Combination Therapy of Bland Transarterial Embolization and Microwave Ablation for Hepatocellular Carcinoma within the Milan Criteria Leads to Significantly Higher Overall Survival
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Data
2.2. TAE
2.3. MWA
2.4. Follow-Up
2.5. Definitions
2.6. Statistics
3. Results
3.1. Preablation Tumor Measurements
3.2. Complications
3.3. Complete Ablation
3.4. Oncological Response
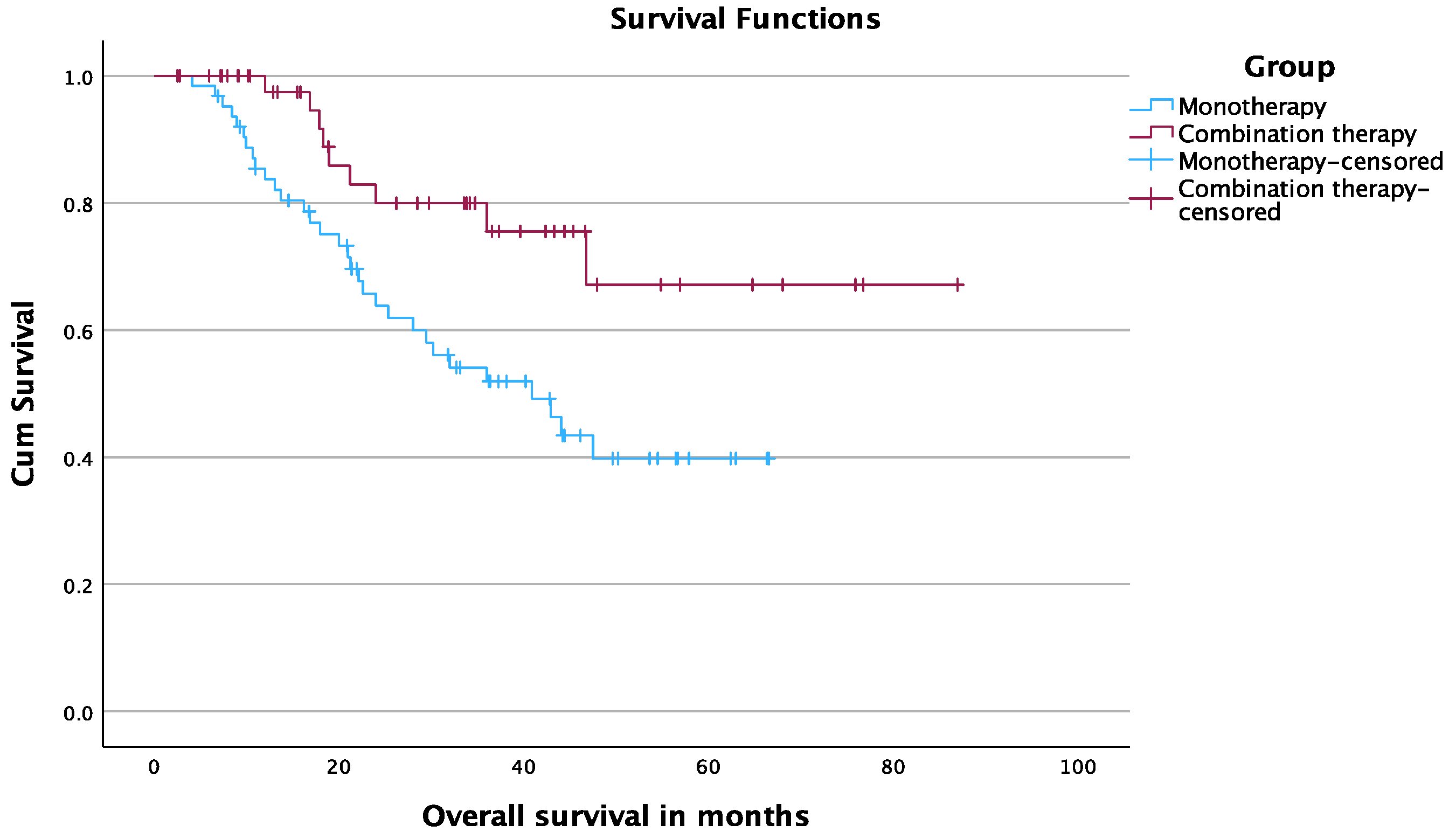
3.5. Overall Survival
3.6. Progression-Free Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Boutros, C.; Somasundar, P.; Garrean, S.; Saied, A.; Espat, N.J. Microwave coagulation therapy for hepatic tumors: Review of the literature and critical analysis. Surg. Oncol. 2010, 19, e22–e32. [Google Scholar] [CrossRef]
- Facciorusso, A.; Di Maso, M.; Muscatiello, N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int. J. Hyperth. 2016, 32, 339–344. [Google Scholar] [CrossRef]
- Bryce, K.; Tsochatzis, E.A. Downstaging for hepatocellular cancer: Harm or benefit? Transl. Gastroenterol. Hepatol. 2017, 2, 106. [Google Scholar] [CrossRef]
- Brown, K.T.; Do, R.K.; Gonen, M.; Covey, A.M.; Getrajdman, G.I.; Sofocleous, C.T.; Jarnagin, W.R.; D’Angelica, M.I.; Allen, P.G.; Erinjeri, J.P.; et al. Randomized Trial of Hepatic Artery Embolization for Hepatocellular Carcinoma Using Doxorubicin-Eluting Microspheres Compared With Embolization With Microspheres Alone. J. Clin. Oncol. 2016, 34, 2046–2053. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Bellanti, F.; Villani, R.; Salvatore, V.; Muscatiello, N.; Piscaglia, F.; Serviddio, G. Transarterial chemoembolization vs bland embolization in hepatocellular carcinoma: A meta-analysis of randomized trials. United Eur. Gastroenterol. J. 2017, 5, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.B.; Ma, L.; Wang, X.B.; Bai, T.; Ye, J.Z.; Zhong, J.H.; Li, L.Q. Transarterial embolization with or without chemotherapy for advanced hepatocellular carcinoma: A systematic review. Tumor Biol. 2014, 35, 8451–8459. [Google Scholar] [CrossRef]
- Vogl, T.J.; Gruber-Rouh, T. HCC: Transarterial Therapies-What the Interventional Radiologist Can Offer. Dig. Dis. Sci. 2019, 64, 959–967. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, J.H.; Won, H.J.; Shin, Y.M.; Yoon, H.K.; Sung, K.B.; Kim, P.N. Hepatocellular carcinomas 2-3 cm in diameter: Transarterial chemoembolization plus radiofrequency ablation vs. radiofrequency ablation alone. Eur. J. Radiol. 2012, 81, e189–e193. [Google Scholar] [CrossRef] [PubMed]
- Song, M.J.; Bae, S.H.; Lee, J.S.; Lee, S.W.; You, C.R.; Choi, J.Y.; Yoon, S.K. Combination transarterial chemoembolization and radiofrequency ablation therapy for early hepatocellular carcinoma. Korean J. Intern. Med. 2016, 31, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.W.; Zhang, Y.J.; Chen, M.S.; Xu, L.; Liang, H.H.; Lin, X.J.; Guo, R.P.; Zhang, Y.Q.; Lau, W.Y. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: A prospective randomized trial. J. Clin. Oncol. 2013, 31, 426–432. [Google Scholar] [CrossRef]
- Peng, Z.W.; Zhang, Y.J.; Liang, H.H.; Lin, X.J.; Guo, R.P.; Chen, M.S. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: A prospective randomized trial. Radiology 2012, 262, 689–700. [Google Scholar] [CrossRef]
- Zaitoun, M.M.; Elsayed, S.B.; Zaitoun, N.A.; Soliman, R.K.; Elmokadem, A.H.; Farag, A.A.; Amer, M.; Hendi, A.M.; Mahmoud, N.E.M.; Deen, D.S.E.; et al. Combined therapy with conventional trans-arterial chemoembolization (cTACE) and microwave ablation (MWA) for hepatocellular carcinoma >3–<5 cm. Int. J. Hyperth. 2021, 38, 248–256. [Google Scholar]
- Kobe, A.; Tselikas, L.; Deschamps, F.; Roux, C.; Delpla, A.; Varin, E.; Hakime, A.; Deschamps, F. Single-session transarterial chemoembolization combined with percutaneous thermal ablation in liver metastases 3 cm or larger. Diagn. Interv. Imaging 2022, 103, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Maluccio, M.; Covey, A.M.; Gandhi, R.; Gonen, M.; Getrajdman, G.I.; Brody, L.A.; Fong, Y.; William, J.; D’Angelica, M.; Blumgart, L.; et al. Comparison of survival rates after bland arterial embolization and ablation versus surgical resection for treating solitary hepatocellular carcinoma up to 7 cm. J. Vasc. Interv. Radiol. 2005, 16, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Elnekave, E.; Erinjeri, J.P.; Brown, K.T.; Thornton, R.H.; Petre, E.N.; Maybody, M.; Maluccio, M.A.; Hsu, M.; Sofocleous, C.T.; Getrajdman, G.I.; et al. Long-term outcomes comparing surgery to embolization-ablation for treatment of solitary HCC < 7 cm. Ann. Surg. Oncol. 2013, 20, 2881–2886. [Google Scholar] [PubMed]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L.; et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef]
- Langenbach, M.C.; Vogl, T.J.; von den Driesch, I.; Kaltenbach, B.; Scholtz, J.E.; Hammerstingl, R.M.; Gruber-Rouh, T. Analysis of Lipiodol uptake in angiography and computed tomography for the diagnosis of malignant versus benign hepatocellular nodules in cirrhotic liver. Eur Radiol. 2019, 29, 6539–6549. [Google Scholar] [CrossRef]
- Adwan, H.; Vogl, T.J.; Balaban, Ü.; Nour-Eldin, N.A. Percutaneous Thermal Ablation Therapy of Hepatocellular Carcinoma (HCC): Microwave Ablation (MWA) versus Laser-Induced Thermotherapy (LITT). Diagnostics 2022, 12, 564. [Google Scholar] [CrossRef] [PubMed]
- Adwan, H.; Hammann, L.; Vogl, T.J. Microwave Ablation of Recurrent Hepatocellular Carcinoma after Curative Surgical Resection. J. Clin. Med. 2023, 12, 2560. [Google Scholar] [CrossRef]
- Sacks, D.; McClenny, T.E.; Cardella, J.F.; Lewis, C.A. Society of Interventional Radiology clinical practice guidelines. J. Vasc. Interv. Radiol. 2003, 14, S199–S202. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Solbiati, L.; Brace, C.L.; Breen, D.J.; Callstrom, M.R.; Charboneau, J.W.; Chen, M.-H.; Choi, B.I.; de Baère, T.; Dodd, G.D.; et al. Image-Guided Tumor Ablation: Standardization of Terminology and Reporting Criteria—A 10-Year Update. J. Vasc. Interv. Radiol. 2014, 25, 1691–1705.e4. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar]
- Del Prete, M.; Fiore, F.; Modica, R.; Marotta, V.; Marciello, F.; Ramundo, V.; Di Sarno, A.; Carratù, A.; de Luca di Roseto, C.; Tafuto, S.; et al. Hepatic arterial embolization in patients with neuroendocrine tumors. J. Exp. Clin. Cancer Res. 2014, 33, 43. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.S.; Kim, K.H.; Seo, G.S.; Cho, E.Y.; Oh, H.J.; Choi, S.C.; Kim, T.H.; Kim, H.C.; Roh, B.S. Cerebral and pulmonary embolisms after transcatheter arterial chemoembolization for hepatocellular carcinoma. World J. Gastroenterol. 2008, 14, 4834–4837. [Google Scholar] [CrossRef]
- Wu, L.; Yang, Y.F.; Liang, J.; Shen, S.Q.; Ge, N.J.; Wu, M.C. Cerebral lipiodol embolism following transcatheter arterial chemoembolization for hepatocellular carcinoma. World J. Gastroenterol. 2010, 16, 398–402. [Google Scholar] [CrossRef]
- Chu, H.J.; Lee, C.W.; Yeh, S.J.; Tsai, L.K.; Tang, S.C.; Jeng, J.S. Cerebral Lipiodol Embolism in Hepatocellular Carcinoma Patients Treated with Transarterial Embolization/Chemoembolization. PLoS ONE 2015, 10, e0129367. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | TAE + MWA | MWA | p-Value |
|---|---|---|---|
| No. of patients | 49 | 63 | |
| Mean age | 63.3 ± 9.6 years | 65.9 ± 10.5 years | 0.136 |
| Sex | |||
| Women (%) | 12 (24.5) | 18 (28.6) | 0.788 |
| Male (%) | 37 (75.5) | 45 (71.4) | |
| Cirrhosis (%) | 43 (88%) | 58 (92%) | 0.53 |
| Child–Pugh class A (%) | 35 (81) | 40 (69) | 0.158 |
| Child–Pugh class B (%) | 8 (19) | 18 (31) | |
| No. of tumors | 55 | 67 | |
| ≤2 cm (%) | 32 (58.2) | 35 (52.2) | |
| 2–3 cm (%) | 18 (32.7) | 23 (34.3) | |
| ≥3 cm (%) | 5 (9.1) | 9 (13.4) | |
| Solitary tumor (%) | 43 (87.8) | 59 (93.7) | 0.329 |
| ≥two tumors (%) | 6 (12.2) | 4 (6.3) | |
| Location of tumor | |||
| Right lobe (%) | 35 (63.6) | 42 (62.7) | |
| Left lobe (%) | 19 (34.5) | 21 (31.3) | |
| Both lobes (%) | 1 (1.8) | 3 (4.5) | |
| Caudate lobe (%) | 0 | 1 (1.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adwan, H.; Adwan, M.; Vogl, T.J. Combination Therapy of Bland Transarterial Embolization and Microwave Ablation for Hepatocellular Carcinoma within the Milan Criteria Leads to Significantly Higher Overall Survival. Cancers 2023, 15, 5076. https://doi.org/10.3390/cancers15205076
Adwan H, Adwan M, Vogl TJ. Combination Therapy of Bland Transarterial Embolization and Microwave Ablation for Hepatocellular Carcinoma within the Milan Criteria Leads to Significantly Higher Overall Survival. Cancers. 2023; 15(20):5076. https://doi.org/10.3390/cancers15205076
Chicago/Turabian StyleAdwan, Hamzah, Moath Adwan, and Thomas J. Vogl. 2023. "Combination Therapy of Bland Transarterial Embolization and Microwave Ablation for Hepatocellular Carcinoma within the Milan Criteria Leads to Significantly Higher Overall Survival" Cancers 15, no. 20: 5076. https://doi.org/10.3390/cancers15205076
APA StyleAdwan, H., Adwan, M., & Vogl, T. J. (2023). Combination Therapy of Bland Transarterial Embolization and Microwave Ablation for Hepatocellular Carcinoma within the Milan Criteria Leads to Significantly Higher Overall Survival. Cancers, 15(20), 5076. https://doi.org/10.3390/cancers15205076









