last RT session - checkups of CIED at 1, 3 and 6 months after RT | - interrogation of CIED after the last RT session - checkups of CIED at 1 and 6 months after RT | - interrogation of CIED after the last RT session - checkup of CIED 3–6 months after RT | - interrogation of CIED after the last RT session - checkups of CIED at 1, 3 and 6 months after RT | - interrogation of CIED after the last RT session | - interrogation of CIED after the last RT session - checkups of CIED at 1 and 6 months after RT | |
| To be additionally considered | - asynchronous mode in stimulation-dependent patients | - device relocation - measurement of RT dose at CIED site | - magnet placement - device reprogramming - device relocation - presence of electrophysiologist/nurse/technician - presence of anesthesiologist | - magnet placement only at the discretion of the cardiologist | - asynchronous mode in stimulation-dependent patients - considering device replacement in case of damage | - deactivation of ATP therapy of ICDs through reprogramming or magnet placement during RT at the discretion of the cardiologist | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisowski, D.; Lutyj, P.; Abazari, A.; Weick, S.; Traub, J.; Polat, B.; Flentje, M.; Kraft, J. Impact of Radiotherapy on Malfunctions and Battery Life of Cardiac Implantable Electronic Devices in Cancer Patients. Cancers 2023, 15, 4830. https://doi.org/10.3390/cancers15194830
Lisowski D, Lutyj P, Abazari A, Weick S, Traub J, Polat B, Flentje M, Kraft J. Impact of Radiotherapy on Malfunctions and Battery Life of Cardiac Implantable Electronic Devices in Cancer Patients. Cancers. 2023; 15(19):4830. https://doi.org/10.3390/cancers15194830
Chicago/Turabian StyleLisowski, Dominik, Paul Lutyj, Arya Abazari, Stefan Weick, Jan Traub, Bülent Polat, Michael Flentje, and Johannes Kraft. 2023. "Impact of Radiotherapy on Malfunctions and Battery Life of Cardiac Implantable Electronic Devices in Cancer Patients" Cancers 15, no. 19: 4830. https://doi.org/10.3390/cancers15194830
APA StyleLisowski, D., Lutyj, P., Abazari, A., Weick, S., Traub, J., Polat, B., Flentje, M., & Kraft, J. (2023). Impact of Radiotherapy on Malfunctions and Battery Life of Cardiac Implantable Electronic Devices in Cancer Patients. Cancers, 15(19), 4830. https://doi.org/10.3390/cancers15194830







