MRI Assessment of Changes in Tumor Vascularization during Neoadjuvant Anti-Angiogenic Treatment in Locally Advanced Breast Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Study Protocol
2.3. MRI Examinations
2.4. Tumor Volumetry
2.5. Tumor Perfusion
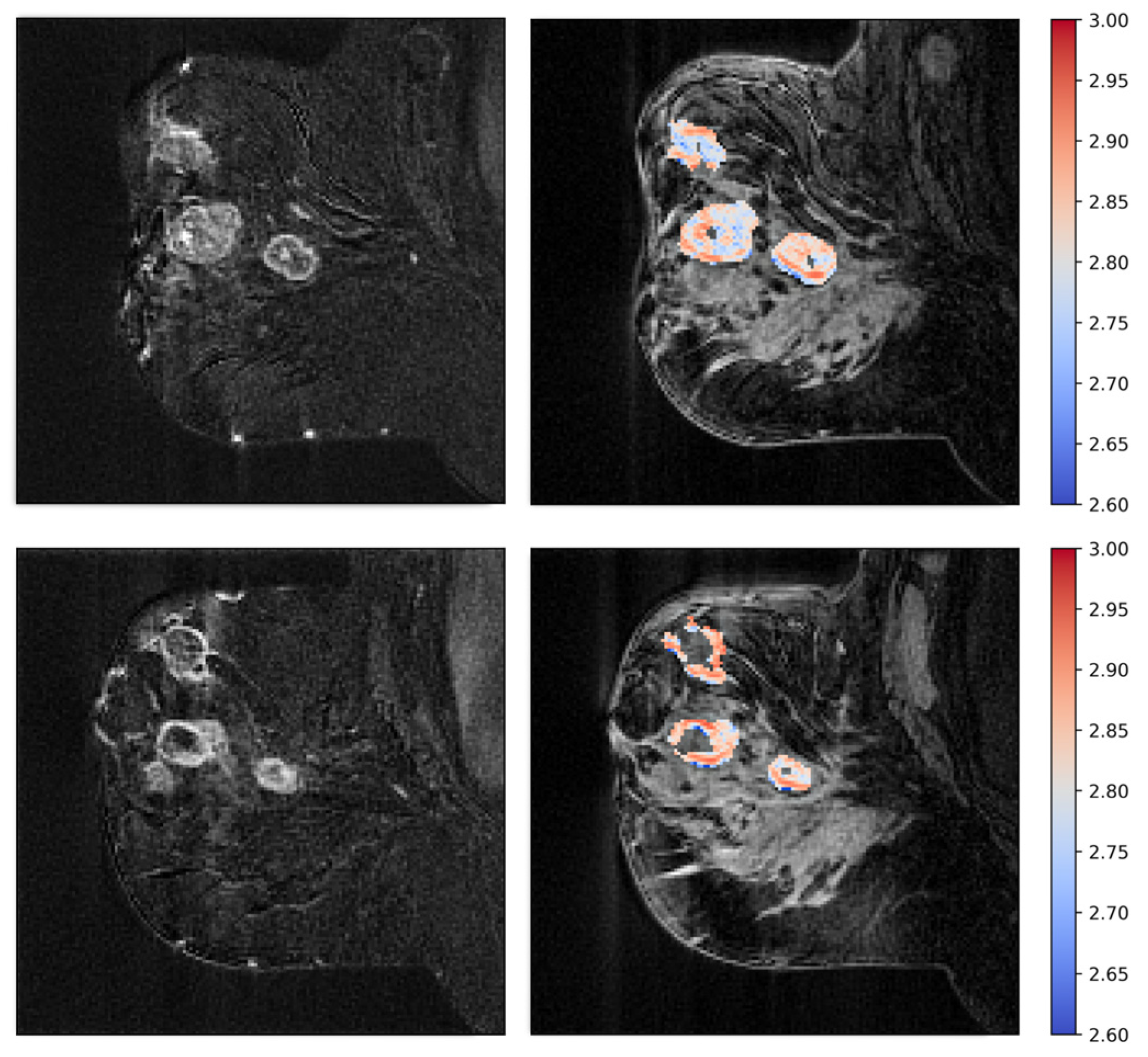
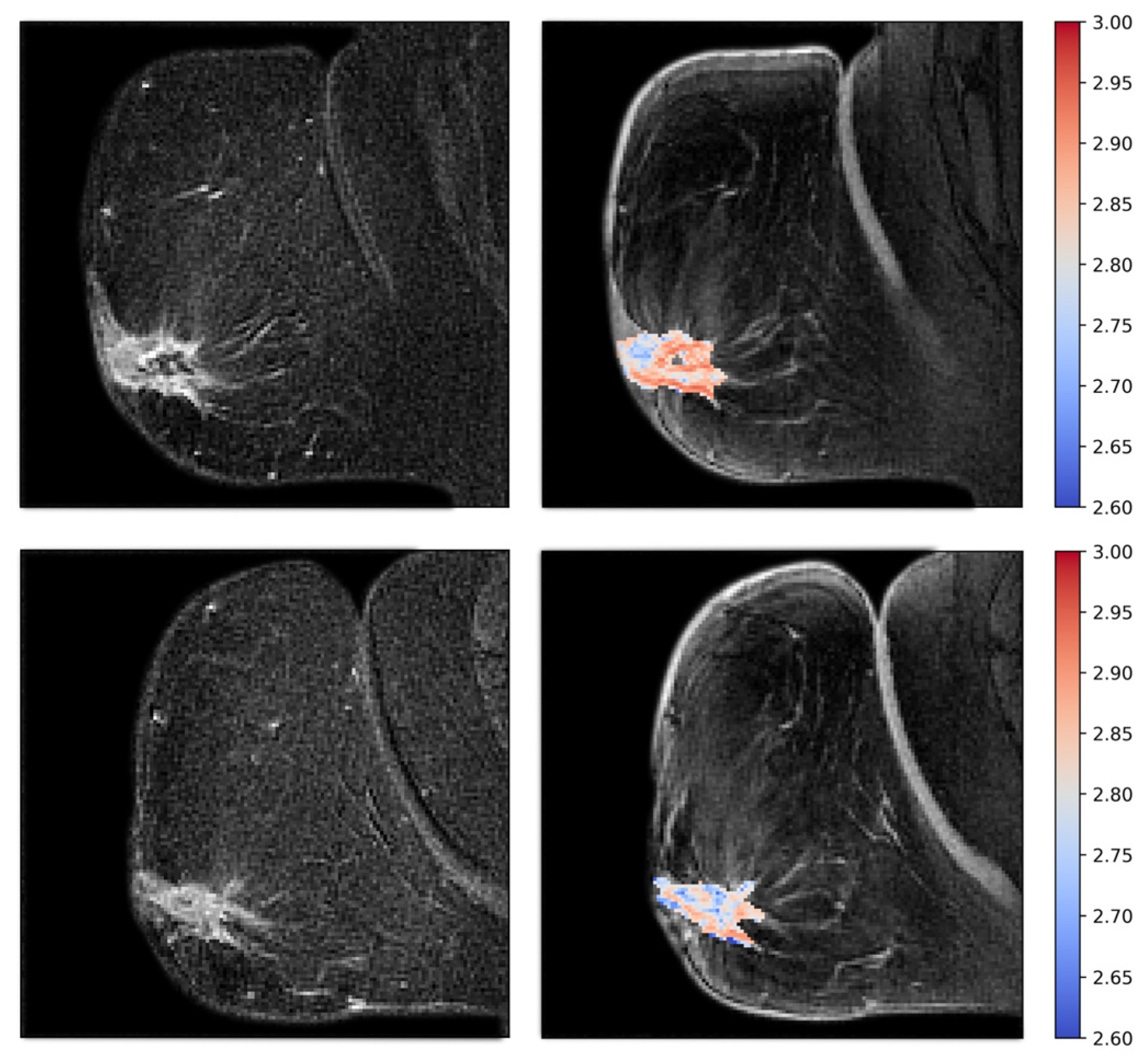
2.6. Tumor Vascular Architecture—Texture Analyses
2.7. Statistics
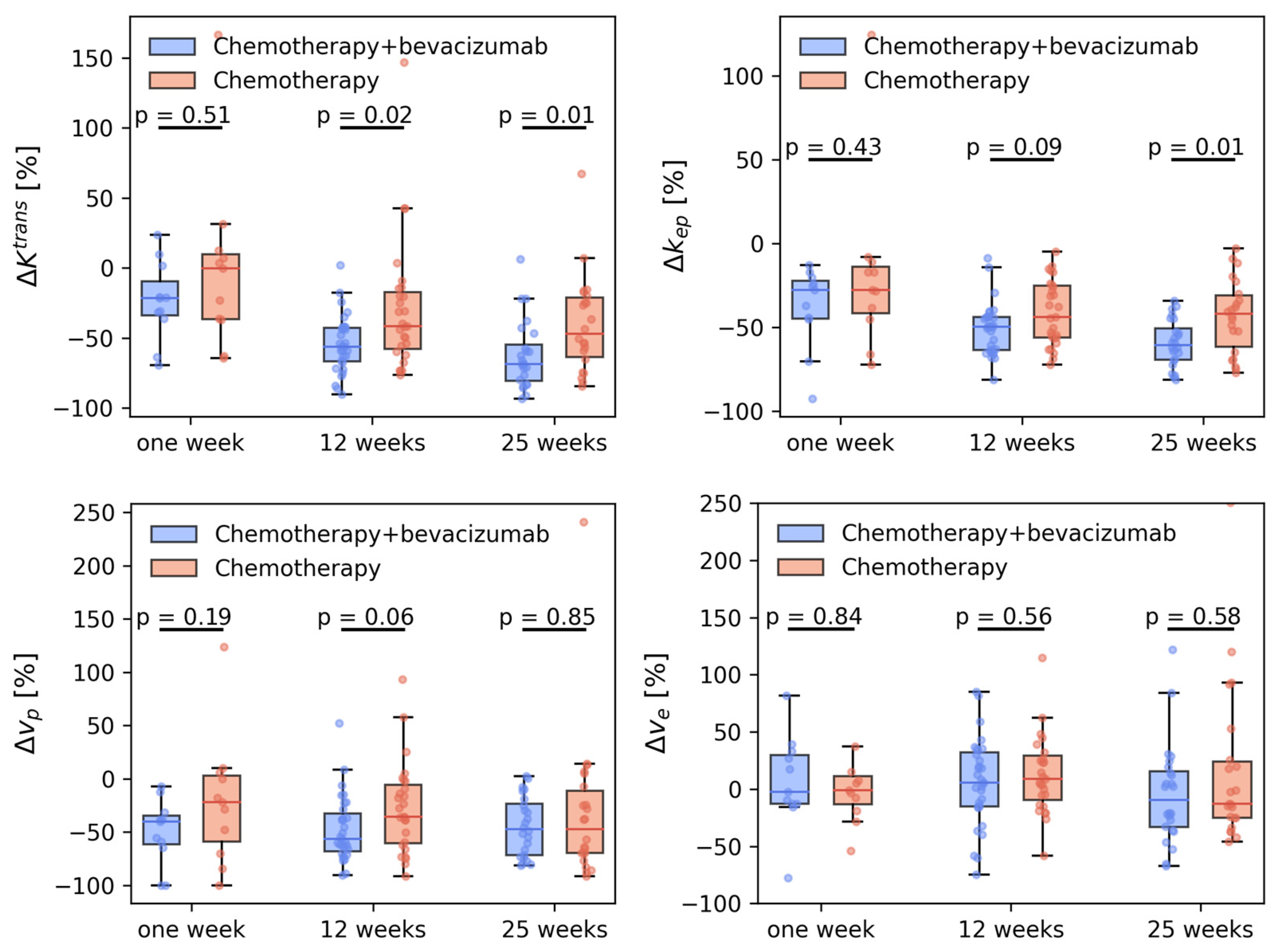
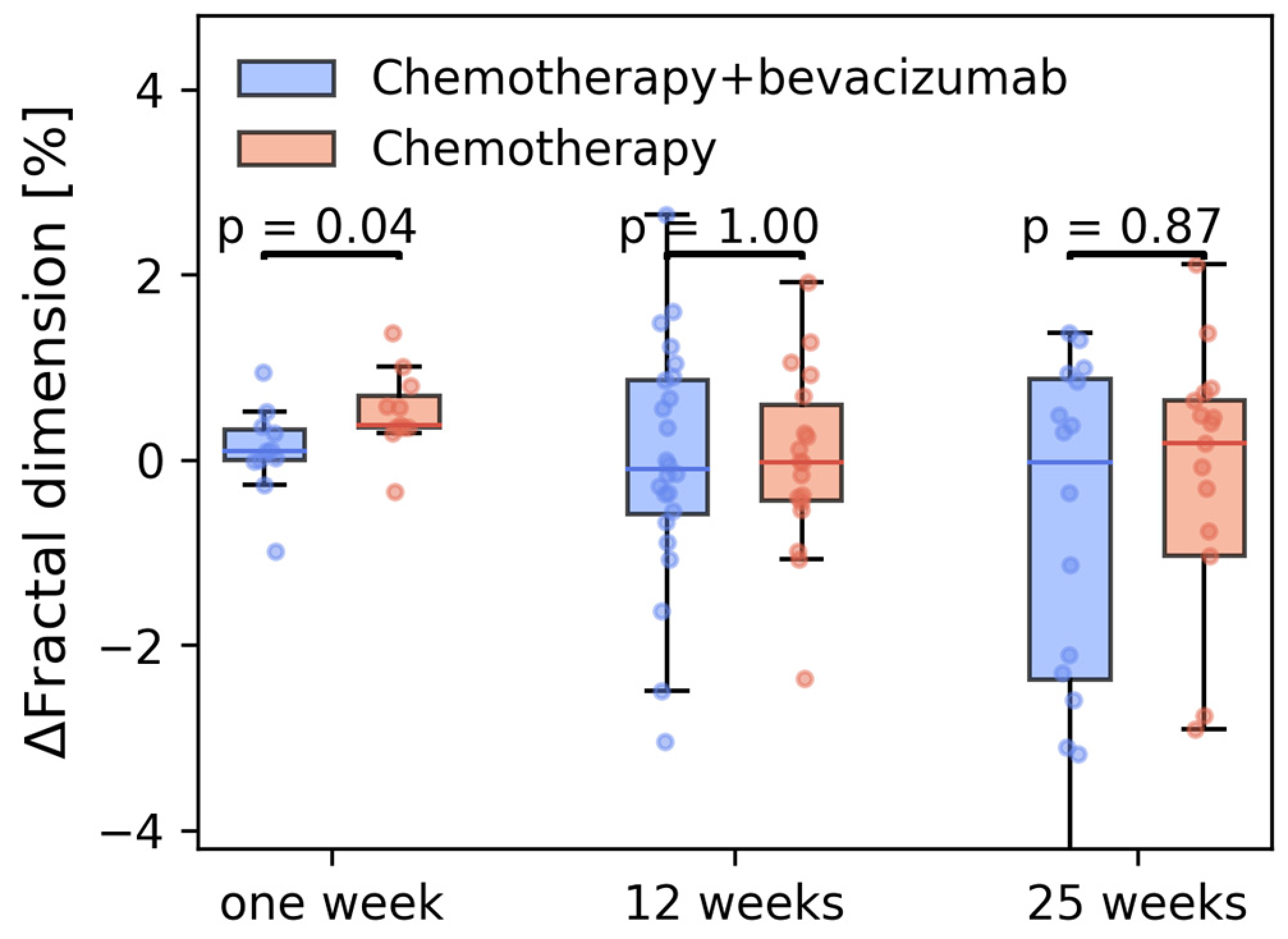
3. Results
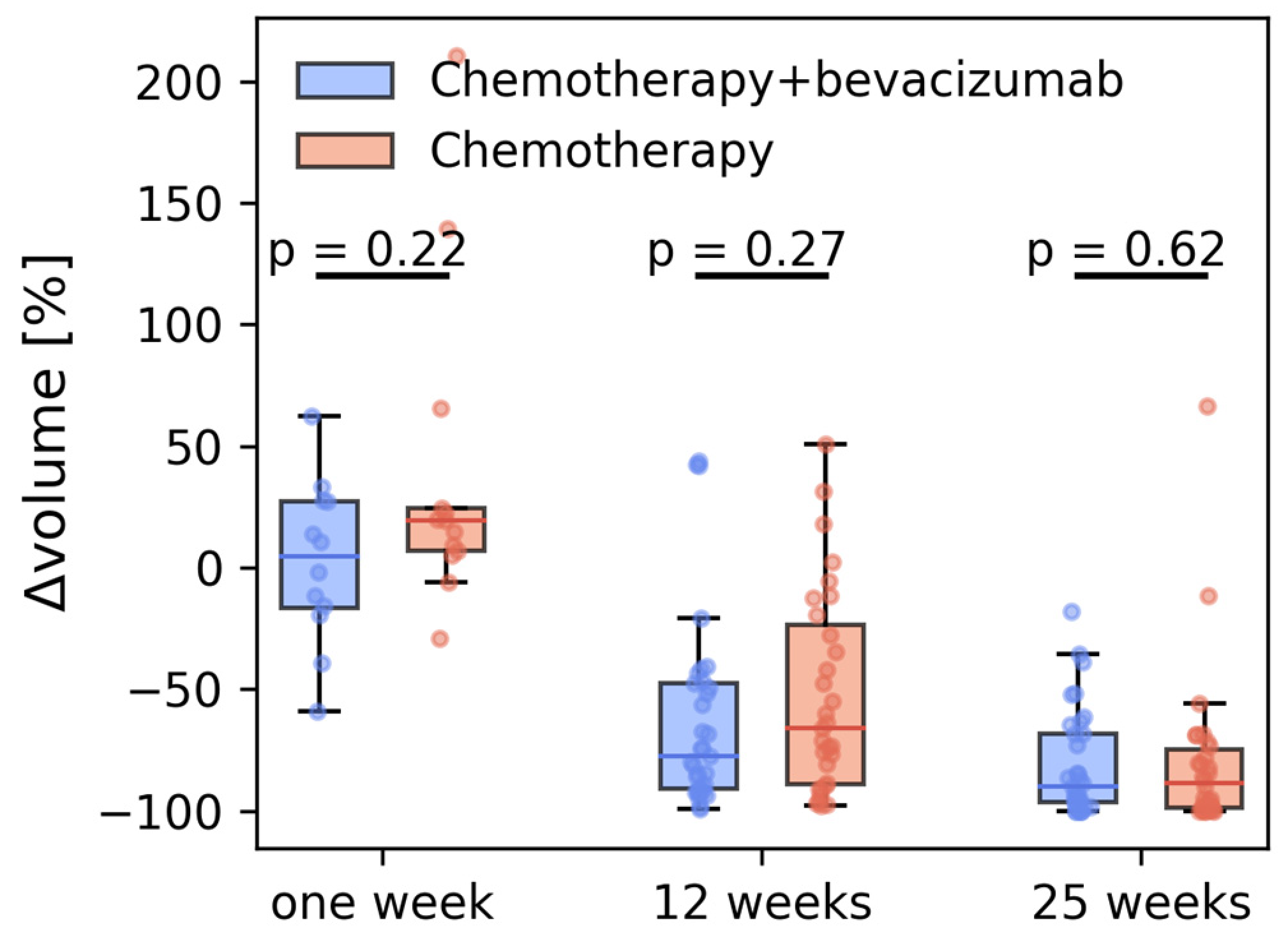
3.1. Tumor Volumetry
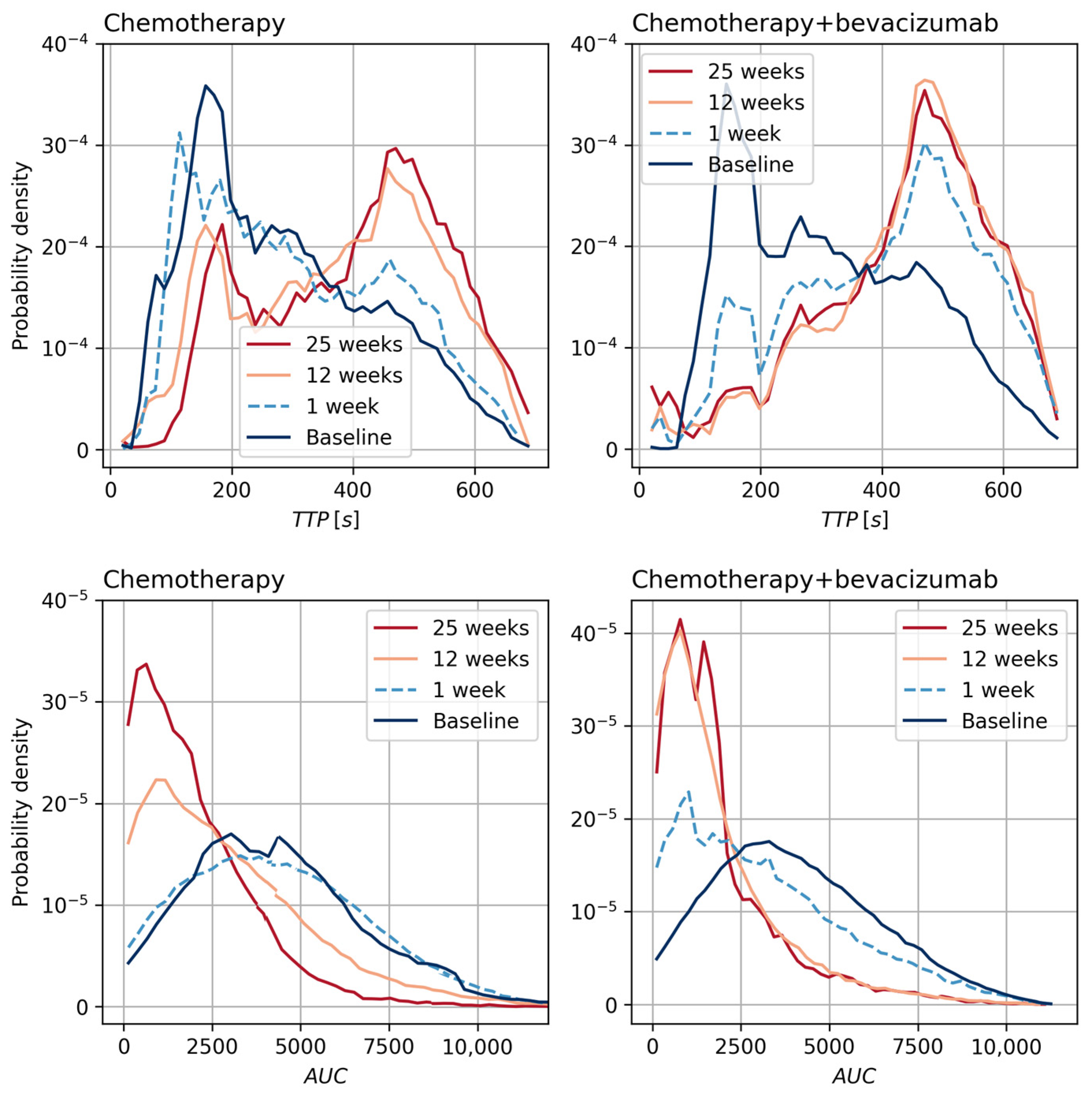
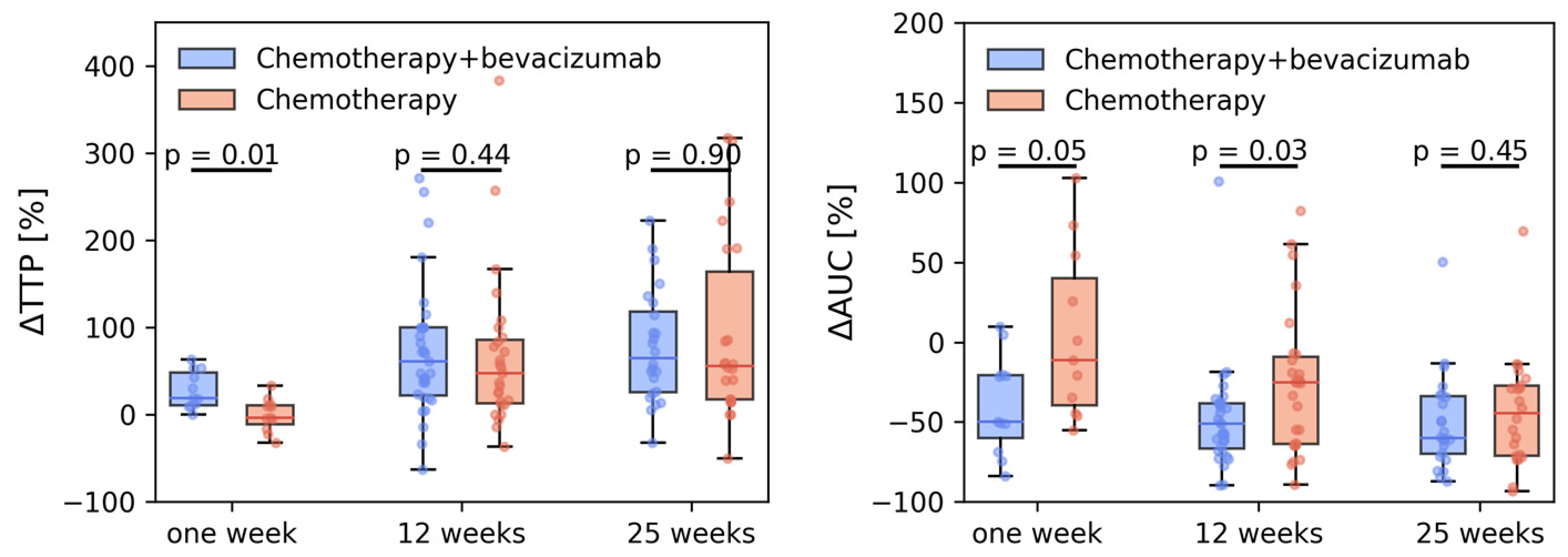
3.2. Tumor Perfusion
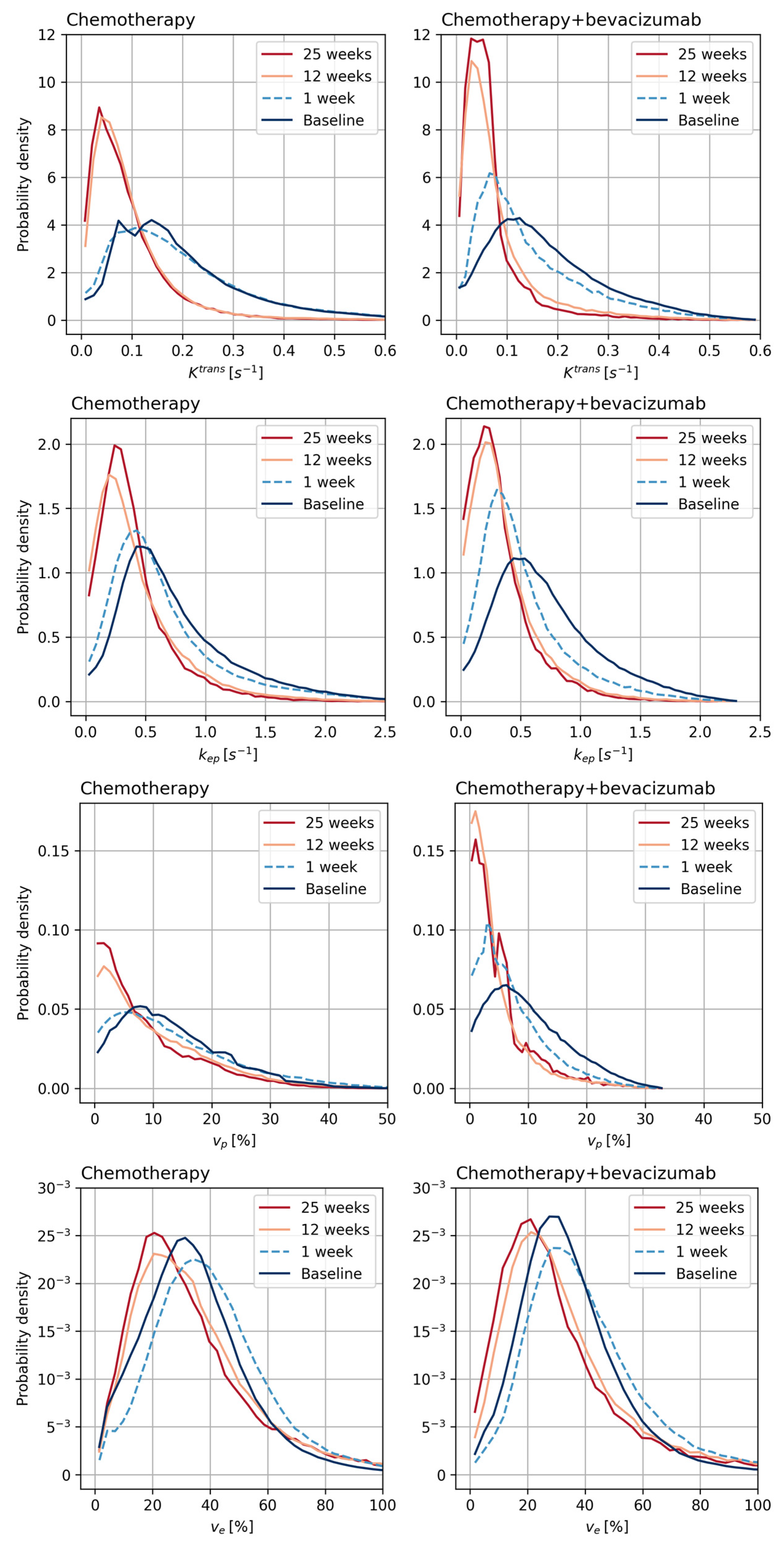
3.3. Tumor Vascular Architecture
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ayoub, N.M.; Jaradat, S.K.; Al-Shami, K.M.; Alkhalifa, A.E. Targeting Angiogenesis in Breast Cancer: Current Evidence and Future Perspectives of Novel Anti-Angiogenic Approaches. Front. Pharmacol. 2022, 13, 838133. [Google Scholar] [CrossRef] [PubMed]
- Tanne, J.H. FDA cancels approval for bevacizumab in advanced breast cancer. BMJ 2011, 343, d7684. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Kümler, I.; Christiansen, O.G.; Nielsen, D.L. A systematic review of bevacizumab efficacy in breast cancer. Cancer Treat. Rev. 2014, 40, 960–973. [Google Scholar] [CrossRef]
- Fakhrejahani, E.; Toi, M. Antiangiogenesis Therapy for Breast Cancer: An Update and Perspectives from Clinical Trials. Jpn. J. Clin. Oncol. 2014, 44, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Silwal-Pandit, L.; Nord, S.; von der Lippe Gythfeldt, H.; Møller, E.K.; Fleischer, T.; Rødland, E.; Krohn, M.; Borgen, E.; Garred, Ø.; Olsen, T.; et al. The Longitudinal Transcriptional Response to Neoadjuvant Chemotherapy with and without Bevacizumab in Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 4662–4670. [Google Scholar] [CrossRef]
- Haugen, M.H.; Lingjærde, O.C.; Hedenfalk, I.; Garred, Ø.; Borgen, E.; Loman, N.; Hatschek, T.; Børresen-Dale, A.-L.; Naume, B.; Mills, G.B.; et al. Protein Signature Predicts Response to Neoadjuvant Treatment With Chemotherapy and Bevacizumab in HER2-Negative Breast Cancers. JCO Precis. Oncol. 2021, 5, 286–306. [Google Scholar] [CrossRef]
- Yang, T.; Xiao, H.; Liu, X.; Wang, Z.; Zhang, Q.; Wei, N.; Guo, X. Vascular Normalization: A New Window Opened for Cancer Therapies. Front. Oncol. 2021, 11, 719836. [Google Scholar] [CrossRef]
- Magnussen, A.L.; Mills, I.G. Vascular normalisation as the stepping stone into tumour microenvironment transformation. Br. J. Cancer 2021, 125, 324–336. [Google Scholar] [CrossRef]
- Baish, J.W.; Jain, R.K. Fractals and cancer. Cancer Res. 2000, 60, 3683–3688. [Google Scholar]
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology 2019, 292, 520–536. [Google Scholar] [CrossRef]
- Song, H.K.; Dougherty, L. Dynamic MRI with projection reconstruction and KWIC processing for simultaneous high spatial and temporal resolution. Magn. Reson. Med. 2004, 52, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Song, H.K.; Dougherty, L. k-space weighted image contrast (KWIC) for contrast manipulation in projection reconstruction MRI. Magn. Reson. Med. 2000, 44, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.O.; Brindle, K.M.; Evelhoch, J.L.; Griffiths, J.R.; Horsman, M.R.; Jackson, A.; Jayson, G.C.; Judson, I.R.; Knopp, M.V.; Maxwell, R.J.; et al. The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: Issues and recommendations. Br. J. Cancer 2005, 92, 1599–1610. [Google Scholar] [CrossRef]
- Leach, M.O.; Brindle, K.M.; Evelhoch, J.L.; Griffiths, J.R.; Horsman, M.R.; Jackson, A.; Jayson, G.; Judson, I.R.; Knopp, M.V.; Maxwell, R.J.; et al. Assessment of antiangiogenic and antivascular therapeutics using MRI: Recommendations for appropriate methodology for clinical trials. Br. J. Radiol. 2003, 76 (Suppl. S1), S87–S91. [Google Scholar] [CrossRef] [PubMed]
- Marstal, K.; Berendsen, F.; Staring, M.; Klein, S. SimpleElastix: A User-Friendly, Multi-lingual Library for Medical Image Registration. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition Workshops (CVPRW), Las Vegas, NV, USA, 26 June–1 July 2016; IEEE: Las Vegas, NV, USA, 2016; pp. 574–582. [Google Scholar]
- Tofts, P.S.; Brix, G.; Buckley, D.L.; Evelhoch, J.L.; Henderson, E.; Knopp, M.V.; Larsson, H.B.; Lee, T.Y.; Mayr, N.A.; Parker, G.J.; et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: Standardized quantities and symbols. J. Magn. Reson. Imaging JMRI 1999, 10, 223–232. [Google Scholar] [CrossRef]
- Sourbron, S.P.; Buckley, D.L. On the scope and interpretation of the Tofts models for DCE-MRI. Magn. Reson. Med. 2011, 66, 735–745. [Google Scholar] [CrossRef]
- Rata, M.; Collins, D.J.; Darcy, J.; Messiou, C.; Tunariu, N.; Desouza, N.; Young, H.; Leach, M.O.; Orton, M.R. Assessment of repeatability and treatment response in early phase clinical trials using DCE-MRI: Comparison of parametric analysis using MR- and CT-derived arterial input functions. Eur. Radiol. 2016, 26, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Tofts, P.S.; Berkowitz, B.; Schnall, M.D. Quantitative analysis of dynamic Gd-DTPA enhancement in breast tumors using a permeability model. Magn. Reson. Med. 1995, 33, 564–568. [Google Scholar] [CrossRef]
- Yankeelov, T.E.; Lepage, M.; Chakravarthy, A.; Broome, E.E.; Niermann, K.J.; Kelley, M.C.; Meszoely, I.; Mayer, I.A.; Herman, C.R.; McManus, K.; et al. Integration of quantitative DCE-MRI and ADC mapping to monitor treatment response in human breast cancer: Initial results. Magn. Reson. Imaging 2007, 25, 1–13. [Google Scholar] [CrossRef]
- Merchant, T.E.; Thelissen, G.R.; de Graaf, P.W.; Nieuwenhuizen, C.W.; Kievit, H.C.; Den Otter, W. Application of a mixed imaging sequence for MR imaging characterization of human breast disease. Acta Radiol. Stockh. Swed. 1987 1993, 34, 356–361. [Google Scholar]
- Michallek, F.; Huisman, H.; Hamm, B.; Elezkurtaj, S.; Maxeiner, A.; Dewey, M. Prediction of prostate cancer grade using fractal analysis of perfusion MRI: Retrospective proof-of-principle study. Eur. Radiol. 2022, 32, 3236–3247. [Google Scholar] [CrossRef] [PubMed]
- Novianto, S.; Suzuki, Y.; Maeda, J. Near optimum estimation of local fractal dimension for image segmentation. Pattern Recognit. Lett. 2003, 24, 365–374. [Google Scholar] [CrossRef]
- Schnall, M.D.; Blume, J.; Bluemke, D.A.; DeAngelis, G.A.; DeBruhl, N.; Harms, S.; Heywang-Köbrunner, S.H.; Hylton, N.; Kuhl, C.K.; Pisano, E.D.; et al. Diagnostic architectural and dynamic features at breast MR imaging: Multicenter study. Radiology 2006, 238, 42–53. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Mielcareck, P.; Klaschik, S.; Leutner, C.; Wardelmann, E.; Gieseke, J.; Schild, H.H. Dynamic Breast MR Imaging: Are Signal Intensity Time Course Data Useful for Differential Diagnosis of Enhancing Lesions? Radiology 1999, 211, 101–110. [Google Scholar] [CrossRef]
- Willett, C.G.; Boucher, Y.; di Tomaso, E.; Duda, D.G.; Munn, L.L.; Tong, R.T.; Chung, D.C.; Sahani, D.V.; Kalva, S.P.; Kozin, S.V.; et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat. Med. 2004, 10, 145–147. [Google Scholar] [CrossRef]
- Willett, C.G.; Duda, D.G.; di Tomaso, E.; Boucher, Y.; Ancukiewicz, M.; Sahani, D.V.; Lahdenranta, J.; Chung, D.C.; Fischman, A.J.; Lauwers, G.Y.; et al. Efficacy, Safety, and Biomarkers of Neoadjuvant Bevacizumab, Radiation Therapy, and Fluorouracil in Rectal Cancer: A Multidisciplinary Phase II Study. J. Clin. Oncol. 2009, 27, 3020–3026. [Google Scholar] [CrossRef]
- López-Vega, J.M.; Álvarez, I.; Antón, A.; Illarramendi, J.J.; Llombart, A.; Boni, V.; García-Velloso, M.J.; Martí-Climent, J.M.; Pina, L.; García-Foncillas, J. Early Imaging and Molecular Changes with Neoadjuvant Bevacizumab in Stage II/III Breast Cancer. Cancers 2021, 13, 3511. [Google Scholar] [CrossRef]
- Yang, J.; Liao, C.; Liu, Y.; Yang, G.; Ke, T.; Ding, Y.; Li, Q. MR imaging biomarkers evaluating vascular normalization window after anti-vessel treatment. Oncotarget 2018, 9, 11964–11976. [Google Scholar] [CrossRef][Green Version]
- Sorensen, A.G.; Emblem, K.E.; Polaskova, P.; Jennings, D.; Kim, H.; Ancukiewicz, M.; Wang, M.; Wen, P.Y.; Ivy, P.; Batchelor, T.T.; et al. Increased Survival of Glioblastoma Patients Who Respond to Antiangiogenic Therapy with Elevated Blood Perfusion. Cancer Res. 2012, 72, 402–407. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | All | Chemotherapy | Chemotherapy+ Bevacizumab | p-Value |
|---|---|---|---|---|
| Patients (N) | 70 | 32 | 38 | |
| Age (years) | 0.86 (t-test) | |||
| Mean | 49.3 | 49.0 | 49.4 | |
| Median | 49.5 | 50.5 | 48.0 | |
| Range | 30–70 | 30–64 | 31–70 | |
| Tumor stage | 0.42 (ANOVA) | |||
| T2 | 20 | 10 | 10 | |
| T3 | 46 | 20 | 26 | |
| T4 | 4 | 2 | 2 | |
| Tumor size [mm] | 0.70 (t-test) | |||
| Mean | 46.8 | 46.1 | 47.5 | |
| Range | 16–92 | 16–92 | 29–76 | |
| Lymph node status 1 | ||||
| cN0 | 36 | 15 | 21 | 0.79 (ANOVA) |
| cN1 | 5 | 4 | 1 | |
| pN1 | 29 | 13 | 16 | |
| Type | ||||
| Ductal carcinoma | 55 | 26 | 30 | 1.00 (Fischer’s exact test) |
| Lobular carcinoma | 14 | 6 | 8 | |
| Grade | ||||
| 1 | 6 | 0 | 6 | 0.41 (ANOVA) |
| 2 | 49 | 24 | 25 | |
| 3 | 14 | 7 | 7 | |
| N/A | 1 | 1 | 0 | |
| Estrogen receptor status | ||||
| Positive | 59 | 27 | 32 | 1.00 (Fischer’s exact test) |
| Negative | 11 | 5 | 6 |
| N | Volume [%] | ||
|---|---|---|---|
| Chemotherapy | |||
| Baseline | 32 | 9.7 (5.8–21.6) | |
| 1 week | 13 | 14.4 (6.6–24.0) | 19.2 (−7.0–24.6) |
| 12 weeks | 31 | 3.8 (2.1–6.5) | −66 (−89.0–23.6) |
| 25 weeks | 29 | 0.9 (0.2–2.5) | −88.5 (−98.6–(−74.7)) |
| Chemotherapy + bevacizumab | |||
| Baseline | 38 | 11.1 (8.5–15.5) | |
| 1 week | 13 | 11.4 (8.0–15.1) | +4.4 (−16.6–27.3) |
| 12 weeks | 35 | 1.9 (1.0–7.1) | −77.8 (−90.8–(−47.7)) |
| 25 weeks | 35 | 1.0 (0.2–3.2) | −89.8 (−96.7–(−68.4)) |
| All | |||
| Baseline | 70 | 10.4 (6.1–18.7) | |
| 1 week | 26 | 11.9 (6.6–21.6) | 14 (−6.1–27.1) |
| 12 weeks | 66 | 2.9 (1.4–7.1) | −73.7 (−90.8–(−41.8) |
| 25 weeks | 64 | 1.0 (0.2–2.9) | −89.8 (−98.3–(−70.2)) |
| Chemotherapy | ||||
| Baseline | 0.13 (0.11, 0.15) | 0.55 (0.48, 0.61) | 9.4 (8.11, 10.69) | 23.92 (20.98, 26.86) |
| 1 week | 0.11 (0.08, 0.14) | 0.36 (0.27, 0.45) | 8.41 (2.14, 14.67) | 24.97 (19.99, 29.94) |
| 12 weeks | 0.08 (0.06, 0.11) | 0.31 (0.25, 0.37) | 6.25 (4.48, 8.02) | 30.6 (18.81, 42.39) |
| 25 weeks | 0.07 (0.05, 0.09) | 0.3 (0.24, 0.35) | 6.61 (3.2, 10.02) | 24.61 (20.25, 28.98) |
| 1 week [%] | −0.3 (−42.4, 41.9) | −18.7 (−53.6, −33.8) | −21.0 (−61.1, 19.1) | 26.0 (−43.7, 95.7) |
| 12 weeks [%] | −31.4 (−50.6, −12.2) | −41.2 (−49.5, −32.9) | −28.9 (−46.9, −10.8) | 40.0 (−18.3, 98.3) |
| 25 weeks [%] | −39.4 (−55.5, −23.3) | −43.7 (−53.6, −33.8) | −28.7 (−59.2, 1.8) | 18.8 (−13.1, 50.7) |
| Chemotherapy + bevacizumab | ||||
| Baseline | 0.14 (0.12, 0.16) | 0.55 (0.5, 0.6) | 7.83 (6.78, 8.87) | 25.98 (23.94, 28.02) |
| 1 week | 0.08 (0.05, 0.11) | 0.27 (0.16, 0.37) | 2.8 (1.38, 4.21) | 22.9 (14.22, 31.57) |
| 12 weeks | 0.06 (0.05, 0.07) | 0.27 (0.24, 0.3) | 3.8 (2.99, 4.61) | 26.12 (22.35, 29.9) |
| 25 weeks | 0.05 (0.04, 0.06) | 0.22 (0.19, 0.25) | 3.39 (2.71, 4.07) | 24.33 (20.34, 28.33) |
| 1 week [%] | −29.8 (−52.6, −7.0) | −44.4 (−64.6, −24.2) | −53.7 (−76.3, −31.1) | −6.6 (−39.8, 26.5) |
| 12 weeks [%] | −54.3 (−61.8, −46.8) | −50.7 (−56.4, −45.0) | −47.2 (−58.4, −36.0) | 6.1 (−7.9, 20.0) |
| 25 weeks [%] | −60.5 (−71.1, −49.9) | −58.9 (−64.5, −53.2) | −46.0 (−57.4, −34.6) | −1.5 (−20.1, 17.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, T.; Brandal, S.H.B.; Geier, O.M.; Engebråten, O.; Nilsen, L.B.; Kristensen, V.N.; Hole, K.H.; Hompland, T.; Fleischer, T.; Seierstad, T. MRI Assessment of Changes in Tumor Vascularization during Neoadjuvant Anti-Angiogenic Treatment in Locally Advanced Breast Cancer Patients. Cancers 2023, 15, 4662. https://doi.org/10.3390/cancers15184662
Mo T, Brandal SHB, Geier OM, Engebråten O, Nilsen LB, Kristensen VN, Hole KH, Hompland T, Fleischer T, Seierstad T. MRI Assessment of Changes in Tumor Vascularization during Neoadjuvant Anti-Angiogenic Treatment in Locally Advanced Breast Cancer Patients. Cancers. 2023; 15(18):4662. https://doi.org/10.3390/cancers15184662
Chicago/Turabian StyleMo, Torgeir, Siri Helene Bertelsen Brandal, Oliver Marcel Geier, Olav Engebråten, Line Brennhaug Nilsen, Vessela N. Kristensen, Knut Håkon Hole, Tord Hompland, Thomas Fleischer, and Therese Seierstad. 2023. "MRI Assessment of Changes in Tumor Vascularization during Neoadjuvant Anti-Angiogenic Treatment in Locally Advanced Breast Cancer Patients" Cancers 15, no. 18: 4662. https://doi.org/10.3390/cancers15184662
APA StyleMo, T., Brandal, S. H. B., Geier, O. M., Engebråten, O., Nilsen, L. B., Kristensen, V. N., Hole, K. H., Hompland, T., Fleischer, T., & Seierstad, T. (2023). MRI Assessment of Changes in Tumor Vascularization during Neoadjuvant Anti-Angiogenic Treatment in Locally Advanced Breast Cancer Patients. Cancers, 15(18), 4662. https://doi.org/10.3390/cancers15184662







