The Risk of Colorectal Polyps after Weight Loss Therapy Versus Obesity: A Propensity-Matched Nationwide Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The MarketScan Database
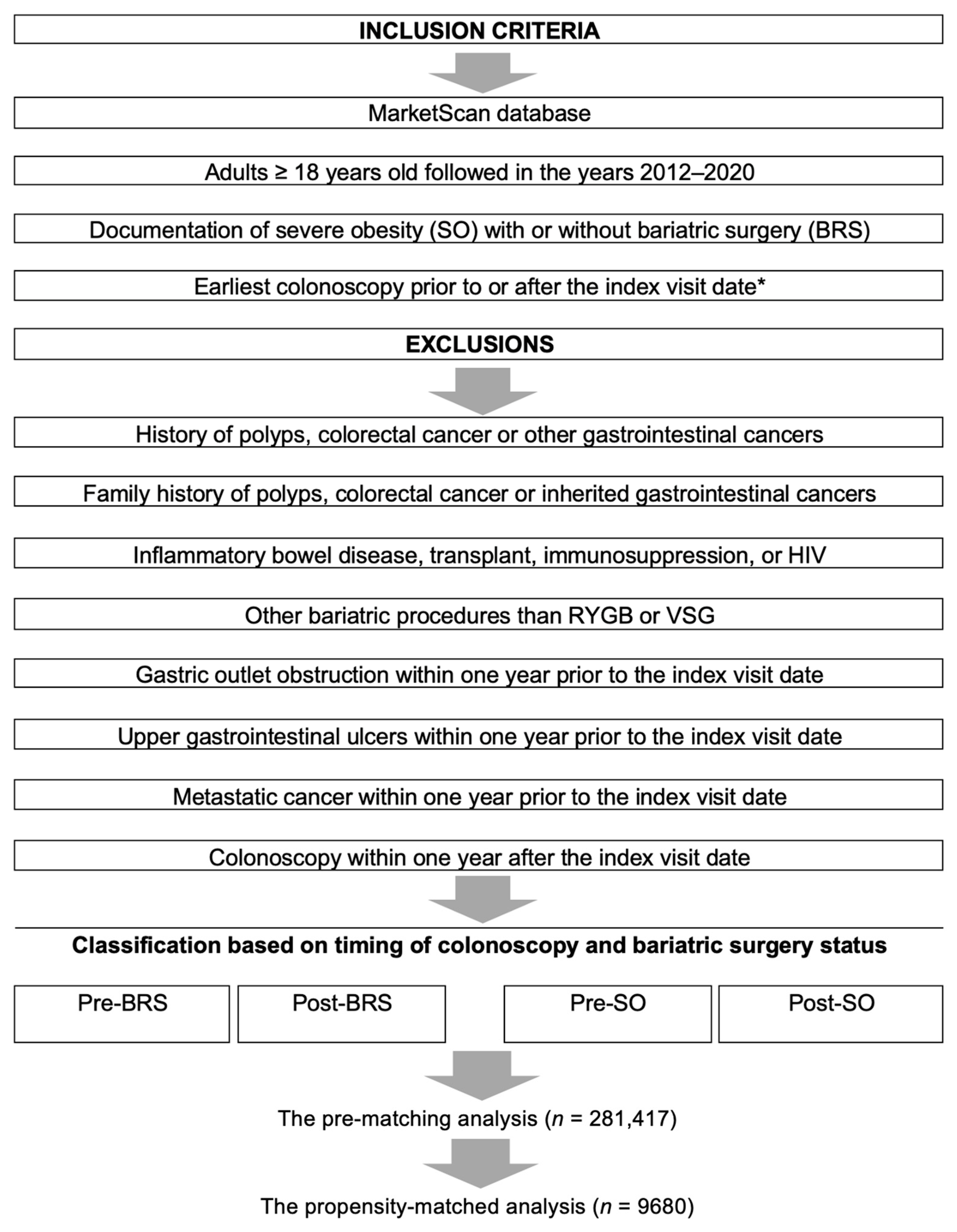
2.2. Study Cohort
2.3. Outcomes
2.4. Definition of Covariates
2.5. Statistical Analysis
2.5.1. The Pre-Matching Cohort
2.5.2. The Propensity-Matched Analysis
3. Results
3.1. The Pre-Matching Population Analysis
3.1.1. General Characteristics
3.1.2. The Risk of Polyps in Adults with or without Bariatric Surgery (the Pre-Matching Cohort)
3.2. The Matched Cohort Analysis
3.2.1. General Characteristics
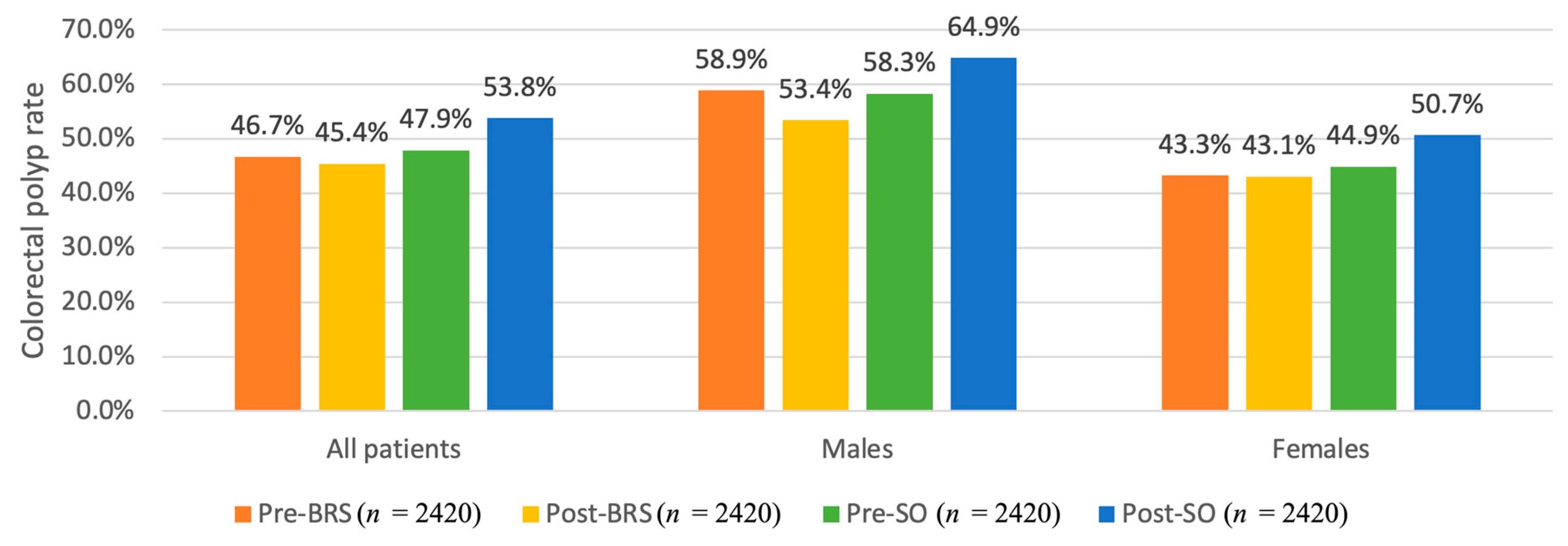
3.2.2. The Propensity-Matched Analysis Comparing the Risk of Colorectal Polyps in Adults with or without Bariatric Surgery
3.2.3. The Propensity-Matched Analysis Comparing the Risk of Rectal Polyps in Adults with or without Bariatric Surgery
3.3. The Impact of Bariatric Surgery on Metabolic Markers and Relation to Polyp Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tran, K.B.; Lang, J.J.; Xu, R.; Compton, k.; Acheson, A.R.; Henrikson, H.J.; Kocarnik, J.M.; Penberthy, L.; Aali, A.; Abbas, Q. The global burden of cancer attributable to risk factors, 2010–19: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 563–591. [Google Scholar] [CrossRef] [PubMed]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Loosen, S.H.; Roderburg, C.; Jördens, M.S.; Fluegen, G.; Luedde, T.; Kostev, K. Overweight and Obesity Determine the Risk for Gastrointestinal Cancer in a Sex-Dependent Manner: A Retrospective Cohort Study of 287,357 Outpatients in Germany. Cancers 2022, 14, 931. [Google Scholar] [CrossRef]
- Demb, J.; Earles, A.; Martínez, M.E.; Bustamante, R.; Bryant, A.K.; Murphy, J.D.; Liu, L.; Gupta, S. Risk factors for colorectal cancer significantly vary by anatomic site. BMJ Open Gastroenterol. 2019, 6, e000313. [Google Scholar] [CrossRef]
- Maciejewski, M.L.; Arterburn, D.E.; Van Scoyoc, L.; Smith, V.A.; Yancy, W.S., Jr.; Weidenbacher, H.J.; Livingston, E.H.; Olsen, M.K. Bariatric Surgery and Long-term Durability of Weight Loss. JAMA Surg. 2016, 151, 1046–1055. [Google Scholar] [CrossRef]
- Kennedy-Dalby, A.; Adam, S.; Ammori, B.J.; Syed, A.A. Weight loss and metabolic outcomes of bariatric surgery in men versus women—A matched comparative observational cohort study. Eur. J. Intern. Med. 2014, 25, 922–925. [Google Scholar] [CrossRef]
- Katsogiannos, P.; Kamble, P.G.; Wiklund, U.; Sundbom, M.; Espes, D.; Hammar, U.; Karlsson, F.A.; Pereira, M.J.; Eriksson, J.W. Rapid changes in neuroendocrine regulation may contribute to reversal of type 2 diabetes after gastric bypass surgery. Endocrine 2020, 67, 344–353. [Google Scholar] [CrossRef]
- Bailly, L.; Fabre, R.; Pradier, C.; Iannelli, A. Colorectal Cancer Risk Following Bariatric Surgery in a Nationwide Study of French Individuals with Obesity. JAMA Surg. 2020, 155, 395–402. [Google Scholar] [CrossRef]
- Schauer, D.P.; Feigelson, H.S.; Koebnick, C.; Caan, B.; Weinmann, S.; Leonard, A.C.; Powers, J.D.; Yenumula, P.R.; Arterburn, D.E. Bariatric Surgery and the Risk of Cancer in a Large Multisite Cohort. Ann. Surg. 2019, 269, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Hussan, H.; Akinyeye, S.; Mihaylova, M.; McLaughlin, E.; Chiang, C.; Clinton, S.K.; Lieberman, D. Colorectal Cancer Risk Is Impacted by Sex and Type of Surgery after Bariatric Surgery. Obes. Surg. 2022, 32, 2880–2890. [Google Scholar] [CrossRef] [PubMed]
- Chierici, A.; Amoretti, P.; Drai, C.; De Fatico, S.; Barriere, J.; Schiavo, L.; Iannelli, A. Does Bariatric Surgery Reduce the Risk of Colorectal Cancer in Individuals with Morbid Obesity? A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 467. [Google Scholar] [CrossRef] [PubMed]
- Risi, R.; Rossini, G.; Tozzi, R.; Pieralice, S.; Monte, L.; Masi, D.; Castagneto-Gissey, L.; Gallo, I.F.; Strigari, L.; Casella, G. Sex difference in the safety and efficacy of bariatric procedures: A systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2022, 18, 983–996. [Google Scholar] [CrossRef]
- Kedrin, D.; Gandhi, S.-C.C.; Wolf, M.; Roper, J.; Yilmaz, O.; Corey, K.; Khalili, H.; Stanford, F.C.; Gala, M. Bariatric Surgery Prior to Index Screening Colonoscopy Is Associated with a Decreased Rate of Colorectal Adenomas in Obese Individuals. Clin. Transl. Gastroenterol. 2017, 8, e73. [Google Scholar] [CrossRef]
- Droney, A.C.; Sellers, W.; Gupta, A.; Johnson, K.R.; Fluck, M.; Petrick, A.; Bannon, J.; Erchinger, T.; Protyniak, B. Incidence of polyp formation following bariatric surgery. Surg. Obes. Relat. Dis. 2021, 17, 1773–1779. [Google Scholar] [CrossRef]
- Peleg, N.; Sapoznikov, S.; Levi, Z.; Dotan, I.; Shamah, S. Incidence of Colorectal Adenomas after Bariatric Surgery: Pre-operative Super Morbid Obesity Is Independently Associated with Increased Risk. Obes. Surg. 2021, 31, 4220–4226. [Google Scholar] [CrossRef]
- IBM. IBM MarketScan Research Databases. Available online: https://www.ibm.com/watson-health/about/truven-health-analytics (accessed on 21 April 2021).
- Gill, K.; Chia, V.M.; Hernandez, R.K.; Navetta, M. Rates of Vascular Events in Patients with Migraine: A MarketScan® Database Retrospective Cohort Study. Headache J. Head Face Pain 2020, 60, 2265–2280. [Google Scholar] [CrossRef]
- Arterburn, D.; Wellman, R.; Emiliano, A.; Smith, S.R.; Odegaard, A.O.; Murali, S.; Williams, N.; Coleman, K.J.; Courcoulas, A.; Coley, R.Y. Comparative Effectiveness and Safety of Bariatric Procedures for Weight Loss: A PCORnet Cohort Study. Ann. Intern. Med. 2018, 169, 741–750. [Google Scholar] [CrossRef]
- Wolfe, B.M.; Kvach, E.; Eckel, R.H. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ. Res. 2016, 118, 1844–1855. [Google Scholar] [CrossRef]
- Gandhi, S.K.; Reynolds, M.W.; Boyer, J.G.; Goldstein, J.L. Recurrence and malignancy rates in a benign colorectal neoplasm patient cohort: Results of a 5-year analysis in a managed care environment. Am. J. Gastroenterol. 2001, 96, 2761–2767. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Naumann, D.N.; Karandikar, S. Differences in screening vs non-screening colonoscopy: Scope for improvement? Color. Dis. 2016, 18, 903–909. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, W.; Song, M.; Smith-Warner, S.A.; Yang, J.; Li, Y.; Ma, W.; Hu, Y.; Ogino, S.; Hu, F.B. Type 2 diabetes and risk of colorectal cancer in two large U.S. prospective cohorts. Br. J. Cancer 2018, 119, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Tian, Z. Dyslipidemia and colorectal cancer risk: A meta-analysis of prospective studies. Cancer Causes Control 2015, 26, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sterling, N.W.; Kong, L.; Lewis, M.M.; Mailman, R.B.; Chen, H.; Leslie, D.; Huang, X. Statins may facilitate Parkinson’s disease: Insight gained from a large, national claims database. Mov. Disord. 2017, 32, 913–917. [Google Scholar] [CrossRef]
- Cho, I.-J.; Shin, J.-H.; Jung, M.-H.; Kang, C.Y.; Hwang, J.; Kwon, C.H.; Kim, W.; Kim, D.-H.; Lee, C.J.; Kang, S.-H. Antihypertensive Drugs and the Risk of Cancer: A Nationwide Cohort Study. J. Clin. Med. 2021, 10, 771. [Google Scholar] [CrossRef]
- Sabatino, M.J.; Burroughs, P.J.; Moore, H.G.; Grauer, J.N. Spine coding transition from ICD-9 to ICD-10: Not taking advantage of the specificity of a more granular system. N. Am. Spine Soc. J. 2020, 4, 100035. [Google Scholar] [CrossRef]
- Hussan, H.; Drosdak, A.; Le Roux, M.; Patel, K.; Porter, K.; Clinton, S.K.; Focht, B.; Noria, S. The Long-term Impact of Roux-en-Y Gastric Bypass on Colorectal Polyp Formation and Relation to Weight Loss Outcomes. Obes. Surg. 2019, 30, 407–415. [Google Scholar] [CrossRef]
- Karahalios, A.; English, D.R.; Simpson, J.A. Weight change and risk of colorectal cancer: A systematic review and meta-analysis. Am. J. Epidemiol. 2015, 181, 832–845. [Google Scholar] [CrossRef]
- Ben, Q.; An, W.; Jiang, Y.; Zhan, X.; Du, Y.; Cai, Q.C.; Gao, J.; Li, Z. Body mass index increases risk for colorectal adenomas based on meta-analysis. Gastroenterology 2012, 142, 762–772. [Google Scholar] [CrossRef]
- Bardou, M.; Barkun, A.N.; Martel, M. Obesity and colorectal cancer. Gut 2013, 62, 933–947. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A. Obesity and colon and rectal cancer risk: A meta-analysis of prospective studies. Am. J. Clin. Nutr. 2007, 86, 556–565. [Google Scholar] [CrossRef]
- Baker, A.-M.; Cross, W.; Curtius, K.; Bakir, I.A.; Choi, C.-H.R.; Davis, H.L.; Temko, D.; Biswas, S.; Martinez, P.; Williams, M.J. Evolutionary history of human colitis-associated colorectal cancer. Gut 2019, 68, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.C.; Merchea, A. Dysplasia and Cancer in Inflammatory Bowel Disease. Surg. Clin. N. Am. 2017, 97, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.D.; Saunders, B.P.; Wilkinson, K.H.; Rumbles, S.; Schofield, G.; Kamm, M.A.; Williams, C.B.; Price, A.B.; Talbot, I.C.; Forbes, A. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology 2006, 130, 1030–1038. [Google Scholar] [CrossRef]
- Itzkowitz, S.H.; Harpaz, N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology 2004, 126, 1634–1648. [Google Scholar] [CrossRef]
- Sainsbury, A.; Goodlad, R.A.; Perry, S.L.; Pollard, S.G.; Robins, G.G.; Hull, M.A. Increased colorectal epithelial cell proliferation and crypt fission associated with obesity and roux-en-Y gastric bypass. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1401–1410. [Google Scholar] [CrossRef]
- Kant, P.; Sainsbury, A.; Reed, K.R.; Pollard, S.G.; Scott, N.; Clarke, A.R.; Coletta, P.L.; Hull, M.A. Rectal epithelial cell mitosis and expression of macrophage migration inhibitory factor are increased 3 years after Roux-en-Y gastric bypass (RYGB) for morbid obesity: Implications for long-term neoplastic risk following RYGB. Gut 2011, 60, 893–901. [Google Scholar] [CrossRef]
- Garibay, D.; Zaborska, K.E.; Shanahan, M.; Zheng, Q.; Kelly, K.M.; Montrose, D.C.; Dannenberg, A.J.; Miller, A.D.; Sethupathy, P.; Cummings, B.P. TGR5 Protects against Colitis in Mice, but Vertical Sleeve Gastrectomy Increases Colitis Severity. Obes. Surg. 2019, 29, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Braga Neto, M.B.; Gregory, M.; Ramos, G.P.; Loftus, E.V., Jr.; Ciorba, M.A.; Bruining, D.H.; Bazerbachi, F.; Abu Dayyeh, B.K.; Kushnir, V.M.; Shah, M. De-novo Inflammatory Bowel Disease after Bariatric Surgery: A Large Case Series. J. Crohn’s Colitis 2018, 12, 452–457. [Google Scholar] [CrossRef]
- Ungaro, R.; Fausel, R.; Chang, H.L.; Chang, S.; Chen, L.A.; Nakad, A.; El Nawar, A.; Prytz Berset, I.; Axelrad, J.; Lawlor, G. Bariatric surgery is associated with increased risk of new-onset inflammatory bowel disease: Case series and national database study. Aliment. Pharmacol. Ther. 2018, 47, 1126–1134. [Google Scholar] [CrossRef]
- Allin, K.H.; Jacobsen, R.K.; Ungaro, R.C.; Colombel, J.F.; Egeberg, A.; Villumsen, M.; Jess, T. Bariatric Surgery and Risk of New-onset Inflammatory Bowel Disease: A Nationwide Cohort Study. J. Crohn’s Colitis 2021, 15, 1474–1480. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Liu, Z.; Jiang, A.; Li, S.; Wu, D.; Zhang, Y.; Zhu, X.; Zhou, E.; Wei, Z. Sodium Butyrate Alleviates Lipopolysaccharide-Induced Inflammatory Responses by Down-Regulation of NF-κB, NLRP3 Signaling Pathway, and Activating Histone Acetylation in Bovine Macrophages. Front. Vet. Sci. 2020, 7, 579674. [Google Scholar] [CrossRef] [PubMed]
- Segain, J.-P.; de la Blétière, D.R.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottière, H.M.; Galmiche, J.-P. Butyrate inhibits inflammatory responses through NFκB inhibition: Implications for Crohn’s disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef]
- Karin, M.; Greten, F.R. NF-κB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Yin, L.; Joshi, S.; Rosenberg, D.W.; Giardina, C. Cyclooxygenase-2 Regulation in Colon Cancer Cells: Modulation of RNA Polymerase II Elongation by Histone Deacetylase Inhibitors *. J. Biol. Chem. 2005, 280, 15503–15509. [Google Scholar] [CrossRef]
- Tong, X.; Yin, L.; Giardina, C. Butyrate suppresses Cox-2 activation in colon cancer cells through HDAC inhibition. Biochem. Biophys. Res. Commun. 2004, 317, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Carretta, M.D.; Quiroga, J.; López, R.; Hidalgo, M.A.; Burgos, R.A. Participation of Short-Chain Fatty Acids and Their Receptors in Gut Inflammation and Colon Cancer. Front. Physiol. 2021, 12, 662739. [Google Scholar] [CrossRef]
- Kurata, N.; Tokashiki, N.; Fukushima, K.; Misao, T.; Hasuoka, N.; Kitagawa, K.; Mashimo, M.; Regan, J.W.; Murayama, T.; Fujino, H. Short chain fatty acid butyrate uptake reduces expressions of prostanoid EP4 receptors and their mediation of cyclooxygenase-2 induction in HCA-7 human colon cancer cells. Eur. J. Pharmacol. 2019, 853, 308–315. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Ouyang, Q.; Gan, H.T. Targeting cyclooxygenase-2 with sodium butyrate and NSAIDs on colorectal adenoma/carcinoma cells. World J. Gastroenterol. 2004, 10, 2954–2957. [Google Scholar] [CrossRef] [PubMed]
- Jahns, F.; Wilhelm, A.; Jablonowski, N.; Mothes, H.; Radeva, M.; Wölfert, A.; Greulich, K.O.; Glei, M. Butyrate suppresses mRNA increase of osteopontin and cyclooxygenase-2 in human colon tumor tissue. Carcinogenesis 2011, 32, 913–920. [Google Scholar] [CrossRef]
- Krawczyk, M.; Emerson, B.M. p50-associated COX-2 extragenic RNA (PACER) activates COX-2 gene expression by occluding repressive NF-κB complexes. eLife 2014, 3, e01776. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Li, D.; Fu, J.; Sun, Y.; Li, Y.; Qu, R.; Jin, X.; Li, D. Upregulation of cyclooxygenase-2 is associated with activation of the alternative nuclear factor kappa B signaling pathway in colonic adenocarcinoma. Am. J. Transl. Res. 2015, 7, 1612–1620. [Google Scholar] [PubMed]
- Yamamoto, K.; Arakawa, T.; Ueda, N.; Yamamoto, S. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J. Biol. Chem. 1995, 270, 31315–31320. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. Chapter Three—The Role of Short-Chain Fatty Acids in Health and Disease. In Advances in Immunology; Alt, F.W., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 91–119. [Google Scholar]
- Schwitalla, S.; Fingerle, A.; Cammareri, P.; Nebelsiek, T.; Göktuna, S.I.; Ziegler, P.K.; Canli, O.; Heijmans, J.; Huels, D.J.; Moreaux, G. Intestinal Tumorigenesis Initiated by Dedifferentiation and Acquisition of Stem-Cell-like Properties. Cell 2013, 152, 25–38. [Google Scholar] [CrossRef]
- Bordonaro, M.; Lazarova, D.L.; Sartorelli, A.C. Butyrate and Wnt signaling: A possible solution to the puzzle of dietary fiber and colon cancer risk? Cell Cycle 2008, 7, 1178–1183. [Google Scholar] [CrossRef]
- Cray, N.; Zhao, Y.; Fang, Y.; Liu, P.; Pollak, L.; Duvick, S.; Birt, D.F.; Whitley, E.M. Effects of Dietary Resistant Starch on the Wnt Signaling Pathway and Preneoplastic Cells in the Colons of Azoxymethane-Treated Rats. Nutr. Cancer 2017, 69, 632–642. [Google Scholar] [CrossRef]
- Malcomson, F.C.; Willis, N.D.; McCallum, I.; Xie, L.; Shivappa, N.; Wirth, M.D.; Hébert, J.R.; Kocaadam-Bozkurt, B.; Özturan-Sirin, A.; Kelly, S.B. Diet-Associated Inflammation Modulates Inflammation and WNT Signaling in the Rectal Mucosa, and the Response to Supplementation with Dietary Fiber. Cancer Prev. Res. 2021, 14, 337–346. [Google Scholar] [CrossRef]
- Malcomson, F.C.; Willis, N.D.; Mathers, J.C. Is resistant starch protective against colorectal cancer via modulation of the WNT signalling pathway? Proc. Nutr. Soc. 2015, 74, 282–291. [Google Scholar] [CrossRef]
- American, D.G.F. Dietary Guidelines for Americans 2015–2020. Available online: https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf (accessed on 15 May 2023).
- World Cancer Research Fund. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Cancer prevention guidelines. Available online: https://www.wcrf.org/dietandcancer. (accessed on 20 May 2023).
- Farias, G.; Silva, R.M.O.; da Silva, P.P.P.; Vilela, R.M.; Bettini, S.C.; Dâmaso, A.R.; Netto, B.D.M. Impact of dietary patterns according to NOVA food groups: 2 y after Roux-en-Y gastric bypass surgery. Nutrition 2020, 74, 110746. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.K.; Andersen, L.F.; Hofsø, D.; Aasheim, E.T.; Holven, K.B.; Sandbu, R.; Røislien, J.; Hjelmesæth, J. Dietary changes in obese patients undergoing gastric bypass or lifestyle intervention: A clinical trial. Br. J. Nutr. 2013, 110, 127–134. [Google Scholar] [CrossRef]
- Ziadlou, M.; Hosseini-Esfahani, F.; Mozaffari Khosravi, H.; Hosseinpanah, F.; Barzin, M.; Khalaj, A.; Valizadeh, M. Dietary macro- and micro-nutrients intake adequacy at 6th and 12th month post-bariatric surgery. BMC Surg. 2020, 20, 232. [Google Scholar] [CrossRef] [PubMed]
- Golzarand; Toolabi, K.; Djafarian, K. Changes in Body Composition, Dietary Intake, and Substrate Oxidation in Patients Underwent Laparoscopic Roux-en-Y Gastric Bypass and Laparoscopic Sleeve Gastrectomy: A Comparative Prospective Study. Obes. Surg. 2019, 29, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.C.; Mota, M.C.; Marot, L.P.; Mattar, L.A.; de Sousa, J.A.G.; Araújo, A.C.T.; da Costa Assis, C.T.; Crispim, C.A. Circadian Misalignment Is Negatively Associated with the Anthropometric, Metabolic and Food Intake Outcomes of Bariatric Patients 6 Months after Surgery. Obes. Surg. 2021, 31, 159–169. [Google Scholar] [CrossRef]
- Novais, P.F.S.; Rasera, I.; Leite, C.V.d.S.; Marin, F.A.; de Oliveira, M.R.M. Food intake in women two years or more after bariatric surgery meets adequate intake requirements. Nutr. Res. 2012, 32, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Verger, E.O.; Aron-Wisnewsky, J.; Dao, M.C.; Kayser, B.D.; Oppert, J.-M.; Bouillot, J.-L.; Torcivia, A.; Clément, K. Micronutrient and Protein Deficiencies after Gastric Bypass and Sleeve Gastrectomy: A 1-year Follow-up. Obes. Surg. 2016, 26, 785–796. [Google Scholar] [CrossRef]
- Jeffreys, R.M.; Hrovat, K.; Woo, J.G.; Schmidt, M.; Inge, T.H.; Xanthakos, S.A. Dietary assessment of adolescents undergoing laparoscopic Roux-en-Y gastric bypass surgery: Macro- and micronutrient, fiber, and supplement intake. Surg. Obes. Relat. Dis. 2012, 8, 331–336. [Google Scholar] [CrossRef]
- Farup, P.G.; Valeur, J. Changes in Faecal Short-Chain Fatty Acids after Weight-Loss Interventions in Subjects with Morbid Obesity. Nutrients 2020, 12, 802. [Google Scholar] [CrossRef]
- Meijer, J.L.; Roderka, M.N.; Chinburg, E.L.; Renier, T.J.; McClure, A.C.; Rothstein, R.I.; Barry, E.L.; Billmeier, S.; Gilbert-Diamond, D. Alterations in Fecal Short-Chain Fatty Acids after Bariatric Surgery: Relationship with Dietary Intake and Weight Loss. Nutrients 2022, 14, 4243. [Google Scholar] [CrossRef]
- Tremaroli, V.; Karlsson, F.; Werling, M.; Ståhlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fändriks, L.; le Roux, C.W.; Nielsen, J.; Bäckhed, F. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015, 22, 228–238. [Google Scholar] [CrossRef]
- Juárez-Fernández, M.; Román-Sagüillo, S.; Porras, D.; García-Mediavilla, M.V.; Linares, P.; Ballesteros-Pomar, M.D.; Urioste-Fondo, A.; Álvarez-Cuenllas, B.; González-Gallego, J.; Sánchez-Campos, S. Long-Term Effects of Bariatric Surgery on Gut Microbiota Composition and Faecal Metabolome Related to Obesity Remission. Nutrients 2021, 13, 2519. [Google Scholar] [CrossRef]
- Ou, J.; DeLany, J.P.; Zhang, M.; Sharma, S.; O’Keefe, S.J.D. Association between low colonic short-chain fatty acids and high bile acids in high colon cancer risk populations. Nutr. Cancer 2012, 64, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Graessler, J.; Qin, Y.; Zhong, H.; Zhang, J.; Licinio, J.; Wong, M.L.; Xu, A.; Chavakis, T.; Bornstein, A.B.; Ehrhart-Bornstein, M. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: Correlation with inflammatory and metabolic parameters. Pharmacogenom. J. 2013, 13, 514–522. [Google Scholar] [CrossRef]
- Sowah, S.A.; Riedl, L.; Damms-Machado, A.; Johnson, T.S.; Schübel, R.; Graf, M.; Kartal, E.; Zeller, G.; Schwingshackl, L.; Stangl, G.I. Effects of Weight-Loss Interventions on Short-Chain Fatty Acid Concentrations in Blood and Feces of Adults: A Systematic Review. Adv. Nutr. 2019, 10, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Kanerva, N.; Larsson, I.; Peltonen, M.; Lindroos, A.K.; Carlsson, L.M. Sociodemographic and lifestyle factors as determinants of energy intake and macronutrient composition: A 10-year follow-up after bariatric surgery. Surg. Obes. Relat. Dis. 2017, 13, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Kashyap, S.R.; Wolski, K.; Brethauer, S.A.; Kirwan, J.P.; Pothier, C.E.; Thomas, S.; Abood, B.; Nissen, S.E.; Bhatt, D.L. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N. Engl. J. Med. 2012, 366, 1567–1576. [Google Scholar] [CrossRef]
- Murphy, B.; Myers, E.; O’Shea, T.; Feeley, K.; Waldron, B. Correlation between adenoma detection rate and polyp detection rate at endoscopy in a non-screening population. Sci. Rep. 2020, 10, 2295. [Google Scholar] [CrossRef]
- Comstock, S.S.; Hortos, K.; Kovan, B.; McCaskey, S.; Pathak, D.R.; Fenton, J.I. Adipokines and obesity are associated with colorectal polyps in adult males: A cross-sectional study. PLoS ONE 2014, 9, e85939. [Google Scholar] [CrossRef]
- Wernli, K.J.; Newcomb, P.A.; Wang, Y.; Makar, K.W.; Shadman, M.; Chia, V.M.; Burnett-Hartman, A.; Wurscher, M.A.; Zheng, Y.; Mandelson, M.T. Body size, IGF and growth hormone polymorphisms, and colorectal adenomas and hyperplastic polyps. Growth Horm. IGF Res. 2010, 20, 305–309. [Google Scholar] [CrossRef]
- Lieberman, D.A.; Prindiville, S.; Weiss, D.G.; Willett, W. Risk Factors for Advanced Colonic Neoplasia and Hyperplastic Polyps in Asymptomatic Individuals. JAMA 2003, 290, 2959–2967. [Google Scholar] [CrossRef] [PubMed]
- Omata, F.; Brown, W.R.; Tokuda, Y.; Takahashi, O.; Fukui, T.; Ueno, F.; Mine, T. Modifiable risk factors for colorectal neoplasms and hyperplastic polyps. Intern. Med. 2009, 48, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Mongraw-Chaffin, M.; Foster, M.C.; Kalyani, R.R.; Vaidya, D.; Burke, G.L.; Woodward, M.; Anderson, C.A. Obesity Severity and Duration Are Associated with Incident Metabolic Syndrome: Evidence against Metabolically Healthy Obesity from the Multi-Ethnic Study of Atherosclerosis. J. Clin. Endocrinol. Metab. 2016, 101, 4117–4124. [Google Scholar] [CrossRef] [PubMed]
| Post- vs. Pre- Colonoscopy | Males | Females | |
|---|---|---|---|
| Adjusted* OR (95% CI) | BRS | 0.86 (0.77–0.97) | 0.90 (0.84–0.96) |
| SO | 1.10 (1.06–1.14) | 1.22 (1.19–1.26) | |
| BRS vs. SO | 0.78 (0.70–0.88) | 0.73 (0.69–0.78) | |
| Variable | Colonoscopy Pre-BRS (n = 2420) | Colonoscopy Post-BRS (n = 2420) | Colonoscopy Pre-SO (n = 2420) | Colonoscopy Post-SO (n = 2420) | p-Value | |
|---|---|---|---|---|---|---|
| Age at Time of Colonoscopy | 52 [49–57] | 52 [49–57] | 52 [49–57] | 52 [49–57] | 0.99 | |
| Sex Male Female | 530 (21.9) 1890 (78.1) | 530 (21.9) 1890 (78.1) | 530 (21.9) 1890 (78.1) | 530 (21.9) 1890 (78.1) | 0.99 | |
| Charlson Comorbidity Index | 3 [2–5] | 3 [2–4] | 3 [[2–4] | 3 [1–4] | <0.001 | |
| Years from Pre-BRS or Pre-SO Colonoscopy to Index Visit Date * | Median [IQR] | 0.5 [0.2–1.1] | N/A | 0.6 [0.2–1.2] | N/A | 0.003 |
| Range | 0–6 | N/A | 0–6 | N/A | ||
| Years from Index Visit Date to Post-BRS or Post-SO Colonoscopy | Median [IQR] | N/A | 3.2 [2.0–4.7] | N/A | 3.0 [1.9–4.6] | 0.19 |
| Range | N/A | 1–8.5 | N/A | 1–8.6 | ||
| Screening Colonoscopy Indication | 1475 (61.0) | 1420 (58.7) | 1380 (57.0) | 1335 (55.2) | <0.001 | |
| Alcohol Use | 39 (1.6) | 11 (0.5) | 34 (1.4) | 23 (1.0) | <0.001 | |
| Tobacco Use | 458 (18.9) | 237 (9.8) | 380 (15.7) | 233 (9.6) | <0.001 | |
| Post- vs. Pre- Colonoscopy | Males | Females | |
|---|---|---|---|
| Adjusted* OR (95% CI) | BRS | 0.83 (0.65–1.07) | 1.02 (0.89–1.17) |
| SO | 1.32 (1.02–1.70) | 1.29 (1.13–1.47) | |
| BRS vs. SO | 0.63 (0.44–0.90) | 0.79 (0.66–0.96) | |
| Post- vs. Pre- Colonoscopy | Males | Females | |
|---|---|---|---|
| Adjusted* OR (95% CI) | BRS | 0.66 (0.43–0.99) | 1.11 (0.86–1.42) |
| SO | 1.08 (0.72–1.61) | 0.86 (0.68–1.09) | |
| BRS vs. SO | 0.61 (0.34–1.08) | 1.29 (0.92–1.81) | |
| Diabetes Medications’ Changes | |||||
|---|---|---|---|---|---|
| Group | N | Index Visit * | Post-BRS or Post-SO Colonoscopy | Difference | p-Value |
| Male, BRS | 530 | 44.7% | 20.4% | −24.3% | <0.001 |
| Male, SO | 530 | 24.2% | 34.9% | +10.7% | <0.001 |
| Female, BRS | 1890 | 30.1% | 13.6% | −16.5% | <0.001 |
| Female, SO | 1890 | 16.1% | 28.2% | +12.1% | <0.001 |
| Cholesterol Medications’ Changes | |||||
| Group | N | Index Visit * | Post-BRS or Post-SO Colonoscopy | Difference | p-Value |
| Male, BRS | 530 | 41.3% | 29.3% | −12.0% | <0.001 |
| Male, SO | 530 | 26.6% | 37.2% | +10.6% | <0.001 |
| Female, BRS | 1890 | 25.4% | 17.6% | −7.8% | <0.001 |
| Female, SO | 1890 | 15.5% | 25.0% | +9.5% | <0.001 |
| Variable | Post-BRS Males (n = 530) | Post-BRS Females (n = 1890) |
|---|---|---|
| Diabetes medications at index (Reference = No) | 0.80 (0.54–1.17) p = 0.25 | 0.88 (0.71–1.10) p = 0.26 |
| Cessation of diabetes medications at post-index colonoscopy (Reference = Still use medication) | 0.83 (0.48–1.42) p = 0.50 | 0.91 (0.63–1.29) p = 0.59 |
| Began diabetes medication after index (Reference = No) | 1.04 (0.26–4.14) p = 0.96 | 1.00 (0.54–1.86) p = 0.99 |
| Diabetes medications at colonoscopy (Reference = No) | 1.04 (0.66–1.64) p = 0.86 | 0.98 (0.74–1.29) p = 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussan, H.; McLaughlin, E.; Chiang, C.; Marsano, J.G.; Lieberman, D. The Risk of Colorectal Polyps after Weight Loss Therapy Versus Obesity: A Propensity-Matched Nationwide Cohort Study. Cancers 2023, 15, 4820. https://doi.org/10.3390/cancers15194820
Hussan H, McLaughlin E, Chiang C, Marsano JG, Lieberman D. The Risk of Colorectal Polyps after Weight Loss Therapy Versus Obesity: A Propensity-Matched Nationwide Cohort Study. Cancers. 2023; 15(19):4820. https://doi.org/10.3390/cancers15194820
Chicago/Turabian StyleHussan, Hisham, Eric McLaughlin, Chienwei Chiang, Joseph G. Marsano, and David Lieberman. 2023. "The Risk of Colorectal Polyps after Weight Loss Therapy Versus Obesity: A Propensity-Matched Nationwide Cohort Study" Cancers 15, no. 19: 4820. https://doi.org/10.3390/cancers15194820
APA StyleHussan, H., McLaughlin, E., Chiang, C., Marsano, J. G., & Lieberman, D. (2023). The Risk of Colorectal Polyps after Weight Loss Therapy Versus Obesity: A Propensity-Matched Nationwide Cohort Study. Cancers, 15(19), 4820. https://doi.org/10.3390/cancers15194820







