PACS-Integrated Tools for Peritumoral Edema Volumetrics Provide Additional Information to RANO-BM-Based Assessment of Lung Cancer Brain Metastases after Stereotactic Radiotherapy: A Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
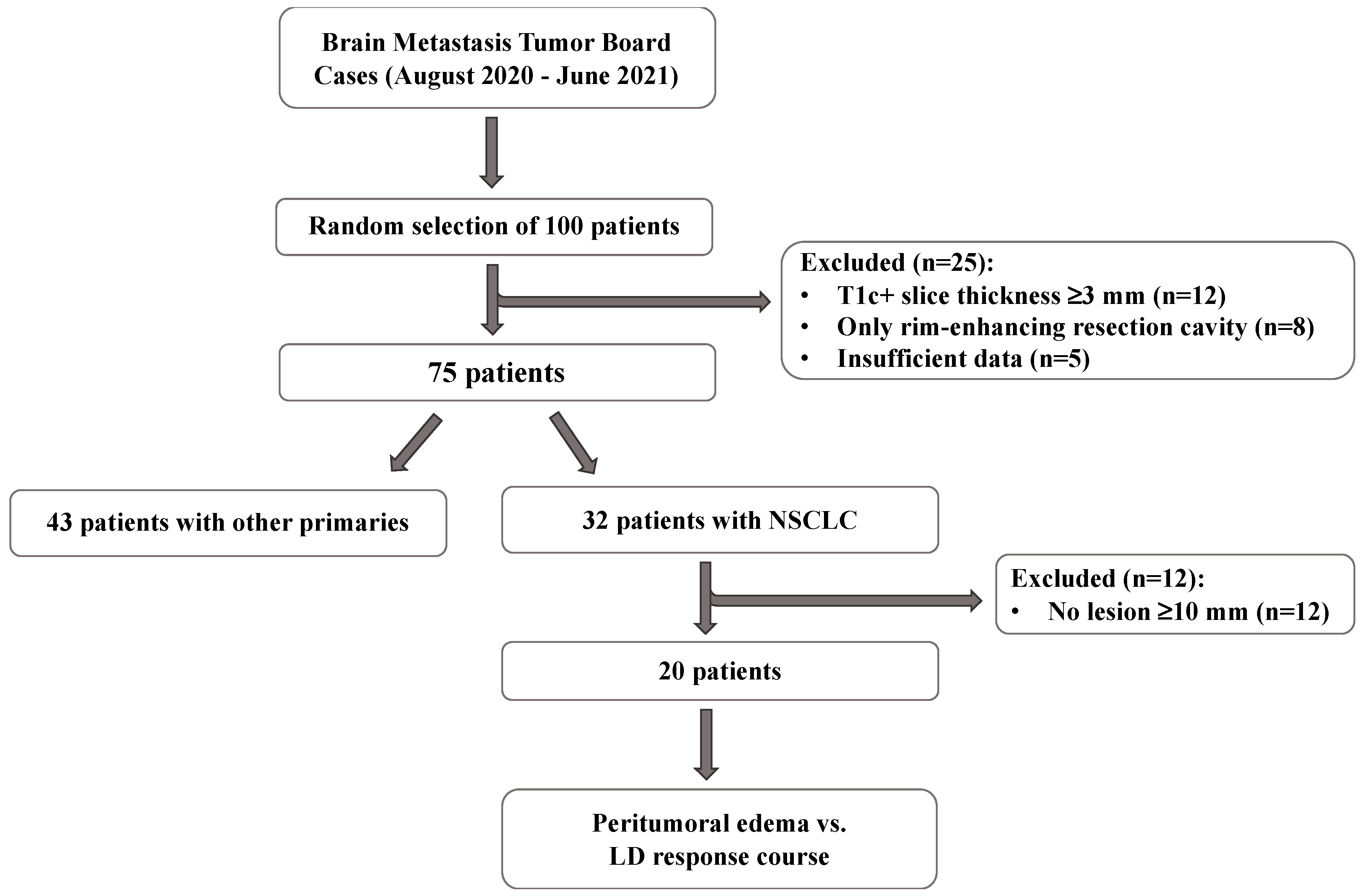
2.1. Patient Selection
2.2. Measurement of 2D Diameters of Contrast-Enhancing Lesions, and Volume of Peritumoral Edema
2.3. Data Analysis
2.4. Comparing LD of Contrast-Enhancing Lesions with Volume of Peritumoral Edema
2.5. Steroid Admission and Peritumoral Edema Course
3. Results
3.1. Patient Characteristics
3.2. LD Assessment of Contrast-Enhancing Tumor Core
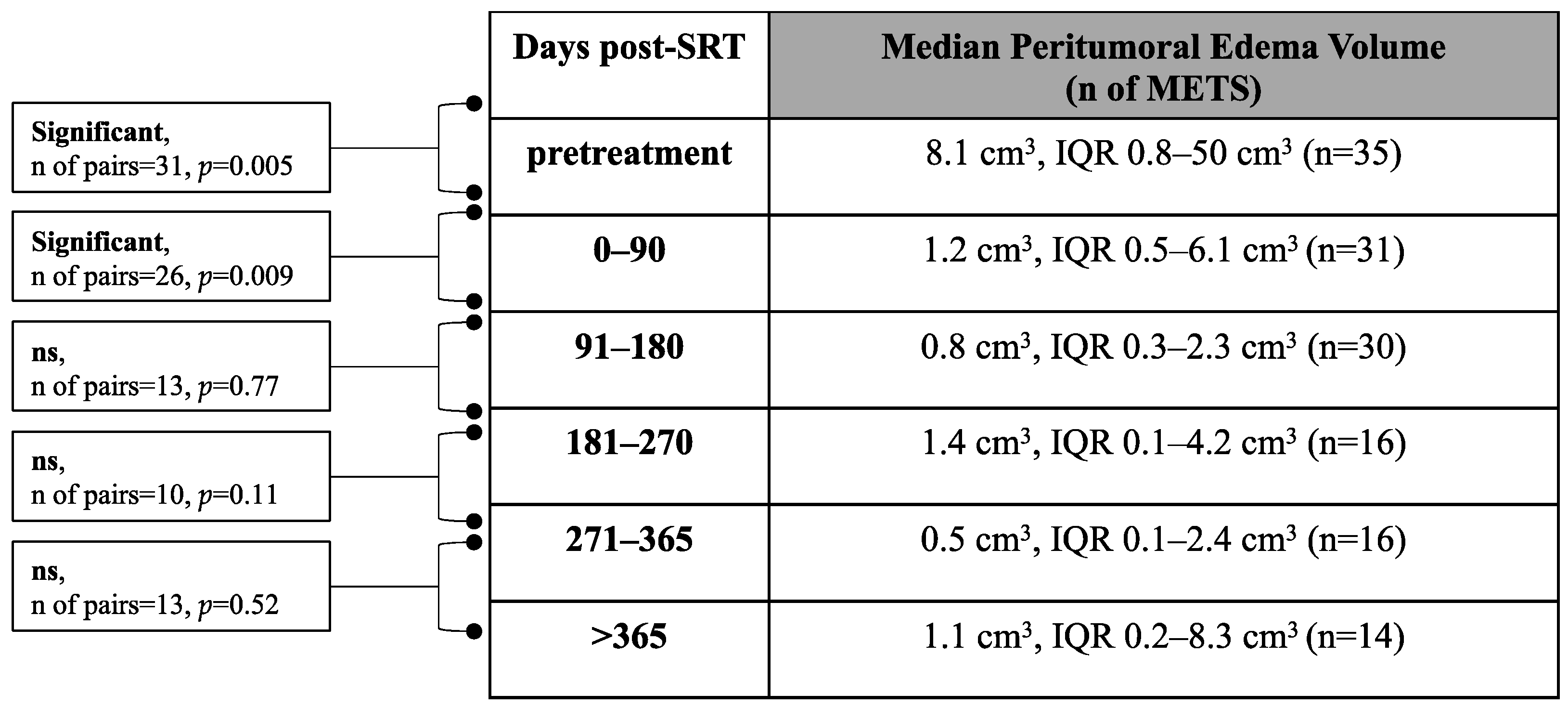
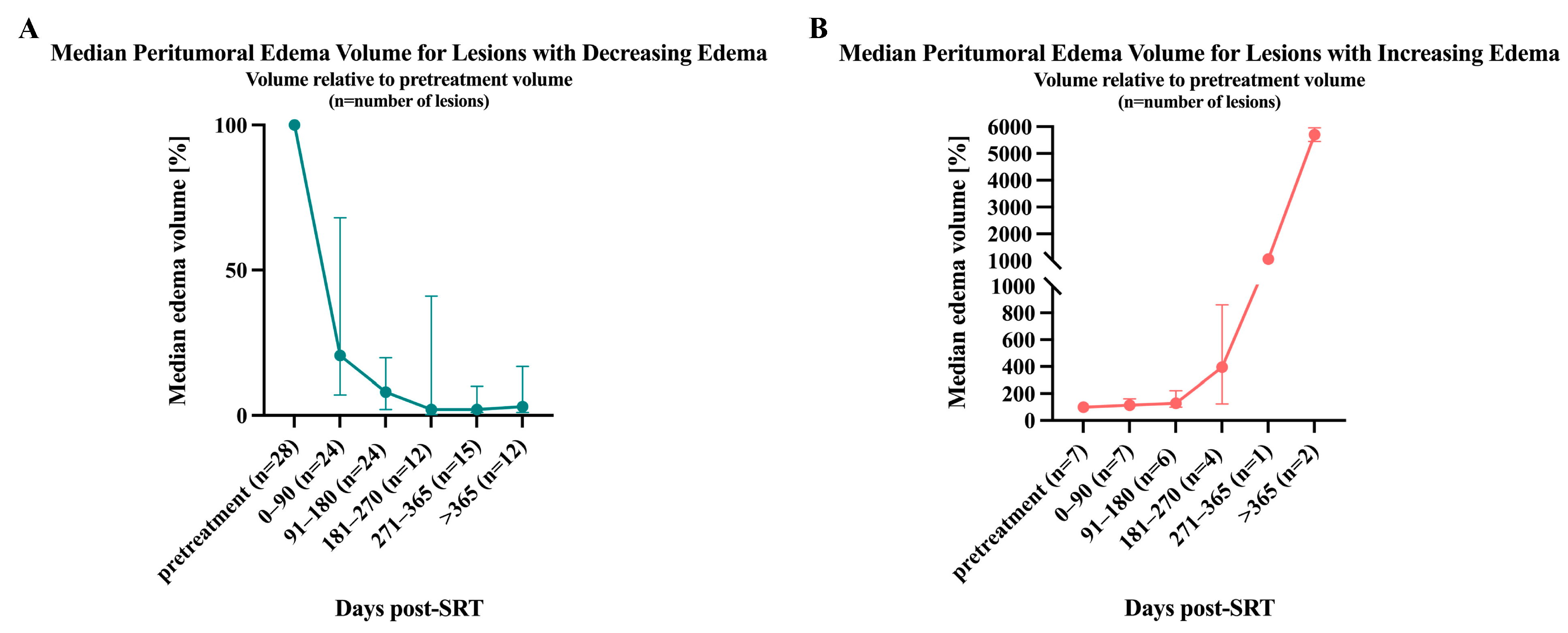
3.3. Volumetric Assessment of Peritumoral Edema
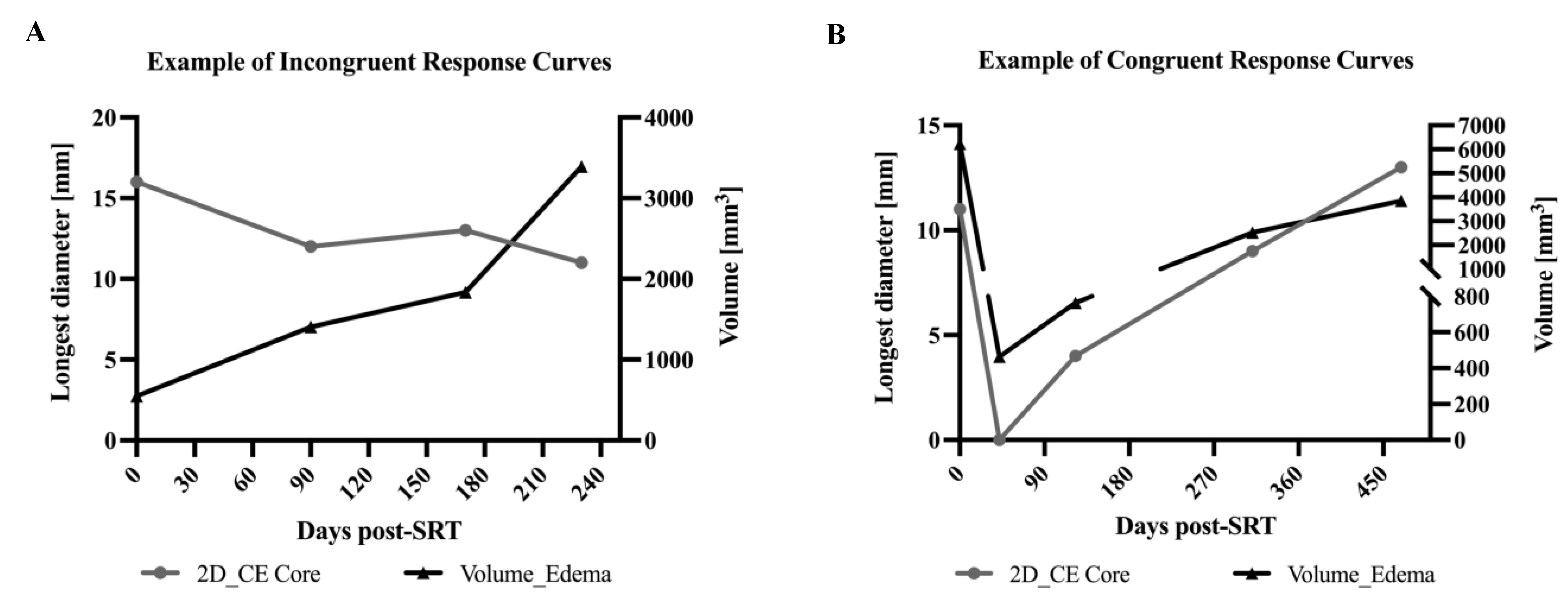
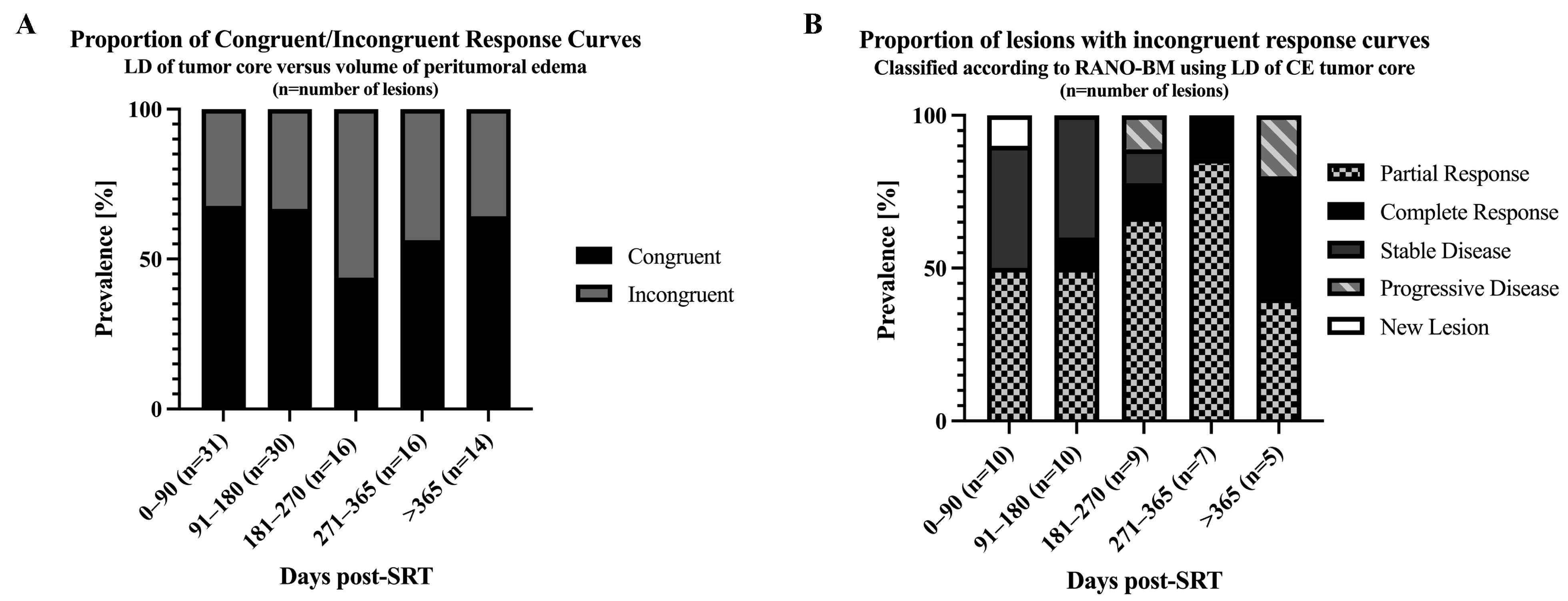
3.4. Correlation of Tumor Core LD Versus Peritumoral Edema Volume
3.5. Effects of Steroid Treatment on Peritumoral Edema Volume
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Lagerwaard, F.J.; Levendag, P.C.; Nowak, P.J.; Eijkenboom, W.M.; Hanssens, P.E.; Schmitz, P.I. Identification of prognostic factors in patients with brain metastases: A review of 1292 patients. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 795–803. [Google Scholar] [CrossRef]
- Nussbaum, E.S.; Djalilian, H.R.; Cho, K.H.; Hall, W.A. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer 1996, 78, 1781–1788. [Google Scholar] [CrossRef]
- Fabi, A.; Felici, A.; Metro, G.; Mirri, A.; Bria, E.; Telera, S.; Moscetti, L.; Russillo, M.; Lanzetta, G.; Mansueto, G.; et al. Brain metastases from solid tumors: Disease outcome according to type of treatment and therapeutic resources of the treating center. J. Exp. Clin. Cancer Res. 2011, 30, 10. [Google Scholar] [CrossRef]
- Hartgerink, D.; van der Heijden, B.; De Ruysscher, D.; Postma, A.; Ackermans, L.; Hoeben, A.; Anten, M.; Lambin, P.; Terhaag, K.; Jochems, A.; et al. Stereotactic Radiosurgery in the Management of Patients with Brain Metastases of Non-Small Cell Lung Cancer: Indications, Decision Tools and Future Directions. Front. Oncol. 2018, 8, 154. [Google Scholar] [CrossRef]
- Aoyama, H.; Shirato, H.; Tago, M.; Nakagawa, K.; Toyoda, T.; Hatano, K.; Kenjyo, M.; Oya, N.; Hirota, S.; Shioura, H.; et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA 2006, 295, 2483–2491. [Google Scholar] [CrossRef]
- Fessart, E.; Mouttet Audouard, R.; Le Tinier, F.; Coche-Dequeant, B.; Lacornerie, T.; Tresch, E.; Scherpereel, A.; Lartigau, E.; Mirabel, X.; Pasquier, D. Stereotactic irradiation of non-small cell lung cancer brain metastases: Evaluation of local and cerebral control in a large series. Sci. Rep. 2020, 10, 11201. [Google Scholar] [CrossRef]
- Lin, N.U.; Lee, E.Q.; Aoyama, H.; Barani, I.J.; Barboriak, D.P.; Baumert, B.G.; Bendszus, M.; Brown, P.D.; Camidge, D.R.; Chang, S.M.; et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015, 16, e270–e278. [Google Scholar] [CrossRef]
- Cassinelli Petersen, G.; Bousabarah, K.; Verma, T.; von Reppert, M.; Jekel, L.; Gordem, A.; Jang, B.; Merkaj, S.; Fadel, S.A.; Owens, R.; et al. Real-time PACS-integrated longitudinal brain metastasis tracking tool provides comprehensive assessment of treatment response to radiosurgery. Neurooncol. Adv. 2022, 4, vdac116. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, A.G.; Patel, S.; Harmath, C.; Bridges, S.; Synnott, J.; Sievers, A.; Yoon, Y.-H.; Lee, E.J.; Yang, M.C.; Lewis, R.F.; et al. Comparison of diameter and perimeter methods for tumor volume calculation. J. Clin. Oncol. 2001, 19, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Bauknecht, H.C.; Romano, V.C.; Rogalla, P.; Klingebiel, R.; Wolf, C.; Bornemann, L.; Hamm, B.; Hein, P.A. Intra- and interobserver variability of linear and volumetric measurements of brain metastases using contrast-enhanced magnetic resonance imaging. Investig. Radiol. 2010, 45, 49–56. [Google Scholar] [CrossRef]
- Pan, H.C.; Sun, M.H.; Chen, C.C.; Chen, C.J.; Lee, C.H.; Sheehan, J. Neuroimaging and quality-of-life outcomes in patients with brain metastasis and peritumoral edema who undergo Gamma Knife surgery. J. Neurosurg. 2008, 109, 90–98. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv192–iv237. [Google Scholar] [CrossRef]
- Sheehan, J.P.; Sun, M.H.; Kondziolka, D.; Flickinger, J.; Lunsford, L.D. Radiosurgery for non-small cell lung carcinoma metastatic to the brain: Long-term outcomes and prognostic factors influencing patient survival time and local tumor control. J. Neurosurg. 2002, 97, 1276–1281. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Untersteiner, H.; Hirschmann, D.; Shaltout, A.; Gobl, P.; Dorfer, C.; Rössler, K.; Marik, W.; Kirchbacher, K.; Kapfhammer, I.; et al. Gamma Knife Radiosurgery for Brain Metastases in Non-Small Cell Lung Cancer Patients Treated with Immunotherapy or Targeted Therapy. Cancers 2020, 12, 3668. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kondziolka, D.; Flickinger, J.C.; Lunsford, L.D. Stereotactic radiosurgery for patients with nonsmall cell lung carcinoma metastatic to the brain. Cancer 1997, 80, 2075–2083. [Google Scholar] [CrossRef]
- Moazami, N.; Rice, T.W.; Rybicki, L.A.; Adelstein, D.J.; Murthy, S.C.; DeCamp, M.M.; Barnett, G.H.; Chidel, M.A.; Suh, J.H.; Blackstone, E.H. Stage III non-small cell lung cancer and metachronous brain metastases. J. Thorac. Cardiovasc. Surg. 2002, 124, 113–122. [Google Scholar] [CrossRef][Green Version]
- Wen, P.Y.; Loeffler, J.S. Management of brain metastases. Oncology 1999, 13, 941–954, 957–961; discussion 961-2, 9. [Google Scholar]
- Tran, T.T.; Mahajan, A.; Chiang, V.L.; Goldberg, S.B.; Nguyen, D.X.; Jilaveanu, L.B.; Kluger, H.M. Perilesional edema in brain metastases: Potential causes and implications for treatment with immune therapy. J. Immunother. Cancer 2019, 7, 200. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Rajky, O.; Winkler, F.; Bartsch, R.; Furtner, J.; Hainfellner, J.A.; Goodman, S.L.; Weller, M.; Schittenhelm, J.; Preusser, M. Invasion patterns in brain metastases of solid cancers. Neuro Oncol. 2013, 15, 1664–1672. [Google Scholar] [CrossRef]
- Yoo, H.; Kim, Y.Z.; Nam, B.H.; Shin, S.H.; Yang, H.S.; Lee, J.S.; Zo, J.I.; Lee, S.H. Reduced local recurrence of a single brain metastasis through microscopic total resection. J. Neurosurg. 2009, 110, 730–736. [Google Scholar] [CrossRef]
- Kerschbaumer, J.; Bauer, M.; Popovscaia, M.; Grams, A.E.; Thome, C.; Freyschlag, C.F. Correlation of Tumor and Peritumoral Edema Volumes with Survival in Patients with Cerebral Metastases. Anticancer Res. 2017, 37, 871–876. [Google Scholar] [CrossRef]
- Xu, G.; Yang, X.; Zhang, L.; Xu, M. Prognostic and Predictive Markers of Limited (1-4) Brain Metastases in Patients with Lung Adenocarcinoma After Stereotactic Radiosurgery: A Retrospective Analysis. World Neurosurg. 2022, 164, e671–e680. [Google Scholar] [CrossRef]
- Nardone, V.; Nanni, S.; Pastina, P.; Vinciguerra, C.; Cerase, A.; Correale, P.; Guida, C.; Giordano, A.; Tini, P.; Reginelli, A.; et al. Role of perilesional edema and tumor volume in the prognosis of non-small cell lung cancer (NSCLC) undergoing radiosurgery (SRS) for brain metastases. Strahlenther. Onkol. 2019, 195, 734–744. [Google Scholar] [CrossRef]
- Calluaud, G.; Terrier, L.M.; Mathon, B.; Destrieux, C.; Velut, S.; Francois, P.; Zemmoura, I.; Amelot, A. Peritumoral Edema/Tumor Volume Ratio: A Strong Survival Predictor for Posterior Fossa Metastases. Neurosurgery 2018, 85, 117–125. [Google Scholar] [CrossRef]
- Sharpton, S.R.; Oermann, E.K.; Moore, D.T.; Schreiber, E.; Hoffman, R.; Morris, D.E.; Ewend, M.G. The volumetric response of brain metastases after stereotactic radiosurgery and its post-treatment implications. Neurosurgery 2014, 74, 9–16, discussion 6; quiz 6. [Google Scholar] [CrossRef]
- Diao, K.; Bian, S.X.; Routman, D.M.; Yu, C.; Kim, P.E.; Wagle, N.A.; Wong, M.K.; Zada, G.; Chang, E.L. Combination ipilimumab and radiosurgery for brain metastases: Tumor, edema, and adverse radiation effects. J. Neurosurg. 2018, 129, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Kim, D.G.; Han, J.H.; Paek, S.H.; Chung, H.T.; Park, C.K.; Kim, C.-Y.; Kim, Y.H.; Kim, J.W.; Jung, H.-W. Early significant tumor volume reduction after radiosurgery in brain metastases from renal cell carcinoma results in long-term survival. Int. J. Radiat. Oncol. 2012, 82, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Fabian, K.; Gyulai, M.; Furak, J.; Varallyay, P.; Jackel, M.; Bogos, K.; Döme, B.; Pápay, J.; Tímár, J.; Szállási, Z.; et al. Significance of Primary Tumor Location and Histology for Brain Metastasis Development and Peritumoral Brain Edema in Lung Cancer. Oncology 2016, 91, 237–242. [Google Scholar] [CrossRef]
- Leeman, J.E.; Clump, D.A.; Flickinger, J.C.; Mintz, A.H.; Burton, S.A.; Heron, D.E. Extent of perilesional edema differentiates radionecrosis from tumor recurrence following stereotactic radiosurgery for brain metastases. Neuro Oncol. 2013, 15, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W. Mechanisms of tumor-related brain edema. Neurosurg. Focus 2007, 22, E8. [Google Scholar] [CrossRef]
- Patel, T.R.; McHugh, B.J.; Bi, W.L.; Minja, F.J.; Knisely, J.P.; Chiang, V.L. A comprehensive review of MR imaging changes following radiosurgery to 500 brain metastases. Am. J. Neuroradiol. 2011, 32, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Aboian, M.; Bousabarah, K.; Kazarian, E.; Zeevi, T.; Holler, W.; Merkaj, S.; Petersen, G.C.; Bahar, R.; Subramanian, H.; Sunku, P.; et al. Clinical implementation of artificial intelligence in neuroradiology with development of a novel workflow-efficient picture archiving and communication system-based automated brain tumor segmentation and radiomic feature extraction. Front. Neurosci. 2022, 16, 860208. [Google Scholar] [CrossRef] [PubMed]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martínez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.E.L.; et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients with Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, M.; Cassinelli Petersen, G.; Jekel, L.; von Reppert, M.; Varghese, S.; Dixe de Oliveira Santo, I.; Avesta, A.; Aneja, S.; Omuro, A.; Chiang, V.; et al. PACS-Integrated Tools for Peritumoral Edema Volumetrics Provide Additional Information to RANO-BM-Based Assessment of Lung Cancer Brain Metastases after Stereotactic Radiotherapy: A Pilot Study. Cancers 2023, 15, 4822. https://doi.org/10.3390/cancers15194822
Kaur M, Cassinelli Petersen G, Jekel L, von Reppert M, Varghese S, Dixe de Oliveira Santo I, Avesta A, Aneja S, Omuro A, Chiang V, et al. PACS-Integrated Tools for Peritumoral Edema Volumetrics Provide Additional Information to RANO-BM-Based Assessment of Lung Cancer Brain Metastases after Stereotactic Radiotherapy: A Pilot Study. Cancers. 2023; 15(19):4822. https://doi.org/10.3390/cancers15194822
Chicago/Turabian StyleKaur, Manpreet, Gabriel Cassinelli Petersen, Leon Jekel, Marc von Reppert, Sunitha Varghese, Irene Dixe de Oliveira Santo, Arman Avesta, Sanjay Aneja, Antonio Omuro, Veronica Chiang, and et al. 2023. "PACS-Integrated Tools for Peritumoral Edema Volumetrics Provide Additional Information to RANO-BM-Based Assessment of Lung Cancer Brain Metastases after Stereotactic Radiotherapy: A Pilot Study" Cancers 15, no. 19: 4822. https://doi.org/10.3390/cancers15194822
APA StyleKaur, M., Cassinelli Petersen, G., Jekel, L., von Reppert, M., Varghese, S., Dixe de Oliveira Santo, I., Avesta, A., Aneja, S., Omuro, A., Chiang, V., & Aboian, M. (2023). PACS-Integrated Tools for Peritumoral Edema Volumetrics Provide Additional Information to RANO-BM-Based Assessment of Lung Cancer Brain Metastases after Stereotactic Radiotherapy: A Pilot Study. Cancers, 15(19), 4822. https://doi.org/10.3390/cancers15194822






