Randomized Trial Evaluating a Self-Guided Lifestyle Intervention Delivered via Evidence-Based Materials versus a Waitlist Group on Changes in Body Weight, Diet Quality, Physical Activity, and Quality of Life among Breast Cancer Survivors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
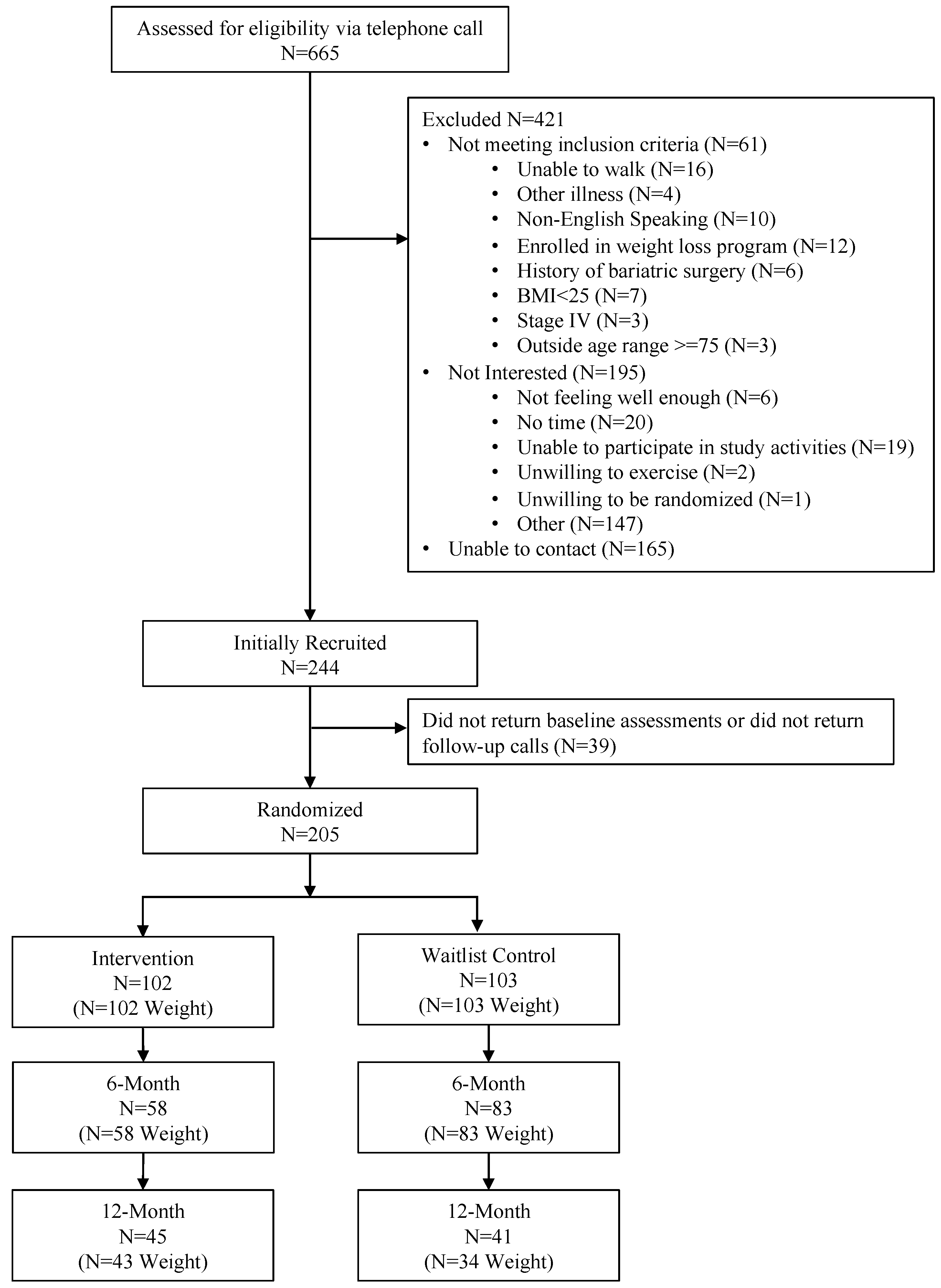
2.1. Study Participants and Recruitment
2.2. Design and Randomization
2.3. Weight Loss Intervention
2.3.1. Primary Outcome—Body Weight
2.3.2. Secondary Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Body Weight at 6-Months
3.3. Changes in Secondary Outcomes at 6-Months
3.4. Twelve-Month Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. LEAN Self-Guided Book Table of Contents
| Week/Section | Topics Covered|Page Number |
| Introduction | How to read the LEAN book|12 LEAN Benefits|13 LEAN Goals|15 Dr. Tara: Weight Gain from Treatment|17 Dr. Tara: Excess Body Fat and Breast Cancer|18 Baseline Timed One Mile Walk|23 |
| Week 1 | Set Your Weight Loss Goals|27 Exercise Guidelines for Cancer Survivors|29 Dr. Tara: What Causes Breast Cancer?|34 |
| Week 2 | Concept of Energy Balance: The “No More Dieting” Approach|37 Shoe Selection and Exercise Wear Tips|41 Counting Steps Using a Pedometer|44 Dr. Tara: Why Do I Feel so Tired?|47 |
| Week 3 | Adopt the New American Plate|49 How to Exercise Safely|50 |
| Week 4 | Pump Up the Phytonutrients|55 Dr. Tara: Tamoxifen|59 Benefits of Exercise|60 Timed One Mile Walk # 2|65 |
| Week 5 | Portion Distortion: Understanding Food Labels and Serving Sizes|67 Make Time for Exercise|73 Dr. Tara: Aromatase Inhibitors|76 |
| Week 6 | Be a Fat Detective|79 Setting Your Daily Fat Gram Goal|82 Reducing Your Sedentary Behavior|91 |
| Week 7 | Be a Sugar Detective: Adding Up the Sugar Grams|95 Dr. Tara: Chemo-brain|98 Exercise Goal Setting|99 |
| Week 8 | Whole Grains: The Other Carbohydrate 105 Fiber: Nature’s Gift to Our Bodies|106 Add Variety—Cross Train!|111 Timed One Mile Walk # 3|113 |
| Week 9 | Alcohol|115 Enlist Support…Exercise Buddies|116 Dr. Tara: Peripheral Neuropathy|117 |
| Week 10 | Practicing Mindful Eating|119 Cardiovascular Disease and Cardiorespiratory Fitness|125 |
| Week 11 | Understanding Food Labels: Sodium Content|129 Dr. Tara: Lymphedema|131 |
| Week 12 | Vitamin and Supplement Use|135 Dr. Tara: Exercise and Bone Maintenance|141 Timed One Mile Walk # 4|144 |
| Week 13 | Mid-Point Self-Assessment|147 Exercise and Weight Loss Improve Breast Cancer Survival|152 |
| Week 14 | Grocery Shopping|155 Exercise and Reducing Cancer Related Fatigue|162 |
| Week 15 | Creating a Healthy Food Environment at Work|165 The LiveSTRONG at the YMCA Exercise Program|168 |
| Week 16 | Organic Foods|173 The Question of Soy Foods|174 Dr. Tara: Yoga and Sleep|178 Timed One Mile Walk # 5|179 |
| Week 17 | Dining Out|181 Exercise and Improved Joint Pain|182 |
| Week 18 | Food Safety and Your Immune System|191 Exercise and Immune Changes|193 |
| Week 19 | Choose to Lose|197 Certified Cancer Exercise Trainer|199 |
| Week 20 | Vacation/Travel Strategies|203 Times One Mile Walk # 6|206 |
| Week 21 | Talk Positively to Yourself|209 Benefits of Interval Training|213 |
| Week 22 | You Can Manage Stress|217 Dr. Tara: Sexual Health|220 |
| Week 23 | The Slippery Slope of Lifestyle Change|223 Coping with Lapses|224 |
| Week 24 | Healthy Communities|229 Physical Activity and Improved Survival|232 Times One Mile Walk # 7|234 |
| Week 25 | Your LEAN Toolkit|237 Reward Yourself|240 |
| Week 26 | Maintaining a Healthy Lifestyle|243 Continue to Challenge Yourself|246 |
| Appendix | Strength Training|244 |
References
- Petrelli, F.; Cortellini, A.; Indini, A.; Tomasello, G.; Ghidini, M.; Nigro, O.; Salati, M.; Dottorini, L.; Iaculli, A.; Varricchio, A.; et al. Association of Obesity With Survival Outcomes in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e213520. [Google Scholar] [CrossRef]
- Chan, D.S.M.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Navarro Rosenblatt, D.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Runowicz, C.D.; Leach, C.R.; Henry, N.L.; Henry, K.S.; Mackey, H.T.; Cowens-Alvarado, R.L.; Cannady, R.S.; Pratt-Chapman, M.L.; Edge, S.B.; Jacobs, L.A.; et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA A Cancer J. Clin. 2016, 66, 43–73. [Google Scholar] [CrossRef]
- Greenlee, H.; Shi, Z.; Sardo Molmenti, C.L.; Rundle, A.; Tsai, W.Y. Trends in Obesity Prevalence in Adults With a History of Cancer: Results From the US National Health Interview Survey, 1997 to 2014. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 3133–3140. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2019, 150, 663–671. [Google Scholar] [CrossRef]
- Patel, A.V.; Friedenreich, C.M.; Moore, S.C.; Hayes, S.C.; Silver, J.K.; Campbell, K.L.; Winters-Stone, K.; Gerber, L.H.; George, S.M.; Fulton, J.E.; et al. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med. Sci. Sports Exerc. 2019, 51, 2391–2402. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.A.; Sullivan, K.R.; Howe, C.L.; Kushi, L.H.; Caan, B.J.; Neuhouser, M.L.; Bandera, E.V.; Wang, Y.; Robien, K.; et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA A Cancer J. Clin. 2022, 72, 230–262. [Google Scholar] [CrossRef]
- Cifu, G.; Arem, H. Adherence to lifestyle-related cancer prevention guidelines and breast cancer incidence and mortality. Ann. Epidemiol. 2018, 28, 767–773.e761. [Google Scholar] [CrossRef]
- Inoue-Choi, M.; Robien, K.; Lazovich, D. Adherence to the WCRF/AICR Guidelines for Cancer Prevention Is Associated with Lower Mortality among Older Female Cancer Survivors. Cancer Epidemiol. Biomark. Prev. 2013, 22, 792–802. [Google Scholar] [CrossRef]
- Hastert, T.A.; Beresford, S.A.; Sheppard, L.; White, E. Adherence to the WCRF/AICR cancer prevention recommendations and cancer-specific mortality: Results from the Vitamins and Lifestyle (VITAL) Study. Cancer Causes Control 2014, 25, 541–552. [Google Scholar] [CrossRef]
- Spei, M.E.; Samoli, E.; Bravi, F.; La Vecchia, C.; Bamia, C.; Benetou, V. Physical activity in breast cancer survivors: A systematic review and meta-analysis on overall and breast cancer survival. Breast 2019, 44, 144–152. [Google Scholar] [CrossRef]
- Lee, J. A Meta-analysis of the Association Between Physical Activity and Breast Cancer Mortality. Cancer Nurs. 2019, 42, 271–285. [Google Scholar] [CrossRef]
- Puklin, L.; Cartmel, B.; Harrigan, M.; Lu, L.; Li, F.-Y.; Sanft, T.; Irwin, M.L. Randomized trial of weight loss on circulating ghrelin levels among breast cancer survivors. NPJ Breast Cancer 2021, 7, 49. [Google Scholar] [CrossRef]
- Harrigan, M.; Cartmel, B.; Loftfield, E.; Sanft, T.; Chagpar, A.B.; Zhou, Y.; Playdon, M.; Li, F.; Irwin, M.L. Randomized Trial Comparing Telephone Versus In-Person Weight Loss Counseling on Body Composition and Circulating Biomarkers in Women Treated for Breast Cancer: The Lifestyle, Exercise, and Nutrition (LEAN) Study. J. Clin. Oncol. 2016, 34, 669–676. [Google Scholar] [CrossRef]
- Rock, C.L.; Flatt, S.W.; Byers, T.E.; Colditz, G.A.; Demark-Wahnefried, W.; Ganz, P.A.; Wolin, K.Y.; Elias, A.; Krontiras, H.; Liu, J.; et al. Results of the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial: A Behavioral Weight Loss Intervention in Overweight or Obese Breast Cancer Survivors. J. Clin. Oncol. 2015, 33, 3169–3176. [Google Scholar] [CrossRef]
- Carayol, M.; Ninot, G.; Senesse, P.; Bleuse, J.P.; Gourgou, S.; Sancho-Garnier, H.; Sari, C.; Romieu, I.; Romieu, G.; Jacot, W. Short- and long-term impact of adapted physical activity and diet counseling during adjuvant breast cancer therapy: The “APAD1” randomized controlled trial. BMC Cancer 2019, 19, 737. [Google Scholar] [CrossRef]
- Swisher, A.K.; Abraham, J.; Bonner, D.; Gilleland, D.; Hobbs, G.; Kurian, S.; Yanosik, M.A.; Vona-Davis, L. Exercise and dietary advice intervention for survivors of triple-negative breast cancer: Effects on body fat, physical function, quality of life, and adipokine profile. Support Care Cancer 2015, 23, 2995–3003. [Google Scholar] [CrossRef]
- Morishita, S.; Hamaue, Y.; Fukushima, T.; Tanaka, T.; Fu, J.B.; Nakano, J. Effect of Exercise on Mortality and Recurrence in Patients With Cancer: A Systematic Review and Meta-Analysis. Integr. Cancer Ther. 2020, 19, 1534735420917462. [Google Scholar] [CrossRef]
- Kennedy, M.A.; Bayes, S.; Newton, R.U.; Zissiadis, Y.; Spry, N.A.; Taaffe, D.R.; Hart, N.H.; Galvão, D.A. Implementation barriers to integrating exercise as medicine in oncology: An ecological scoping review. J. Cancer Surviv. 2022, 16, 865–881. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Peterson, B.; McBride, C.; Lipkus, I.; Clipp, E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer 2000, 88, 674–684. [Google Scholar] [CrossRef]
- Kriska, A. Modifiable activity questionnaire. Med. Sci. Sports Exerc. 1997, 29, S73–S78. [Google Scholar]
- Patterson, R.E.; Kristal, A.R.; Tinker, L.F.; Carter, R.A.; Bolton, M.P.; Agurs-Collins, T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann. Epidemiol. 1999, 9, 178–187. [Google Scholar] [CrossRef]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J.; et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef]
- Brady, M.J.; Cella, D.F.; Mo, F.; Bonomi, A.E.; Tulsky, D.S.; Lloyd, S.R.; Deasy, S.; Cobleigh, M.; Shiomoto, G. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J. Clin. Oncol. 1997, 15, 974–986. [Google Scholar] [CrossRef]
- FACT-B Functional Assessment of Cancer Therapy-Breast. Available online: https://www.facit.org/measures/FACT-B (accessed on 15 August 2023).
- Fallowfield, L.J.; Leaity, S.K.; Howell, A.; Benson, S.; Cella, D. Assessment of quality of life in women undergoing hormonal therapy for breast cancer: Validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res. Treat. 1999, 55, 189–199. [Google Scholar] [CrossRef]
- Yellen, S.B.; Cella, D.F.; Webster, K.; Blendowski, C.; Kaplan, E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J. Pain Symptom Manag. 1997, 13, 63–74. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef]
- Luo, J.; Hendryx, M.; Manson, J.E.; Figueiredo, J.C.; LeBlanc, E.S.; Barrington, W.; Rohan, T.E.; Howard, B.V.; Reding, K.; Ho, G.Y.; et al. Intentional Weight Loss and Obesity-Related Cancer Risk. JNCI Cancer Spectr. 2019, 3, pkz054. [Google Scholar] [CrossRef]
- Ryan, D.H.; Yockey, S.R. Weight Loss and Improvement in Comorbidity: Differences at 5%, 10%, 15%, and Over. Curr. Obes. Rep. 2017, 6, 187–194. [Google Scholar] [CrossRef]
- Deslippe, A.L.; Soanes, A.; Bouchaud, C.C.; Beckenstein, H.; Slim, M.; Plourde, H.; Cohen, T.R. Barriers and facilitators to diet, physical activity and lifestyle behavior intervention adherence: A qualitative systematic review of the literature. Int. J. Behav. Nutr. Phys. Act. 2023, 20, 14. [Google Scholar] [CrossRef]
- Meneses-Echávez, J.F.; González-Jiménez, E.; Ramírez-Vélez, R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: A systematic review and meta-analysis. BMC Cancer 2015, 15, 77. [Google Scholar] [CrossRef]
- Vetrovsky, T.; Borowiec, A.; Juřík, R.; Wahlich, C.; Śmigielski, W.; Steffl, M.; Tufano, J.J.; Drygas, W.; Stastny, P.; Harris, T.; et al. Do physical activity interventions combining self-monitoring with other components provide an additional benefit compared with self-monitoring alone? A systematic review and meta-analysis. Br. J. Sports Med. 2022, 56, 1366–1374. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; McCabe, M.S. Models for Delivering Survivorship Care. J. Clin. Oncol. 2006, 24, 5117–5124. [Google Scholar] [CrossRef]
- Gordon, L.G.; DiSipio, T.; Battistutta, D.; Yates, P.; Bashford, J.; Pyke, C.; Eakin, E.; Hayes, S.C. Cost-effectiveness of a pragmatic exercise intervention for women with breast cancer: Results from a randomized controlled trial. Psycho-Oncology 2017, 26, 649–655. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Clipp, E.C.; Lipkus, I.M.; Lobach, D.; Snyder, D.C.; Sloane, R.; Peterson, B.; Macri, J.M.; Rock, C.L.; McBride, C.M.; et al. Main outcomes of the FRESH START trial: A sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J. Clin. Oncol. 2007, 25, 2709–2718. [Google Scholar] [CrossRef]
- Vallance, J.K.; Courneya, K.S.; Plotnikoff, R.C.; Yasui, Y.; Mackey, J.R. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. J. Clin. Oncol. 2007, 25, 2352–2359. [Google Scholar] [CrossRef]
- Park, C.L.; Cho, D.; Salner, A.L.; Dornelas, E. A randomized controlled trial of two mail-based lifestyle interventions for breast cancer survivors. Support Care Cancer 2016, 24, 3037–3046. [Google Scholar] [CrossRef]
- Heideman, W.H.; Russell, N.S.; Gundy, C.; Rookus, M.A.; Voskuil, D.W. The frequency, magnitude and timing of post-diagnosis body weight gain in Dutch breast cancer survivors. Eur. J. Cancer 2009, 45, 119–126. [Google Scholar] [CrossRef]
- Nyrop, K.A.; Deal, A.M.; Shachar, S.S.; Park, J.; Choi, S.K.; Lee, J.T.; O’Hare, E.A.; Wheless, A.; Carey, L.A.; Muss, H.B. Weight trajectories in women receiving systemic adjuvant therapy for breast cancer. Breast Cancer Res. Treat. 2020, 179, 709–720. [Google Scholar] [CrossRef]
- Raghavendra, A.; Sinha, A.K.; Valle-Goffin, J.; Shen, Y.; Tripathy, D.; Barcenas, C.H. Determinants of Weight Gain During Adjuvant Endocrine Therapy and Association of Such Weight Gain With Recurrence in Long-term Breast Cancer Survivors. Clin. Breast Cancer 2018, 18, e7–e13. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, P.J.; Ennis, M.; Pritchard, K.I.; McCready, D.; Koo, J.; Sidlofsky, S.; Trudeau, M.; Hood, N.; Redwood, S. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. J. Clin. Oncol. 1999, 17, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.; Braybrooke, J.; Gray, R.; Hills, R.K.; Liu, Z.; Pan, H.; Peto, R.; Dodwell, D.; McGale, P.; Taylor, C.; et al. Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: A patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol. 2022, 23, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, C.H.; Chen, W.Y.; Rosner, B.; Holmes, M.D. Weight, weight gain, and survival after breast cancer diagnosis. J. Clin. Oncol. 2005, 23, 1370–1378. [Google Scholar] [CrossRef]
- Partridge, A.H. Cancer survivorship and the young breast cancer patient: Addressing the important issues. Oncologist 2013, 18, e19–e20. [Google Scholar] [CrossRef]
- Greenlee, H.A.; Crew, K.D.; Mata, J.M.; McKinley, P.S.; Rundle, A.G.; Zhang, W.; Liao, Y.; Tsai, W.Y.; Hershman, D.L. A pilot randomized controlled trial of a commercial diet and exercise weight loss program in minority breast cancer survivors. Obesity 2013, 21, 65–76. [Google Scholar] [CrossRef]
- Harris, M.N.; Swift, D.L.; Myers, V.H.; Earnest, C.P.; Johannsen, N.M.; Champagne, C.M.; Parker, B.D.; Levy, E.; Cash, K.C.; Church, T.S. Cancer Survival Through Lifestyle Change (CASTLE): A Pilot Study of Weight Loss. Int. J. Behav. Med. 2013, 20, 403–412. [Google Scholar] [CrossRef]
- Lisevick, A.; Cartmel, B.; Harrigan, M.; Li, F.; Sanft, T.; Fogarasi, M.; Irwin, M.L.; Ferrucci, L.M. Effect of the Lifestyle, Exercise, and Nutrition (LEAN) Study on Long-Term Weight Loss Maintenance in Women with Breast Cancer. Nutrients 2021, 13, 3265. [Google Scholar] [CrossRef]
- Sanft, T.; Harrigan, M.; Cartmel, B.; Ferrucci, L.M.; Li, F.-Y.; McGowan, C.; Zupa, M.; Nguyen, T.H.; Ligibel, J.; Neuhouser, M.L.; et al. Effect of healthy diet and exercise on chemotherapy completion rate in women with breast cancer: The Lifestyle, Exercise and Nutrition Early after Diagnosis (LEANer) study: Study protocol for a randomized clinical trial. Contemp. Clin. Trials 2021, 109, 106508. [Google Scholar] [CrossRef]
| Characteristic | Total Population a,b N = 205 | Intervention a,b N = 102 | Waitlist Group a,b N = 103 | p-Value c |
|---|---|---|---|---|
| Age (years) | 57.4 ± 10.4 | 57.0 ± 10.7 | 57.9 ± 10.0 | 0.54 |
| Marital Status | 0.58 | |||
| Married or Living with Partner | 140 (68.3) | 69 (67.7) | 71 (68.9) | |
| Divorced or Separated/Never Married/Widowed | 64 (31.2) | 33 (32.4) | 31 (30.1) | |
| Prefer not to answer | 1 (1.0) | 0 (0.0) | 1 (1.0) | |
| Education | 0.60 | |||
| College and above | 171 (83.4) | 85 (83.3) | 86 (83.5) | |
| Less than College | 33 (16.1) | 17 (16.7) | 16 (15.5) | |
| Prefer not to answer | 1 (0.5) | 0 (0) | 1 (1.0) | |
| Race and Ethnicity | 0.08 | |||
| Non-Hispanic White | 177 (86.3) | 84 (82.4) | 93 (90.3) | |
| Non-Hispanic Black | 16 (7.8) | 13 (12.8) | 3 (2.9) | |
| Hispanic | 6 (2.9) | 3 (2.9) | 3 (2.9) | |
| Asian/Pacific Islander | 1 (0.5) | 0 (0.0) | 1 (1.0) | |
| Prefer not to answer | 1 (0.5) | 1 (1.0) | 0 (0.0) | |
| Other d | 4 (2.0) | 1 (1.0) | 3 (2.9) | |
| Employment | 0.74 | |||
| Full Time (≥35 h/wk) | 100 (48.8) | 48 (47.1) | 52 (50.5) | |
| Part time (<35 h/wk) | 35 (17.1) | 16 (15.7) | 18 (17.5) | |
| Unemployed/Retired | 67 (32.7) | 36 (35.3) | 31 (30.1) | |
| Prefer not to answer | 1 (0.5) | 0 (0.0) | 1 (1.0) | |
| Other e | 2 (1.0) | 2 (2.0) | 1 (1.0) | |
| Weight (kg) | 85.6 ± 14.8 | 85.6 ± 13.9 | 85.6 ± 15.7 | 0.98 |
| BMI (kg/m2) | 32.3 ± 5.0 | 32.3 ± 4.8 | 32.2 ± 5.3 | 0.88 |
| Postmenopausal | 177 (86.8) | 86 (85.2) | 91 (88.4) | 0.42 |
| Time since diagnosis (years) | 3.7 ± 3.6 | 3.6 ± 3.1 | 3.8 ± 4.0 | 0.70 |
| Recurrence before randomization | 11 (5.5) | 5 (5.0) | 6 (6.0) | 0.74 |
| Cancer Stage | 0.37 | |||
| 0 | 27 (13.9) | 11 (11.6) | 16 (16.0) | |
| I | 85 (43.6) | 45 (47.4) | 40 (40.0) | |
| II | 61 (31.3) | 26 (27.4) | 35 (35.0) | |
| III | 22 (11.3) | 13 (13.7) | 9 (9.0) | |
| Radiotherapy | 151 (42.1) | 74 (74.0) | 77 (76.2) | 0.71 |
| Chemotherapy | 107 (53.2) | 54 (54.0) | 53 (54.3) | 0.83 |
| Surgery | 199 (98.0) | 98 (97.0) | 101 (99.0) | 0.31 |
| Exercise (min/week) | 94.0 ± 133.3 | 82.0 ± 118.5 | 106.1 ± 146.4 | 0.20 |
| Healthy Eating Index (HEI) | 66.5 ± 11.7 | 65.0 ± 12.4 | 68.0 ± 10.9 | 0.07 |
| Fiber intake (g) | 18.3 ± 8.9 | 17.4 ± 7.9 | 19.2 ± 9.7 | 0.17 |
| Fruit (servings/day) | 1.3 ± 1.2 | 1.2 ± 1.2 | 1.4 ± 1.2 | 0.44 |
| Vegetable (servings/day) | 2.3 ± 1.5 | 2.2 ± 1.4 | 2.4 ± 1.5 | 0.30 |
| % Fat | 34.9 ± 6.7 | 35.4 ± 7.1 | 34.4 ± 6.2 | 0.30 |
| FACT-G | 84.9 ± 15.0 | 84.8 ± 15.4 | 85.1 ± 14.8 | 0.91 |
| FACT-B | 107.0 ± 19.1 | 106.7 ± 19.8 | 107.2 ± 18.5 | 0.84 |
| FACT-ES | 55.6 ± 11.2 | 54.3 ± 11.5 | 56.9 ± 10.7 | 0.11 |
| FACIT-F | 37.4 ± 10.5 | 36.9 ± 9.6 | 37.8 ± 11.4 | 0.54 |
| Time Period | Intervention a N = 102 | Waitlist Group a N = 103 | Effect Size, Least Square Mean (95% CI) a | p-Value a |
|---|---|---|---|---|
| Baseline to 6-Month Period | ||||
| Common baseline weight (kg) | 85.6 (83.6, 87.6) | |||
| Weight change (kg) | −2.1 (−3.0, −1.1) | −0.73 (−1.5, 0.05) | −1.3 (−2.5, −0.13) | 0.03 |
| p value b | <0.001 | 0.07 | ||
| 6-Month to 12-Month Follow-up Period (Intervention Only) | Intervention N = 58 | Waitlist Group N = 83 | ||
| Weight change (kg) | −0.21 (−1.5, 1.1) | -- | -- | |
| p value b | 0.75 | -- | -- | |
| 6-Month to 12-Month Delayed Intervention Period (Waitlist Group Only) | Intervention N = 58 | Waitlist Group N = 83 | ||
| Weight change (kg) | -- | −2.9 (−4.3, −1.5) | -- | |
| p value b | -- | <0.001 | -- | |
| 6-Month Change | N | Weight (kg) | |||||
|---|---|---|---|---|---|---|---|
| Intervention | Waitlist Group | Intervention LSmean (SE) a | Waitlist Group LSmean (SE) a | Effect Size (95% CI) | p | Pinteraction | |
| BMI (kg/m2) | 0.18 | ||||||
| ≥30 | 67 | 61 | −2.1 (0.61) | −0.48 (0.51) | −1.7 (−3.2, −0.10) | 0.04 | |
| <30 | 35 | 42 | 0.14 (0.71) | 0.18 (0.59) | −0.04 (−1.8, 1.8) | 0.97 | |
| Age, years (median) | 0.42 | ||||||
| ≥57 | 53 | 56 | −2.3 (0.55) | −0.68 (0.49) | −1.7 (−3.1, −0.23) | 0.02 | |
| <57 | 49 | 47 | −0.27 (0.67) | 0.47 (0.57) | −0.74 (−2.5, 0.99) | 0.40 | |
| Clinical Stage | 0.31 | ||||||
| 0/I | 56 | 56 | −2.6 (0.60) | −0.50 (0.49) | −2.1 (−3.6, −0.52) | 0.01 | |
| II/III | 39 | 44 | −1.0 (0.71) | −0.18 (0.60) | −0.84 (−2.7, 0.98) | 0.37 | |
| Education | 0.47 | ||||||
| College or above | 85 | 86 | −1.6 (0.50) | −0.46 (0.41) | −1.2 (−2.4, 0.12) | 0.07 | |
| Below college | 17 | 16 | −1.4 (0.91) | 0.82 (1.01) | −2.2 (−4.9, 0.42) | 0.10 | |
| Employed | 0.19 | ||||||
| Employed | 64 | 70 | −0.79 (0.57) | −0.15 (0.46) | −0.64 (−2.1, 0.81) | 0.38 | |
| Not Employed | 36 | 31 | −2.4 (0.70) | −0.20 (0.66) | −2.2 (−4.1, −0.32) | 0.02 | |
| Marriage | 0.83 | ||||||
| Married/Living with someone | 69 | 71 | −1.7 (0.54) | −0.26 (0.45) | −1.4 (−2.8, −0.01) | 0.05 | |
| Live Alone | 33 | 31 | −1.4 (0.76) | −0.23 (0.72) | −1.1 (−3.2, 0.94) | 0.28 | |
| Menopausal Status | 0.03 | ||||||
| Postmenopausal | 86 | 91 | −1.7 (0.46) | −0.58 (0.38) | −1.1 (−2.3, 0.06) | 0.06 | |
| Premenopausal | 15 | 11 | −1.1 (1.1) | 4.1 (1.4) | −5.1 (−8.6, −1.7) | 0.003 | |
| Chemotherapy | 0.45 | ||||||
| Yes | 54 | 53 | −1.0 (0.60) | −0.01 (0.51) | −1.0 (−2.6, 0.52) | 0.19 | |
| No | 46 | 48 | −2.4 (0.62) | −0.49 (0.55) | −1.9 (−3.5, −0.28) | 0.02 | |
| Radiation | 0.72 | ||||||
| Yes | 74 | 77 | −1.8 (0.50) | −0.24 (0.43) | −1.6 (−2.9, −0.30) | 0.02 | |
| No | 26 | 24 | −1.2 (0.91) | −0.10 (0.82) | −1.1 (−3.5, 1.3) | 0.37 | |
| Time Since Diagnosis, years (median) | 0.80 | ||||||
| ≥2.75 | 54 | 47 | −2.0 (0.55) | −0.74 (0.56) | −1.3 (−2.8, 0.27) | 0.11 | |
| <2.75 | 48 | 56 | −0.80 (0.70) | 0.18 (0.50) | −0.98 (−2.7, 0.71) | 0.25 | |
| Baseline FACT-B (median) | 0.18 | ||||||
| ≥109 | 51 | 53 | −2.5 (0.59) | −0.46 (0.51) | −2.0 (−3.5, 0.49) | 0.01 | |
| <109 | 51 | 50 | −0.52 (0.64) | −0.03 (0.54) | −0.49 (−2.1, 1.2) | 0.56 | |
| N | Intervention a | N | Waitlist Group a | Effect Size, Least Square Mean (95% CI) a | p-Value a | |
|---|---|---|---|---|---|---|
| Weekly Exercise (min/week) | 100 | 99 | ||||
| Combined baseline | 95.7 (77.1, 114.4) | |||||
| Baseline to 6-Month change | 30.8 (−1.7, 63.3) | 12.1 (−16.0, 40.1) | 18.7 (−24.2, 61.6) | 0.39 | ||
| HEI-2010 Score | 99 | 103 | ||||
| Combined baseline | 66.5 (64.8, 68.1) | |||||
| Baseline to 6-Month change | 4.6 (2.0, 7.3) | 1.4 (−0.64, 3.5) | 3.2 (−0.20, 6.5) | 0.07 | ||
| Fiber intake (g/1000 kcal) | 99 | 103 | ||||
| Combined baseline | 18.3 (17.1, 19.5) | |||||
| Baseline to 6-Month change | 1.1 (−0.66, 2.8) | 0.44 (−0.86, 1.7) | 0.62 (−1.5, 2.8) | 0.57 | ||
| Fruit (servings/day) | 98 | 99 | ||||
| Combined baseline | 1.3 (1.1, 1.5) | |||||
| Baseline to 6-Month change | 0.36 (0.02, 0.70) | 0.09 (−0.17, 0.34) | 0.27 (−0.15, 0.70) | 0.21 | ||
| Vegetable (servings/day) | 97 | 99 | ||||
| Combined baseline | 2.3 (2.1, 2.6) | |||||
| Baseline to 6-Month change | 0.54 (0.16, 0.91) | −0.13 (−0.41, 0.15) | 0.67 (0.20, 1.13) | 0.01 | ||
| Fat (%) | 99 | 103 | ||||
| Combined baseline | 34.9 (34.0, 35.8) | |||||
| Baseline to 6-Month change | −3.0 (−4.7, −1.2) | −1.2 (−2.6, 0.18) | −1.8 (−4.0, 0.47) | 0.12 | ||
| FACT-G | 101 | 103 | ||||
| Combined baseline | 84.9 (82.8, 86.9) | |||||
| Baseline to 6-Month change | 1.5 (−1.0, 4.1) | 2.2 (0.12, 4.2) | −0.63 (−3.9, 2.7) | 0.71 | ||
| FACT-B | 101 | 103 | ||||
| Combined baseline | 106.9 (104.2, 109.5) | |||||
| Baseline to 6-Month change | 1.8 (−1.4, 5.1) | 3.5 (0.93, 6.0) | −1.6 (−5.7, 2.5) | 0.44 | ||
| FACT-ES | 101 | 103 | ||||
| Combined baseline | 55.6 (54.1, 57.1) | |||||
| Baseline to 6-Month change | 2.3 (0.48, 4.2) | 0.59 (−0.88, 2.1) | 1.7 (−0.6, 4.1) | 0.15 | ||
| FACIT-F | 101 | 103 | ||||
| Combined baseline | 37.4 (36.0, 38.9) | |||||
| Baseline to 6-Month change | 2.7 (0.77, 4.7) | 1.3 (−0.28, 2.8) | 1.4 (−1.1, 3.9) | 0.26 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puklin, L.S.; Harrigan, M.; Cartmel, B.; Sanft, T.; Gottlieb, L.; Zhou, B.; Ferrucci, L.M.; Li, F.-Y.; Spiegelman, D.; Sharifi, M.; et al. Randomized Trial Evaluating a Self-Guided Lifestyle Intervention Delivered via Evidence-Based Materials versus a Waitlist Group on Changes in Body Weight, Diet Quality, Physical Activity, and Quality of Life among Breast Cancer Survivors. Cancers 2023, 15, 4719. https://doi.org/10.3390/cancers15194719
Puklin LS, Harrigan M, Cartmel B, Sanft T, Gottlieb L, Zhou B, Ferrucci LM, Li F-Y, Spiegelman D, Sharifi M, et al. Randomized Trial Evaluating a Self-Guided Lifestyle Intervention Delivered via Evidence-Based Materials versus a Waitlist Group on Changes in Body Weight, Diet Quality, Physical Activity, and Quality of Life among Breast Cancer Survivors. Cancers. 2023; 15(19):4719. https://doi.org/10.3390/cancers15194719
Chicago/Turabian StylePuklin, Leah S., Maura Harrigan, Brenda Cartmel, Tara Sanft, Linda Gottlieb, Bin Zhou, Leah M. Ferrucci, Fang-Yong Li, Donna Spiegelman, Mona Sharifi, and et al. 2023. "Randomized Trial Evaluating a Self-Guided Lifestyle Intervention Delivered via Evidence-Based Materials versus a Waitlist Group on Changes in Body Weight, Diet Quality, Physical Activity, and Quality of Life among Breast Cancer Survivors" Cancers 15, no. 19: 4719. https://doi.org/10.3390/cancers15194719
APA StylePuklin, L. S., Harrigan, M., Cartmel, B., Sanft, T., Gottlieb, L., Zhou, B., Ferrucci, L. M., Li, F.-Y., Spiegelman, D., Sharifi, M., & Irwin, M. L. (2023). Randomized Trial Evaluating a Self-Guided Lifestyle Intervention Delivered via Evidence-Based Materials versus a Waitlist Group on Changes in Body Weight, Diet Quality, Physical Activity, and Quality of Life among Breast Cancer Survivors. Cancers, 15(19), 4719. https://doi.org/10.3390/cancers15194719







