Simple Summary
This study investigates cancer in migrants in Southern Italy, who represent a neglected but vulnerable population. We used data from the Eastern Sicily Cancer Registry collected between 2004 and 2019 to compare the adjusted proportionate morbidity ratio for the most common cancer types in migrants and non-migrants, and we calculated the odds of migrant status for one cancer compared to all cancers. The migrants/non-migrants odds of cancer was 2.1%, with most cancers occurring in migrant women. We observed increased proportions in cervical and lung cancer, with higher odds of cervical cancer and lower odds of colorectal cancer in migrants. Measures should be implemented to enhance the access of migrants to prevention, early diagnosis and care for cancer. These interventions should account for the migrant’s country of origin. Particular attention should be given to HPV vaccination, cervical cancer screening and tobacco control to reduce the cancer burden in this population.
Abstract
Background: Migrants are a vulnerable and neglected population. We aimed at investigating cancer proportionate rates in migrants in Sicily, Southern Italy. Methods: We extracted data on new cancer cases diagnosed between 2004 and 2019 from the Eastern Sicily cancer registry. We compared the adjusted proportionate morbidity ratio (PMR) for the most common cancer types among migrants and non-migrants. We fitted multivariate logistic regression models comparing one cancer to all other cancers to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for migration status. The analysis was stratified by region of origin. Results: Overall, 4726 new cancer cases occurred in migrants between 2004 and 2019, 63.5% of those among women and 224,211 in non-migrants, including 54.5% among men, with odds for migrants/non-migrants of 2.1%. Migrants had an increased proportion of cervical (PMR = 2.68, 95% CI = 2.29–3.10) and lung cancer (PMR = 1.20, 95% CI = 1.07–1.33). The highest OR in migrants was observed for cervical cancer (OR = 3.54, 95% CI = 2.99–4.20). Colorectal cancer was decreased among migrants (OR = 0.86, 95% CI = 0.77–0.96). Conclusions: Migrants to Sicily have higher odds of cervical cancer and a decreased risk of colorectal cancer compared to non-migrants. Increased odds were also detected for lung cancer, in particular in women. Different cancer patterns could be observed based on the region of origin. HPV-related cancers need targeted attention in migrants living in Sicily.
1. Introduction
Migrants represent a vulnerable subgroup of the population from multiple points of view [1], including socioeconomic condition (job position, income and educational level) [2] as well as health status (vaccination history, participation in screening programs, disease diagnosis and care). Migrants often suffer disease disparities [3,4], such as poor disease management, lack of check-ups and screening tests [5], delayed diagnosis [6] and inadequate treatments. A meta-analysis reported that foreign-born women were less likely to be diagnosed with localized-stage breast cancer compared to native women [6], including a relationship between the magnitude of the disparity and the level of development of the country of origin. On the other hand, reports have been published in which migrants registered a lower risk of cancer and lower cancer mortality compared to non-migrants [7,8,9], possibly due to a healthy migrant effect and low incidence of some cancers in the country of origin.
The number and proportion of cancer deaths and incident cases in migrants have been estimated in several countries, providing quantitative evidence of cancer epidemiology in this population group [8,9,10,11]. Cancer epidemiology in migrants is important for several reasons [3,4,6]. First, the description of cancer in this special population offers valuable information to understand the determinants of cancer in different ethnic groups and to disentangle the role played by environmental and genetic factors. Additionally, the investigation of cancer in migrants can highlight patterns of cancer occurrence in different ethnic groups, possibly identifying subjects to be targeted for specific preventive actions [3,12,13]. Moreover, studies on cancer in migrants may help in understanding the causes of cancer also in native populations [12]. These results may also have implications for national regulations and health policies, providing useful information derived from the comparison between the epidemiologic data of cancer in people born in different countries [14]. Indeed, migrants may acquire the same risk profile of the population of the host country [15]. An example comes from a population-based study conducted in Norway, which reported higher overall cancer incidence rates in native people than in migrants and observed higher liver cancer incidence in Asians than in Norwegians, as well as higher lung cancer incidence in male migrants from other Nordic countries and from Eastern Europe than in native men [9].
The Mediterranean countries of Europe are subject to migration from Northern Africa, Eastern Europe and West Asia, given the geographical position [14]. A recent study reported declining trends in cancer mortality in migrants in Spain between 2000 and 2016 [14]. In general, however, data on cancer incidence rates in migrants to Mediterranean countries in the last decade are scarce.
Sicily is an island in Southern Italy, which has experienced an increase in the migrant population in recent years. The official proportion of foreign subjects over the total population in Sicily in 2021 was 3.9%. The main countries of origin were Romania. Tunisia, Morocco, Sri Lanka, Albania and Bangladesh [16]. The actual number of migrants, however, is likely to be higher because of the presence of illegal and seasonal migrants. This is particularly true for Sicily, because of (i) the widespread use of seasonal migrants in agriculture and (ii) the role of Sicily as an entry point from the Mediterranean and the presence of numerous temporary transit camps for undocumented and illegal immigrants.
In order to estimate cancer proportion in migrants at a population-base level, we analyzed data of a cancer registry in Sicily, Italy. We focused on major areas of origin of the migrants and on the cancer sites with the highest occurrence in this special population.
2. Materials and Methods
2.1. Study Design and Population
This study is designed as a case–control study, where migrant status is the exposure and cancer types are the controls.
We analyzed data from the Eastern Sicily Cancer Registry covering 2.5 million people from four provinces (Catania, Enna, Messina and Syracuse) [17]. The registry is considered to be complete and is included in the Cancer Incidence in Five Continents, a collection of high-quality registries maintained by the International Agency for Research on Cancer [18]. This registry includes cases identified only from death certificates. Data are validated and periodically checked by the Associazione Italiana Registro Tumori (AIRTUM) through different programs (e.g., CheckAIRTUM and IARC CRG Tools). We selected new cases of the most frequent cancers diagnosed between 2004 and 2019 in migrants and identified new cases of the same cancers occurring among non-migrants during the same time period. The Cancer Registry collects data on cancer diagnosed mostly based on histological confirmation of the primary tumor and, for a minority of cases, clinical based on data or imaging; during 2009–2012, for only 1.6% of new cancer cases in men and 2.1% in women, the site of origin was classified as ‘other or unspecified’ [18].
2.2. Data Sources
Data derived from the Eastern Sicily Cancer Registry. We extracted the following information from the Cancer Registry: sex, age, country of birth, basis for diagnosis (histology/citology; clinical; death certificate/other), date of diagnosis and treatment (chemotherapy, radiotherapy and surgery). Information on the three treatment modalities was missing for a proportion of subjects. Since we were not able to distinguish between missing information or no therapy, we did not include these data in the analysis. Country of birth was categorized as Northern/Western Europe, Eastern Europe and Balkans, Northern Africa, Sub-Saharan Africa, Western Asia, other Asian countries excluding Japan, North America/Oceania/Japan and Latin America.
2.3. Statistical Analysis
Information on the total number of migrants living in the four provinces covered by the Cancer Registry was not available. We, therefore, could not calculate incidence rates and ratios directly comparing migrants and non-migrants but, rather, used the proportions of cancer occurring in the two populations. Specifically, we calculated the proportion of new cases of each cancer over total cancers among migrants and compared this with the same proportion among non-migrants. We then calculated the proportionate morbidity ratio (PMR) for each cancer type as the ratio of new observed cases in migrants over new expected cases, based on the proportion in non-migrants after adjusting for sex, age group and calendar year. Further, 95% confidence intervals (CIs) of PMR were calculated based on the Poisson distribution of new expected cases. We stratified the analyses by region of origin, sex and age. We tested heterogeneity in PIR between geographic region, sex and age categories using the Cochrane Q-test [19].
In addition, we fitted multivariate logistic regression models comparing one cancer to all other cancers to calculate ORs and 95% CIs of migration status (overall and by sex), after adjustment for sex, age category, basis of diagnosis and period of diagnosis. We repeated the analysis by geographic region of origin by fitting separate models, including migrants from one specific region and all non-migrants, and after stratification, by period of diagnosis. We tested heterogeneity between strata of sex and age period by adding interaction terms to the regression models.
For all the aforementioned analyses, p <0.05 was considered statistically significant.
3. Results
Table 1 illustrates the main characteristics of the study population. Overall, a total of 4726 new cases of cancer were registered among migrants between 2004 and 2019, including 1724 (36.5%) new cases among men and 3002 (63.5%) new cases among women. In the same period, 224,211 new cases were registered among non-migrants, including 122,241 (54.5%) among men and 101,970 (45.5%) among women. The overall odds of new cases in migrants to non-migrants was 2.1% and increased from 1.7% in 2004–2007 to 2.5% in 2016–2019. The countries of origin with the largest number of new cases of cancer among migrants were Germany (N = 968), Libya (N = 625) and Romania (N = 442).

Table 1.
Distribution of new cases of cancer by migrant status and selected characteristics.
Overall and sex-specific PMR for the main cancer types is shown in Table 2. We observed an increased proportion of cervical cancer (PIR = 2.68, 95% CI = 2.29–3.10) and lung cancer (PIR = 1.20, 95% CI = 1.07–1.33) among migrants. The result of lung cancer for both sexes (PMR = 1.20, 95% CI = 1.07–1.33) was driven by the pattern in women (PMR = 1.32, 95% CI = 1.11–1.56), with no increased proportion in men (p-value of test of heterogeneity between sexes = 0.09). The PMR of leukemia was decreased, with a stronger result among women (PMR = 0.77, 95% CI = 0.61–0.95, p-heterogeneity between sexes = 0.07).

Table 2.
Proportionate morbidity ratios of cancer among migrants by sex.
The results of the multivariate logistic regression analysis (Table 3) are consistent with the previous ones. The cancer with the highest OR in migrants was cervical cancer (OR = 3.54, 95% CI = 2.99–4.20), and an increase was also detected for lung cancer, in particular in women (OR = 1.23, 95% CI = 1.03–1.47). Colorectal cancer was the only neoplasm whose OR was decreased among migrants (OR = 0.86, 95% CI = 0.77–0.96), and a decreased OR of borderline statistical significance was observed for liver, breast and prostate cancer and NHL. Liver cancer and leukemia were the two neoplasms for which there was evidence of heterogeneity in OR between men and women (p = 0.02 and 0.03, respectively).

Table 3.
Odds ratio of selected cancer for migrant status, overall and by gender—results of multivariate logistic regression analysis.
In the analysis by region of origin in migrants (Figure 1, detailed results are available in Supplementary Table S1), migrants from Northern and Western Europe, including the European Union, showed a decreased OR for colorectal and breast cancer as well as NHL and leukemia, and an increased OR for cervical cancer. Migrants from Eastern Europe showed a decreased OR for prostate cancer and an increased OR for lung and cervical cancer. Migrants from North Africa experienced an increased OR for bladder cancer, whereas migrants from Sub-Saharan Africa experienced an increased OR of liver, breast cancer and leukemia and a decreased OR for colorectal cancer. The results on migrants from West Asia were hampered by small numbers. Migrants from other Asian countries had a decreased OR for bladder cancer and an increased OR for leukemia. Migrants from North America, Oceania and Japan did not have a statistically significant increased or decreased OR for any cancer. Finally, the OR for breast cancer was increased among migrants from Latin America.
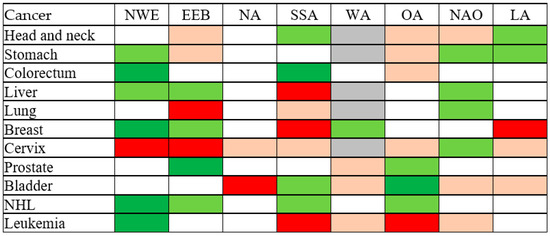
Figure 1.
Odds ratio of selected cancer among migrants, by area of origin—results of multivariate logistic regression analysis. NWE, Northern and Western Europe and European Union, excluding Bulgaria and Romania. EEB, Eastern Europe and the Balkans. NA, North Africa. SSA, Sub-Saharan Africa. WA, West Asia. OA, Other Asia, excluding Japan. NAO, North America and Oceania, including Japan. LA, Latin America. Light green: OR < 0.8, p > 0.05. Dark green: OR < 1, p < 0.05. Light red: OR > 1.25, p > 0.05. Dark red: OR > 1, p < 0.05. Grey: Model did not converge. NHL, non-Hodgkin lymphoma. OR, odds ratio adjusted for sex, age, type of diagnosis, year of diagnosis.
The analysis by period of diagnosis suggested a trend in OR of liver cancer (OR increased from 0.55 (95% CI 0.30–0.98) in 2004–2007 to 1.13 (95% CI 0.78–1.65) in 2016–2019), lung cancer (OR increased from 0.89 (95% CI 0.67–1.19) to 1.22 (95% CI 1.00–1.49)) and breast cancer (OR increased from 0.84 (95% CI 0.68–1.04) to 1.08 (95% CI 0.94–1.25)), although none of these trends were statistically significant (results not shown in detail).
4. Discussion
Our analysis revealed several patterns of cancer incidence among migrants in a Southern Italy population, including a higher proportion of cervical and lung cancers and a borderline statistically significant lower proportion of breast and prostate cancers compared to non-migrants. The stratification by geographical region of origin revealed that these patterns were mainly due to migration from Europe.
The results we describe are impaired by the lack of population data on the number of migrants in Sicily, thus preventing us from calculating cancer incidence rates in this population and incidence ratios in the comparisons with non-migrants. We tried to address this problem by using official data on the number of migrants present in four provinces during the study period [20] but obtained unreliable results, likely due to an undercount of migrants in official statistics. Despite this important limitation, ours remains one of the few studies to provide data on the neglected issue of cancer incidence in migrants in Italy and one of the first to provide a comprehensive analysis of different cancer types in migrants.
Although there was no increased proportion of head and neck cancer among migrants, an increase was suggested for migrants from Eastern Europe, the Balkans and from Asia, and a decrease was found among migrants from sub-Saharan Africa and Latin America. Possible explanations are relatable to the different distribution of the risk factors of head and neck cancer in Italy-born people and migrants, specifically HPV, tobacco smoking and alcohol [21]. Central and Eastern Europe is one of the regions with the highest incidence of this group of cancers [22].
Incidence of gastric cancer is elevated in Eastern European and East Asian countries [23], and migrants from these regions had a higher proportion of gastric cancer, although the difference was not statistically significant. Conversely, the incidence of colorectal cancer is relatively low in sub-Saharan Africa [24], and migrants from these countries had a non-significantly lower proportion of colorectal cancer. We observed a reduced risk of liver cancer in migrant men but not in women. Despite not having the information to assess the reason of this sex difference, we may hypothesize that it depends on sociocultural factors, leading to a better management of chronic liver disease (which can be a precursor of cancer) in men than in women, e.g., more frequent clinical visits and medical exams. However, this neoplasm was increased among migrants from Sub-Saharan Africa, a region at high risk for hepatitis B infection and liver cancer [25,26].
We observed an increased proportion of lung cancer among migrants, which was primarily related to migrants from Eastern European countries. The high proportion of lung cancer in migrants could be explained by higher exposure to risk factors, including tobacco smoking, indoor and outdoor air pollution and occupational risk factors during their lifetime. The difference between women and men is likely due to the low incidence of lung cancer among women in Southern Italy [18], which was explained by low tobacco consumption in past decades. Thus, the sex pattern observed in migrants may be an artifact rather than reflect particular risk factors in migrant women, despite the fact that we could not exclude potential confounders.
The proportion of breast cancer was reduced among migrants from Eastern Europe and the Balkans, a region with a lower incidence of these neoplasms compared to Italy [27]. A similar pattern was shown for migrants from other countries of Europe, while migrants from sub-Saharan Africa and Latin America had a higher proportion of this neoplasm. These differences by region of origin may derive from different approaches to cancer screening in addition to different exposure circumstances. In particular, among the factors which may affect breast cancer risk, oral contraceptives have been reported to be higher in women from Eastern Europe.
Cervical cancer showed the highest difference between migrants and non-migrants of all cancers, which was statistically significant for migrants from Europe. This is not unexpected, given the low incidence of this disease in Southern Italy and Sicily in particular [18]. The fact that the greatest proportion of new cases was seen among migrants from Eastern Europe might be explained by the high prevalence of HPV infection in that region [28]. An Italian study reported that 58% Eastern European and African women vs. 19% of Italy-born women to be HPV-positive [29]. Campari et al. reported a higher prevalence of preneoplastic cervical lesions and a lower participation in cervical cancer screening among migrants than Italian women [30].
The proportion of prostate cancer was lower in migrants from Eastern Europe and the Balkans and, although not significantly so, from Asian countries, excluding West Asia, which is consistent with previous findings [31,32]. This difference may be attributable to different levels of “westernization” of the lifestyle habits in different geographical areas of the Asian continent [33]; however, random fluctuation may also explain the difference we observed. Further, the results on the higher incidence rate of bladder cancer in migrants from North Africa than non-migrants are consistent with worldwide patterns of this disease [34].
These heterogeneous patterns of risk of specific cancers in migrants indicate the need for tailored cancer control programs based on the specific cancer predisposition in different populations. Overall, these results agree with a review by Arnold and colleagues, which described a higher proportion of infectious-related cancer (e.g., gastric and cervical) and a lower proportion of lifestyle-related cancer (e.g., colorectum, breast and prostate) in migrants to Europe [35].
Our results provide novel evidence on the different incidence of cancer in migrants in Italy and Sicily in particular, and they relate the pattern of cancer occurrence by geographical area [27]. The results obtained reflect lifestyle, environmental and genetic factors, which underlie the occurrence of specific cancers, such as cervical and prostate cancer in the Balkans [36] and colorectal cancer in Africa [24,37]. Compared to non-migrants, migrants from Eastern Europe and Africa may be less sedentary [38] and have a healthier lifestyle [39], including diet [40,41,42], and a lower prevalence of dysmetabolic diseases, such as diabetes and hypercholesterolemia [43], factors which may be associated with a lower incidence of prostate [44] and colorectal cancer [45]. Conversely, non-migrants may be less exposed than migrants to unsafe sex, resulting in a lower prevalence of HPV infection and HPV-related cancers [46,47,48], a pattern which has also been observed in other countries [49,50].
Migrants represent, in large part, a vulnerable group, connoted by a higher prevalence of unhealthy lifestyle habits (e.g., tobacco smoking, alcohol drinking [22], poor diet [51]), occupational disparities [2] and reduced access to healthcare services (e.g., vaccination, screening) [52,53]. These aspects are ultimately related to socioeconomic status. Most of the migrant populations, in Italy as well as in other countries, belong to low socioeconomic status. The relationship between low socioeconomic status and cancer is well described [54], especially with regard to some types of cancers, including those for which we evidenced an increased PIR, namely cervical [55,56] and lung [57,58,59].
Our analysis addresses the issue of cancer in migrants in Sicily through two approaches, namely PIR estimates and multivariate logistic regression analysis. This latter could account for several aspects, including treatment, which has been reported to differ between migrant and non-migrant populations. Different cancer treatment causes cancer disparities in migrants and non-migrants and is linked to barriers experienced by migrants, such as lack of language proficiency and not being familiar with the health system [60]. Our data on treatment, however, were impaired by missing data. This limitation is unlikely to have introduced bias, because even if missing data could have led to misclassification of treatment, we observed no confounding effect by this variable in the multivariate model.
When focusing on results by geographical area, an important observation is the increased proportion of breast cancer in women from sub-Saharan Africa. This result is unexpected, as African populations (incidence rates around 50/100,000) usually have lower incidence rates of breast cancer compared to European populations (incidence rates 80–90/100,000) [61]. The reason for this result is not clear. Breast cancer screening is usually associated with higher numbers of diagnoses [62]; thus, disparities in participation for cancer screening among migrants do not seem to explain our finding [63]. In addition to this, the migrant population may not precisely reflect incidence rates of the country of origin because they do not represent a random sample of that population. Interestingly, the increased proportion of breast cancer in this migrant subgroup is homogeneously distributed by age.
This study has some limitations. First, as already mentioned, the number of migrants in Sicily was not available, preventing us from calculating the incidence of cancer in migrants and comparing it with that in non-migrants. For this reason, the study design is that of a case–control study where controls are cancers in non-migrants. This approach has been used by other authors [64]. In addition, no information was available on the duration of residence of the migrants: analyses by duration of stay in the host country help to clarify the role of factors affecting cancer risk [65]. Moreover, the lack of information on lifestyle, occupational and sociodemographic factors prevented us from adjusting and stratifying for important variables. Additionally, results by area of origin of the migrant population showed patterns of risk which were not fully consistent with previous literature. Last, small numbers impaired the stratified analyses for some areas of origin.
The present study also has several strengths. We provided updated data on an under-investigated topic, focusing on a special population which is particularly vulnerable and affected by health inequalities. We identified interesting cancer patterns, and, because of the population-based nature of our data, we added valuable data to the current literature on cancer epidemiology in migrants and offered new important information on cancer incidence in different groups of migrants in Italy compared to non-migrants. Further, the data we used were from a high-quality cancer registry, increasing the reliability of our results [66,67].
5. Conclusions
In conclusion, migrants to Sicily appear to have an increased OR of cervical and lung cancer than non-migrants, although the comparison is based on proportionate ratios. Different cancer patterns could be observed based on the area of origin of the migrants, with lifestyle and socioeconomic factors in migrants from Centra/Eastern Europe, the Balkans and Africa possibly explaining several results. Differences were identified in particular for women, regarding HPV-related cancers as well as lung cancer and hematologic malignancies. These data may be a useful source of information for understanding cancer epidemiology in migrants in Italy. Cancer control in this special population requires public attention, despite its future trends being unpredictable given the acute nature of migration in Sicily, where most migrants move quickly to other countries.
Given the vulnerability of migrants to cancer, and the discrimination which they might be subjected to, measures should be implemented to enhance their access to prevention, diagnosis and care for cancer. Tailored intervention may be developed based on migrants’ country of origin, given the different cancer risk by geographical area. Targeted attention to HPV vaccination and cervical cancer screening participation in migrant women would help to better control infection-related cancers. Tobacco control interventions targeting migrants would also be important to reduce the cancer burden in this population. Cancer control and early detection in migrants may improve, while it is difficult to establish a follow-up for such a special population, which is quickly moving from Sicily to other countries.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15123103/s1, Table S1: Odds ratio of selected neoplasms among migrants, by region of origin.
Author Contributions
Conceptualization, G.C., P.B. and S.S. (Salvatore Sciacca); methodology, G.C. and P.B.; formal analysis, A.I., A.D.P. and P.B.; resources, M.F., C.C., S.S. (Salvatore Scarpulla) and S.S. (Salvatore Sciacca); data curation, M.F., C.C., S.S. (Salvatore Scarpulla) and S.S. (Salvatore Sciacca); writing—original draft preparation, G.C. and P.B.; writing—review and editing, A.I. and A.D.P.; supervision, M.F., P.B. and S.S. (Salvatore Sciacca); project administration, M.F. and S.S. (Salvatore Sciacca). All authors have read and agreed to the published version of the manuscript.
Funding
The project was conducted with internal resources of the institutions involved.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the public nature of the de-identified data.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be provided to external investigators upon reasonable request and agreement of the institutions involved.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bo, A.; Zinckernagel, L.; Krasnik, A.; Petersen, J.H.; Norredam, M. Coronary heart disease incidence among non-Western immigrants compared to Danish-born people: Effect of country of birth, migrant status, and income. Eur. J. Prev. Cardiol. 2015, 22, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Collatuzzo, G.; Teglia, F.; Boffetta, P. Role of Occupation in Shaping Cancer Disparities. Cancers 2022, 14, 4259. [Google Scholar] [CrossRef] [PubMed]
- Taylor, V.M.; Ko, L.K.; Hwang, J.H.; Sin, M.K.; Inadomi, J.M. Gastric cancer in Asian American populations: A neglected health disparity. Asian Pac. J. Cancer Prev. 2014, 15, 10565–10571. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, S.L.; Tiro, J.A.; Xuan, L.; Lee, S.J. Hispanic and Immigrant Paradoxes in U.S. Breast Cancer Mortality: Impact of Neighborhood Poverty and Hispanic Density. Int. J. Environ. Res. Public Health 2016, 13, 1238. [Google Scholar] [CrossRef]
- Bhargava, S.; Moen, K.; Qureshi, S.A.; Hofvind, S. Mammographic screening attendance among immigrant and minority women: A systematic review and meta-analysis. Acta Radiol. 2018, 59, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Herbach, E.L.; Weeks, K.S.; O’Rorke, M.; Novak, N.L.; Schweizer, M.L. Disparities in breast cancer stage at diagnosis between immigrant and native-born women: A meta-analysis. Ann. Epidemiol. 2021, 54, 64–72. [Google Scholar] [CrossRef]
- Stevenson, J.K.; Cheung, M.C.; Earle, C.C.; Fischer, H.D.; Camacho, X.; Saskin, R.; Shah, B.R.; Austin, P.C.; Singh, S. Chinese and South Asian ethnicity, immigration status, and clinical cancer outcomes in the Ontario Cancer System. Cancer 2018, 124, 1473–1482. [Google Scholar] [CrossRef]
- Shah, B.R.; Griffiths, R.; Hall, S.F. Thyroid cancer incidence among Asian immigrants to Ontario, Canada: A population-based cohort study. Cancer 2017, 123, 3320–3325. [Google Scholar] [CrossRef]
- Hjerkind, K.V.; Larsen, I.K.; Aaserud, S.; Møller, B.; Ursin, G. Cancer incidence in non-immigrants and immigrants in Norway. Acta Oncol. 2020, 59, 1275–1283. [Google Scholar] [CrossRef]
- Noel, C.W.; Sutradhar, R.; Li, Q.; Forner, D.; Hallet, J.; Cheung, M.; Singh, S.; Coburn, N.G.; Eskander, A. Association of Immigration Status and Chinese and South Asian Ethnicity with Incidence of Head and Neck Cancer. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 1125–1135. [Google Scholar] [CrossRef]
- Bates, J.H.; Hofer, B.M.; Parikh-Patel, A. Cervical cancer incidence, mortality, and survival among Asian subgroups in California, 1990–2004. Cancer 2008, 113 (Suppl. 10), 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Mousavi, S.M.; Sundquist, J.; Brandt, A. Does the breast cancer age at diagnosis differ by ethnicity? A study on immigrants to Sweden. Oncologist 2011, 16, 146–154. [Google Scholar] [CrossRef]
- Huang, R.J.; Sharp, N.; Talamoa, R.O.; Ji, H.P.; Hwang, J.H.; Palaniappan, L.P. One Size Does Not Fit All: Marked Heterogeneity in Incidence of and Survival from Gastric Cancer among Asian American Subgroups. Cancer Epidemiol. Biomark. Prev. 2020, 29, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Oliva-Arocas, A.; Pereyra-Zamora, P.; Copete, J.M.; Nolasco, A. Cancer Mortality Trends in Spain (2000–2016): Differences between Immigrant and Native Populations. Int. J. Environ. Res. Public Health 2020, 17, 5127. [Google Scholar] [CrossRef]
- Benchimol, E.I.; Mack, D.R.; Guttmann, A.; Nguyen, G.C.; To, T.; Mojaverian, N.; Quach, P.; Manuel, D.G. Inflammatory bowel disease in immigrants to Canada and their children: A population-based cohort study. Am. J. Gastroenterol. 2015, 110, 553–563. [Google Scholar] [CrossRef]
- lstat, Movimento e Calcolo Annuale Della Popolazione Straniera Residente e Struttura per Cittadinanza. Rome, ISTAT. 2021. Available online: https://noi-italia.istat.it (accessed on 5 December 2022). (In Italian).
- Benedetto, G.; Prima, A.D.; Sciacca, S.; Grosso, G. Design, functionality, and validity of the SWInCaRe, a web-based application used to administer cancer registry records. Health Inform. J. 2019, 25, 149–160. [Google Scholar] [CrossRef]
- Bray, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Zanetti, R.; Ferlay, J. (Eds.) Cancer Incidence in Five Continents, Vol. XI (Electronic Version); International Agency for Research on Cancer: Lyon, France, 2017. Available online: https://ci5.iarc.fr (accessed on 5 December 2022).
- Cochran, W.G. The comparison of percentages in matched samples. Biometrika 1950, 37, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://dati.istat.it/Index.aspx?DataSetCode=DCIS_POPSTRRES1 (accessed on 5 December 2022). (In Italian).
- Roman, B.R.; Aragones, A. Epidemiology and incidence of HPV-related cancers of the head and neck. J. Surg. Oncol. 2021, 124, 920–922. [Google Scholar] [CrossRef]
- Peacock, A.; Leung, J.; Larney, S.; Colledge, S.; Hickman, M.; Rehm, J.; Giovino, G.A.; West, R.; Hall, W.; Griffiths, P.; et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 2018, 113, 1905–1926. [Google Scholar] [CrossRef]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef]
- Bray, F.; Parkin, D.M.; African Cancer Registry Network. Cancer in sub-Saharan Africa in 2020: A review of current estimates of the national burden, data gaps, and future needs. Lancet Oncol. 2022, 23, 719–728. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, F.; Ferrigno, L.; Mele, A.; Alfonsi, V.; Declich, S.; De Ponte, G.; Crateri, S.; Burgio, A.; Caminada, S.; Tosti, M.E.; et al. Differences in Incidence of Acute Viral Hepatitis between Foreigners and Autochthonous Population in Italy. Int. J. Environ. Res. Public Health 2021, 18, 7944. [Google Scholar] [CrossRef] [PubMed]
- Coppola, N.; Alessio, L.; Gualdieri, L.; Pisaturo, M.; Sagnelli, C.; Minichini, C.; Di Caprio, G.; Starace, M.; Onorato, L.; Signoriello, G.; et al. Hepatitis B virus infection in undocumented immigrants and refugees in Southern Italy: Demographic, virological, and clinical features. Infect. Dis. Poverty 2017, 6, 33. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020. Available online: https://gco.iarc.fr/today (accessed on 5 December 2022).
- Bruni, L.; Diaz, M.; Castellsagué, X.; Ferrer, E.; Bosch, F.X.; de Sanjosé, S. Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J. Infect. Dis. 2010, 202, 1789–1799. [Google Scholar] [CrossRef]
- Tornesello, M.L.; Cassese, R.; De Rosa, N.; Buonaguro, L.; Masucci, A.; Vallefuoco, G.; Palmieri, S.; Schiavone, V.; Piccoli, R.; Buonaguro, F.M. High prevalence of human papillomavirus infection in Eastern European and West African women immigrants in South Italy. APMIS 2011, 119, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Campari, C.; Fedato, C.; Petrelli, A.; Zorzi, M.; Cogo, C.; Caprioglio, A.; Gallo, F.; Giordano, L.; Domenighini, S.; Pasquale, L.; et al. HPV prevalence and risk of pre-cancer and cancer in regular immigrants in Italy: Results from HPV DNA test-based screening pilot programs. Infect. Agent Cancer 2015, 10, 14. [Google Scholar] [CrossRef]
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef]
- Roshandel, G.; Boreiri, M.; Sadjadi, A.; Malekzadeh, R. A diversity of cancer incidence and mortality in West Asian populations. Ann. Glob. Health 2014, 80, 346–357. [Google Scholar] [CrossRef]
- Kimura, T.; Egawa, S. Epidemiology of prostate cancer in Asian countries. Int. J. Urol. 2018, 25, 524–531. [Google Scholar] [CrossRef]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Arnold, M.; Razum, O.; Coebergh, J.W. Cancer risk diversity in non-western migrants to Europe: An overview of the literature. Eur. J. Cancer 2010, 46, 2647–2659. [Google Scholar] [CrossRef] [PubMed]
- Rebbeck, T.R. Prostate Cancer Genetics: Variation by Race, Ethnicity, and Geography. Semin. Radiat. Oncol. 2017, 27, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Katsidzira, L.; Gangaidzo, I.; Thomson, S.; Rusakaniko, S.; Matenga, J.; Ramesar, R. The shifting epidemiology of colorectal cancer in sub-Saharan Africa. Lancet Gastroenterol. Hepatol. 2017, 2, 377–383. [Google Scholar] [CrossRef]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob. Health 2018, 6, e1077–e1086. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.; Anderson, C.; Lippman, S.M. Physical activity, sedentary behaviour, diet, and cancer: An update and emerging new evidence. Lancet Oncol. 2017, 18, e457–e471. [Google Scholar] [CrossRef]
- Roswall, N.; Olsen, A.; Boll, K.; Christensen, J.; Halkjær, J.; Sørensen, T.I.; Dahm, C.C.; Overvad, K.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; et al. Consumption of predefined ‘Nordic’ dietary items in ten European countries—An investigation in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Public Health Nutr. 2014, 17, 2650–2659. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Vitale, M.; Masulli, M.; Calabrese, I.; Rivellese, A.A.; Bonora, E.; Signorini, S.; Perriello, G.; Squatrito, S.; Buzzetti, R.; Sartore, G.; et al. Impact of a Mediterranean Dietary Pattern and Its Components on Cardiovascular Risk Factors, Glucose Control, and Body Weight in People with Type 2 Diabetes: A Real-Life Study. Nutrients 2018, 10, 1067. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- Oczkowski, M.; Dziendzikowska, K.; Pasternak-Winiarska, A.; Włodarek, D.; Gromadzka-Ostrowska, J. Dietary Factors and Prostate Cancer Development, Progression, and Reduction. Nutrients 2021, 13, 496. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Liivlaid, H.; Uusküla, A. Changes in high-risk sexual behaviour among Estonian adults between 1996 and 2006. Sex. Transm. Infect. 2013, 89, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Panatto, D.; Amicizia, D.; Lugarini, J.; Sasso, T.; Sormani, M.P.; Badolati, G.; Gasparini, R. Sexual behaviour in Ligurian (Northern Italy) adolescents and young people: Suggestions for HPV vaccination policies. Vaccine 2009, 27 (Suppl. 1), A6–A10. [Google Scholar] [CrossRef]
- Panatto, D.; Amicizia, D.; Trucchi, C.; Casabona, F.; Lai, P.L.; Bonanni, P.; Boccalini, S.; Bechini, A.; Tiscione, E.; Zotti, C.M.; et al. Sexual behaviour and risk factors for the acquisition of human papillomavirus infections in young people in Italy: Suggestions for future vaccination policies. BMC Public Health 2012, 12, 623. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M.; Reiter, P.L.; McRee, A.L. Nativity status and genital HPV infection among adults in the U.S. Hum. Vaccin. Immunother. 2019, 15, 1897–1903. [Google Scholar] [CrossRef]
- Causevic, S.; Salazar, M.; Orsini, N.; Kågesten, A.; Ekström, A.M. Sexual risk-taking behaviors among young migrant population in Sweden. BMC Public Health 2022, 22, 625. [Google Scholar] [CrossRef]
- Gilbert, P.A.; Khokhar, S. Changing dietary habits of ethnic groups in Europe and implications for health. Nutr. Rev. 2008, 66, 203–215. [Google Scholar] [CrossRef]
- Bao, H.; Zhang, L.; Wang, L.; Zhang, M.; Zhao, Z.; Fang, L.; Cong, S.; Zhou, M.; Wang, L. Significant variations in the cervical cancer screening rate in China by individual-level and geographical measures of socioeconomic status: A multilevel model analysis of a nationally representative survey dataset. Cancer Med. 2018, 7, 2089–2100. [Google Scholar] [CrossRef]
- Kurani, S.; MacLaughlin, K.L.; Jacobson, R.M.; St Sauver, J.L.; Jenkins, G.D.; Fan, C.; Jacobson, D.J.; Inselman, J.; Zhu, X.; Griffin, J.M.; et al. Socioeconomic disadvantage and human papillomavirus (HPV) vaccination uptake. Vaccine 2022, 40, 471–476. [Google Scholar] [CrossRef]
- Vaccarella, S.; Lortet-Tieulent, J.; Saracci, R.; Conway, D.I.; Straif, K.; Wild, C.P. (Eds.) Reducing Social Inequalities in Cancer: Evidence and Priorities for Research; International Agency for Research on Cancer: Lyon, France, 2019.
- Morriscey, C.; Hajizadeh, M. Income and education inequalities in cervical cancer incidence in Canada, 1992–2010. J. Public Health 2021, 43, 814–823. [Google Scholar] [CrossRef]
- Froment, M.A.; Gomez, S.L.; Roux, A.; DeRouen, M.C.; Kidd, E.A. Impact of socioeconomic status and ethnic enclave on cervical cancer incidence among Hispanics and Asians in California. Gynecol. Oncol. 2014, 133, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, M.; Johnston, G.M.; Manos, D. Socio-economic inequalities in lung cancer incidence in Canada, 1992–2010: Results from the Canadian Cancer Registry. Public Health 2020, 185, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Hovanec, J.; Siemiatycki, J.; Conway, D.I.; Olsson, A.; Stücker, I.; Guida, F.; Jöckel, K.-H.; Pohlabeln, H.; Ahrens, W.; Brüske, I.; et al. Lung cancer and socioeconomic status in a pooled analysis of case-control studies. PLoS ONE 2018, 13, e0192999. [Google Scholar] [CrossRef] [PubMed]
- Sidorchuk, A.; Agardh, E.E.; Aremu, O.; Hallqvist, J.; Allebeck, P.; Moradi, T. Socioeconomic differences in lung cancer incidence: A systematic review and meta-analysis. Cancer Causes Control 2009, 20, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, B.; Brough, M.; Wyld, D.; Durham, J. Equity across the cancer care continuum for culturally and linguistically diverse migrants living in Australia: A scoping review. Global Health 2021, 17, 87. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: An independent review. Lancet 2012, 380, 1778–1786. [Google Scholar] [CrossRef]
- Petrelli, A.; Giorgi Rossi, P.; Francovich, L.; Giordani, B.; Di Napoli, A.; Zappa, M.; Mirisola, C.; Gargiulo, L. Geographical and socioeconomic differences in uptake of Pap test and mammography in Italy: Results from the National Health Interview Survey. BMJ Open 2018, 8, e021653. [Google Scholar] [CrossRef]
- Siemiatycki, J.; Day, N.E.; Fabry, J.; Cooper, J.A. Discovering carcinogens in the occupational environment: A novel epidemiologic approach. J. Natl. Cancer Inst. 1981, 66, 217–225. [Google Scholar]
- Parkin, D.M. Studies of Cancer in Migrant Populations; International Agency for Research on Cancer: Lyon, France, 1993.
- Ragusa, R.; Torrisi, A.; Di Prima, A.A.; Torrisi, A.A.; Ippolito, A.; Ferrante, M.; Madeddu, A.; Guardabasso, V. Cancer Prevention for Survivors: Incidence of Second Primary Cancers and Sex Differences—A Population-Based Study from an Italian Cancer Registry. Int. J. Environ. Res. Public Health 2022, 19, 12201. [Google Scholar] [CrossRef]
- AIRTUM Working Group. Italian cancer figures, report 2013: Multiple tumours. Epidemiol. Prev. 2013, 37 (Suppl. 1), 1–152. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).