Elevated Glycated Haemoglobin (HbA1c) Is Associated with an Increased Risk of Pancreatic Ductal Adenocarcinoma: A UK Biobank Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
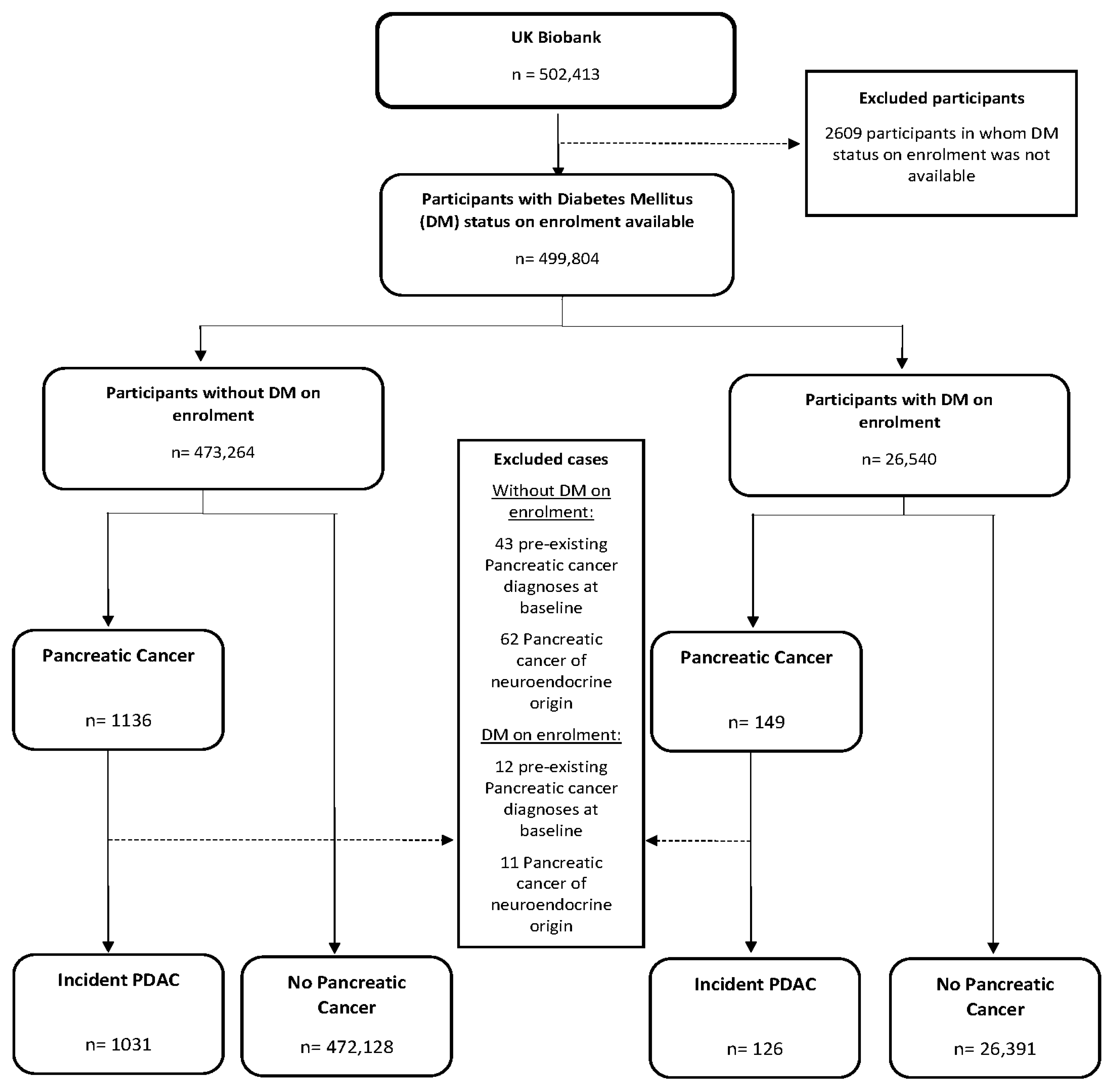
2.1. Identification of Incident PDAC Participants and the Main Exposures
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carioli, G.; Malvezzi, M.; Bertuccio, P.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2021 with focus on pancreatic and female lung cancer. Ann. Oncol. 2021, 32, 478–487. [Google Scholar] [CrossRef]
- Hidalgo, M.; Cascinu, S.; Kleeff, J.; Labianca, R.; Lohr, J.M.; Neoptolemos, J.; Real, F.X.; Van Laethem, J.L.; Heinemann, V. Addressing the challenges of pancreatic cancer: Future directions for improving outcomes. Pancreatology 2015, 15, 8–18. [Google Scholar] [CrossRef]
- La Salvia, A.; Persano, I.; Parlagreco, E.; Audisio, A.; Cani, M.; Brizzi, M.P. Pancreatic adenocarcinoma and pancreatic high-grade neuroendocrine carcinoma: Two sides of the moon. Med. Oncol. 2022, 39, 168. [Google Scholar] [CrossRef] [PubMed]
- Alexakis, N.; Neoptolemos, J.P. Pancreatic neuroendocrine tumours. Best Pract. Res. Clin. Gastroenterol. 2008, 22, 183–205. [Google Scholar] [CrossRef] [PubMed]
- Cabasag, C.J.; Arnold, M.; Rutherford, M.; Bardot, A.; Ferlay, J.; Morgan, E.; Little, A.; De, P.; Dixon, E.; Woods, R.R.; et al. Pancreatic cancer survival by stage and age in seven high-income countries (ICBP SURVMARK-2): A population-based study. Br. J. Cancer 2022, 126, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Green, R.C., Jr.; Baggenstoss, A.H.; Sprague, R.G. Diabetes mellitus in association with primary carcinoma of the pancreas. Diabetes 1958, 7, 308–311. [Google Scholar] [CrossRef]
- Ben, Q.; Xu, M.; Ning, X.; Liu, J.; Hong, S.; Huang, W.; Zhang, H.; Li, Z. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur. J. Cancer 2011, 47, 1928–1937. [Google Scholar] [CrossRef]
- Andersen, D.K.; Korc, M.; Petersen, G.M.; Eibl, G.; Li, D.; Rickels, M.R.; Chari, S.T.; Abbruzzese, J.L. Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. Diabetes 2017, 66, 1103–1110. [Google Scholar] [CrossRef]
- Pannala, R.; Leirness, J.B.; Bamlet, W.R.; Basu, A.; Petersen, G.M.; Chari, S.T. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology 2008, 134, 981–987. [Google Scholar] [CrossRef]
- Aggarwal, G.; Ramachandran, V.; Javeed, N.; Arumugam, T.; Dutta, S.; Klee, G.G.; Klee, E.W.; Smyrk, T.C.; Bamlet, W.; Han, J.J.; et al. Adrenomedullin is up-regulated in patients with pancreatic cancer and causes insulin resistance in beta cells and mice. Gastroenterology 2012, 143, 1510–1517.e1. [Google Scholar] [CrossRef]
- Everhart, J.; Wright, D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA 1995, 273, 1605–1609. [Google Scholar]
- Shen, B.; Li, Y.; Sheng, C.S.; Liu, L.; Hou, T.; Xia, N.; Sun, S.; Miao, Y.; Pang, Y.; Gu, K.; et al. Association between age at diabetes onset or diabetes duration and subsequent risk of pancreatic cancer: Results from a longitudinal cohort and mendelian randomization study. Lancet Reg. Health West Pac. 2023, 30, 100596. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Vittinghoff, E.; Bertenthal, D.; Corley, D.; Shen, H.; Walter, L.C.; McQuaid, K. New-onset diabetes and pancreatic cancer. Clin. Gastroenterol. Hepatol. 2006, 4, 1366–1372; quiz 1301. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, G.; Rabe, K.G.; Petersen, G.M.; Chari, S.T. New-onset diabetes in pancreatic cancer: A study in the primary care setting. Pancreatology 2012, 12, 156–161. [Google Scholar] [CrossRef]
- Chari, S.T.; Leibson, C.L.; Rabe, K.G.; Timmons, L.J.; Ransom, J.; de Andrade, M.; Petersen, G.M. Pancreatic cancer-associated diabetes mellitus: Prevalence and temporal association with diagnosis of cancer. Gastroenterology 2008, 134, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Molina-Montes, E.; Coscia, C.; Gomez-Rubio, P.; Fernandez, A.; Boenink, R.; Rava, M.; Marquez, M.; Molero, X.; Lohr, M.; Sharp, L.; et al. Deciphering the complex interplay between pancreatic cancer, diabetes mellitus subtypes and obesity/BMI through causal inference and mediation analyses. Gut 2020, 70, 319–329. [Google Scholar] [CrossRef]
- Chari, S.T.; Leibson, C.L.; Rabe, K.G.; Ransom, J.; de Andrade, M.; Petersen, G.M. Probability of pancreatic cancer following diabetes: A population-based study. Gastroenterology 2005, 129, 504–511. [Google Scholar] [CrossRef]
- Farmer, A. Use of HbA1c in the diagnosis of diabetes. BMJ 2012, 345, e7293. [Google Scholar] [CrossRef]
- Lemanska, A.; Price, C.A.; Jeffreys, N.; Byford, R.; Dambha-Miller, H.; Fan, X.; Hinton, W.; Otter, S.; Rice, R.; Stunt, A.; et al. BMI and HbA1c are metabolic markers for pancreatic cancer: Matched case-control study using a UK primary care database. PLoS ONE 2022, 17, e0275369. [Google Scholar] [CrossRef]
- Tan, P.S.; Garriga, C.; Clift, A.; Liao, W.; Patone, M.; Coupland, C.; Bashford-Rogers, R.; Sivakumar, S.; Hippisley-Cox, J. Temporality of body mass index, blood tests, comorbidities and medication use as early markers for pancreatic ductal adenocarcinoma (PDAC): A nested case-control study. Gut 2023, 72, 512–521. [Google Scholar] [CrossRef]
- Ross, J.A.D.; Barron, E.; McGough, B.; Valabhji, J.; Daff, K.; Irwin, J.; Henley, W.E.; Murray, E. Uptake and impact of the English National Health Service digital diabetes prevention programme: Observational study. BMJ Open Diabetes Res. Care 2022, 10, e002736. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.E.; Sudlow, C.; Peakman, T.; Collins, R.; Biobank, U.K. UK biobank data: Come and get it. Sci. Transl. Med. 2014, 6, 224ed224. [Google Scholar] [CrossRef] [PubMed]
- Au Yeung, S.L.; Schooling, C.M. Impact of glycemic traits, type 2 diabetes and metformin use on breast and prostate cancer risk: A Mendelian randomization study. BMJ Open Diabetes Res. Care 2019, 7, e000872. [Google Scholar] [CrossRef] [PubMed]
- Ortega, L.S.; Bradbury, K.E.; Cross, A.J.; Morris, J.S.; Gunter, M.J.; Murphy, N. A Prospective Investigation of Body Size, Body Fat Composition and Colorectal Cancer Risk in the UK Biobank. Sci. Rep. 2017, 7, 17807. [Google Scholar] [CrossRef]
- Ke, T.M.; Lophatananon, A.; Muir, K.R. Risk Factors Associated with Pancreatic Cancer in the UK Biobank Cohort. Cancers 2022, 14, 4991. [Google Scholar] [CrossRef]
- Rentsch, C.T.; Farmer, R.E.; Eastwood, S.V.; Mathur, R.; Garfield, V.; Farmaki, A.E.; Bhaskaran, K.; Chaturvedi, N.; Smeeth, L. Risk of 16 cancers across the full glycemic spectrum: A population-based cohort study using the UK Biobank. BMJ Open Diabetes Res. Care 2020, 8, e001600. [Google Scholar] [CrossRef]
- Shinoda, S.; Nakamura, N.; Roach, B.; Bernlohr, D.A.; Ikramuddin, S.; Yamamoto, M. Obesity and Pancreatic Cancer: Recent Progress in Epidemiology, Mechanisms and Bariatric Surgery. Biomedicines 2022, 10, 1284. [Google Scholar] [CrossRef]
- Cassano, P.A.; Rosner, B.; Vokonas, P.S.; Weiss, S.T. Obesity and body fat distribution in relation to the incidence of non-insulin-dependent diabetes mellitus. A prospective cohort study of men in the normative aging study. Am. J. Epidemiol. 1992, 136, 1474–1486. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nguyen, X.M.; Lane, J.; Wang, P. Relationship between obesity and diabetes in a US adult population: Findings from the National Health and Nutrition Examination Survey, 1999–2006. Obes. Surg. 2011, 21, 351–355. [Google Scholar] [CrossRef]
- Nicholson, B.D.; Thompson, M.J.; Hobbs, F.D.R.; Nguyen, M.; McLellan, J.; Green, B.; Chubak, J.; Oke, J.L. Measured weight loss as a precursor to cancer diagnosis: Retrospective cohort analysis of 43 302 primary care patients. J. Cachexia Sarcopenia Muscle 2022, 13, 2492–2503. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Peakman, T.C.; Biobank, U.K. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int. J. Epidemiol. 2008, 37, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Hue, J.J.; Sugumar, K.; Kyasaram, R.K.; Shanahan, J.; Lyons, J.; Ocuin, L.M.; Rothermel, L.D.; Hardacre, J.M.; Ammori, J.B.; Rao, G.; et al. Weight Loss as an Untapped Early Detection Marker in Pancreatic and Periampullary Cancer. Ann. Surg. Oncol. 2021, 28, 6283–6292. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Lucenteforte, E.; Silverman, D.T.; Petersen, G.; Bracci, P.M.; Ji, B.T.; Negri, E.; Li, D.; Risch, H.A.; Olson, S.H.; et al. Cigarette smoking and pancreatic cancer: An analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann. Oncol. 2012, 23, 1880–1888. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Colditz, G.A.; Stampfer, M.J.; Giovannucci, E.L.; Hunter, D.J.; Rimm, E.B.; Willett, W.C.; Speizer, F.E. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch. Intern. Med. 1996, 156, 2255–2260. [Google Scholar] [CrossRef]
- Jiao, L.; Silverman, D.T.; Schairer, C.; Thiebaut, A.C.; Hollenbeck, A.R.; Leitzmann, M.F.; Schatzkin, A.; Stolzenberg-Solomon, R.Z. Alcohol use and risk of pancreatic cancer: The NIH-AARP Diet and Health Study. Am. J. Epidemiol. 2009, 169, 1043–1051. [Google Scholar] [CrossRef]
- Tramacere, I.; Scotti, L.; Jenab, M.; Bagnardi, V.; Bellocco, R.; Rota, M.; Corrao, G.; Bravi, F.; Boffetta, P.; La Vecchia, C. Alcohol drinking and pancreatic cancer risk: A meta-analysis of the dose-risk relation. Int. J. Cancer 2010, 126, 1474–1486. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. 2018. Available online: https://www.wcrf.org/dietandcancer (accessed on 1 February 2022).
- Wu, B.U.; Butler, R.K.; Lustigova, E.; Lawrence, J.M.; Chen, W. Association of Glycated Hemoglobin Levels With Risk of Pancreatic Cancer. JAMA Netw. Open 2020, 3, e204945. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Obesity: Identification, Assessment and Management. Clinical Guideline [CG 189]. Available online: https://www.nice.org.uk/guidance/cg189/resources/obesity-identification-assessment-and-management-pdf-35109821097925 (accessed on 1 October 2022).
- Grote, V.A.; Rohrmann, S.; Nieters, A.; Dossus, L.; Tjonneland, A.; Halkjaer, J.; Overvad, K.; Fagherazzi, G.; Boutron-Ruault, M.C.; Morois, S.; et al. Diabetes mellitus, glycated haemoglobin and C-peptide levels in relation to pancreatic cancer risk: A study within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Diabetologia 2011, 54, 3037–3046. [Google Scholar] [CrossRef]
- Jacobson, S.; Dahlqvist, P.; Johansson, M.; Svensson, J.; Billing, O.; Sund, M.; Franklin, O. Hyperglycemia as a risk factor in pancreatic cancer: A nested case-control study using prediagnostic blood glucose levels. Pancreatology 2021, 12, 1112–1118. [Google Scholar] [CrossRef]
- Ben, Q.; Cai, Q.; Li, Z.; Yuan, Y.; Ning, X.; Deng, S.; Wang, K. The relationship between new-onset diabetes mellitus and pancreatic cancer risk: A case-control study. Eur. J. Cancer 2011, 47, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Dankner, R.; Freedman, L.S.; Gerstein, H.C.; Roth, J.; Keinan-Boker, L. Newly diagnosed type 2 diabetes may serve as a potential marker for pancreatic cancer. Diabetes Metab. Res. Rev. 2018, 34, e3018. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, L.; Stott, M.; Hanson, R.; Jackson, R.J.; Reynolds, W.; Chandran-Gorner, V.; Van Der Meer, R.; Alison, L.; Tejeiro, R.; Purewal, T.; et al. United Kingdom Early Detection Initiative (UK-EDI): Protocol for establishing a national multicentre cohort of individuals with new-onset diabetes for early detection of pancreatic cancer. BMJ Open 2022, 12, e068010. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kandlakunta, H.; Nagpal, S.J.S.; Feng, Z.; Hoos, W.; Petersen, G.M.; Chari, S.T. Model to Determine Risk of Pancreatic Cancer in Patients With New-Onset Diabetes. Gastroenterology 2018, 155, 730–739.e733. [Google Scholar] [CrossRef] [PubMed]
- ONS. 2011 Census Analysis: Ethnicity and Religion of the Non-UK Born Population in England and Wales: 2011. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity/articles/2011censusanalysisethnicityandreligionofthenonukbornpopulationinenglandandwales/2015-06-18 (accessed on 25 July 2023).
- Fry, A.; Littlejohns, T.J.; Sudlow, C.; Doherty, N.; Adamska, L.; Sprosen, T.; Collins, R.; Allen, N.E. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants with Those of the General Population. Am. J. Epidemiol. 2017, 186, 1026–1034. [Google Scholar] [CrossRef]

| Participant Characteristics | No Diabetes Mellitus on Enrolment | Diabetes Mellitus on Enrolment | Total | p Value |
|---|---|---|---|---|
| Number of Participants, n (%) | 473,264 (94.7%) | 26,540 (5.3%) | 499,804 | <0.001 b |
| Age at attendance (years) a | 56.4 (8.1) | 59.5 (7.2) | 56.5 (8.1) | <0.001 b |
| Men, n (%) | 211,583 (44.7%) | 16,095 (60.6%) | 227,678 (45.6%) | <0.001 c |
| Glycated haemoglobin (HbA1c) category (mmol/mol), n (%) | <0.001 c | |||
| <42 mmol/mol | 420,780 (95.6%) | 5208 (21.3%) | 425,997 (91.7%) | |
| 42–47 mmol/mol | 15,852 (3.6%) | 5186 (21.2%) | 21,038 (4.5%) | |
| ≥48 mmol/mol | 3360 (0.8%) | 14,038 (57.5%) | 17,398 (3.8%) | |
| Body mass index (BMI) a | 27.2 (4.6) | 31.3 (5.9) | 27.4 (4.8) | <0.001 b |
| Body mass index (BMI) category, n (%) | <0.001 c | |||
| Underweight d | 2574 (0.6%) | 36 (0.1%) | 2610 (0.5%) | |
| Normal d | 156,970 (33.3%) | 2838 (10.8%) | 159,808 (32.1%) | |
| Overweight d | 202,425 (43.0%) | 8961 (34.2%) | 211,386 (42.5%) | |
| Obese d | 108,990 (23.1%) | 14,381 (54.9%) | 123,371 (24.8%) | |
| Variable | Diabetes Mellitus on Enrolment | Participants | Incident PDAC (n, %) | Incidence (per 1000 Person-Years) | Multivariable Hazard Ratio (95% CI) b | p Value | Proportional Hazards Assumption |
|---|---|---|---|---|---|---|---|
| Glycated haemoglobin (HbA1c) | |||||||
| <42 mmol/mol | No | 420,789 | 869 (91.1%) | 0.17 | 1 (Reference) (N/A c) | <0.001 | |
| 42–47 mmol/mol | 15,872 | 66 (6.9%) | 0.3 | 1.39 (1.07–1.81) (N/A c) | 0.015 | ||
| ≥48 mmol/mol | 3360 | 19 (2.0%) | 0.33 | 2.17 (1.37–3.44) (N/A c) | 0.001 | ||
| <42 mmol/mol | Yes | 5208 | 16 (13.8%) | 0.28 | 1 (Reference) | 0.2263 | |
| 42–47 mmol/mol | 5186 | 22 (19.0%) | 0.39 | 1.28 (0.66–2.46) | 0.463 | ||
| ≥48 mmol/mol | 14,038 | 78 (67.2%) | 0.51 | 1.95 (1.12–3.37) | 0.017 |
| Time Since Enrolment in UKBB | Glycaemic Category at Enrolment and Cox Proportional Hazard Ratio for Incident PDAC | |||||||
|---|---|---|---|---|---|---|---|---|
| <42 mmol/mol | 42–47 mmol/mol | |||||||
| Total at Risk During Time Interval (n) | Incident PDAC during Time Interval (n) | Hazard Ratio (95% CI) b | p Value | Total at Risk During Time Interval (n) | Incident PDAC during Time Interval (n) | Hazard Ratio (95% CI) b | p Value | |
| 12 months | 420,707 | 36 | 1 (Reference) | 15,840 | 9 | 2.10 (1.31–3.37) | 0.002 | |
| 24 months | 419,980 | 50 | 1 (Reference) | 15,752 | 3 | 1.92 (1.29–2.88) | 0.001 | |
| 36 months | 418,816 | 66 | 1 (Reference) | 15,645 | 5 | 1.76 (1.26–2.49) | 0.001 | |
| 48 months | 417,318 | 71 | 1 (Reference) | 15,532 | 9 | 1.62 (1.21–2.17) | 0.001 | |
| 60 months | 415,620 | 78 | 1 (Reference) | 15,394 | 5 | 1.49 (1.14–1.94) | 0.004 | |
| 72 months | 413,705 | 86 | 1 (Reference) | 15,260 | 5 | 1.36 (1.04–1.78) | 0.024 | |
| 84 months | 411,526 | 103 | 1 (Reference) | 15,118 | 7 | 1.25 (0.93–1.68) | 0.141 | |
| Time Since Enrolment in UKBB | Glycaemic Category at Enrolment and Cox Proportional Hazard Ratio for Incident PDAC | |||||||
|---|---|---|---|---|---|---|---|---|
| <42 mmol/mol | ≥48 mmol/mol | |||||||
| Total at Risk During Time Interval (n) | Incident PDAC during Time Interval (n) | Hazard Ratio (95% CI) b | p Value | Total at Risk during Time Interval (n) | Incident PDAC during Time Interval (n) | Hazard Ratio (95% CI) b | p Value | |
| 12 months | 420,707 | 36 | 1 (Reference) | 3356 | 6 | 8.55 (4.58–15.99) | <0.001 | |
| 24 months | 419,980 | 50 | 1 (Reference) | 3330 | 2 | 5.80 (3.45–9.75) | <0.001 | |
| 36 months | 418,816 | 66 | 1 (Reference) | 3312 | 3 | 3.94 (2.46–6.29) | <0.001 | |
| 48 months | 417,318 | 71 | 1 (Reference) | 3290 | 3 | 2.67 (1.63–4.37) | <0.001 | |
| 60 months | 415,620 | 78 | 1 (Reference) | 3265 | 1 | 1.81 (1.01–3.24) | 0.045 | |
| 72 months | 413,705 | 86 | 1 (Reference) | 3232 | 1 | 1.23 (0.60–2.50) | 0.568 | |
| 84 months | 411,526 | 103 | 1 (Reference) | 3204 | 1 | 0.82 (0.35–1.98) | 0.681 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDonnell, D.; Cheang, A.W.E.; Wilding, S.; Wild, S.H.; Frampton, A.E.; Byrne, C.D.; Hamady, Z.Z. Elevated Glycated Haemoglobin (HbA1c) Is Associated with an Increased Risk of Pancreatic Ductal Adenocarcinoma: A UK Biobank Cohort Study. Cancers 2023, 15, 4078. https://doi.org/10.3390/cancers15164078
McDonnell D, Cheang AWE, Wilding S, Wild SH, Frampton AE, Byrne CD, Hamady ZZ. Elevated Glycated Haemoglobin (HbA1c) Is Associated with an Increased Risk of Pancreatic Ductal Adenocarcinoma: A UK Biobank Cohort Study. Cancers. 2023; 15(16):4078. https://doi.org/10.3390/cancers15164078
Chicago/Turabian StyleMcDonnell, Declan, Adrian W. E. Cheang, Sam Wilding, Sarah H. Wild, Adam E. Frampton, Christopher D. Byrne, and Zaed Z. Hamady. 2023. "Elevated Glycated Haemoglobin (HbA1c) Is Associated with an Increased Risk of Pancreatic Ductal Adenocarcinoma: A UK Biobank Cohort Study" Cancers 15, no. 16: 4078. https://doi.org/10.3390/cancers15164078
APA StyleMcDonnell, D., Cheang, A. W. E., Wilding, S., Wild, S. H., Frampton, A. E., Byrne, C. D., & Hamady, Z. Z. (2023). Elevated Glycated Haemoglobin (HbA1c) Is Associated with an Increased Risk of Pancreatic Ductal Adenocarcinoma: A UK Biobank Cohort Study. Cancers, 15(16), 4078. https://doi.org/10.3390/cancers15164078






