The Role of the Multiparametric MRI LiverMultiScanTM in the Quantitative Assessment of the Liver and Its Predicted Clinical Applications in Patients Undergoing Major Hepatic Resection for Colorectal Liver Metastasis
Abstract
:Simple Summary
Abstract
1. Introduction
2. The LiverMultiScanTM
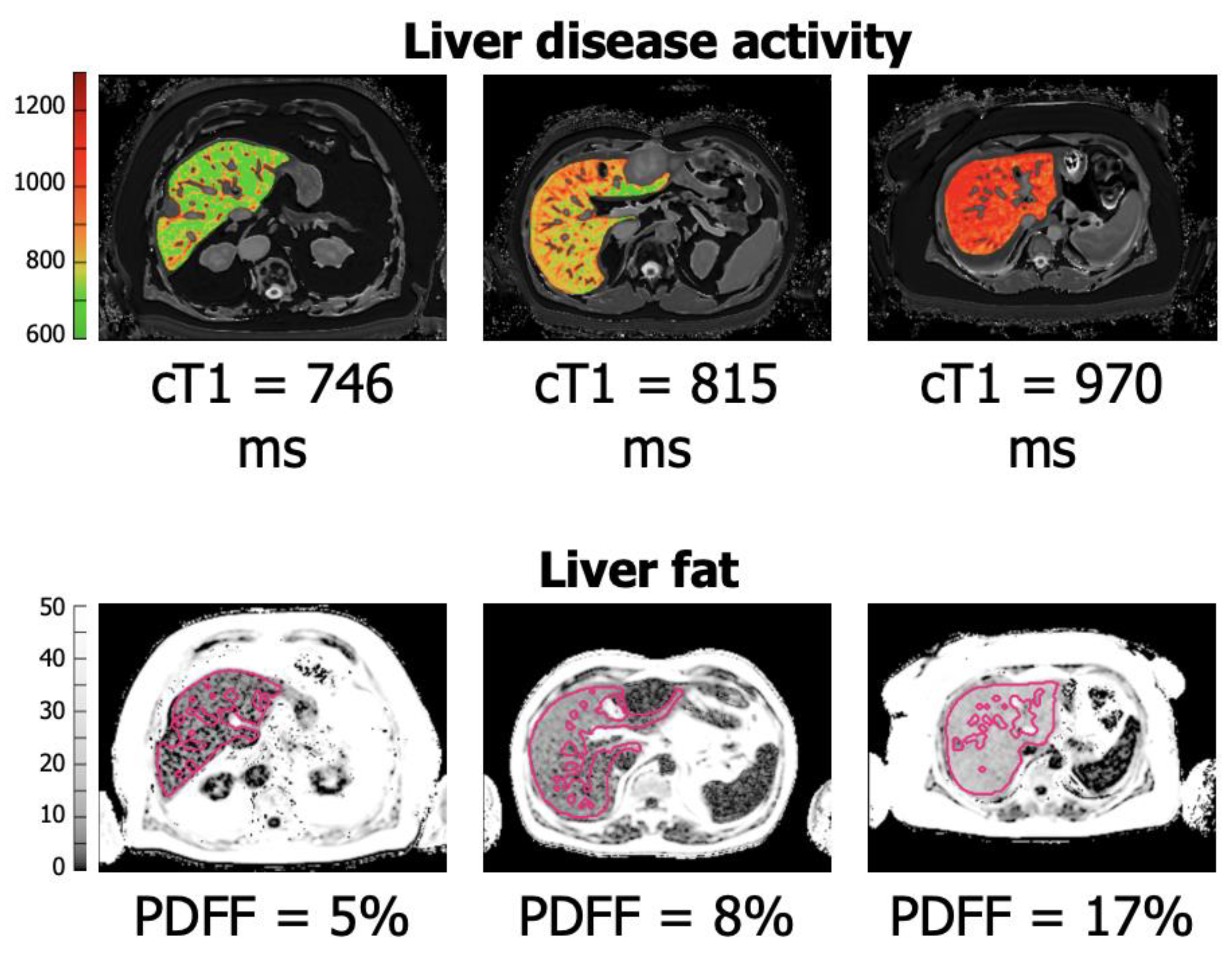
2.1. LiverMultiScan TM: The Grading of Hepatic Fibrosis and Future Applications in Hepatic Surgery
2.2. Alternative Imaging Markers of Liver Fibrosis and Fibrosis Grading
2.3. LiverMultiScanTM: Grading of Hepatic Steatosis/Steatohepatitis and Applications in Hepatic Surgery
2.4. Future Clinical Applications of the LiverMultiScanTM in Neoadjuvant Chemotherapy Setting in CRLM and Decision Making Regarding Optimum Treatment Modalities
2.5. Future Application of Alternative Biomarkers in the Pre-Operative Setting and LiverMultiScanTM
2.6. Pertinent Current Trials Examining the Clinical Applications of LiverMultiScanTM in Liver Surgery for CRLM and Other Hepatic Malignancies
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ratziu, V.; Charlotte, F.; Heurtier, A.; Gombert, S.; Giral, P.; Bruckert, E.; Grimaldi, A.; Capron, F.; Poynard, T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005, 128, 1898–1906. [Google Scholar] [CrossRef]
- Poynard, T.; Lenaour, G.; Vaillant, J.C.; Capron, F.; Munteanu, M.; Eyraud, D.; Ngo, Y.; M’Kada, H.; Ratziu, V.; Hannoun, L.; et al. Liver biopsy analysis has a low level of performance for diagnosis of intermediate stages of fibrosis. Clin. Gastroenterol. Hepatol. 2012, 10, 657–663.e7. [Google Scholar] [CrossRef] [PubMed]
- Regev, A.; Berho, M.; Jeffers, L.J.; Milikowski, C.; Molina, E.G.; Pyrsopoulos, N.T.; Feng, Z.Z.; Reddy, K.R.; Schiff, E.R. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am. J. Gastroenterol. 2002, 97, 2614–2618. [Google Scholar] [CrossRef] [PubMed]
- McGill, D.B.; Rakela, J.; Zinsmeister, A.R.; Ott, B.J. A 21-year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology 1990, 99, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P.; Carrat, F. Liver biopsy: The best, not the gold standard. J. Hepatol. 2009, 50, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stål, P.; Hultcrantz, R.; Kechagias, S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 2017, 67, 1265–1273. [Google Scholar] [CrossRef]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015, 149, 389–397.e10. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Wai-Sun Wong, V.; Castellanos, M.; Aller-de la Fuente, R.; Metwally, M.; Eslam, M.; Gonzalez-Fabian, L.; Alvarez-Quiñones Sanz, M.; Conde-Martin, A.F.; et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients with Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018, 155, 443–457.e417. [Google Scholar] [CrossRef]
- Banerjee, R.; Pavlides, M.; Tunnicliffe, E.M.; Piechnik, S.K.; Sarania, N.; Philips, R.; Collier, J.D.; Booth, J.C.; Schneider, J.E.; Wang, L.M.; et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J. Hepatol. 2014, 60, 69–77. [Google Scholar] [CrossRef]
- Tunnicliffe, E.M.; Banerjee, R.; Pavlides, M.; Neubauer, S.; Robson, M.D. A model for hepatic fibrosis: The competing effects of cell loss and iron on shortened modified Look-Locker inversion recovery T1 (shMOLLI-T1 in the liver. J. Magn. Reson. Imaging 2017, 45, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Mole, D.J.; Fallowfield, J.A.; Sherif, A.E.; Kendall, T.; Semple, S.; Kelly, M.; Ridgway, G.; Connell, J.J.; McGonigle, J.; Banerjee, R.; et al. Quantitative magnetic resonance imaging predicts individual future liver performance after liver resection for cancer. PLoS ONE 2020, 15, e0238568. [Google Scholar] [CrossRef] [PubMed]
- Bajre, M.; Moawad, M.; Shumbayawonda, E.; Carolan, J.E.; Hart, J.; Culver, E.; Heneghan, M. LiverMultiScan as an alternative to liver biopsy to monitor autoimmune hepatitis in the National Health Service in England: An economic evaluation. BMJ Open 2022, 12, e058999. [Google Scholar] [CrossRef] [PubMed]
- Eddowes, P.J.; McDonald, N.; Davies, N.; Semple, S.I.K.; Kendall, T.J.; Hodson, J.; Newsome, P.N.; Flintham, R.B.; Wesolowski, R.; Blake, L.; et al. Utility and cost evaluation of multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2018, 47, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.R.; Cox, E.F.; Scott, R.A.; James, M.W.; Kaye, P.; Aithal, G.P.; Francis, S.T.; Guha, I.N. Multi-organ assessment of compensated cirrhosis patients using quantitative magnetic resonance imaging. J. Hepatol. 2018, 69, 1015–1024. [Google Scholar] [CrossRef]
- McDonald, N.; Eddowes, P.J.; Hodson, J.; Semple, S.I.K.; Davies, N.P.; Kelly, C.J.; Kin, S.; Phillips, M.; Herlihy, A.H.; Kendall, T.J.; et al. Multiparametric magnetic resonance imaging for quantitation of liver disease: A two-centre cross-sectional observational study. Sci. Rep. 2018, 8, 9189. [Google Scholar] [CrossRef]
- Singh, A.; Reddy, D.; Haris, M.; Cai, K.; Rajender Reddy, K.; Hariharan, H.; Reddy, R. T1ρ MRI of healthy and fibrotic human livers at 1.5 T. J. Transl. Med. 2015, 13, 292. [Google Scholar] [CrossRef]
- Allkemper, T.; Sagmeister, F.; Cicinnati, V.; Beckebaum, S.; Kooijman, H.; Kanthak, C.; Stehling, C.; Heindel, W. Evaluation of fibrotic liver disease with whole-liver T1ρ MR imaging: A feasibility study at 1.5 T. Radiology 2014, 271, 408–415. [Google Scholar] [CrossRef]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef]
- Marti-Aguado, D.; Rodríguez-Ortega, A.; Alberich-Bayarri, A.; Marti-Bonmati, L. Magnetic Resonance imaging analysis of liver fibrosis and inflammation: Overwhelming gray zones restrict clinical use. Abdom. Radiol. 2020, 45, 3557–3568. [Google Scholar] [CrossRef]
- Wang, Q.; Fiel, M.I.; Blank, S.; Luan, W.; Kadri, H.; Kim, K.W.; Manizate, F.; Rosenblatt, A.G.; Labow, D.M.; Schwartz, M.E.; et al. Impact of liver fibrosis on prognosis following liver resection for hepatitis B-associated hepatocellular carcinoma. Br. J. Cancer 2013, 109, 573–581. [Google Scholar] [CrossRef]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]
- Aierken, Y.; Kong, L.X.; Li, B.; Liu, X.J.; Lu, S.; Yang, J.Y. Liver fibrosis is a major risk factor for liver regeneration: A comparison between healthy and fibrotic liver. Medicine 2020, 99, e20003. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Takasaki, K.; Yamamoto, M.; Tsugita, M.; Otsubo, T. Liver regeneration and restoration of liver function after partial hepatectomy: The relation of fibrosis of the liver parenchyma. Hepatogastroenterology 1999, 46, 2919–2924. [Google Scholar]
- Jang, S.; Lee, J.M.; Lee, D.H.; Joo, I.; Yoon, J.H.; Chang, W.; Han, J.K. Value of MR elastography for the preoperative estimation of liver regeneration capacity in patients with hepatocellular carcinoma. J. Magn. Reson. Imaging 2017, 45, 1627–1636. [Google Scholar] [CrossRef]
- Shirabe, K.; Motomura, T.; Takeishi, K.; Morita, K.; Kayashima, H.; Taketomi, A.; Ikegami, T.; Soejima, Y.; Yoshizumi, T.; Maehara, Y. Human early liver regeneration after hepatectomy in patients with hepatocellular carcinoma: Special reference to age. Scand. J. Surg. 2013, 102, 101–105. [Google Scholar] [CrossRef]
- Kondo, T.; Okabayashi, K.; Hasegawa, H.; Tsuruta, M.; Shigeta, K.; Kitagawa, Y. The impact of hepatic fibrosis on the incidence of liver metastasis from colorectal cancer. Br. J. Cancer 2016, 115, 34–39. [Google Scholar] [CrossRef]
- Mozes, F.E.; Tunnicliffe, E.M.; Moolla, A.; Marjot, T.; Levick, C.K.; Pavlides, M.; Robson, M.D. Mapping tissue water T(1) in the liver using the MOLLI T(1) method in the presence of fat, iron and B(0) inhomogeneity. NMR Biomed. 2019, 32, e4030. [Google Scholar] [CrossRef]
- Levelt, E.; Pavlides, M.; Banerjee, R.; Mahmod, M.; Kelly, C.; Sellwood, J.; Ariga, R.; Thomas, S.; Francis, J.; Rodgers, C.; et al. Ectopic and Visceral Fat Deposition in Lean and Obese Patients With Type 2 Diabetes. J. Am. Coll. Cardiol. 2016, 68, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Pavlides, M.; Banerjee, R.; Tunnicliffe, E.M.; Kelly, C.; Collier, J.; Wang, L.M.; Fleming, K.A.; Cobbold, J.F.; Robson, M.D.; Neubauer, S.; et al. Multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease severity. Liver Int. 2017, 37, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Hoad, C.L.; Palaniyappan, N.; Kaye, P.; Chernova, Y.; James, M.W.; Costigan, C.; Austin, A.; Marciani, L.; Gowland, P.A.; Guha, I.N.; et al. A study of T₁ relaxation time as a measure of liver fibrosis and the influence of confounding histological factors. NMR Biomed. 2015, 28, 706–714. [Google Scholar] [CrossRef]
- Dennis, A.; Kelly, M.D.; Fernandes, C.; Mouchti, S.; Fallowfield, J.A.; Hirschfield, G.; Pavlides, M.; Harrison, S.; Chakravarthy, M.V.; Banerjee, R.; et al. Correlations Between MRI Biomarkers PDFF and cT1 With Histopathological Features of Non-Alcoholic Steatohepatitis. Front. Endocrinol. 2020, 11, 575843. [Google Scholar] [CrossRef]
- McKay, A.; Pantoja, C.; Hall, R.; Matthews, S.; Spalding, P.; Banerjee, R. Patient understanding and experience of non-invasive imaging diagnostic techniques and the liver patient pathway. J. Patient-Rep. Outcomes 2021, 5, 89. [Google Scholar] [CrossRef]
- Pavlides, M.; Banerjee, R.; Sellwood, J.; Kelly, C.J.; Robson, M.D.; Booth, J.C.; Collier, J.; Neubauer, S.; Barnes, E. Multiparametric magnetic resonance imaging predicts clinical outcomes in patients with chronic liver disease. J. Hepatol. 2016, 64, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Wong, V.W.; Cammà, C.; Hiriart, J.B.; Wong, G.L.; Vergniol, J.; Chan, A.W.; Di Marco, V.; Merrouche, W.; Chan, H.L.; et al. Serial combination of non-invasive tools improves the diagnostic accuracy of severe liver fibrosis in patients with NAFLD. Aliment. Pharmacol. Ther. 2017, 46, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Ang, B.; Haufe, W.; Hernandez, C.; Verna, E.C.; Sirlin, C.B.; Loomba, R. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: A prospective study. Aliment. Pharmacol. Ther. 2015, 41, 1271–1280. [Google Scholar] [CrossRef]
- Chin, J.L.; Pavlides, M.; Moolla, A.; Ryan, J.D. Non-invasive Markers of Liver Fibrosis: Adjuncts or Alternatives to Liver Biopsy? Front. Pharmacol. 2016, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Cassinotto, C.; Feldis, M.; Vergniol, J.; Mouries, A.; Cochet, H.; Lapuyade, B.; Hocquelet, A.; Juanola, E.; Foucher, J.; Laurent, F.; et al. MR relaxometry in chronic liver diseases: Comparison of T1 mapping, T2 mapping, and diffusion-weighted imaging for assessing cirrhosis diagnosis and severity. Eur. J. Radiol. 2015, 84, 1459–1465. [Google Scholar] [CrossRef]
- Levick, C.; Phillips-Hughes, J.; Collier, J.; Banerjee, R.; Cobbold, J.F.; Wang, L.M.; Piechnik, S.K.; Robson, M.D.; Neubauer, S.; Barnes, E.; et al. Non-invasive assessment of portal hypertension by multi-parametric magnetic resonance imaging of the spleen: A proof of concept study. PLoS ONE 2019, 14, e0221066. [Google Scholar] [CrossRef]
- Dillman, J.R.; Serai, S.D.; Trout, A.T.; Singh, R.; Tkach, J.A.; Taylor, A.E.; Blaxall, B.C.; Fei, L.; Miethke, A.G. Diagnostic performance of quantitative magnetic resonance imaging biomarkers for predicting portal hypertension in children and young adults with autoimmune liver disease. Pediatr. Radiol. 2019, 49, 332–341. [Google Scholar] [CrossRef]
- van den Broek, M.A.; Olde Damink, S.W.; Dejong, C.H.; Lang, H.; Malagó, M.; Jalan, R.; Saner, F.H. Liver failure after partial hepatic resection: Definition, pathophysiology, risk factors and treatment. Liver Int. 2008, 28, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Parkes, J.; Roderick, P.; Harris, S.; Day, C.; Mutimer, D.; Collier, J.; Lombard, M.; Alexander, G.; Ramage, J.; Dusheiko, G.; et al. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut 2010, 59, 1245–1251. [Google Scholar] [CrossRef]
- Sethi, P.; Thavanesan, N.; Welsh, F.K.; Connell, J.; Pickles, E.; Kelly, M.; Fallowfield, J.A.; Kendall, T.J.; Mole, D.J.; Rees, M. Quantitative multiparametric MRI allows safe surgical planning in patients undergoing liver resection for colorectal liver metastases: Report of two patients. BJR Case Rep. 2021, 7, 20200172. [Google Scholar] [CrossRef]
- Friedrich-Rust, M.; Ong, M.F.; Martens, S.; Sarrazin, C.; Bojunga, J.; Zeuzem, S.; Herrmann, E. Performance of transient elastography for the staging of liver fibrosis: A meta-analysis. Gastroenterology 2008, 134, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Wolfson, T.; Ang, B.; Hooker, J.; Behling, C.; Peterson, M.; Valasek, M.; Lin, G.; Brenner, D.; Gamst, A.; et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: A prospective study. Hepatology 2014, 60, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Asbach, P.; Klatt, D.; Schlosser, B.; Biermer, M.; Muche, M.; Rieger, A.; Loddenkemper, C.; Somasundaram, R.; Berg, T.; Hamm, B.; et al. Viscoelasticity-based staging of hepatic fibrosis with multifrequency MR elastography. Radiology 2010, 257, 80–86. [Google Scholar] [CrossRef]
- Tsochatzis, E.A.; Gurusamy, K.S.; Ntaoula, S.; Cholongitas, E.; Davidson, B.R.; Burroughs, A.K. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: A meta-analysis of diagnostic accuracy. J. Hepatol. 2011, 54, 650–659. [Google Scholar] [CrossRef]
- Castéra, L.; Foucher, J.; Bernard, P.H.; Carvalho, F.; Allaix, D.; Merrouche, W.; Couzigou, P.; de Lédinghen, V. Pitfalls of liver stiffness measurement: A 5-year prospective study of 13,369 examinations. Hepatology 2010, 51, 828–835. [Google Scholar] [CrossRef]
- Arena, U.; Vizzutti, F.; Corti, G.; Ambu, S.; Stasi, C.; Bresci, S.; Moscarella, S.; Boddi, V.; Petrarca, A.; Laffi, G.; et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 2008, 47, 380–384. [Google Scholar] [CrossRef]
- Coco, B.; Oliveri, F.; Maina, A.M.; Ciccorossi, P.; Sacco, R.; Colombatto, P.; Bonino, F.; Brunetto, M.R. Transient elastography: A new surrogate marker of liver fibrosis influenced by major changes of transaminases. J. Viral Hepat. 2007, 14, 360–369. [Google Scholar] [CrossRef]
- Sagir, A.; Erhardt, A.; Schmitt, M.; Häussinger, D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology 2008, 47, 592–595. [Google Scholar] [CrossRef]
- Millonig, G.; Reimann, F.M.; Friedrich, S.; Fonouni, H.; Mehrabi, A.; Büchler, M.W.; Seitz, H.K.; Mueller, S. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology 2008, 48, 1718–1723. [Google Scholar] [CrossRef]
- Pavlov, C.S.; Casazza, G.; Nikolova, D.; Tsochatzis, E.; Burroughs, A.K.; Ivashkin, V.T.; Gluud, C. Transient elastography for diagnosis of stages of hepatic fibrosis and cirrhosis in people with alcoholic liver disease. Cochrane Database Syst. Rev. 2015, 1, Cd010542. [Google Scholar] [CrossRef] [PubMed]
- Nascimbeni, F.; Lebray, P.; Fedchuk, L.; Oliveira, C.P.; Alvares-da-Silva, M.R.; Varault, A.; Ingiliz, P.; Ngo, Y.; de Torres, M.; Munteanu, M.; et al. Significant variations in elastometry measurements made within short-term in patients with chronic liver diseases. Clin. Gastroenterol. Hepatol. 2015, 13, 763–771.e6. [Google Scholar] [CrossRef] [PubMed]
- Bachtiar, V.; Kelly, M.D.; Wilman, H.R.; Jacobs, J.; Newbould, R.; Kelly, C.J.; Gyngell, M.L.; Groves, K.E.; McKay, A.; Herlihy, A.H.; et al. Repeatability and reproducibility of multiparametric magnetic resonance imaging of the liver. PLoS ONE 2019, 14, e0214921. [Google Scholar] [CrossRef] [PubMed]
- Trout, A.T.; Serai, S.; Mahley, A.D.; Wang, H.; Zhang, Y.; Zhang, B.; Dillman, J.R. Liver Stiffness Measurements with MR Elastography: Agreement and Repeatability across Imaging Systems, Field Strengths, and Pulse Sequences. Radiology 2016, 281, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Dennis, A.; Fiore, M.M.; Kelly, M.D.; Kelly, C.J.; Paredes, A.H.; Whitehead, J.M.; Neubauer, S.; Traber, P.G.; Banerjee, R. Utility and variability of three non-invasive liver fibrosis imaging modalities to evaluate efficacy of GR-MD-02 in subjects with NASH and bridging fibrosis during a phase-2 randomized clinical trial. PLoS ONE 2018, 13, e0203054. [Google Scholar] [CrossRef]
- Yoshimitsu, K.; Mitsufuji, T.; Shinagawa, Y.; Fujimitsu, R.; Morita, A.; Urakawa, H.; Hayashi, H.; Takano, K. MR elastography of the liver at 3.0 T in diagnosing liver fibrosis grades; preliminary clinical experience. Eur. Radiol. 2016, 26, 656–663. [Google Scholar] [CrossRef]
- Peppercorn, P.D.; Reznek, R.H.; Wilson, P.; Slevin, M.L.; Gupta, R.K. Demonstration of hepatic steatosis by computerized tomography in patients receiving 5-fluorouracil-based therapy for advanced colorectal cancer. Br. J. Cancer 1998, 77, 2008–2011. [Google Scholar] [CrossRef]
- Sørensen, P.; Edal, A.L.; Madsen, E.L.; Fenger, C.; Poulsen, M.R.; Petersen, O.F. Reversible hepatic steatosis in patients treated with interferon alfa-2a and 5-fluorouracil. Cancer 1995, 75, 2592–2596. [Google Scholar] [CrossRef]
- Inaba, Y.; Furutani, T.; Kimura, K.; Watanabe, H.; Haga, S.; Kido, Y.; Matsumoto, M.; Yamamoto, Y.; Harada, K.; Kaneko, S.; et al. Growth arrest and DNA damage-inducible 34 regulates liver regeneration in hepatic steatosis in mice. Hepatology 2015, 61, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Behrns, K.E.; Tsiotos, G.G.; DeSouza, N.F.; Krishna, M.K.; Ludwig, J.; Nagorney, D.M. Hepatic steatosis as a potential risk factor for major hepatic resection. J. Gastrointest. Surg. 1998, 2, 292–298. [Google Scholar] [CrossRef]
- Vauthey, J.N.; Pawlik, T.M.; Ribero, D.; Wu, T.T.; Zorzi, D.; Hoff, P.M.; Xiong, H.Q.; Eng, C.; Lauwers, G.Y.; Mino-Kenudson, M.; et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J. Clin. Oncol. 2006, 24, 2065–2072. [Google Scholar] [CrossRef]
- Karoui, M.; Penna, C.; Amin-Hashem, M.; Mitry, E.; Benoist, S.; Franc, B.; Rougier, P.; Nordlinger, B. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann. Surg. 2006, 243, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, L.S.; Babcock, E.E.; Schick, F.; Dobbins, R.L.; Garg, A.; Burns, D.K.; McGarry, J.D.; Stein, D.T. Measurement of intracellular triglyceride stores by H spectroscopy: Validation in vivo. Am. J. Physiol. 1999, 276, E977–E989. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Jonsson, J.R.; Cowin, G.J.; O’Rourke, P.; Clouston, A.D.; Volp, A.; Horsfall, L.; Jothimani, D.; Fawcett, J.; Galloway, G.J.; et al. Magnetic resonance imaging and spectroscopy accurately estimate the severity of steatosis provided the stage of fibrosis is considered. J. Hepatol. 2009, 51, 389–397. [Google Scholar] [CrossRef]
- Reeder, S.B.; Sirlin, C.B. Quantification of liver fat with magnetic resonance imaging. Magn. Reson. Imaging Clin. N. Am. 2010, 18, 337-ix. [Google Scholar] [CrossRef]
- Bohte, A.E.; van Werven, J.R.; Bipat, S.; Stoker, J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: A meta-analysis. Eur. Radiol. 2011, 21, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Van Werven, J.R.; Hoogduin, J.M.; Nederveen, A.J.; van Vliet, A.A.; Wajs, E.; Vandenberk, P.; Stroes, E.S.; Stoker, J. Reproducibility of 3.0 Tesla magnetic resonance spectroscopy for measuring hepatic fat content. J. Magn. Reson. Imaging 2009, 30, 444–448. [Google Scholar] [CrossRef]
- Valls, C.; Iannacconne, R.; Alba, E.; Murakami, T.; Hori, M.; Passariello, R.; Vilgrain, V. Fat in the liver: Diagnosis and characterization. Eur. Radiol. 2006, 16, 2292–2308. [Google Scholar] [CrossRef] [PubMed]
- Longo, R.; Ricci, C.; Masutti, F.; Vidimari, R.; Crocé, L.S.; Bercich, L.; Tiribelli, C.; Dalla Palma, L. Fatty infiltration of the liver. Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Investig. Radiol. 1993, 28, 297–302. [Google Scholar] [CrossRef]
- Longo, R.; Pollesello, P.; Ricci, C.; Masutti, F.; Kvam, B.J.; Bercich, L.; Crocè, L.S.; Grigolato, P.; Paoletti, S.; de Bernard, B.; et al. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J. Magn. Reson. Imaging 1995, 5, 281–285. [Google Scholar] [CrossRef]
- Thomsen, C.; Becker, U.; Winkler, K.; Christoffersen, P.; Jensen, M.; Henriksen, O. Quantification of liver fat using magnetic resonance spectroscopy. Magn. Reson. Imaging 1994, 12, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, T.; Serai, S.D.; Pirasteh, A.; Bashir, M.R.; Hamilton, G.; Hernando, D.; Hu, H.H.; Hetterich, H.; Kühn, J.P.; Kukuk, G.M.; et al. Linearity, Bias, and Precision of Hepatic Proton Density Fat Fraction Measurements by Using MR Imaging: A Meta-Analysis. Radiology 2018, 286, 486–498. [Google Scholar] [CrossRef]
- Kang, G.H.; Cruite, I.; Shiehmorteza, M.; Wolfson, T.; Gamst, A.C.; Hamilton, G.; Bydder, M.; Middleton, M.S.; Sirlin, C.B. Reproducibility of MRI-determined proton density fat fraction across two different MR scanner platforms. J. Magn. Reson. Imaging 2011, 34, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, M.; Lam, J.; Peterson, M.R.; Middleton, M.; Hamilton, G.; Le, T.A.; Bettencourt, R.; Changchien, C.; Brenner, D.A.; Sirlin, C.; et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013, 58, 1930–1940. [Google Scholar] [CrossRef]
- França, M.; Martí-Bonmatí, L.; Alberich-Bayarri, Á.; Oliveira, P.; Guimaraes, S.; Oliveira, J.; Amorim, J.; Gonzalez, J.S.; Vizcaíno, J.R.; Miranda, H.P. Evaluation of fibrosis and inflammation in diffuse liver diseases using intravoxel incoherent motion diffusion-weighted MR imaging. Abdom. Radiol. 2017, 42, 468–477. [Google Scholar] [CrossRef]
- Allen, A.M.; Shah, V.H.; Therneau, T.M.; Venkatesh, S.K.; Mounajjed, T.; Larson, J.J.; Mara, K.C.; Kellogg, T.A.; Kendrick, M.L.; McKenzie, T.J.; et al. Multiparametric Magnetic Resonance Elastography Improves the Detection of NASH Regression Following Bariatric Surgery. Hepatol. Commun. 2019, 4, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.J., Jr.; Mills, J.B.; Suriawinata, A.A.; Putra, J.; Tosteson, T.D.; Axelrod, D.; Freeman, R.; Whalen, G.F.; LaFemina, J.; Tarczewski, S.M.; et al. Short-term Preoperative Diet Decreases Bleeding After Partial Hepatectomy: Results From a Multi-institutional Randomized Controlled Trial. Ann. Surg. 2019, 269, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Folprecht, G.; Grothey, A.; Alberts, S.; Raab, H.R.; Köhne, C.H. Neoadjuvant treatment of unresectable colorectal liver metastases: Correlation between tumour response and resection rates. Ann. Oncol. 2005, 16, 1311–1319. [Google Scholar] [CrossRef]
- Tzeng, C.W.; Aloia, T.A. Colorectal liver metastases. J. Gastrointest. Surg. 2013, 17, 195–201, quiz pp. 201–192. [Google Scholar] [CrossRef] [PubMed]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013, 14, 1208–1215. [Google Scholar] [CrossRef]
- Adam, R.; Delvart, V.; Pascal, G.; Valeanu, A.; Castaing, D.; Azoulay, D.; Giacchetti, S.; Paule, B.; Kunstlinger, F.; Ghémard, O.; et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: A model to predict long-term survival. Ann. Surg. 2004, 240, 644–657, discussion 657–648. [Google Scholar] [CrossRef]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): A randomised controlled trial. Lancet 2008, 371, 1007–1016. [Google Scholar] [CrossRef]
- Duwe, G.; Knitter, S.; Pesthy, S.; Beierle, A.S.; Bahra, M.; Schmelzle, M.; Schmuck, R.B.; Lohneis, P.; Raschzok, N.; Öllinger, R.; et al. Hepatotoxicity following systemic therapy for colorectal liver metastases and the impact of chemotherapy-associated liver injury on outcomes after curative liver resection. Eur. J. Surg. Oncol. 2017, 43, 1668–1681. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.M.; Wilson, C.H.; Burt, A.D.; Manas, D.M.; White, S.A. Chemotherapy-associated liver injury in patients with colorectal liver metastases: A systematic review and meta-analysis. Ann. Surg. Oncol. 2012, 19, 4287–4299. [Google Scholar] [CrossRef]
- Rubbia-Brandt, L.; Audard, V.; Sartoretti, P.; Roth, A.D.; Brezault, C.; Le Charpentier, M.; Dousset, B.; Morel, P.; Soubrane, O.; Chaussade, S.; et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann. Oncol. 2004, 15, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, D.; Laurent, A.; Pawlik, T.M.; Lauwers, G.Y.; Vauthey, J.N.; Abdalla, E.K. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br. J. Surg. 2007, 94, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Kooby, D.A.; Fong, Y.; Suriawinata, A.; Gonen, M.; Allen, P.J.; Klimstra, D.S.; DeMatteo, R.P.; D’Angelica, M.; Blumgart, L.H.; Jarnagin, W.R. Impact of steatosis on perioperative outcome following hepatic resection. J. Gastrointest. Surg. 2003, 7, 1034–1044. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Twelves, C.; Cassidy, J.; Allman, D.; Bajetta, E.; Boyer, M.; Bugat, R.; Findlay, M.; Frings, S.; Jahn, M.; et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: Results of a large phase III study. J. Clin. Oncol. 2001, 19, 4097–4106. [Google Scholar] [CrossRef]
- Fernandez, F.G.; Ritter, J.; Goodwin, J.W.; Linehan, D.C.; Hawkins, W.G.; Strasberg, S.M. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J. Am. Coll. Surg. 2005, 200, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Rubbia-Brandt, L.; Tauzin, S.; Brezault, C.; Delucinge-Vivier, C.; Descombes, P.; Dousset, B.; Majno, P.E.; Mentha, G.; Terris, B. Gene expression profiling provides insights into pathways of oxaliplatin-related sinusoidal obstruction syndrome in humans. Mol. Cancer Ther. 2011, 10, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Agostini, J.; Benoist, S.; Seman, M.; Julié, C.; Imbeaud, S.; Letourneur, F.; Cagnard, N.; Rougier, P.; Brouquet, A.; Zucman-Rossi, J.; et al. Identification of molecular pathways involved in oxaliplatin-associated sinusoidal dilatation. J. Hepatol. 2012, 56, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Oussoultzoglou, E.; Rosso, E.; Casnedi, S.; Chenard-Neu, M.P.; Dufour, P.; Bachellier, P.; Jaeck, D. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann. Surg. 2008, 247, 118–124. [Google Scholar] [CrossRef]
- Soubrane, O.; Brouquet, A.; Zalinski, S.; Terris, B.; Brézault, C.; Mallet, V.; Goldwasser, F.; Scatton, O. Predicting high grade lesions of sinusoidal obstruction syndrome related to oxaliplatin-based chemotherapy for colorectal liver metastases: Correlation with post-hepatectomy outcome. Ann. Surg. 2010, 251, 454–460. [Google Scholar] [CrossRef]
- Welsh, F.K.; Tilney, H.S.; Tekkis, P.P.; John, T.G.; Rees, M. Safe liver resection following chemotherapy for colorectal metastases is a matter of timing. Br. J. Cancer 2007, 96, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Kishi, Y.; Zorzi, D.; Contreras, C.M.; Maru, D.M.; Kopetz, S.; Ribero, D.; Motta, M.; Ravarino, N.; Risio, M.; Curley, S.A.; et al. Extended Preoperative Chemotherapy Does Not Improve Pathologic Response and Increases Postoperative Liver Insufficiency After Hepatic Resection for Colorectal Liver Metastases. Ann. Surg. Oncol. 2010, 17, 2870–2876. [Google Scholar] [CrossRef]
- Urata, K.; Kawasaki, S.; Matsunami, H.; Hashikura, Y.; Ikegami, T.; Ishizone, S.; Momose, Y.; Komiyama, A.; Makuuchi, M. Calculation of child and adult standard liver volume for liver transplantation. Hepatology 1995, 21, 1317–1321. [Google Scholar] [CrossRef]
- Vauthey, J.N.; Chaoui, A.; Do, K.A.; Bilimoria, M.M.; Fenstermacher, M.J.; Charnsangavej, C.; Hicks, M.; Alsfasser, G.; Lauwers, G.; Hawkins, I.F.; et al. Standardized measurement of the future liver remnant prior to extended liver resection: Methodology and clinical associations. Surgery 2000, 127, 512–519. [Google Scholar] [CrossRef]
- Kubota, K.; Makuuchi, M.; Kusaka, K.; Kobayashi, T.; Miki, K.; Hasegawa, K.; Harihara, Y.; Takayama, T. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology 1997, 26, 1176–1181. [Google Scholar] [CrossRef]
- Ferrero, A.; Viganò, L.; Polastri, R.; Muratore, A.; Eminefendic, H.; Regge, D.; Capussotti, L. Postoperative liver dysfunction and future remnant liver: Where is the limit? Results of a prospective study. World J. Surg. 2007, 31, 1643–1651. [Google Scholar] [CrossRef]
- Chuang, Y.H.; Ou, H.Y.; Lazo, M.Z.; Chen, C.L.; Chen, M.H.; Weng, C.C.; Cheng, Y.F. Predicting post-hepatectomy liver failure by combined volumetric, functional MR image and laboratory analysis. Liver Int. 2018, 38, 868–874. [Google Scholar] [CrossRef]
- Kishi, Y.; Abdalla, E.K.; Chun, Y.S.; Zorzi, D.; Madoff, D.C.; Wallace, M.J.; Curley, S.A.; Vauthey, J.N. Three hundred and one consecutive extended right hepatectomies: Evaluation of outcome based on systematic liver volumetry. Ann. Surg. 2009, 250, 540–548. [Google Scholar] [CrossRef]
- Vauthey, J.N.; Pawlik, T.M.; Abdalla, E.K.; Arens, J.F.; Nemr, R.A.; Wei, S.H.; Kennamer, D.L.; Ellis, L.M.; Curley, S.A. Is extended hepatectomy for hepatobiliary malignancy justified? Ann. Surg. 2004, 239, 722–730, discussion 730–722. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, R.A.; Marroquin, C.E.; Bute, B.P.; Khuri, S.; Henderson, W.G.; Kuo, P.C. Predictive indices of morbidity and mortality after liver resection. Ann. Surg. 2006, 243, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Dinant, S.; de Graaf, W.; Verwer, B.J.; Bennink, R.J.; van Lienden, K.P.; Gouma, D.J.; van Vliet, A.K.; van Gulik, T.M. Risk assessment of posthepatectomy liver failure using hepatobiliary scintigraphy and CT volumetry. J. Nucl. Med. 2007, 48, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, D.; Heijnen, B.H.; Bennink, R.J.; Kok, M.; Dinant, S.; Straatsburg, I.H.; Gouma, D.J.; van Gulik, T.M. Preoperative assessment of liver function: A comparison of 99mTc-Mebrofenin scintigraphy with indocyanine green clearance test. Liver Int. 2004, 24, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Schindl, M.J.; Redhead, D.N.; Fearon, K.C.H.; Garden, O.J.; Wigmore, S.J. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut 2005, 54, 289–296. [Google Scholar] [CrossRef]
- Farges, O.; Malassagne, B.; Flejou, J.F.; Balzan, S.; Sauvanet, A.; Belghiti, J. Risk of major liver resection in patients with underlying chronic liver disease: A reappraisal. Ann. Surg. 1999, 229, 210–215. [Google Scholar] [CrossRef]
- Farges, O.; Belghiti, J.; Kianmanesh, R.; Regimbeau, J.M.; Santoro, R.; Vilgrain, V.; Denys, A.; Sauvanet, A. Portal vein embolization before right hepatectomy: Prospective clinical trial. Ann. Surg. 2003, 237, 208–217. [Google Scholar] [CrossRef]
- Poon, R.T.; Fan, S.T.; Lo, C.M.; Liu, C.L.; Lam, C.M.; Yuen, W.K.; Yeung, C.; Wong, J. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: Is it justified? Ann. Surg. 2002, 236, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Amptoulach, S.; Gross, G.; Kalaitzakis, E. Differential impact of obesity and diabetes mellitus on survival after liver resection for colorectal cancer metastases. J. Surg. Res. 2015, 199, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Little, S.A.; Jarnagin, W.R.; DeMatteo, R.P.; Blumgart, L.H.; Fong, Y. Diabetes is associated with increased perioperative mortality but equivalent long-term outcome after hepatic resection for colorectal cancer. J. Gastrointest. Surg. 2002, 6, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Rahnemai-Azar, A.A.; Cloyd, J.M.; Weber, S.M.; Dillhoff, M.; Schmidt, C.; Winslow, E.R.; Pawlik, T.M. Update on Liver Failure Following Hepatic Resection: Strategies for Prediction and Avoidance of Post-operative Liver Insufficiency. J. Clin. Transl. Hepatol. 2018, 6, 97–104. [Google Scholar] [CrossRef]
- Lafaro, K.; Buettner, S.; Maqsood, H.; Wagner, D.; Bagante, F.; Spolverato, G.; Xu, L.; Kamel, I.; Pawlik, T.M. Defining Post Hepatectomy Liver Insufficiency: Where do We stand? J. Gastrointest. Surg. 2015, 19, 2079–2092. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.B.; Yu, C.Y.; Tzao, C.; Chu, H.C.; Chen, T.W.; Hsieh, H.F.; Liu, Y.C.; Yu, J.C. Prediction of the risk of hepatic failure in patients with portal vein invasion hepatoma after hepatic resection. Eur. J. Surg. Oncol. 2006, 32, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Hotta, T.; Kobayashi, Y.; Taniguchi, K.; Johata, K.; Sahara, M.; Ochiai, M.; Watanabe, T.; Tanimura, H. Liver functional analysis by total bile acid level of C-tube bile after hepatectomy. Hepatogastroenterology 2005, 52, 1211–1215. [Google Scholar]
- Nanashima, A.; Tobinaga, S.; Abo, T.; Nonaka, T.; Takeshita, H.; Hidaka, S.; Sawai, T.; Nagayasu, T. Reducing the incidence of post-hepatectomy hepatic complications by preoperatively applying parameters predictive of liver function. J. Hepatobiliary Pancreat. Sci. 2010, 17, 871–878. [Google Scholar] [CrossRef]
- Du, Z.G.; Wei, Y.G.; Chen, K.F.; Li, B. An accurate predictor of liver failure and death after hepatectomy: A single institution’s experience with 478 consecutive cases. World J. Gastroenterol. 2014, 20, 274–281. [Google Scholar] [CrossRef]
- Osada, S.; Saji, S. The clinical significance of monitoring alkaline phosphatase level to estimate postoperative liver failure after hepatectomy. Hepatogastroenterology 2004, 51, 1434–1438. [Google Scholar]
- Oussoultzoglou, E.; Jaeck, D.; Addeo, P.; Fuchshuber, P.; Marzano, E.; Rosso, E.; Pessaux, P.; Bachellier, P. Prediction of mortality rate after major hepatectomy in patients without cirrhosis. Arch. Surg. 2010, 145, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Nishida, M.; Takao, T.; Mori, N.; Tamesa, T.; Tangoku, A.; Oka, M. Risk factors for postoperative liver failure after hepatectomy for hepatocellular carcinoma. Hepatogastroenterology 2004, 51, 1792–1796. [Google Scholar]
- Rassam, F.; Olthof, P.B.; Richardson, H.; van Gulik, T.M.; Bennink, R.J. Practical guidelines for the use of technetium-99m mebrofenin hepatobiliary scintigraphy in the quantitative assessment of liver function. Nucl. Med. Commun. 2019, 40, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Haimerl, M.; Probst, U.; Poelsterl, S.; Beyer, L.; Fellner, C.; Selgrad, M.; Hornung, M.; Stroszczynski, C.; Wiggermann, P. Hepatobiliary MRI: Signal intensity based assessment of liver function correlated to 13C-Methacetin breath test. Sci. Rep. 2018, 8, 9078. [Google Scholar] [CrossRef]
- Wang, L.; Xie, L.; Zhang, N.; Zhu, W.; Zhou, J.; Pan, Q.; Mao, A.; Lin, Z.; Wang, L.; Zhao, Y. Predictive Value of Intraoperative Indocyanine Green Clearance Measurement on Postoperative Liver Function After Anatomic Major Liver Resection. J. Gastrointest. Surg. 2020, 24, 1342–1351. [Google Scholar] [CrossRef]
- Mullen, J.T.; Ribero, D.; Reddy, S.K.; Donadon, M.; Zorzi, D.; Gautam, S.; Abdalla, E.K.; Curley, S.A.; Capussotti, L.; Clary, B.M.; et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J. Am. Coll. Surg. 2007, 204, 854–862, discussion 862–854. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, D.; Rumpf, B.; Ammann, M.; Perrodin, S.F.; Tamandl, D.; Haselmann, C.; Stift, J.; Brostjan, C.; Laengle, F.; Beldi, G. The combination of APRI and ALBI facilitates preoperative risk stratification for patients undergoing liver surgery after neoadjuvant chemotherapy. Ann. Surg. Oncol. 2019, 26, 791–799. [Google Scholar] [CrossRef]
- Starlinger, P.; Ubl, D.; Hackl, H.; Starlinger, J.; Nagorney, D.; Smoot, R.; Habermann, E.; Cleary, S. Combined APRI/ALBI score to predict mortality after hepatic resection. BJS Open 2021, 5, zraa043. [Google Scholar] [CrossRef]
- Hackl, M.; Heilmeier, U.; Weilner, S.; Grillari, J. Circulating microRNAs as novel biomarkers for bone diseases—Complex signatures for multifactorial diseases? Mol. Cell Endocrinol. 2016, 432, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Montani, F.; Marzi, M.J.; Dezi, F.; Dama, E.; Carletti, R.M.; Bonizzi, G.; Bertolotti, R.; Bellomi, M.; Rampinelli, C.; Maisonneuve, P.; et al. miR-Test: A blood test for lung cancer early detection. J. Natl. Cancer Inst. 2015, 107, djv063. [Google Scholar] [CrossRef]
- Starlinger, P.; Hackl, H.; Pereyra, D.; Skalicky, S.; Geiger, E.; Finsterbusch, M.; Tamandl, D.; Brostjan, C.; Grünberger, T.; Hackl, M.; et al. Predicting Postoperative Liver Dysfunction Based on Blood-Derived MicroRNA Signatures. Hepatology 2019, 69, 2636–2651. [Google Scholar] [CrossRef] [PubMed]
- Parmar, K.L.; O’Reilly, D.; Valle, J.W.; Braun, M.; Naish, J.H.; Williams, S.R.; Lloyd, W.K.; Malcomson, L.; Cresswell, K.; Bamford, C.; et al. Prospective study of change in liver function and fat in patients with colorectal liver metastases undergoing preoperative chemotherapy: Protocol for the CLiFF Study. BMJ Open 2020, 10, e027630. [Google Scholar] [CrossRef]
- Mojtahed, A.; Núñez, L.; Connell, J.; Fichera, A.; Nicholls, R.; Barone, A.; Marieiro, M.; Puddu, A.; Arya, Z.; Ferreira, C.; et al. Repeatability and reproducibility of deep-learning-based liver volume and Couinaud segment volume measurement tool. Abdom. Radiol. 2022, 47, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Welsh, F.K.; Connell, J.J.; Kelly, M.; Gooding, S.; Banerjee, R.; Rees, M. Precision medicine for liver tumours with quantitative MRI and whole genome sequencing (Precision1 trial): Study protocol for observational cohort study. BMJ Open 2022, 12, e057163. [Google Scholar] [CrossRef] [PubMed]
- Sundaravadanan, S.; Welsh, F.; Sethi, P.; Cresswell, B.; Connell, J.; Knapp, S.; Nunez, L.; Kelly, M.; Brady, M.; Banerjee, R.; et al. HPB P68 Multimetric MRI detects improved quality of the future liver remnant post-dual vein embolization—A novel finding. Br. J. Surg. 2022, 109, znac404.162. [Google Scholar] [CrossRef]
- Welsh, F.; Sethi, P.; Sundaravadnan, S.; Cresswell, B.; Connell, J.; Knapp, S.; Brady, M.; Banerjee, R.; Rees, M. Quantitative liver health imaging impacts surgical decision making and improves clinical outcomes in colorectal liver metastasis surgery. medRxiv 2023. [Google Scholar] [CrossRef]

| Current Studies | ||||||
|---|---|---|---|---|---|---|
| Reference | Title | Study Design | Cohort and Study Information | Findings | Status | Possible Future Applications |
| Mole et al. 2020 [12] | HepaT1ca (NCT03213314) | An observational clinical cohort study in two tertiary referral HPB centres. | Included 149 participants. Combined 3D volumetric assessment of FLR with cT1 score prior to treatment. Total of 135 participants underwent liver resection. Majority of participants had CRLM (n = 114). The remaining had HCC (n = 6), CCA (n = 1) or other secondary malignancies (n = 14). Imaging biomarkers (cT1 and PDFF) correlated with histological assessment from intra-operative tissue samples. |
| Completed and published study | Correlates with histological assays of fibroinflammation and steatosis and may circumvent need for a pre-operative biopsy in select patients in the pre-operative setting. Abnormal cT1 score can help Identify patients at risk of prolonged hospital stay and poor liver performance post operatively. Informed risk stratification of patients and personalised pre-operative decision making. Potential value of composite scoring systems in predicting post-operative outcomes and liver regeneration capacity. |
| Sethi et al. 2021 [43] | Quantitative multiparametric MRI allows safe surgical planning in patients undergoing liver resection for colorectal liver metastases: report of two patients | Retrospective case presentation of 2 patients included in the observational clinical trial, HepaT1ca (NCT03213314) | Both patients had CRLM and underwent extended right hepatectomy with estimated FLR 30%. Comparable pre-operative characteristics in terms of demographics, imaging, and baseline laboratory values. |
| Completed and published study | Potential objective evaluation of liver parenchyma, which can reveal significant underlying liver disease. This may aid/change decision making regarding pre-operative optimisation of the FLR in order to improve post-operative outcomes. |
| McKay et al. 2021 [33] | Patient understanding and experience of non-invasive imaging diagnostic techniques and the liver patient pathway | Cross-sectional study. Pre- and post- LiverMultiScan self-rated questionnaire on understanding of liver health. Post- LiverMultiScan semi-structured qualitative interview re. patient experience, understanding of the report and how to improve experience and delivery of information. | 101 participants included with a spectrum of liver disease diagnosis, including cancer. |
| Completed and published study | Visual reports of liver health may increase patient understanding of their disease care and overall experience. Potential for improving patient engagement with care. |
| Sundaravadanan et al. 2022 [135] | Multimetric MRI detects improved quality of the future liver remnant post-dual vein embolization—a novel finding. | Presentation abstract | Analysis of 81 patients with CRLM considered for liver resection, recruited in Precision1 trial (NCT04597710). Seven patients with CRLM had multiparametric MRI (including LiverMultiScan and volumetric assay) pre- and post- DVE. |
| Presented with published abstract | Demonstrating potential role in clinical trials for interventions aimed at optimising FLR. Aids in surgical decision making in patients with borderline FLR in order to optimise FLR and improve outcomes. |
| Welsh et al. 2023 [136] | Quantitative liver health imaging impacts surgical decision making and improves clinical outcomes in colorectal liver metastasis surgery | Comparative observational cohort study, including prospective cohort from Precision1 trial vs. analysis of a historical similar cohort | Analysis of the clinical utility of mpMRI in 81 patients with CRLM considered for liver resection (recruited in the Precision1 trial, NCT04597710). Clinical utility as measured by a change in the surgical pan. Post operative clinical outcomes of the cohort were compared with a similar historical cohort including 97 patients with CRLM, as well as other hepatic cancers. Both cohorts underwent mpMRI, including cT1, T2, and PDFF. However, information obtained from mpMRI was not used to alter surgical plans in the comparator cohort. |
| Preprint article awaiting peer review | mpMRI utilising LiverMultiScan in pre-operative planning may improve LoS. mpMRI may alter surgical strategy or provide confidence with the proposed treatment strategy. mpMRI may pick up underestimated liver health using conventional assays of liver health and volume. |
| Future studies | ||||||
| Reference | Title | Aim | Study design | Primary objective/end points | Secondary objective/end points | |
| Welsh et al. 2022 [134] | Precision1 Trial: Precision medicine for liver tumours with quantitative MRI and whole genome sequencing. NCT04597710. | Whole genome sequencing (WGS) integration with quantitative MRI and histopathology data to produce a software product to inform management of patients with liver tumours. | A single centre prospective observational cohort study of up to 200 adult participants being considered for liver resection of a primary or secondary liver cancer. | To determine the utility of WGS to aid clinical decision making in patients referred for liver resection. Evaluated retrospectively, with clinically actionable data defined as data resulting in clinicians choosing a different medical intervention to the current standard of care. |
| |
| Parmar et al. 2023 [132] | CoNoR Study: A prospective multi-step study of the potential added benefit of two novel assessment tools in colorectal liver metastases technical resectability decision-making | To evaluate the potential added value of two novel assessment tools (Hepatica, i.e., LiverMultiScan with 3D volumetric assay, and LiMax) in CRLM resectability decision making | A multistep systematic approach of systematic review, international expert interviews, international questionnaire and internation case-based surveys. Including international HPB senior community. | The added value of Hepatica and LiMAx in CRLM technical resectability decision making, assessed by measuring the following in HPB experts:
|
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chouari, T.; Merali, N.; La Costa, F.; Santol, J.; Chapman, S.; Horton, A.; Aroori, S.; Connell, J.; Rockall, T.A.; Mole, D.; et al. The Role of the Multiparametric MRI LiverMultiScanTM in the Quantitative Assessment of the Liver and Its Predicted Clinical Applications in Patients Undergoing Major Hepatic Resection for Colorectal Liver Metastasis. Cancers 2023, 15, 4863. https://doi.org/10.3390/cancers15194863
Chouari T, Merali N, La Costa F, Santol J, Chapman S, Horton A, Aroori S, Connell J, Rockall TA, Mole D, et al. The Role of the Multiparametric MRI LiverMultiScanTM in the Quantitative Assessment of the Liver and Its Predicted Clinical Applications in Patients Undergoing Major Hepatic Resection for Colorectal Liver Metastasis. Cancers. 2023; 15(19):4863. https://doi.org/10.3390/cancers15194863
Chicago/Turabian StyleChouari, Tarak, Nabeel Merali, Francesca La Costa, Jonas Santol, Shelley Chapman, Alex Horton, Somaiah Aroori, John Connell, Timothy A. Rockall, Damian Mole, and et al. 2023. "The Role of the Multiparametric MRI LiverMultiScanTM in the Quantitative Assessment of the Liver and Its Predicted Clinical Applications in Patients Undergoing Major Hepatic Resection for Colorectal Liver Metastasis" Cancers 15, no. 19: 4863. https://doi.org/10.3390/cancers15194863
APA StyleChouari, T., Merali, N., La Costa, F., Santol, J., Chapman, S., Horton, A., Aroori, S., Connell, J., Rockall, T. A., Mole, D., Starlinger, P., Welsh, F., Rees, M., & Frampton, A. E. (2023). The Role of the Multiparametric MRI LiverMultiScanTM in the Quantitative Assessment of the Liver and Its Predicted Clinical Applications in Patients Undergoing Major Hepatic Resection for Colorectal Liver Metastasis. Cancers, 15(19), 4863. https://doi.org/10.3390/cancers15194863






