A Comparison of the Prognostic Effects of Fine Needle Aspiration and Core Needle Biopsy in Patients with Breast Cancer: A Nationwide Multicenter Prospective Registry
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Definitions
2.3. Statistical Analysis
2.4. Subgroup Analysis
3. Results
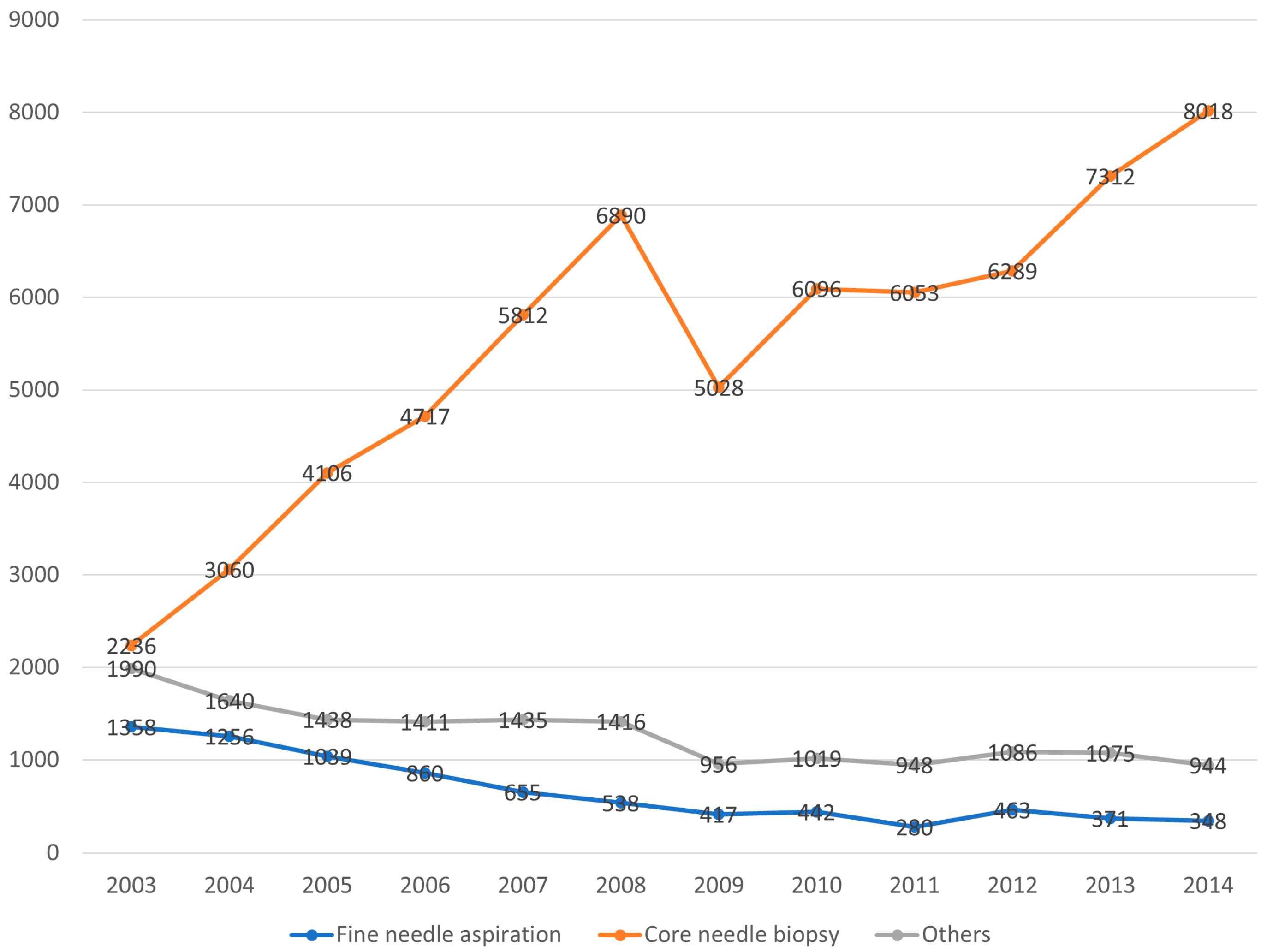
3.1. Time Trends in Breast Biopsy Practice
3.2. Patient Characteristics
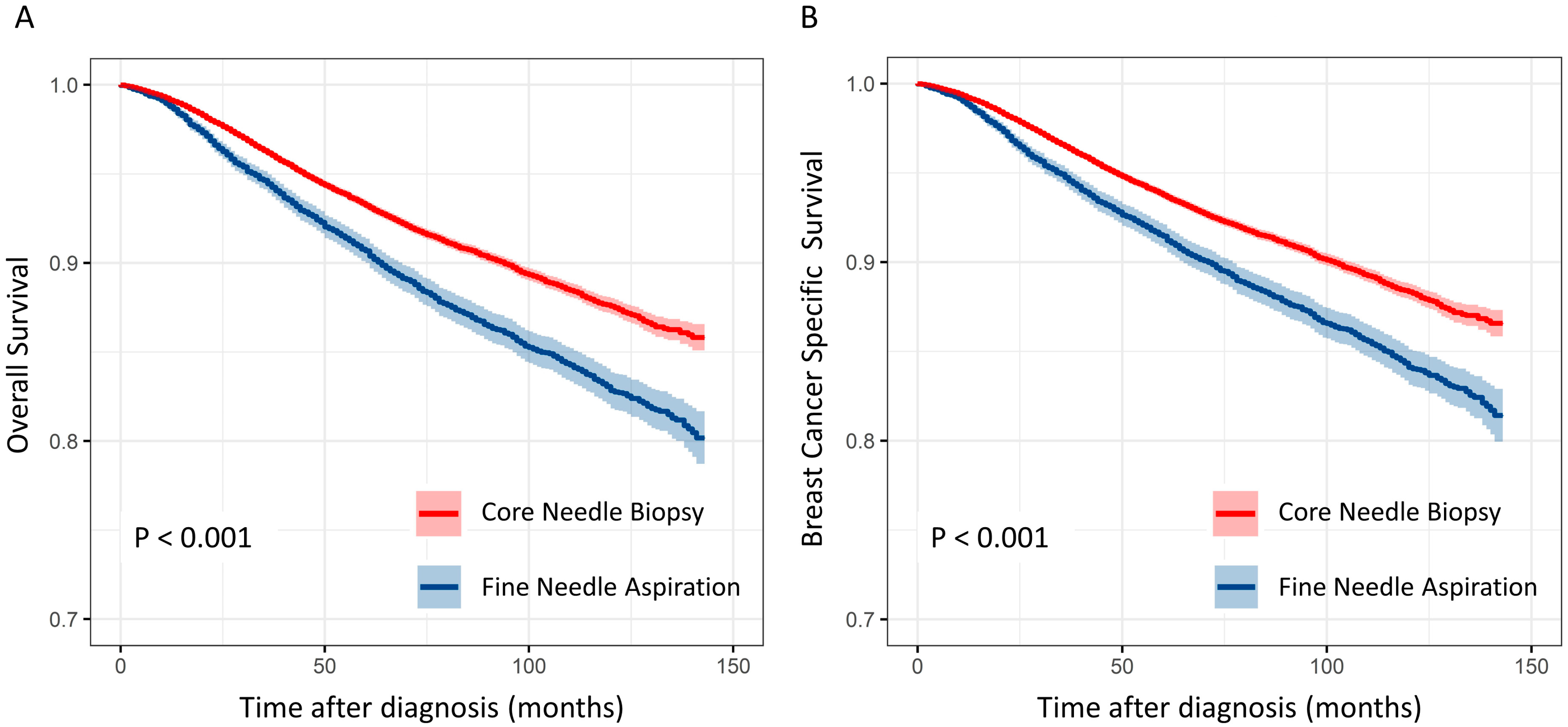
3.3. Survival Analysis
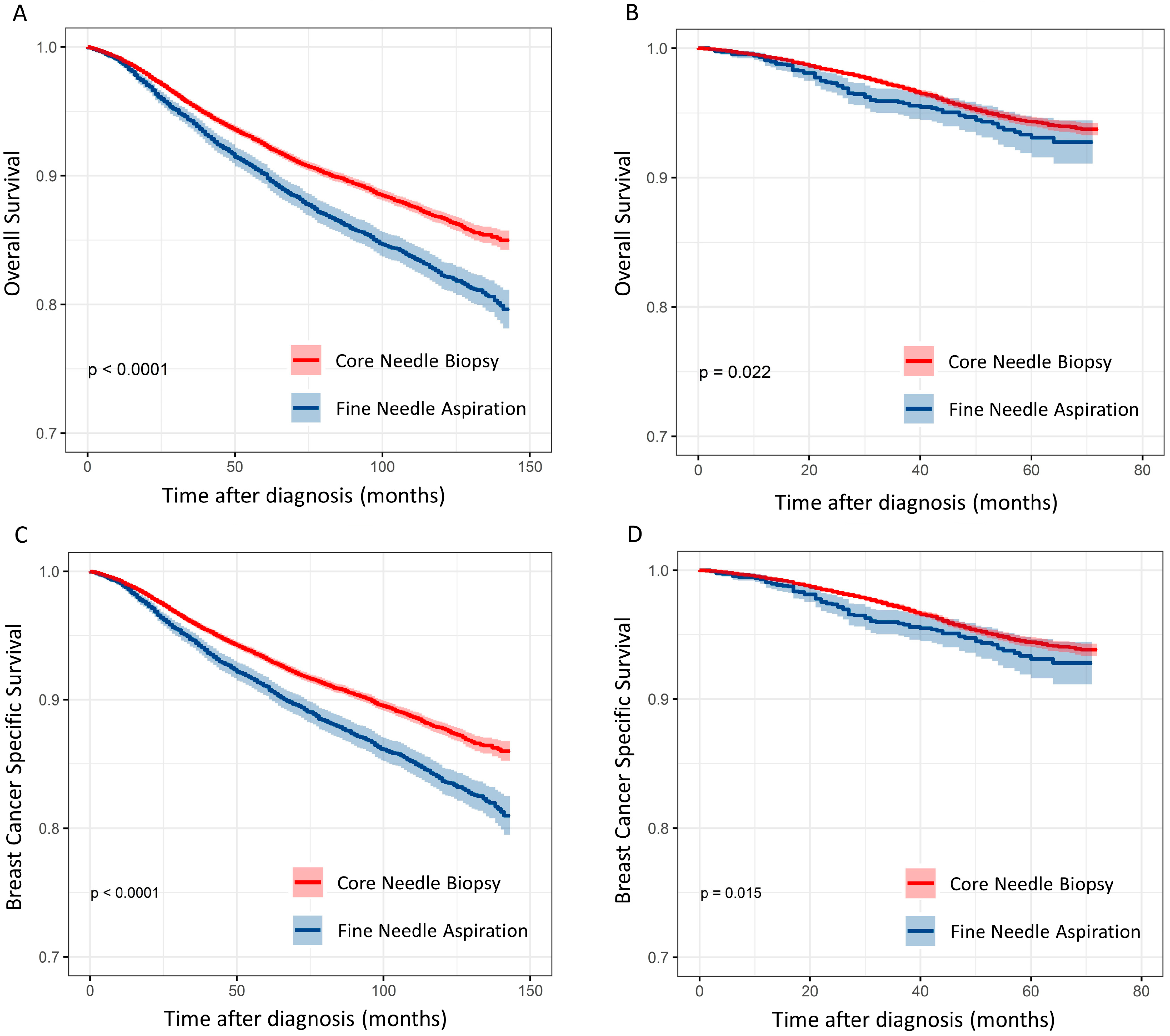
3.4. Subgroup Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Salzman, B.; Collins, E.; Hersh, L. Common Breast Problems. Am. Fam. Physician 2019, 99, 505–514. [Google Scholar] [PubMed]
- Mazari, F.A.K.; Sharma, N.; Reid, D.; Horgan, K. The need for triple assessment and predictors for diagnosis of breast cancer in patients < 40 years of age. Clin. Radiol. 2018, 73, 758.e19–758.e25. [Google Scholar] [CrossRef]
- Willems, S.M.; van Deurzen, C.H.; van Diest, P.J. Diagnosis of breast lesions: Fine-needle aspiration cytology or core needle biopsy? A review. J. Clin. Pathol. 2012, 65, 287–292. [Google Scholar] [CrossRef]
- Teng, R.; Wei, Q.; Zhou, J.; Dong, M.; Jin, L.; Hu, W.; Chen, J.; Wang, L.; Zhao, W. The influence of preoperative biopsy on the surgical method in breast cancer patients: A single-center experience of 3966 cases in China. Gland Surg. 2021, 10, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Ahn, S.G.; Oh, S.J.; Kim, H.; Kang, E.; Jung, Y.; Do Byun, K.; Lee, J.; Sun, W.Y.; Korean Breast Cancer Society. Survival Outcomes of Patients with Breast Cancer Diagnosed Using Vacuum-Assisted Biopsy: A Nationwide Study from the Korean Breast Cancer Society. J. Breast Cancer 2022, 25, 13–24. [Google Scholar] [CrossRef]
- Ly, A.; Ono, J.C.; Hughes, K.S.; Pitman, M.B.; Balassanian, R. Fine-Needle Aspiration Biopsy of Palpable Breast Masses: Patterns of Clinical Use and Patient Experience. J. Natl. Compr. Canc. Netw. 2016, 14, 527–536. [Google Scholar] [CrossRef]
- Li, Z.; Souers, R.J.; Tabbara, S.O.; Natale, K.E.; Nguyen, L.N.; Booth, C.N. Breast Fine-Needle Aspiration Practice in 2019: Results of a College of American Pathologists National Survey. Arch. Pathol. Lab. Med. 2021, 145, 825–833. [Google Scholar] [CrossRef]
- King, T.A.; Hayes, D.H.; Cederbom, G.J.; Champaign, J.L.; Smetherman, D.H.; Farr, G.H.; Bolton, J.S.; Fuhrman, G.M. Biopsy technique has no impact on local recurrence after breast-conserving therapy. Breast J. 2001, 7, 19–24. [Google Scholar] [CrossRef]
- Kong, Y.C.; Bhoo-Pathy, N.; O’Rorke, M.; Subramaniam, S.; Bhoo-Pathy, N.T.; See, M.H.; Jamaris, S.; Teoh, K.H.; Bustam, A.Z.; Looi, L.M.; et al. The association between methods of biopsy and survival following breast cancer: A hospital registry based cohort study. Medicine 2020, 99, e19093. [Google Scholar] [CrossRef]
- Oyama, T.; Koibuchi, Y.; McKee, G. Core needle biopsy (CNB) as a diagnostic method for breast lesions: Comparison with fine needle aspiration cytology (FNA). Breast Cancer 2004, 11, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Sustova, P.; Klijanienko, J. Value of combined use of fine-needle aspiration and core needle biopsy in palpable breast tumors performed by pathologist: Institut Curie experience. Diagn. Cytopathol. 2020, 48, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Gallicchio, L.; McSorley, M.A.; Newschaffer, C.J.; Thuita, L.W.; Huang, H.Y.; Hoffman, S.C.; Helzlsouer, K.J. Nonsteroidal antiinflammatory drugs, cyclooxygenase polymorphisms, and the risk of developing breast carcinoma among women with benign breast disease. Cancer 2006, 106, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Mathenge, E.G.; Dean, C.A.; Clements, D.; Vaghar-Kashani, A.; Photopoulos, S.; Coyle, K.M.; Giacomantonio, M.; Malueth, B.; Nunokawa, A.; Jordan, J.; et al. Core needle biopsy of breast cancer tumors increases distant metastases in a mouse model. Neoplasia 2014, 16, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Nathanson, S.D.; Shah, R.; Rosso, K. Sentinel lymph node metastases in cancer: Causes, detection and their role in disease progression. Semin. Cell Dev. Biol. 2015, 38, 106–116. [Google Scholar] [CrossRef]
- Rautiainen, S.; Masarwah, A.; Sudah, M.; Sutela, A.; Pelkonen, O.; Joukainen, S.; Sironen, R.; Karja, V.; Vanninen, R. Axillary lymph node biopsy in newly diagnosed invasive breast cancer: Comparative accuracy of fine-needle aspiration biopsy versus core-needle biopsy. Radiology 2013, 269, 54–60. [Google Scholar] [CrossRef]
- Sherman, M.E.; Vierkant, R.A.; Kaggal, S.; Hoskin, T.L.; Frost, M.H.; Denison, L.; Visscher, D.W.; Carter, J.M.; Winham, S.J.; Jensen, M.R.; et al. Breast Cancer Risk and Use of Nonsteroidal Anti-inflammatory Agents After a Benign Breast Biopsy. Cancer Prev. Res. 2020, 13, 967–976. [Google Scholar] [CrossRef]
- Liikanen, J.; Leidenius, M.; Joensuu, H.; Vironen, J.; Heikkila, P.; Meretoja, T. Breast cancer prognosis and isolated tumor cell findings in axillary lymph nodes after core needle biopsy and fine needle aspiration cytology: Biopsy method and breast cancer outcome. Eur. J. Surg. Oncol. 2016, 42, 64–70. [Google Scholar] [CrossRef]
- Burnside, E.S.; Sickles, E.A.; Bassett, L.W.; Rubin, D.L.; Lee, C.H.; Ikeda, D.M.; Mendelson, E.B.; Wilcox, P.A.; Butler, P.F.; D’Orsi, C.J. The ACR BI-RADS experience: Learning from history. J. Am. Coll. Radiol. 2009, 6, 851–860. [Google Scholar] [CrossRef]
- Field, A.S.; Raymond, W.A.; Rickard, M.; Schmitt, F. Breast fine needle aspiration biopsy cytology: The potential impact of the International Academy of Cytology Yokohama System for Reporting Breast Fine Needle Aspiration Biopsy Cytopathology and the use of rapid on-site evaluation. J. Am. Soc. Cytopathol. 2020, 9, 103–111. [Google Scholar] [CrossRef]
- Ljung, B.M.; Drejet, A.; Chiampi, N.; Jeffrey, J.; Goodson, W.H., III; Chew, K.; Moore, D.H., II; Miller, T.R. Diagnostic accuracy of fine-needle aspiration biopsy is determined by physician training in sampling technique. Cancer 2001, 93, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Montezuma, D.; Malheiros, D.; Schmitt, F.C. Breast Fine Needle Aspiration Biopsy Cytology Using the Newly Proposed IAC Yokohama System for Reporting Breast Cytopathology: The Experience of a Single Institution. Acta Cytol. 2019, 63, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Ryan, D.; Berney, D.; Calaminici, M.; Sheaff, M.T.; Wells, C.A. Inadequate rates are lower when FNAC samples are taken by cytopathologists. Cytopathology 2003, 14, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Field, A.S. Breast FNA biopsy cytology: Current problems and the International Academy of Cytology Yokohama standardized reporting system. Cancer Cytopathol. 2017, 125, 229–230. [Google Scholar] [CrossRef]
- Ibrahim, A.E.; Bateman, A.C.; Theaker, J.M.; Low, J.L.; Addis, B.; Tidbury, P.; Rubin, C.; Briley, M.; Royle, G.T. The role and histological classification of needle core biopsy in comparison with fine needle aspiration cytology in the preoperative assessment of impalpable breast lesions. J. Clin. Pathol. 2001, 54, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Pisano, E.D.; Fajardo, L.L.; Tsimikas, J.; Sneige, N.; Frable, W.J.; Gatsonis, C.A.; Evans, W.P.; Tocino, I.; McNeil, B.J. Rate of insufficient samples for fine-needle aspiration for nonpalpable breast lesions in a multicenter clinical trial: The Radiologic Diagnostic Oncology Group 5 Study. The RDOG5 investigators. Cancer 1998, 82, 679–688. [Google Scholar] [CrossRef]
- Wojtyla, C.; Bertuccio, P.; Wojtyla, A.; La Vecchia, C. European trends in breast cancer mortality, 1980–2017 and predictions to 2025. Eur. J. Cancer 2021, 152, 4–17. [Google Scholar] [CrossRef]
- Verras, G.I.; Tchabashvili, L.; Mulita, F.; Grypari, I.M.; Sourouni, S.; Panagodimou, E.; Argentou, M.I. Micropapillary Breast Carcinoma: From Molecular Pathogenesis to Prognosis. Breast Cancer 2022, 14, 41–61. [Google Scholar] [CrossRef]
- Verras, G.I.; Mulita, F.; Tchabashvili, L.; Grypari, I.M.; Sourouni, S.; Panagodimou, E.; Argentou, M.I. A rare case of invasive micropapillary carcinoma of the breast. Prz Menopauzalny 2022, 21, 73–80. [Google Scholar] [CrossRef]
- Akrida, I.; Mulita, F. The clinical significance of HER2 expression in DCIS. Med. Oncol. 2022, 40, 16. [Google Scholar] [CrossRef]

| Characteristics | Biopsy Method (n = 73,644) | ||
|---|---|---|---|
| FNA (n = 8027) | CNB (n = 65,617) | p-Value | |
| Age, years (mean ± SD) | 50.1 ± 10.7 | 50.3 ± 10.5 | 0.127 |
| Age, years n (%) | 0.368 | ||
| <55 | 5633 (70.2) | 45,923 (70.0) | |
| ≥55 | 2394 (29.8) | 19,694 (30.0) | |
| Tumor size (cm) | 2.5 ± 3.1 | 2.0± 2.9 | <0.001 |
| Nodal involvement n (%) | <0.001 | ||
| No | 4633 (58.4) | 43,011 (66.2) | |
| Yes | 3305 (41.6) | 21,948 (33.8) | |
| Unknown | 89 | 658 | |
| Stage n (%) | <0.001 | ||
| I | 2377 (31.7) | 25,657 (43.6) | |
| II | 3512 (46.9) | 23,828 (40.4) | |
| III | 1484 (19.8) | 8562 (14.5) | |
| IV | 115 (1.5) | 863 (1.5) | |
| Palpation n (%) | <0.001 | ||
| Yes | 6839 (92.6) | 45,458 (81.7) | |
| No | 543 (7.4) | 10,162 (18.3) | |
| Unknown | 645 | 9997 | |
| Tumor Count n (%) | <0.001 | ||
| Single | 6074 (84.7) | 54,384 (87.2) | |
| Multiple | 1100 (15.3) | 7515 (12.8) | |
| Type n (%) | <0.001 | ||
| IDC | 6984 (93.3) | 54,906 (93.2) | |
| ILC | 144 (1.9) | 1995 (3.4) | |
| Others | 360 (438) | 2009 (3.4) | |
| Location n (%) | <0.001 | ||
| Lateral | 4530 (62.5) | 38,193 (64.8) | |
| Medial | 1742 (24.0) | 14,797 (25.1) | |
| Central | 977 (13.5) | 5953 (10.1) | |
| Unknown | 2346 | 6674 | |
| Histologic grade n (%) | <0.001 | ||
| Grades 1 and 2 | 3586 (54.7) | 34,976 (63.9) | |
| Grade 3 | 2970 (45.3) | 19,755 (36.1) | |
| Unknown | 1471 | 10,886 | |
| Surgery | <0.001 | ||
| Breast conserving | 3531 (44.0%) | 37,791 (57.6) | |
| Mastectomy | 4292 (53.5%) | 26,697 (40.7) | |
| Other | 204 (2.5) | 1129 (1.7) | |
| BI-RADS category n (%) | <0.001 | ||
| 0–3 | 187 (3.0) | 1036 (2.0) | |
| 4 | 369 (6.0) | 6451 (12.5) | |
| 5 | 5600 (91.0) | 44,282 (85.5) | |
| Hormonal receptor n (%) | <0.001 | ||
| Positive | 5331 (68.7) | 47,523 (74.2) | |
| Negative | 2429 (31.3) | 16,506 (25.8) | |
| Unknown | 267 | 1588 | |
| HER2 n (%) | <0.001 | ||
| Positive | 1667 (28.6) | 13,144 (25.9) | |
| Negative | 4158 (71.4) | 37,647 (74.1) | |
| Unknown | 4902 | 14,826 | |
| Univariate Analysis | p-Value | Multivariate Analysis | p-Value | |
|---|---|---|---|---|
| Age | ||||
| <55 | 1 | 1 | ||
| ≥55 | 1.637 (1.547–1.733) | <0.001 | 1.545 (1.429–1.670) | <0.001 |
| Surgery | ||||
| Breast conserving | 1 | 1 | ||
| Mastectomy | 2.722 (2.558–2.897) | <0.001 | 1.517 (1.386–1.661) | <0.001 |
| Others | 4.614 (4.022–5.293) | <0.001 | 2.222 (1.699–2.907) | <0.001 |
| Tumor type | ||||
| IDC | 1 | 1 | ||
| ILC | 0.924 (0.773–1.104) | 0.219 | 1.172 (0.884–1.554) | 0.269 |
| Others | 1.677 (1.479–1.901) | 0.060 | 1.316 (1.813–0.983) | 0.039 |
| Stage | ||||
| I | 1 | 1 | ||
| II | 2.264 (2.081–2.464) | <0.001 | 1.751 (1.559–1.967) | <0.001 |
| III | 7.334 (6.747–7.971) | <0.001 | 5.192 (4.610–5.846) | <0.001 |
| IV | 28.587 (25.488–32.063) | <0.001 | 18.795 (15.629–22.603) | <0.001 |
| Location | ||||
| Peripheral | 1 | 1 | ||
| Central | 1.938 (1.804–2.082) | <0.001 | 1.271 (1.153–1.400) | <0.001 |
| Palpation | ||||
| No | 1 | 1 | ||
| Yes | 2.283 (2.008–2.595) | <0.001 | 1.215 (1.012–1.459) | 0.001 |
| Tumor count | ||||
| Single | 1 | 1 | ||
| Multiple | 1.272 (1.176–1.375) | 0.260 | 1.018 (0.914–1.134) | 0.743 |
| Histologic grade | ||||
| G 1–2 | 1 | 1 | ||
| G 3 | 2.084 (1.964–2.212) | <0.001 | 1.531 (1.411–1.661) | <0.001 |
| BI-RADS | ||||
| 0–3 | 1 | 1 | ||
| 4 | 0.648 (0.504–0.834) | 0.001 | 1.040 (0.742–1.459) | 0.819 |
| 5 | 1.125 (0.921–1.375) | 0.250 | 1.146 (0.866–1.516) | 0.342 |
| Biopsy | ||||
| CNB | 1 | 1 | ||
| FNA | 1.415 (1.322–1.515) | <0.001 | 1.123 (1.026–1.228) | 0.012 |
| Estrogen Receptor | ||||
| Positive | 1 | 1 | ||
| Negative | 1.934 (1.828–2.046) | <0.001 | 1.603 (1.483–1.733) | <0.001 |
| HER2 | ||||
| Negative | 1 | 1 | ||
| Positive | 1.261 (1.178–1.349) | <0.001 | 1.100 (1.016–1.191) | 0.019 |
| Univariate Analysis | p-Value | Multivariate Analysis | p-Value | |
|---|---|---|---|---|
| Age | ||||
| <55 | 1 | 1 | ||
| ≥55 | 1.506 (1.419–1.59) | <0.001 | 1.439 (1.332–1.555) | <0.001 |
| Surgery | ||||
| Breast conserving | 1 | 1 | ||
| Mastectomy | 2.771 (2.597–2.957) | <0.001 | 1.509 (1.378–1.652) | <0.001 |
| Others | 4.755 (4.127–5.478) | <0.001 | 2.216 (1.772–2.772) | <0.001 |
| Tumor type | ||||
| IDC | 1 | 1 | ||
| ILC | 0.889 (0.738–1.072) | 0.219 | 0.952 (0.744–1.220) | 0.700 |
| Others | 1.236 (0.991–1.542) | 0.060 | 1.323 (0.938–1.866) | 0.111 |
| Stage | ||||
| I | 1 | 1 | ||
| II | 2.452 (2.238–2.686) | <0.001 | 2.020 (1.793–2.277) | <0.001 |
| III | 8.416 (7.697–9.203) | <0.001 | 6.474 (5.741–7.300) | <0.001 |
| IV | 33.503 (29.728–37.757) | <0.001 | 24.706 (20.789–29.359) | <0.001 |
| Location | ||||
| Peripheral | 1 | 1 | ||
| Central | 2.006 (1.863–2.159) | <0.001 | 1.267 (1.153–1.393) | <0.001 |
| Palpation | ||||
| Yes | 1 | 1 | ||
| No | 2.323 (2.031–2.657) | <0.001 | 1.264 (1.053–1.519) | 0.012 |
| Tumor count | ||||
| Single | 1 | 1 | ||
| Multiple | 1.311 (1.210–1.421) | <0.001 | 1.066 (0.960–1.183) | 0.231 |
| Histologic grade | ||||
| G 1–2 | 1 | 1 | ||
| G 3 | 2.187 (2.055–2.326) | <0.001 | 1.576 (1.448–1.715) | <0.001 |
| BI-RADS | ||||
| 0–3 | 1 | 1 | ||
| 4 | 0.666 (0.513–0.863) | 0.002 | 1.068 (0.754–1.514) | 0.711 |
| 5 | 1.123 (0.912–1.383) | 0.274 | 1.135 (0.850–1.515) | 0.390 |
| Biopsy | ||||
| CNB | 1 | 1 | ||
| FNA | 1.342 (1.249–1.443) | <0.001 | 1.099 (1.001–1.206) | 0.047 |
| Estrogen Receptor | ||||
| Positive | 1 | 1 | ||
| Negative | 2.003 (1.888–2.124) | <0.001 | 1.668 (1.531–1.816) | <0.001 |
| HER2 | ||||
| Negative | 1 | 1 | ||
| Positive | 1.354 (1.261–1.454) | <0.001 | 1.081 (0.992–1.180) | 0.077 |
| CNB | FNA (Univariate) | p-Value | FNA (Multivariate) | p-Value | |
|---|---|---|---|---|---|
| Age (years) | |||||
| <55 | 1 | 1.396 (1.278–1.524) | <0.001 | 1.083 (0.968–1.212) | 0.165 |
| ≥55 | 1 | 1.410 (1.265–1.572) | <0.001 | 1.146 (0.994–1.321) | 0.061 |
| Size | |||||
| <2 cm | 1 | 1.385 (1.213–1.582) | <0.001 | 1.134 (0.947–1.358) | 0.171 |
| 2–4 cm | 1 | 1.216 (1.097–1.349) | <0.001 | 1.073 (0.943–1.220) | 0.283 |
| ≥4 cm | 1 | 1.257 (1.107–1.428) | <0.001 | 1.135 (0.963–1.338) | 0.132 |
| Location | |||||
| Peripheral | 1 | 1.386 (1.280–1.501) | <0.001 | 1.066 (0.964–1.179) | 0.213 |
| Central | 1 | 1.395 (1.200–1.621) | <0.001 | 1.309 (1.089–1.574) | 0.004 |
| BI-RADS | |||||
| 0–3 | 1 | 1.726 (1.116–2.672) | 0.014 | 1.180(0.667–2.090) | 0.569 |
| 4 | 1 | 1.629 (1.031–2.575) | 0.037 | 0.881 (0.450–1.723) | 0.711 |
| 5 | 1 | 1.338 (1.239–1.445) | <0.001 | 1.122 (1.025–1.228) | 0.012 |
| Palpable | |||||
| Yes | 1 | 1.488 (0.986–2.247) | 0.059 | 0.963 (0.557–1.666) | 0.893 |
| No | 1 | 1.302 (1.211–1.400) | <0.001 | 1.224 (1.126–1.331) | <0.001 |
| CNB | FNA (Univariate) | p-Value | FNA (Multivariate) | p-Value | |
|---|---|---|---|---|---|
| Age (years) | |||||
| <55 | 1 | 1.336 (1.219–1.463) | <0.001 | 1.045 (0.949–1.151) | 0.374 |
| ≥55 | 1 | 1.330 (1.181–1.498) | <0.001 | 1.113 (0.981–1.264) | 0.097 |
| Size | |||||
| <2 cm | 1 | 1.287 (1.113–1.488) | 0.001 | 1.104 (0.950–1.282) | 0.199 |
| 2–4 cm | 1 | 1.149 (1.030–1.281) | 0.013 | 1.020 (0.912–1.140) | 0.731 |
| ≥4 cm | 1 | 1.205 (1.057–1.374) | 0.005 | 1.105 (0.966–1.262) | 0.145 |
| Location | |||||
| Peripheral | 1 | 1.312 (1.206–1.428) | <0.001 | 1.049 (0.961–1.146) | 0.283 |
| Central | 1 | 1.323 (1.130–1.547) | <0.001 | 1.201 (1.018–1.416) | 0.030 |
| BI-RADS | |||||
| 0–3 | 1 | 1.708 (1.082–2.698) | 0.022 | 1.221 (0.677–2.199) | 0.507 |
| 4 | 1 | 1.366 (0.822–2.269) | 0.228 | 1.191 (0.711–1.995) | 0.507 |
| 5 | 1 | 1.274 (1.175–1.383) | <0.001 | 1.113 (1.023–1.211) | 0.013 |
| Palpable | |||||
| Yes | 1 | 1.218 (0.751–1.975) | 0.424 | 0.932 (0.557–1.559) | 0.788 |
| No | 1 | 1.266 (1.173–1.367) | <0.001 | 1.120 (1.034–1.212) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwak, H.; Woo, S.S.; Oh, S.J.; Kim, J.Y.; Shin, H.-C.; Youn, H.J.; Chun, J.W.; Lee, D.; Kim, S.H. A Comparison of the Prognostic Effects of Fine Needle Aspiration and Core Needle Biopsy in Patients with Breast Cancer: A Nationwide Multicenter Prospective Registry. Cancers 2023, 15, 4638. https://doi.org/10.3390/cancers15184638
Gwak H, Woo SS, Oh SJ, Kim JY, Shin H-C, Youn HJ, Chun JW, Lee D, Kim SH. A Comparison of the Prognostic Effects of Fine Needle Aspiration and Core Needle Biopsy in Patients with Breast Cancer: A Nationwide Multicenter Prospective Registry. Cancers. 2023; 15(18):4638. https://doi.org/10.3390/cancers15184638
Chicago/Turabian StyleGwak, Hongki, Sang Seok Woo, Se Jeong Oh, Jee Ye Kim, Hee-Chul Shin, Hyun Jo Youn, Jung Whan Chun, Dasom Lee, and Seong Hwan Kim. 2023. "A Comparison of the Prognostic Effects of Fine Needle Aspiration and Core Needle Biopsy in Patients with Breast Cancer: A Nationwide Multicenter Prospective Registry" Cancers 15, no. 18: 4638. https://doi.org/10.3390/cancers15184638
APA StyleGwak, H., Woo, S. S., Oh, S. J., Kim, J. Y., Shin, H.-C., Youn, H. J., Chun, J. W., Lee, D., & Kim, S. H. (2023). A Comparison of the Prognostic Effects of Fine Needle Aspiration and Core Needle Biopsy in Patients with Breast Cancer: A Nationwide Multicenter Prospective Registry. Cancers, 15(18), 4638. https://doi.org/10.3390/cancers15184638






