Locoregional Therapy for Intrahepatic Cholangiocarcinoma: The Role of Intra-Arterial Therapies
Abstract
Simple Summary
Abstract
1. Introduction
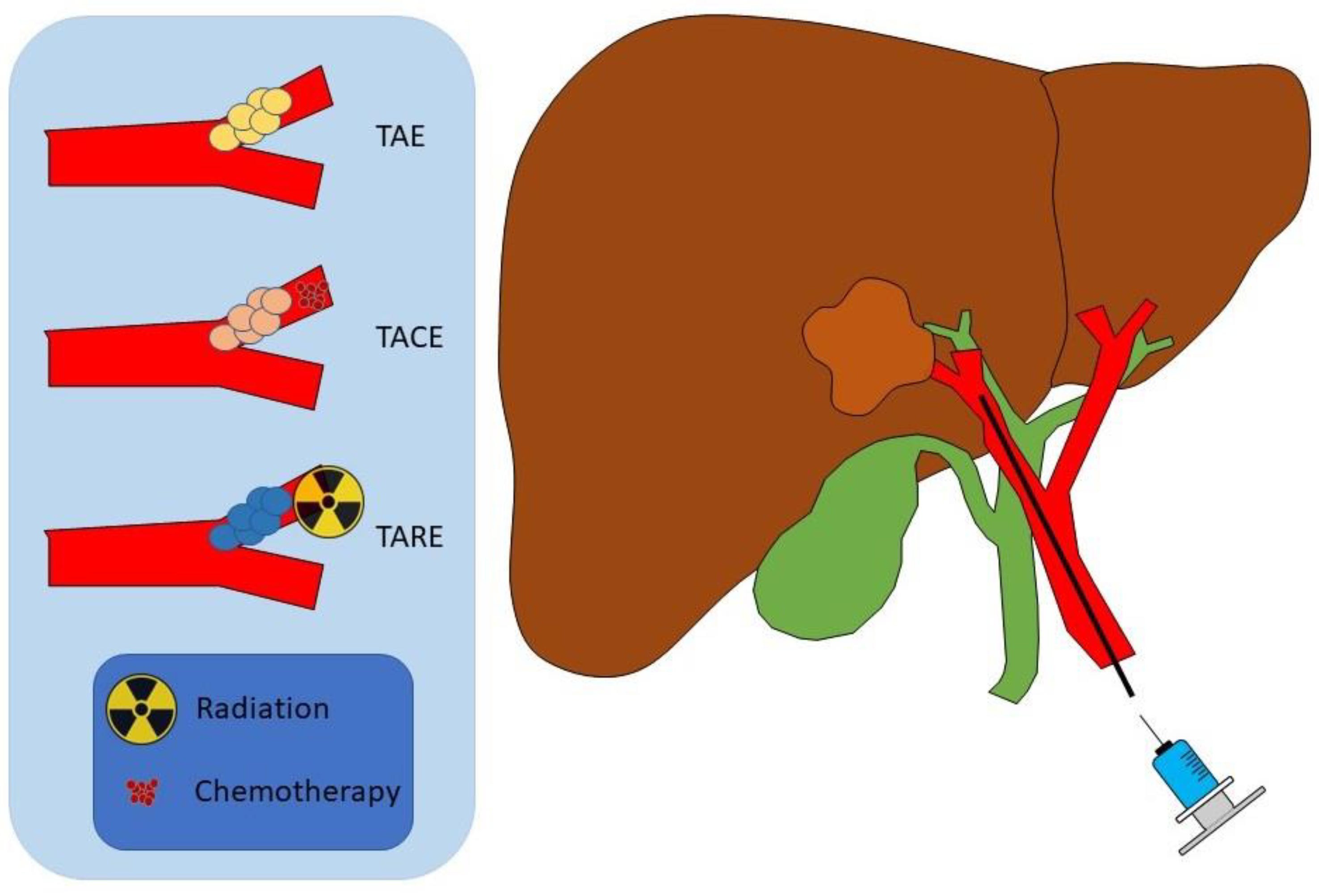
2. Transarterial Embolization
3. Transarterial Chemoembolization
4. Yittrium-90 Radioembolization
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Zhao, Y.Y.; Chen, S.H.; Wan, Q.S. A Prognostic Nomogram for Distal Bile Duct Cancer from Surveillance, Epidemiology, and End Results (SEER) Database Based on the STROBE Compliant. Medicine 2019, 98, e17903. [Google Scholar] [CrossRef] [PubMed]
- Garikipati, S.C.; Roy, P. Biliary Tract Cholangiocarcinoma; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Gorji, L.; Beal, E.W. Surgical Treatment of Distal Cholangiocarcinoma. Curr. Oncol. 2022, 29, 6674–6687. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, Y.; Liu, S. The New Insight of Treatment in Cholangiocarcinoma. J. Cancer 2022, 13, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-R.; Lee, Y.-K.; Chiang, C.-J.; Yang, Y.-W.; Chang, H.-C.; You, S.-L. Secular Trends of Intrahepatic Cholangiocarcinoma in a High Endemic Area: A Population-Based Study. World J. Gastroenterol. 2022, 28, 3695–3705. [Google Scholar] [CrossRef] [PubMed]
- Antwi, S.O.; Patel, T. Increasing Mortality of Intrahepatic Cholangiocarcinoma in the US: Are Gender-Specific Risk Factors Important? Hepatobiliary Surg. Nutr. 2019, 8, 635–636. [Google Scholar] [CrossRef]
- Raoof, M.; Singh, G. Rising Trends in Intrahepatic Cholangiocarcinoma Incidence and Mortality: Getting at the Root Cause. Hepatobiliary Surg. Nutr. 2019, 8, 301–303. [Google Scholar] [CrossRef]
- Bertuccio, P.; Malvezzi, M.; Carioli, G.; Hashim, D.; Boffetta, P.; El-Serag, H.B.; La Vecchia, C.; Negri, E. Global Trends in Mortality from Intrahepatic and Extrahepatic Cholangiocarcinoma. J. Hepatol. 2019, 71, 104–114. [Google Scholar] [CrossRef]
- Krenzien, F.; Nevermann, N.; Krombholz, A.; Benzing, C.; Haber, P.; Fehrenbach, U.; Lurje, G.; Pelzer, U.; Pratschke, J.; Schmelzle, M.; et al. Treatment of Intrahepatic Cholangiocarcinoma—A Multidisciplinary Approach. Cancers 2022, 14, 362. [Google Scholar] [CrossRef]
- Lim, J.H. Cholangiocarcinoma: Morphologic Classification According to Growth Pattern and Imaging Findings. Am. J. Roentgenol. 2003, 181, 819–827. [Google Scholar] [CrossRef]
- Patel, T. Cholangiocarcinoma. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 33–42. [Google Scholar] [CrossRef]
- Lee, A.J.; Chun, Y.S. Intrahepatic Cholangiocarcinoma: The AJCC/UICC 8th Edition Updates. Chin. Clin. Oncol. 2018, 7, 52. [Google Scholar] [CrossRef]
- Goere, D.; Wagholikar, G.D.; Pessaux, P.; Carrère, N.; Sibert, A.; Vilgrain, V.; Sauvanet, A.; Belghiti, J. Utility of Staging Laparoscopy in Subsets of Biliary Cancers: Laparoscopy Is a Powerful Diagnostic Tool in Patients with Intrahepatic and Gallbladder Carcinoma. Surg. Endosc. Other Interv. Tech. 2006, 20, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Franken, L.C.; Coelen, R.J.S.; Roos, E.; Verheij, J.; Phoa, S.S.; Besselink, M.G.; Busch, O.R.C.; van Gulik, T.M. Staging Laparoscopy in Patients with Intrahepatic Cholangiocarcinoma: Is It Still Useful? Visc. Med. 2020, 36, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.T.; Javle, M.M.; Xiao, L.; Kaseb, A.O.; Varadhachary, G.R.; Wolff, R.A.; Raghav, K.P.S.; Iwasaki, M.; Masci, P.; Ramanathan, R.K.; et al. Gemcitabine, Cisplatin, and Nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.W.; Cheung, T.T.; Leung, B.; She, B.W.H.; Chok, K.S.H.; Chan, A.C.Y.; Dai, W.C.; Lo, C.M. Adjuvant Chemotherapy Improves Oncological Outcomes of Resectable Intrahepatic Cholangiocarcinoma: A Meta-Analysis. Medicine 2019, 98, e14013. [Google Scholar] [CrossRef]
- Edeline, J.; Benabdelghani, M.; Bertaut, A.; Watelet, J.; Hammel, P.; Joly, J.-P.; Boudjema, K.; Fartoux, L.; Bouhier-Leporrier, K.; Jouve, J.-L.; et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J. Clin. Oncol. 2019, 37, 658–667. [Google Scholar] [CrossRef]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine Compared with Observation in Resected Biliary Tract Cancer (BILCAP): A Randomised, Controlled, Multicentre, Phase 3 Study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Anaya, D.A.; Anders, R.; Are, C.; Bachini, M.; Borad, M.; Brown, D.; Burgoyne, A.; et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 541–565. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Mackenzie, N.J.; Nicholls, C.; Templeton, A.R.; Perera, M.P.; Jeffery, P.L.; Zimmermann, K.; Kulasinghe, A.; Kenna, T.J.; Vela, I.; Williams, E.D.; et al. Modelling the Tumor Immune Microenvironment for Precision Immunotherapy. Clin. Transl. Immunol. 2022, 11, e1400. [Google Scholar] [CrossRef]
- Cho, S.M.; Esmail, A.; Raza, A.; Dacha, S.; Abdelrahim, M. Timeline of FDA-Approved Targeted Therapy for Cholangiocarcinoma. Cancers 2022, 14, 2641. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. A Phase 3 Randomized, Double-Blind, Placebo-Controlled Study of Durvalumab in Combination with Gemcitabine plus Cisplatin (GemCis) in Patients (Pts) with Advanced Biliary Tract Cancer (BTC): TOPAZ-1. J. Clin. Oncol. 2022, 40 (Suppl. S4), 378. [Google Scholar] [CrossRef]
- Piha-Paul, S.A.; Oh, D.-Y.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Efficacy and Safety of Pembrolizumab for the Treatment of Advanced Biliary Cancer: Results from the KEYNOTE-158 and KEYNOTE-028 Studies. Int. J. Cancer 2020, 147, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Edeline, J.; Lamarca, A.; McNamara, M.G.; Jacobs, T.; Hubner, R.A.; Palmer, D.; Groot Koerkamp, B.; Johnson, P.; Guiu, B.; Valle, J.W. Locoregional Therapies in Patients with Intrahepatic Cholangiocarcinoma: A Systematic Review and Pooled Analysis. Cancer Treat. Rev. 2021, 99, 102258. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.; Makary, M.S.; Beal, E.W. Locoregional Therapy for Intrahepatic Cholangiocarcinoma. Cancers 2023, 15, 2384. [Google Scholar] [CrossRef]
- Sakamoto, I.; Aso, N.; Nagaoki, K.; Matsuoka, Y.; Uetani, M.; Ashizawa, K.; Iwanaga, S.; Mori, M.; Morikawa, M.; Fukuda, T.; et al. Complications Associated with Transcatheter Arterial Embolization for Hepatic Tumors. Radiographics 1998, 18, 605–619. [Google Scholar] [CrossRef]
- Lencioni, R.; Petruzzi, P.; Crocetti, L. Chemoembolization of Hepatocellular Carcinoma. Semin. Interv. Radiol. 2013, 30, 3–11. [Google Scholar] [CrossRef]
- Kennedy, A.; Brown, D.B.; Feilchenfeldt, J.; Marshall, J.; Wasan, H.; Fakih, M.; Gibbs, P.; Knuth, A.; Sangro, B.; Soulen, M.C.; et al. Safety of Selective Internal Radiation Therapy (SIRT) with Yttrium-90 Microspheres Combined with Systemic Anticancer Agents: Expert Consensus. J. Gastrointest. Oncol. 2017, 8, 1079–1099. [Google Scholar] [CrossRef]
- Yamada, R.; Nakatsuka, H.; Nakamura, K.; Sato, M.; Itami, M.; Kobayashi, N.; Minakuchi, K.; Onoyama, T.; Kanno, T.; Monna, T.; et al. Hepatic Artery Embolization in 32 Patients with Unresectable Hepatoma. Osaka City Med. J. 1980, 26, 81–96. [Google Scholar]
- Scaffaro, L.A.; Kruel, C.D.P.; Stella, S.F.; Gravina, G.L.; Machado Filho, G.; de Almeida, C.P.B.; Pinto, L.C.P.F.; Alvares-da-Silva, M.R.; Kruel, C.R.P. Transarterial Embolization for Hepatocellular Carcinoma: A Comparison between Nonspherical PVA and Microspheres. Biomed. Res. Int. 2015, 2015, 435120. [Google Scholar] [CrossRef]
- Vaidya, S.; Tozer, K.R.; Chen, J. An Overview of Embolic Agents. Semin. Interv. Radiol. 2008, 25, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.S.; Benhamou, M.; Teyssier, Y.; Seigneurin, A.; Abousalihac, M.; Sengel, C.; Seror, O.; Ghelfi, J.; Ganne-Carrié, N.; Blaise, L.; et al. Comparison of Trans-Arterial Chemoembolization and Bland Embolization for the Treatment of Hepatocellular Carcinoma: A Propensity Score Analysis. Cancers 2021, 13, 812. [Google Scholar] [CrossRef] [PubMed]
- Kishore, S.A.; Bajwa, R.; Madoff, D.C. Embolotherapeutic Strategies for Hepatocellular Carcinoma: 2020 Update. Cancers 2020, 12, 791. [Google Scholar] [CrossRef] [PubMed]
- Hyder, O.; Marsh, J.W.; Salem, R.; Petre, E.N.; Kalva, S.; Liapi, E.; Cosgrove, D.; Neal, D.; Kamel, I.; Zhu, A.X.; et al. Intra-Arterial Therapy for Advanced Intrahepatic Cholangiocarcinoma: A Multi-Institutional Analysis. Ann. Surg. Oncol. 2013, 20, 3779–3786. [Google Scholar] [CrossRef]
- Niu, H.; Du, T.; Xiao, Q.; Hu, X.; Li, D.; Wang, C.; Gao, W.; Xing, T.; Xu, X. Application of Embolization Microspheres in Interventional Therapy of Malignant Non-Hypervascular Tumor of Liver. Oncotarget 2017, 8, 55593–55599. [Google Scholar] [CrossRef][Green Version]
- Mason, M.C.; Massarweh, N.N.; Salami, A.; Sultenfuss, M.A.; Anaya, D.A. Post-Embolization Syndrome as an Early Predictor of Overall Survival after Transarterial Chemoembolization for Hepatocellular Carcinoma. HPB 2015, 17, 1137–1144. [Google Scholar] [CrossRef]
- Agrawal, R.; Majeed, M.; Aqeel, S.-B.; Wang, Y.; Haque, Z.; Omar, Y.A.; Upadhyay, S.B.; Gast, T.; Attar, B.M.; Gandhi, S. Identifying Predictors and Evaluating the Role of Steroids in the Prevention of Post-Embolization Syndrome after Transarterial Chemoembolization and Bland Embolization. Ann. Gastroenterol. 2021, 34, 241–246. [Google Scholar] [CrossRef]
- Kis, B.; El-Haddad, G.; Sheth, R.A.; Parikh, N.S.; Ganguli, S.; Shyn, P.B.; Choi, J.; Brown, K.T. Liver-Directed Therapies for Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017, 24, 1073274817729244. [Google Scholar] [CrossRef]
- Elsayed-Ali, O.H.; Lipnik, A.J.; Brown, D.B. Bland Liver Tumor Embolization Complicated by Hepatic Abscess. Semin. Interv. Radiol. 2015, 32, 323–328. [Google Scholar] [CrossRef][Green Version]
- Young, M.; John, S. Hepatic Chemoembolization; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Melchiorre, F.; Patella, F.; Pescatori, L.; Pesapane, F.; Fumarola, E.; Biondetti, P.; Brambillasca, P.; Monaco, C.; Ierardi, A.M.; Franceschelli, G.; et al. DEB-TACE: A Standard Review. Future Oncol. 2018, 14, 2969–2984. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, W.; Chen, L.; Ren, Y.; Liu, Y.; Zheng, C. A Comparative Study of Efficacy and Safety of Transarterial Chemoembolization with CalliSpheres and Conventional Transarterial Chemoembolization in Treating Unresectable Intrahepatic Cholangiocarcinoma Patients. J. Cancer 2022, 13, 1282–1288. [Google Scholar] [CrossRef]
- Luo, J.; Zheng, J.; Shi, C.; Fang, J.; Peng, Z.; Huang, J.; Sun, J.; Zhou, G.; Li, T.; Zhu, D.; et al. Drug-Eluting Beads Transarterial Chemoembolization by CalliSpheres Is Effective and Well Tolerated in Treating Intrahepatic Cholangiocarcinoma Patients: A Preliminary Result from CTILC Study. Medicine 2020, 99, e19276. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; de Baere, T.; Soulen, M.C.; Rilling, W.S.; Geschwind, J.-F.H. Lipiodol Transarterial Chemoembolization for Hepatocellular Carcinoma: A Systematic Review of Efficacy and Safety Data. Hepatology 2016, 64, 106–116. [Google Scholar] [CrossRef]
- Ajit, Y.; Sudarsan, H.; Saumya, G.; Abhishek, A.; Navneet, R.; Piyush, R.; Anil, A.; Arun, G. Transarterial Chemoembolization in Unresectable Hepatocellular Carcinoma with Portal Vein Thrombosis: A Perspective on Survival. Oman Med. J. 2014, 29, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Aliberti, C.; Carandina, R.; Sarti, D.; Pizzirani, E.; Ramondo, G.; Mulazzani, L.; Mattioli, G.M.; Fiorentini, G. Chemoembolization with Drug-Eluting Microspheres Loaded with Doxorubicin for the Treatment of Cholangiocarcinoma. Anticancer Res. 2017, 37, 1859–1863. [Google Scholar] [CrossRef]
- Ge, Y.; Jeong, S.; Luo, G.-J.; Ren, Y.-B.; Zhang, B.-H.; Zhang, Y.-J.; Shen, F.; Cheng, Q.-B.; Sui, C.-J.; Wang, H.-Y.; et al. Transarterial Chemoembolization versus Percutaneous Microwave Coagulation Therapy for Recurrent Unresectable Intrahepatic Cholangiocarcinoma: Development of a Prognostic Nomogram. Hepatobiliary Pancreat. Dis. Int. 2020, 19, 138–146. [Google Scholar] [CrossRef]
- Gusani, N.J.; Balaa, F.K.; Steel, J.L.; Geller, D.A.; Marsh, J.W.; Zajko, A.B.; Carr, B.I.; Gamblin, T.C. Treatment of Unresectable Cholangiocarcinoma with Gemcitabine-Based Transcatheter Arterial Chemoembolization (TACE): A Single-Institution Experience. J. Gastrointest. Surg. 2008, 12, 129–137. [Google Scholar] [CrossRef]
- Kuhlmann, J.B.; Euringer, W.; Spangenberg, H.C.; Breidert, M.; Blum, H.E.; Harder, J.; Fischer, R. Treatment of Unresectable Cholangiocarcinoma: Conventional Transarterial Chemoembolization Compared with Drug Eluting Bead-Transarterial Chemoembolization and Systemic Chemotherapy. Eur. J. Gastroenterol. Hepatol. 2012, 24, 437–443. [Google Scholar] [CrossRef]
- Poggi, G.; Quaretti, P.; Minoia, C.; Bernardo, G.; Bonora, M.R.; Gaggeri, R.; Ronchi, A.; Saluzzo, C.M.; Azzaretti, A.; Rodolico, G.; et al. Transhepatic Arterial Chemoembolization with Oxaliplatin-Eluting Microspheres (OEM-TACE) for Unresectable Hepatic Tumors. Anticancer Res. 2008, 28, 3835–3842. [Google Scholar]
- Shen, W.F.; Zhong, W.; Liu, Q.; Sui, C.J.; Huang, Y.Q.; Yang, J.M. Adjuvant Transcatheter Arterial Chemoembolization for Intrahepatic Cholangiocarcinoma after Curative Surgery: Retrospective Control Study. World J. Surg. 2011, 35, 2083–2091. [Google Scholar] [CrossRef]
- Wang, L.; Lin, Z.-G.; Ke, Q.; Lou, J.-Y.; Zheng, S.-G.; Bi, X.-Y.; Wang, J.-M.; Guo, W.; Li, F.-Y.; Wang, J.; et al. Adjuvant Transarterial Chemoembolization Following Radical Resection for Intrahepatic Cholangiocarcinoma: A Multi-Center Retrospective Study. J. Cancer 2020, 11, 4115–4122. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Naguib, N.N.N.; Nour-Eldin, N.-E.A.; Bechstein, W.O.; Zeuzem, S.; Trojan, J.; Gruber-Rouh, T. Transarterial Chemoembolization in the Treatment of Patients with Unresectable Cholangiocarcinoma: Results and Prognostic Factors Governing Treatment Success. Int. J. Cancer 2012, 131, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, J.; Ma, Z.; Zhang, N.; Zhao, Y.; Yang, X.; Wen, Z.; Xie, H. Treatment of Unresectable Intrahepatic Cholangiocarcinoma Using Transarterial Chemoembolisation with Irinotecan-Eluting Beads: Analysis of Efficacy and Safety. Cardiovasc. Interv. Radiol. 2022, 45, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hao, M.; Chen, Q.; Chen, Z.; Lin, H. Comparison of the Efficacy and Safety among Apatinib plus Drug-Eluting Bead Transarterial Chemoembolization (TACE), Apatinib plus Conventional TACE and Apatinib Alone in Advanced Intrahepatic Cholangiocarcinoma. Am. J. Transl. Res. 2020, 12, 6584–6598. [Google Scholar]
- Zhou, T.-Y.; Zhou, G.-H.; Zhang, Y.-L.; Nie, C.-H.; Zhu, T.-Y.; Wang, H.-L.; Chen, S.-Q.; Wang, B.-Q.; Yu, Z.-N.; Wu, L.-M.; et al. Drug-Eluting Beads Transarterial Chemoembolization with CalliSpheres Microspheres for Treatment of Unresectable Intrahepatic Cholangiocarcinoma. J. Cancer 2020, 11, 4534–4541. [Google Scholar] [CrossRef]
- Cheng, Z.; Lei, Z.; Jin, X.; Zhang, Q.; Si, A.; Yang, P.; Zhou, J.; Hartmann, D.; Hüser, N.; Shen, F. Postoperative Adjuvant Transarterial Chemoembolization for Intrahepatic Cholangiocarcinoma Patients with Microvascular Invasion: A Propensity Score Analysis. J. Gastrointest. Oncol. 2021, 12, 819–830. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Lei, Z.; Wu, D.; Si, A.; Wang, K.; Wan, X.; Wang, Y.; Yan, Z.; Xia, Y.; et al. Adjuvant Transarterial Chemoembolization Following Liver Resection for Intrahepatic Cholangiocarcinoma Based on Survival Risk Stratification. Oncologist 2015, 20, 640–647. [Google Scholar] [CrossRef]
- Han, K.; Kim, J.H. Transarterial Chemoembolization in Hepatocellular Carcinoma Treatment: Barcelona Clinic Liver Cancer Staging System. World J. Gastroenterol. 2015, 21, 10327–10335. [Google Scholar] [CrossRef]
- Tong, A.K.T.; Kao, Y.H.; Too, C.W.; Chin, K.F.W.; Ng, D.C.E.; Chow, P.K.H. Yttrium-90 Hepatic Radioembolization: Clinical Review and Current Techniques in Interventional Radiology and Personalized Dosimetry. Br. J. Radiol. 2016, 89, 20150943. [Google Scholar] [CrossRef]
- Kennedy, A.; Nag, S.; Salem, R.; Murthy, R.; McEwan, A.J.; Nutting, C.; Benson, A.; Espat, J.; Bilbao, J.I.; Sharma, R.A.; et al. Recommendations for Radioembolization of Hepatic Malignancies Using Yttrium-90 Microsphere Brachytherapy: A Consensus Panel Report from the Radioembolization Brachytherapy Oncology Consortium. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 13–23. [Google Scholar] [CrossRef]
- Lewandowski, R.J.; Salem, R. Yttrium-90 Radioembolization of Hepatocellular Carcinoma and Metastatic Disease to the Liver. Semin. Interv. Radiol. 2006, 23, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Worsley, D.; Klass, D.; Liu, D.M. Technical Note: Simultaneous 90Y and 99mTc-MAA Injection for Two-Stage Selective Internal Radiation Therapy (SIRT) of Liver Metastases. Transl. Cancer Res. 2014, 3, 138–145. [Google Scholar] [CrossRef]
- Kallini, J.R.; Gabr, A.; Thorlund, K.; Balijepalli, C.; Ayres, D.; Kanters, S.; Ebrahim, S.; Mills, E.; Lewandowski, R.J.; Salem, R. Comparison of the Adverse Event Profile of TheraSphere® with SIR-Spheres® for the Treatment of Unresectable Hepatocellular Carcinoma: A Systematic Review. Cardiovasc. Interv. Radiol. 2017, 40, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Buettner, S.; Braat, A.J.A.T.; Margonis, G.A.; Brown, D.B.; Taylor, K.B.; Borgmann, A.J.; Kappadath, S.C.; Mahvash, A.; IJzermans, J.N.M.; Weiss, M.J.; et al. Yttrium-90 Radioembolization in Intrahepatic Cholangiocarcinoma: A Multicenter Retrospective Analysis. J. Vasc. Interv. Radiol. 2020, 31, 1035–1043.e2. [Google Scholar] [CrossRef] [PubMed]
- Edeline, J.; Touchefeu, Y.; Guiu, B.; Farge, O.; Tougeron, D.; Baumgaertner, I.; Ayav, A.; Campillo-Gimenez, B.; Beuzit, L.; Pracht, M.; et al. Radioembolization Plus Chemotherapy for First-Line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 51–59. [Google Scholar] [CrossRef]
- Bargellini, I.; Mosconi, C.; Pizzi, G.; Lorenzoni, G.; Vivaldi, C.; Cappelli, A.; Vallati, G.E.; Boni, G.; Cappelli, F.; Paladini, A.; et al. Yttrium-90 Radioembolization in Unresectable Intrahepatic Cholangiocarcinoma: Results of a Multicenter Retrospective Study. Cardiovasc. Interv. Radiol. 2020, 43, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Carolan-Rees, G.; Dale, M.; Patrick, H.E.; See, T.C.; Bell, J.K.; Manas, D.M.; Crellin, A.; Slevin, N.J.; Sharma, R.A. Yttrium-90 Transarterial Radioembolization for Chemotherapy-Refractory Intrahepatic Cholangiocarcinoma: A Prospective, Observational Study. J. Vasc. Interv. Radiol. 2019, 30, 1185–1192. [Google Scholar] [CrossRef]
- Levillain, H.; Duran Derijckere, I.; Ameye, L.; Guiot, T.; Braat, A.; Meyer, C.; Vanderlinden, B.; Reynaert, N.; Hendlisz, A.; Lam, M.; et al. Personalised Radioembolization Improves Outcomes in Refractory Intra-Hepatic Cholangiocarcinoma: A Multicenter Study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2270–2279. [Google Scholar] [CrossRef]
- Gangi, A.; Shah, J.; Hatfield, N.; Smith, J.; Sweeney, J.; Choi, J.; El-Haddad, G.; Biebel, B.; Parikh, N.; Arslan, B.; et al. Intrahepatic Cholangiocarcinoma Treated with Transarterial Yttrium-90 Glass Microsphere Radioembolization: Results of a Single Institution Retrospective Study. J. Vasc. Interv. Radiol. 2018, 29, 1101–1108. [Google Scholar] [CrossRef]
- Paz-Fumagalli, R.; Core, J.; Padula, C.; Montazeri, S.; McKinney, J.; Frey, G.; Devcic, Z.; Lewis, A.; Ritchie, C.; Mody, K.; et al. Safety and Initial Efficacy of Ablative Radioembolization for the Treatment of Unresectable Intrahepatic Cholangiocarcinoma. Oncotarget 2021, 12, 2075–2088. [Google Scholar] [CrossRef]
- Schatka, I.; Jochens, H.V.; Rogasch, J.M.M.; Walter-Rittel, T.C.; Pelzer, U.; Benckert, J.; Graef, J.; Feldhaus, F.W.; Gebauer, B.; Amthauer, H. Transarterial Yttrium-90 Radioembolization in Intrahepatic Cholangiocarcinoma Patients: Outcome Assessment Applying a Prognostic Score. Cancers 2022, 14, 5324. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, A.; Ali, A.; Ljuboja, D.; Weinstein, J.L.; Shenoy-Bhangle, A.S.; Nasser, I.A.; Morrow, M.K.; Faintuch, S.; Curry, M.P.; Bullock, A.J.; et al. Neoadjuvant Yttrium-90 Transarterial Radioembolization with Resin Microspheres Prescribed Using the Medical Internal Radiation Dose Model for Intrahepatic Cholangiocarcinoma. J. Vasc. Interv. Radiol. 2021, 32, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Riby, D.; Mazzotta, A.D.; Bergeat, D.; Verdure, L.; Sulpice, L.; Bourien, H.; Lièvre, A.; Rolland, Y.; Garin, E.; Boudjema, K.; et al. Downstaging with Radioembolization or Chemotherapy for Initially Unresectable Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2020, 27, 3729–3737. [Google Scholar] [CrossRef]
- Paprottka, K.J.; Galiè, F.; Ingrisch, M.; Geith, T.; Ilhan, H.; Todica, A.; Michl, M.; Nadjiri, J.; Paprottka, P.M. Outcome and Safety after 103 Radioembolizations with Yttrium-90 Resin Microspheres in 73 Patients with Unresectable Intrahepatic Cholangiocarcinoma-An Evaluation of Predictors. Cancers 2021, 13, 5399. [Google Scholar] [CrossRef]
- Depalo, T.; Traino, A.C.; Bargellini, I.; Lorenzoni, G.; Bozzi, E.; Vivaldi, C.; Lamastra, R.; Masi, G.; Cioni, R.; Boni, G.; et al. Assessment of Radiation Sensitivity of Unresectable Intrahepatic Cholangiocarcinoma in a Series of Patients Submitted to Radioembolization with Yttrium-90 Resin Microspheres. Sci. Rep. 2021, 11, 19745. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.-T.; Paprottka, P.M.; Schön, A.; Bamberg, F.; Haug, A.; Dürr, E.-M.; Rauch, B.; Trumm, C.T.; Jakobs, T.F.; Helmberger, T.K.; et al. Transarterial Hepatic Yttrium-90 Radioembolization in Patients with Unresectable Intrahepatic Cholangiocarcinoma: Factors Associated with Prolonged Survival. Cardiovasc. Interv. Radiol. 2012, 35, 105–116. [Google Scholar] [CrossRef]
- Filippi, L.; Di Costanzo, G.G.; Tortora, R.; Pelle, G.; Saltarelli, A.; Marino Marsilia, G.; Cianni, R.; Schillaci, O.; Bagni, O. Prognostic Value of Neutrophil-to-Lymphocyte Ratio and Its Correlation with Fluorine-18-Fluorodeoxyglucose Metabolic Parameters in Intrahepatic Cholangiocarcinoma Submitted to 90Y-Radioembolization. Nucl. Med. Commun. 2020, 41, 78–86. [Google Scholar] [CrossRef]
- Filippi, L.; Pelle, G.; Cianni, R.; Scopinaro, F.; Bagni, O. Change in Total Lesion Glycolysis and Clinical Outcome after 90Y Radioembolization in Intrahepatic Cholangiocarcinoma. Nucl. Med. Biol. 2015, 42, 59–64. [Google Scholar] [CrossRef]
- Camacho, J.C.; Kokabi, N.; Xing, M.; Prajapati, H.J.; El-Rayes, B.; Kim, H.S. Modified Response Evaluation Criteria in Solid Tumors and European Association for The Study of the Liver Criteria Using Delayed-Phase Imaging at an Early Time Point Predict Survival in Patients with Unresectable Intrahepatic Cholangiocarcinoma Following Yttrium-90 Radioembolization. J. Vasc. Interv. Radiol. 2014, 25, 256–265. [Google Scholar] [CrossRef]
- Gupta, A.N.; Gordon, A.C.; Gabr, A.; Kalyan, A.; Kircher, S.M.; Mahalingam, D.; Mulcahy, M.F.; Merkow, R.P.; Yang, A.D.; Bentrem, D.J.; et al. Yttrium-90 Radioembolization of Unresectable Intrahepatic Cholangiocarcinoma: Long-Term Follow-up for a 136-Patient Cohort. Cardiovasc. Interv. Radiol. 2022, 45, 1117–1128. [Google Scholar] [CrossRef]
- Rafi, S.; Piduru, S.M.; El-Rayes, B.; Kauh, J.S.; Kooby, D.A.; Sarmiento, J.M.; Kim, H.S. Yttrium-90 Radioembolization for Unresectable Standard-Chemorefractory Intrahepatic Cholangiocarcinoma: Survival, Efficacy, and Safety Study. Cardiovasc. Interv. Radiol. 2013, 36, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Garin, E.; Tselikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; de Baere, T.; Assenat, E.; Tacher, V.; Robert, C.; Terroir-Cassou-Mounat, M.; et al. Personalised versus Standard Dosimetry Approach of Selective Internal Radiation Therapy in Patients with Locally Advanced Hepatocellular Carcinoma (DOSISPHERE-01): A Randomised, Multicentre, Open-Label Phase 2 Trial. Lancet Gastroenterol. Hepatol. 2021, 6, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, M.; Yao, W.; Lin, M.; Becker, S.J.; Molitoris, J.K.; Vedam, S.; Yi, B. Validation of a Commercial Software Dose Calculation for Y-90 Microspheres. Brachytherapy 2022, 21, 561–566. [Google Scholar] [CrossRef]
- Skanjeti, A.; Magand, N.; Defez, D.; Tordo, J.; Rode, A.; Manichon, A.F.; Hallouard, F.; Clave-Darcissac, C.; Dhomps, A.; Townsend, D.M.; et al. Selective Internal Radiation Therapy of Hepatic Tumors: Morphologic and Functional Imaging for Voxel-Based Computer-Aided Dosimetry. Biomed. Pharmacother. 2020, 132, 110865. [Google Scholar] [CrossRef] [PubMed]
- Al-Adra, D.P.; Gill, R.S.; Axford, S.J.; Shi, X.; Kneteman, N.; Liau, S.-S. Treatment of Unresectable Intrahepatic Cholangiocarcinoma with Yttrium-90 Radioembolization: A Systematic Review and Pooled Analysis. Eur. J. Surg. Oncol. 2015, 41, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Dhondt, E.; Lambert, B.; Hermie, L.; Huyck, L.; Vanlangenhove, P.; Geerts, A.; Verhelst, X.; Aerts, M.; Vanlander, A.; Berrevoet, F.; et al. 90Y Radioembolization versus Drug-Eluting Bead Chemoembolization for Unresectable Hepatocellular Carcinoma: Results from the TRACE Phase II Randomized Controlled Trial. Radiology 2022, 303, 699–710. [Google Scholar] [CrossRef]
- Stella, M.; Braat, A.J.A.T.; van Rooij, R.; de Jong, H.W.A.M.; Lam, M.G.E.H. Holmium-166 Radioembolization: Current Status and Future Prospective. Cardiovasc. Interv. Radiol. 2022, 45, 1634–1645. [Google Scholar] [CrossRef]
- Radosa, C.G.; Radosa, J.C.; Grosche-Schlee, S.; Zöphel, K.; Plodeck, V.; Kühn, J.P.; Kotzerke, J.; Hoffmann, R.-T. Holmium-166 Radioembolization in Hepatocellular Carcinoma: Feasibility and Safety of a New Treatment Option in Clinical Practice. Cardiovasc. Interv. Radiol. 2019, 42, 405–412. [Google Scholar] [CrossRef]
- Braat, A.J.A.T.; Bruijnen, R.C.G.; van Rooij, R.; Braat, M.N.G.J.A.; Wessels, F.J.; van Leeuwaarde, R.S.; van Treijen, M.J.C.; de Herder, W.W.; Hofland, J.; Tesselaar, M.E.T.; et al. Additional Holmium-166 Radioembolisation after Lutetium-177-Dotatate in Patients with Neuroendocrine Tumour Liver Metastases (HEPAR PLuS): A Single-Centre, Single-Arm, Open-Label, Phase 2 Study. Lancet Oncol. 2020, 21, 561–570. [Google Scholar] [CrossRef]
- Padia, S.A.; Lewandowski, R.J.; Johnson, G.E.; Sze, D.Y.; Ward, T.J.; Gaba, R.C.; Baerlocher, M.O.; Gates, V.L.; Riaz, A.; Brown, D.B.; et al. Radioembolization of Hepatic Malignancies: Background, Quality Improvement Guidelines, and Future Directions. J. Vasc. Interv. Radiol. 2017, 28, 1–15. [Google Scholar] [CrossRef]
- Woerner, A.J.; Johnson, G.E. Advances in Y-90 Radioembolization for the Treatment of Hepatocellular Carcinoma. Hepatoma Res. 2022, 8, 2. [Google Scholar] [CrossRef]
- Gupta, A.; Dixon, E. Epidemiology and Risk Factors: Intrahepatic Cholangiocarcinoma. Hepatobiliary Surg. Nutr. 2017, 6, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next Horizon in Mechanisms and Management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Spolverato, G.; Kim, Y.; Alexandrescu, S.; Marques, H.P.; Lamelas, J.; Aldrighetti, L.; Clark Gamblin, T.; Maithel, S.K.; Pulitano, C.; Bauer, T.W.; et al. Management and Outcomes of Patients with Recurrent Intrahepatic Cholangiocarcinoma Following Previous Curative-Intent Surgical Resection. Ann. Surg. Oncol. 2016, 23, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Sota, Y.; Einama, T.; Kobayashibayashi, K.; Fujinuma, I.; Tsunenari, T.; Takihata, Y.; Iwasaki, T.; Miyata, Y.; Okamoto, K.; Kajiwara, Y.; et al. Recurrent Cholangiocarcinoma with Long-Term Survival by Multimodal Treatment: A Case Report. Mol. Clin. Oncol. 2021, 14, 72. [Google Scholar] [CrossRef]
- Akinwande, O.; Shah, V.; Mills, A.; Noda, C.; Weiner, E.; Foltz, G.; Saad, N. Chemoembolization versus Radioembolization for the Treatment of Unresectable Intrahepatic Cholangiocarcinoma in a Single Institution Image-Based Efficacy and Comparative Toxicity. Hepat. Oncol. 2017, 4, 75–81. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The Entry of Nanoparticles into Solid Tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef]
- Ebeling Barbier, C.; Heindryckx, F.; Lennernäs, H. Limitations and Possibilities of Transarterial Chemotherapeutic Treatment of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 13051. [Google Scholar] [CrossRef]

| Primary Tumor (T) | Nodal Involvement | Metastasis |
|---|---|---|
| T1a: Solitary tumor ≤5 cm without vascular involvement. T1b: Solitary tumor >5 cm without vascular involvement. | N1: Regional lymph node metastasis. | M1: Distant metastases. |
| T2: Solitary tumor with intrahepatic vascular involvement; multiple tumors +/− vascular involvement. | ||
| T3: Tumor invading the visceral peritoneum. | ||
| T4: Tumor invading local extrahepatic structures. |
| Author | Study Period | Location | Patient Population | Approach | Outcomes | Toxicities |
|---|---|---|---|---|---|---|
| Unresectable Disease | ||||||
| Aliberti et al. [47] | 2000–2016 | Italy | Unresectable ICC | N = 127 (N = 109 DEBDOX, N = 18 LIFDOX) | PR 15%, PD 5%, SD 80%, median OS 13.2 mo in patients with unresectable ICC | Abdominal pain, fever, nausea, and transaminitis. No grade 4 adverse events observed. |
| Liu et al. [55] | 2016–2020 | China | Unresectable ICC | N = 39, DEB-TACE | Median OS 11 mo, PFS 8 mo | Nausea, vomiting, abdominal pain, transaminitis, fever, and fatigue. One grade 3 AE of hepatic abscess development. No grade 4 AEs. |
| Ge et al. [48] | 2008–2015 | China | Median age 55 (20–85), Recurrent ICC | N = 275, N = 183 TACE, N = 92 PMCT | 5-year OS improved TACE vs. PMCT, 21.4% vs. 6.1% (p = 0.034) | - |
| Gusani et al. [49] | 2001–2007 | USA | Median age 59 (36–86); 88% w/central ICC, 12% w/peripheral ICC; 45% with extrahepatic dsx. | N = 42 | Median OS gem-cisTACE median OS 13.8 mo vs. gem-alone TACE 6.3 mo, respectively | Hyperbilirubinemia, elevated creatinine, thrombocytopenia, hyperglycemia, hypertension, pulmonary edema, and pancreatitis. Five pts had grade 3 AEs and 2 pts had grade 4 AEs. |
| Hu et al. [56] | 2015–2019 | China | Unresectable or progressive ICC | N = 35, apatinib plus DEB-TACE group (n = 10), apatinib plus cTACE group (n = 12), apatinib group (n = 13) | Apatinib plus DEB-TACE group: PFS 17 mo; OS 19.3 mo, apatinib plus cTACE group: PFS 10.3 mo; OS 14 mo, apatinib group: PFS 4.5 mo; OS 6.5 mo | Nausea, vomiting, abdominal pain, fever, and transaminitis. |
| Kuhlmann et al. [50] | 2002–2010 | Germany | Unresectable ICC | N = 46 with ICC, 23 pts treated with iDEB-TACE, 9 pts with cTACE with mitomycin C, 14 pts with ChT | iDEB-TACE PFS 3.9 mo, median OS 11.7 mo | Abdominal pain (34%), nausea (26.8%), fever (4.4%), hypertension (5.9%), alopecia (2.9%), and urticaria (1.5%) occurred in the cTACE and iDEB-TACE groups. Nine pts had grade 3 or 4 AEs. One death occurred in a cirrhotic, Child–Pugh A pt. |
| Luo et al. [44] | 2015–2016 | China | Primary HCC, ICC (n = 37), or secondary liver metastases | N = 37, DEB-TACE | Mean OS was 376 days, CR 8.1%, and ORR 67.6% | Nausea, vomiting, bone marrow toxicity, and fever. Grading severity not reported. |
| Poggi et al. [51] | 2006 | Italy | 15 pts (8 with CRC LM, 7 with ICC), treatment with GEMOX prior to TACE | N = 7 patients with unresectable ICC treated with OEM-TACE. | SD 53.3%, PR 13.3%, PD 33.3% at a median FU 34 (6–92) mo median OS of 40 mo | Abdominal pain, low-grade fever, and nausea occurred in 53.2% of pts. Cholecystitis was seen in 2 pts, rash in 1 pt, and pancreatitis in 1 pt. There were no grade 4 AEs or deaths. |
| Vogl et al. [54] | 1999–2010 | Germany | Unresectable ICC, median age of 60.4 (37–87), Child–Pugh A or B. | N = 155 underwent TACE—24 pts Mitomycin C, 8 with Gemcitabine only, 54 with Mitomycin C + Gemcitabine, 29 in the Mitomycin C + Gemcitabine + Cisplatin. | 1-, 2-, 3-year OS 52%, 29%, and 10% with no significant survival difference between groups, 8.7% PR, 57.4% SD, 33.9% PD | Abdominal pain, nausea, and vomiting in 9.6% of pts. No grade 3 or 4 complications. |
| Adjuvant TACE | ||||||
| Cheng et al. [58] | 2002–2015 | China | resectable ICC with MVI | N = 223, p-TACE | p-TACE for ICC with MVI demonstrated benefit for OS and TTR in subgroup of patients with elevated CA19-9 and those w/o lymphadenopathy; otherwise, no association between p-TACE and OS or DFS | - |
| Shen et al. [52] | 2002–2003 | China | Recurrent ICC | N = 125, 53 pts underwent p-TACE vs. 72 pts in the non-TACE group | Median FU 18 (3–96) mo, 1-,3-, 5- year OS was higher in the adjuvant TACE after surgical resection group vs. non-TACE group 69.8 vs. 54.2, 37.7 vs. 25.0, and 28.3 vs. 20.8 (p = 0.045), respectively | Abdominal pain (35.8%), nausea/vomiting (47.1%), and fever (11.3%). |
| Wang et al. [53] | 2014–2017 | China | Pts with ICC who underwent curative-intent resection for ICC | N = 335, 39 with p-TACE vs. 296 non-TACE group | Median OS p-TACE 63 mo vs. 18 mo w/o p-TACE (p = 0.041) | - |
| Zhou et al. [57] | 2015–2018 | China | Unresectable or recurrent ICC who underwent DEB-TACE | N = 88 (58 without surgical intervention, 30 adjuvant) | Median PFS and OS 3 mo and 9 mo, respectively. | Nausea, vomiting, abdominal pain, transaminitis, low-grade fever, and cerebral infarct. |
| Author | Study Period | Location | Patient Population | Approach | Outcomes | Toxicities |
|---|---|---|---|---|---|---|
| Bargellini et al. [68] | 2008–2017 | Italy | Unresectable ICC | N = 81, 3 treatment groups (a: 35 chemotherapy-naïve pts, b: 19 pts with disease control after first-line chemo, c: 27 pts with disease progression after first-line chemo) | Median OS 14.5 mo did not differ significantly among the treatment groups. TB > 50%, N/L ratio ≥ 3, and radiologic progression independent, negative factors for OS (p < 0.05) | Abdominal pain, nausea, vomiting |
| Buettner et al. [66] | 2006–2017 | Netherlands, UK, USA | Unresectable ICC | N = 115, 92 pts treated with resin microspheres, 22 pts treated with glass microspheres, 1 treated with both | Median OS 29 mo, and 1-, 3-, and 5- year survival 85%, 31%, 8% | Fatigue, pain, nausea, vomiting, DVT, generalized weakness, gastrointestinal hemorrhage, REILD, neuropathy |
| Camacho et al. [81] | 2009–2012 | USA | Unresectable, chemorefractory ICC | N = 21, treatment with Y-90 resin microspheres | Median OS from Y-90 tx was 16.3 mo | - |
| Depalo et al. [77] | 2013–2018 | Italy | Unresectable ICC | N = 15 | Median of tumor average absorbed dose was 93 Gy, median of α and α3D parameters was 0.005 Gy−1 and 0.007 Gy−1, respectively. Tumor volume and tumor absorbed dose were prognostic indicators of TTP | - |
| Edeline et al. [67] | 2013–2016 | France | Unresectable ICC, chemotherapy, and intra-arterial therapy naïve | N = 41, Y-90 therapy, Phase 2 clinical trial | Combination of chemotherapy (cis+gem) and RE median PFS 14 mo (8–17 mo) and median OS 22 mo (14–52 mo) | Abdominal pain (41%), nausea (49%), diarrhea (29%), constipation (17%), diarrhea (29%), dysphagia (5%), neutropenia (73%), thrombocytopenia (63%) |
| Filippi et al. [80] | Italy | Unresectable, chemorefractory ICC | N = 17, treatment with Y-90 glass or resin microspheres | FDG-PET CT was performed 6 weeks following Y-90 tx. Fourteen pts had a PR and 3 pts with SD. No pts demonstrated CR; Pts with ΔTLG > 50% and ΔTLG < 50% had a mean OS of 79.6 and 43.1 weeks, respectively (p < 0.001) | Abdominal pain (35.3%), moderate gastritis (11.7%), severe gastritis (5.8%) | |
| Gangi et al. [71] | 2009–2016 | USA | Unresectable ICC | N = 85, treatment with Y-90 glass microspheres | Median OS 12 mo, increased with ECOG score < 2 compared to ECOG ≥ 2 (18.5 vs. 5.5 mo p = 0.0012), well-differentiated histology (18.6 vs. 9.7 mo p = 0.012), and solitary tumors vs. multifocal (25 vs. 6.1 mo p = 0.006) | Abdominal pain (18.8%), weight loss (7.1%), ascites (5.9%), biochemical toxicities (hyperbilirubinemia, transaminitis) (53%) |
| Gupta et al. [82] | 2004–2020 | USA | Unresectable ICC | N = 136, treated with Y-90 glass microspheres | Median OS 14.2 mo; At 3 mo, 24.4% had a PR, 74.4% had SD, and 1.2% had PD | Fatigue (72%), abdominal pain (31.1%), hypoalbuminemia (43.9%), elevated alkaline phosphatase (30.9%) |
| Hoffman et al. [78] | 2007–2010 | Germany | Unresectable ICC | N = 33, treatment with Y-90 resin microsphere | Median OS 22 mo posttreatment | Abdominal pain (84.8%), nausea (60.6%), vomiting (27.3%), hyperbilirubinemia (69.7%) |
| Levillain et al. [70] | 2004–2018 | Belgium | Unresectable, chemorefractory ICC | N = 58, 30 pts with previous curative-intent liver resection, 28 pts w/o previous resection treated with Y-90 resin microspheres | Median OS 10.3 mo, 1- and 2-year survival rates after Y-90 were 40% and 22% | - |
| Paprottka et al. [76] | - | Germany | Unresectable ICC | N = 73, treatment with Y-90 resin microspheres | Median PFS 6.4 mo OS 18.9 mo, respectively; Patients with a tumor burden ≤25% had a significantly longer OS (15.2 vs. 6.6 mo; p = 0.036); Median PFS longer for patients with multiple TARE cycles (24.4 vs. 5.8 mo; p = 0.04) | Nausea, vomiting, pain, fever, gastritis, pancreatitis |
| Paz-Fumagalli et al. [72] | 2016–2020 | USA | Unresectable ICC | N = 28, treatment with Y-90 glass microspheres | 30 mo OS of 59% in patients with unresectable ICC; 6 patients were downsized to resection post-Y-90 therapy | Abdominal pain, fever, perforated cholecystitis |
| Rafi et al. [83] | 2002–2010 | USA | Unresectable, chemorefractory ICC | N = 19, treatment with Y-90 resin microspheres | Median OS from diagnosis and first Y90 tx was 752 [95% CI374–1130] and 345 (95% CI 95–595) days, respectively. Higher ECOG scores and extrahepatic metastasis were associated with worse outcomes | Fatigue (21%), abdominal pain (32%), thrombocytopenia (5%) |
| Riby et al. [75] | 1997–2017 | France | Resectable ICC and unresectable ICC (underwent neoadjuvant therapy for downstaging) | N = 169, 137 surgically resectable, 32 with downstaging intervention (13 with neoadjuvant chemotherapy, and 19 with Y-90) | Median OS not statistically significant; 32.3 mo in the primary surgery group, and 45.9 mo in the downstaging group (p = 0.54) | - |
| Sarwar et al. [74] | 2015–2020 | USA | Unresectable ICC | N = 31, treatment with Y-90 resin microspheres; Neoadjuvant use for patients with tumor proximity to middle hepatic vein or insufficient liver remnant in 21 patients | Median PFS 5.4 mo; Median OS 22 mo | Nausea, vomiting, abdominal pain, pneumonia, transaminitis; 9 patients experienced grade 3 events, and 1 patient experienced a grade 4 event (obstructive jaundice) |
| Schatka et al. [73] | 2009–2016 | Germany | Unresectable ICC with hepatic metastases; Additional nodal (19 pts), bone (2 pts), and lung (2 pts) metastases included | N = 39, treatment with Y-90 resin microspheres | Median OS 8 mo. ECOG ≥1 (HR 3.8), high ggt (HR 1.002), AST/ALT quotient (HR 1.86), high CA19-9 (HR 1.00), and dose reduction ≥40% (HR 3.8) were poor prognostic indicators of OS; Median OS 15.3 mo with 0 risk factors, 7.6 mo with 1 risk factor, and 1.8 months with 2 risk factors (p < 0.001) | Nausea, vomiting, fever, abdominal pain, angina |
| White et al. [69] | 2013–2017 | UK | Unresectable ICC | N = 61, treatment with Y-90 microspheres | Median OS was 8.7 mo (5.2–12.1 mo); PFS was 2.8 mo (2.6–3.1 mo) | Abdominal pain, fatigue, fever, diarrhea, tumor lysis syndrome, portal vein thrombosis, liver decompensation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorji, L.; Aoun, H.; Critchfield, J.; Al Hallak, N.; Beal, E.W. Locoregional Therapy for Intrahepatic Cholangiocarcinoma: The Role of Intra-Arterial Therapies. Cancers 2023, 15, 4727. https://doi.org/10.3390/cancers15194727
Gorji L, Aoun H, Critchfield J, Al Hallak N, Beal EW. Locoregional Therapy for Intrahepatic Cholangiocarcinoma: The Role of Intra-Arterial Therapies. Cancers. 2023; 15(19):4727. https://doi.org/10.3390/cancers15194727
Chicago/Turabian StyleGorji, Leva, Hussein Aoun, Jeffrey Critchfield, Najeeb Al Hallak, and Eliza W. Beal. 2023. "Locoregional Therapy for Intrahepatic Cholangiocarcinoma: The Role of Intra-Arterial Therapies" Cancers 15, no. 19: 4727. https://doi.org/10.3390/cancers15194727
APA StyleGorji, L., Aoun, H., Critchfield, J., Al Hallak, N., & Beal, E. W. (2023). Locoregional Therapy for Intrahepatic Cholangiocarcinoma: The Role of Intra-Arterial Therapies. Cancers, 15(19), 4727. https://doi.org/10.3390/cancers15194727






