Association between AHR Expression and Immune Dysregulation in Pancreatic Ductal Adenocarcinoma: Insights from Comprehensive Immune Profiling of Peripheral Blood Mononuclear Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
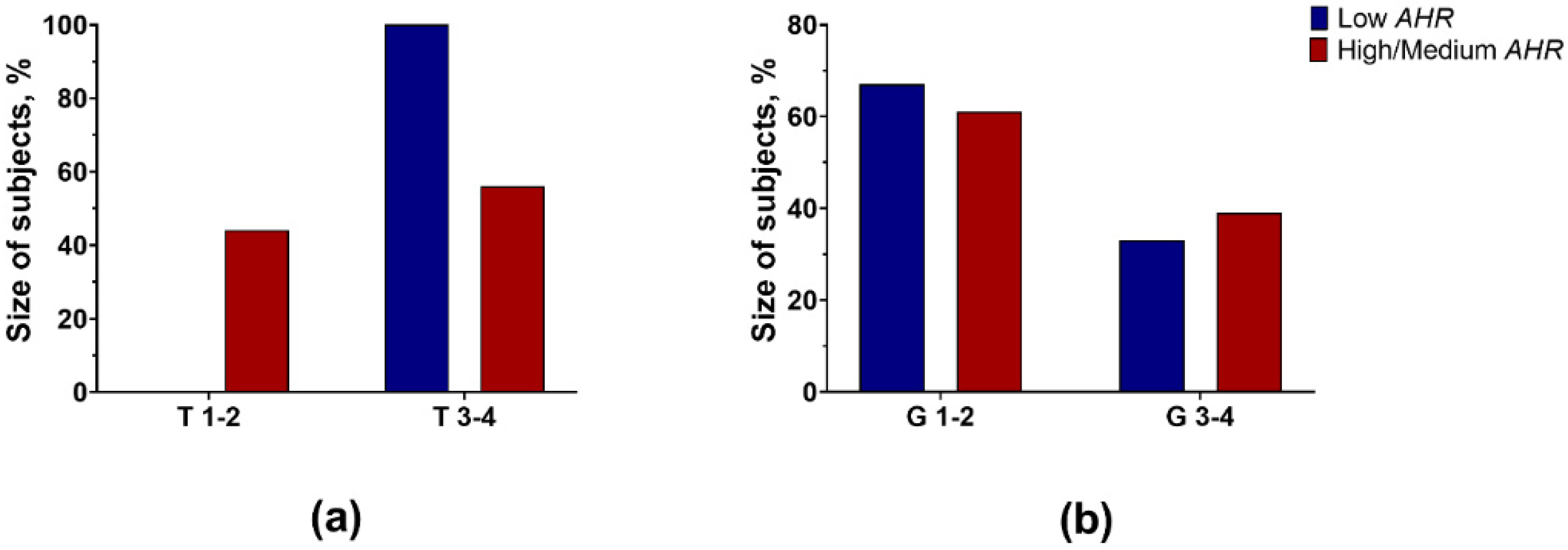
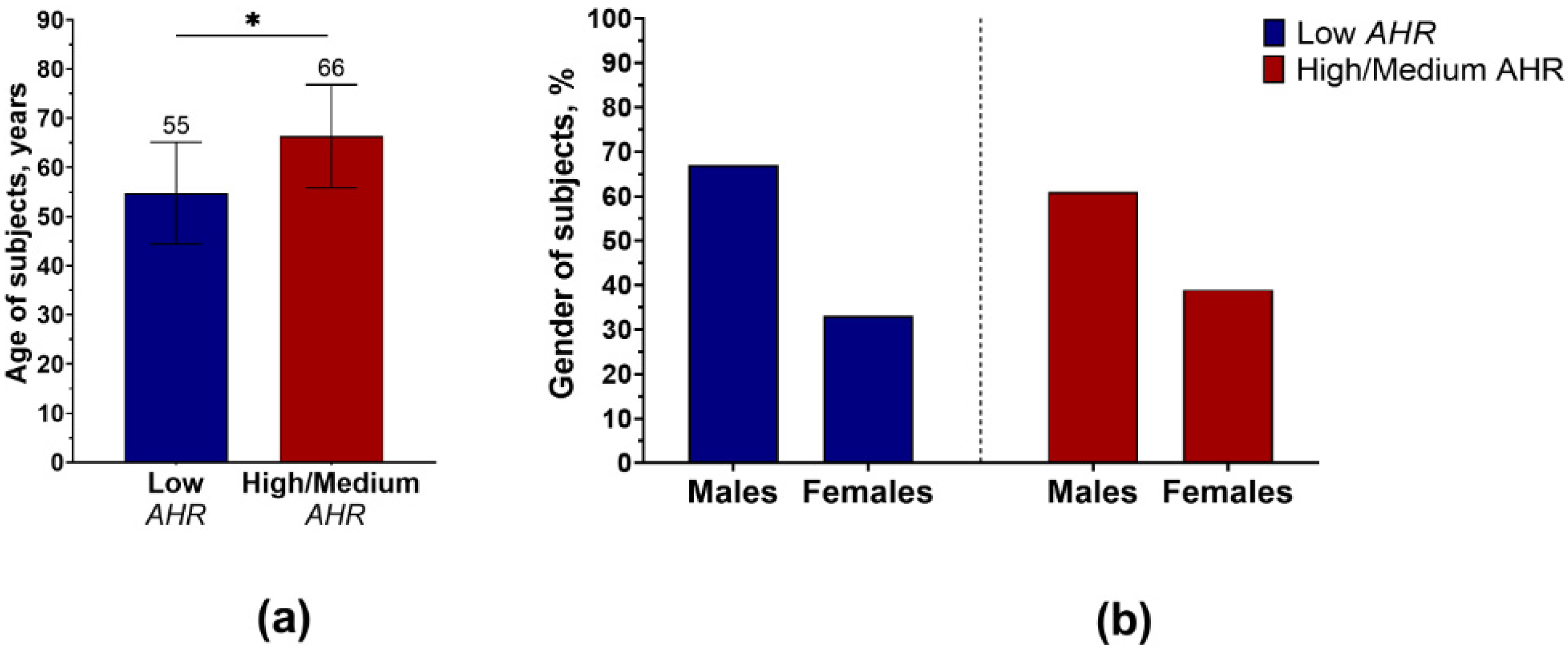
2.1. Patient Population
- Clinically, radiologically or cytologically/histologically diagnosed pancreatic cancer;
- Planned surgical treatment of cancer.
- The patient is treated at LUHS Kaunas clinics.
- Age range of 20–90 years.
- No cancer diagnosed clinically, radiologically or cytologically/histologically.
- Not currently suffering from a viral or bacterial infection, have no signs of an inflammatory disease (not have a fever, not feel pain, fatigue or weakness).
- Not taking medications to affect the immune system: anti-inflammatory drugs, pain relievers or antibiotics.
- Age range of 20–90 years.
2.2. PBMC Isolation and Cultivation
2.3. Phagocytosis
2.4. NO Production
2.5. ROS Production
2.6. RNA Extraction and Real-Time Polymerase Chain Reaction (RT-PCR)
2.7. Western Blot Analysis
2.8. Flow Cytometry (Lymphocyte Subsets, Monocyte/Macrophage Subsets)
2.9. Luminex (Concentrations of Serum Cytokines)
2.10. ELISA (Concentrations of PD1, PDL1 and KYN)
2.11. Statistical Analysis
3. Results
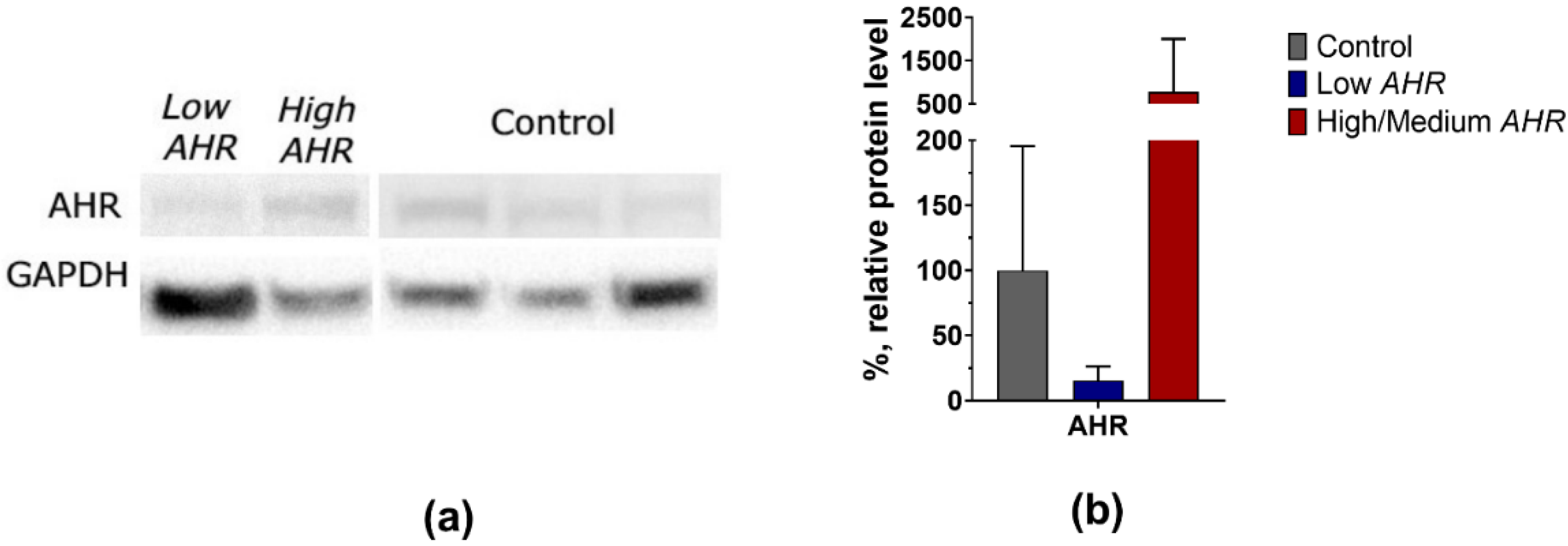
3.1. AHR Level in PBMCs of PDAC Patients
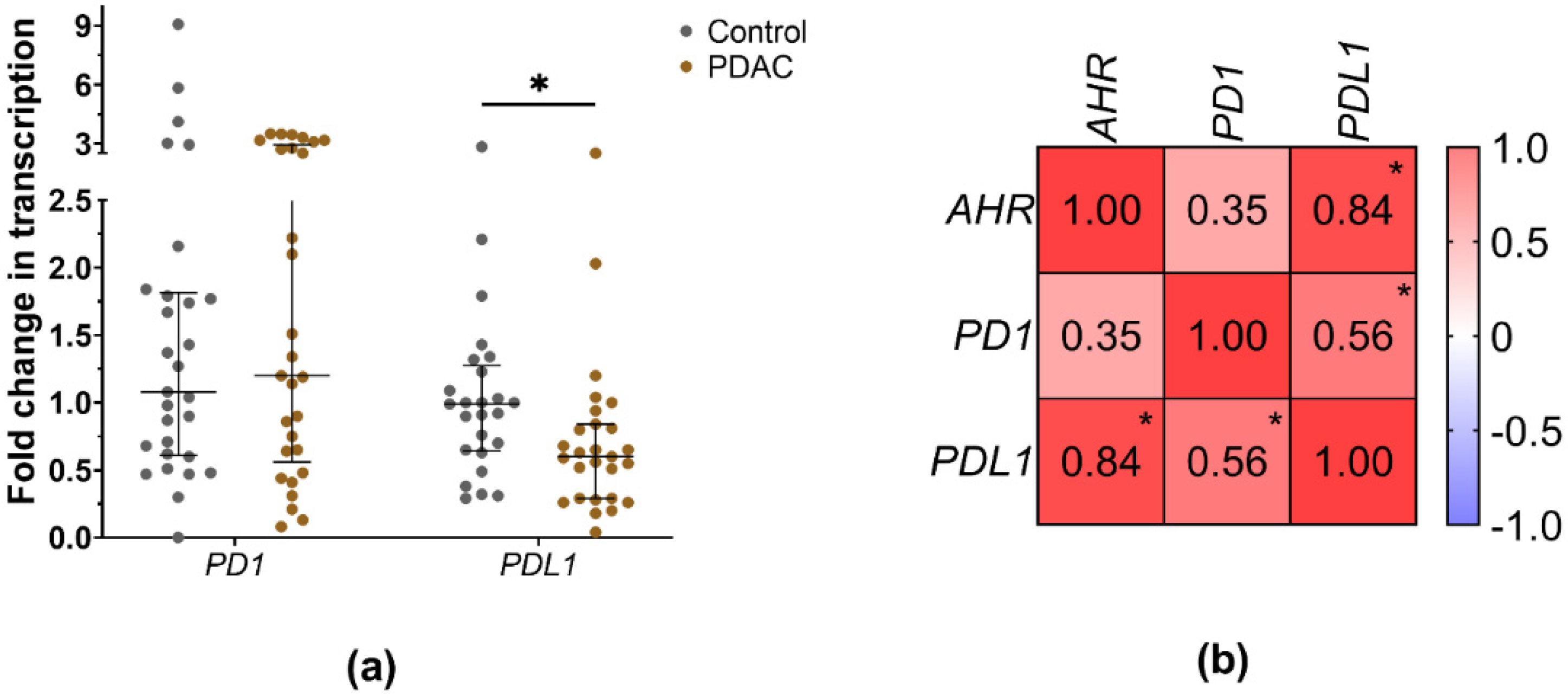
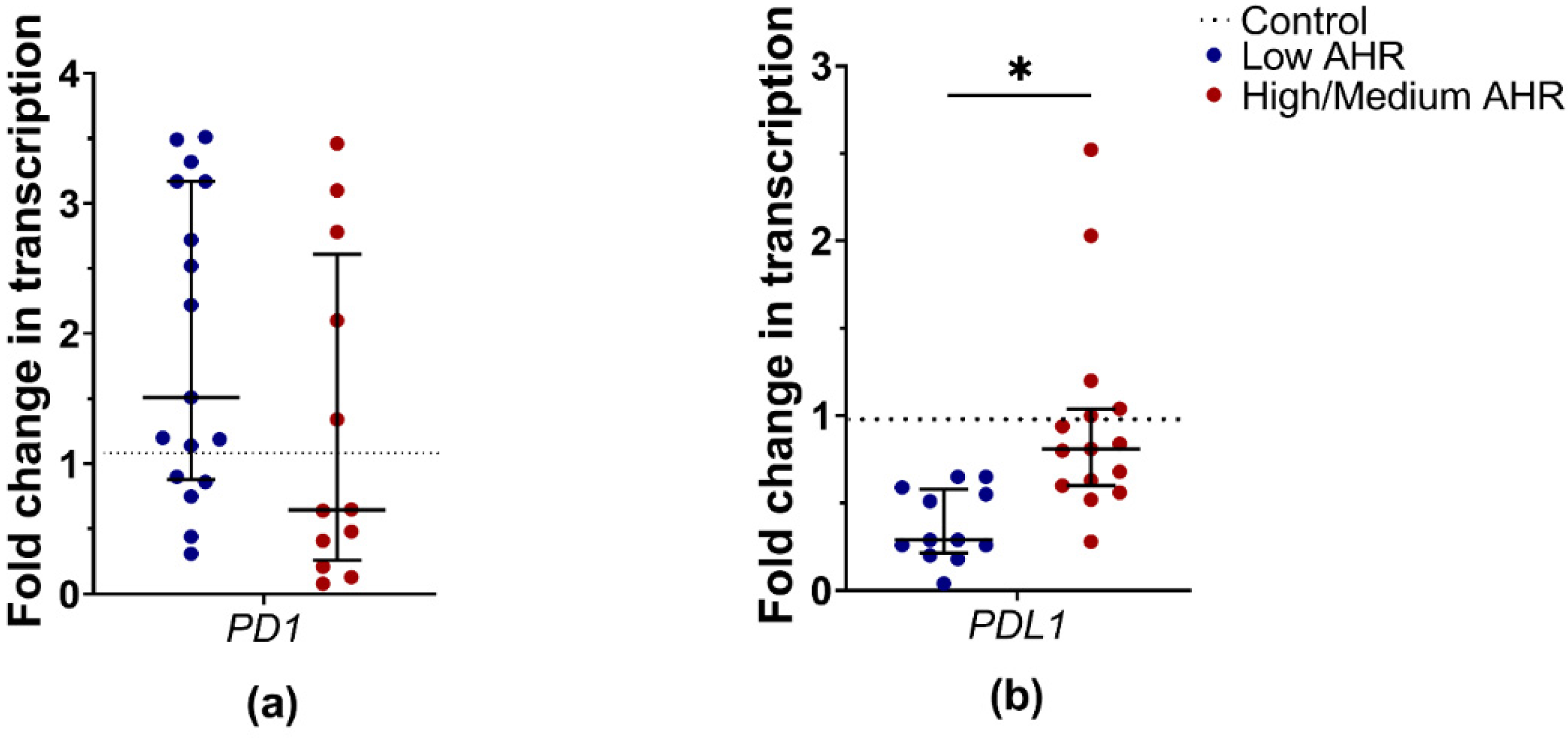
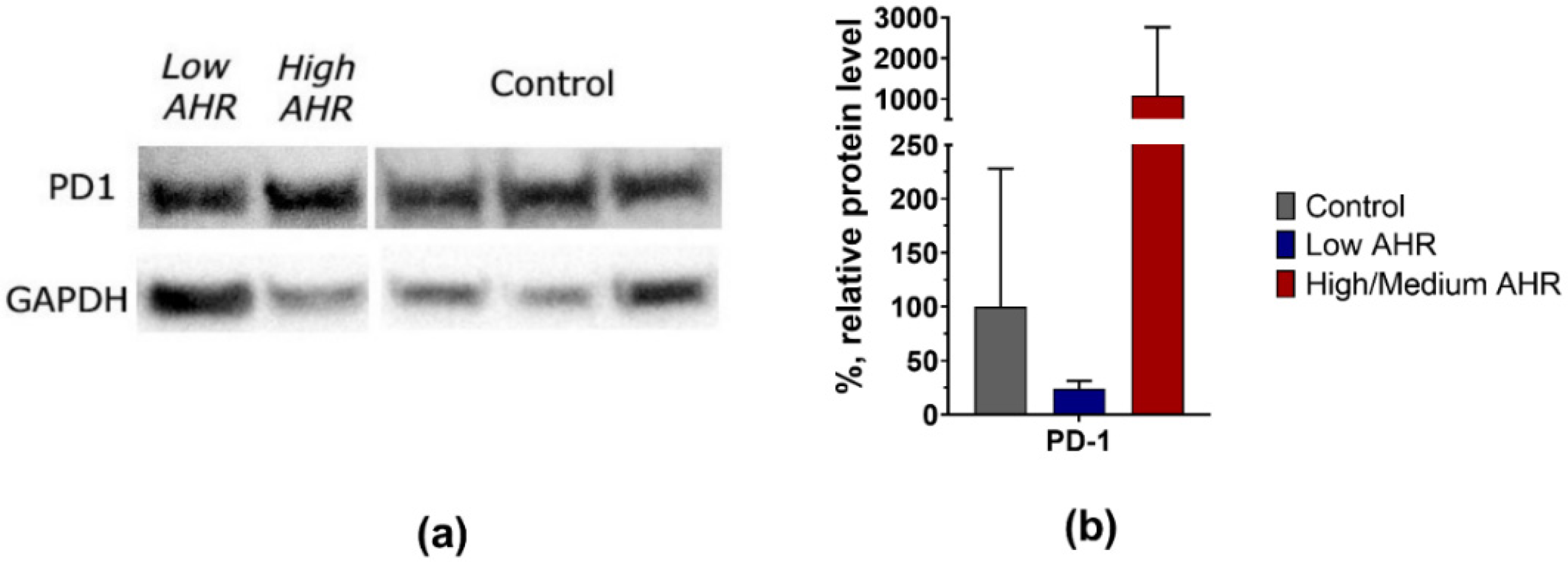
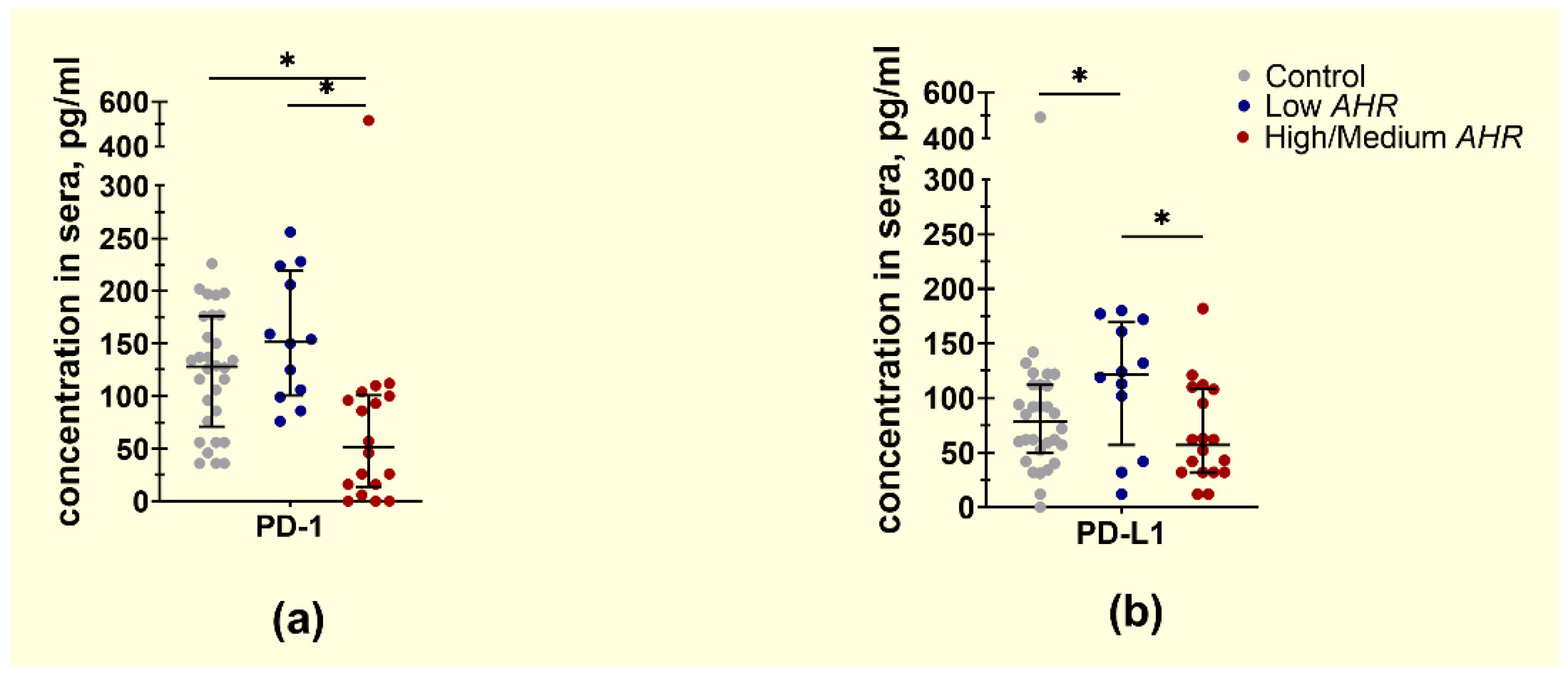
3.2. PD1 and PDL1 Levels in PDAC Patients’ Blood Depending on AHR Level
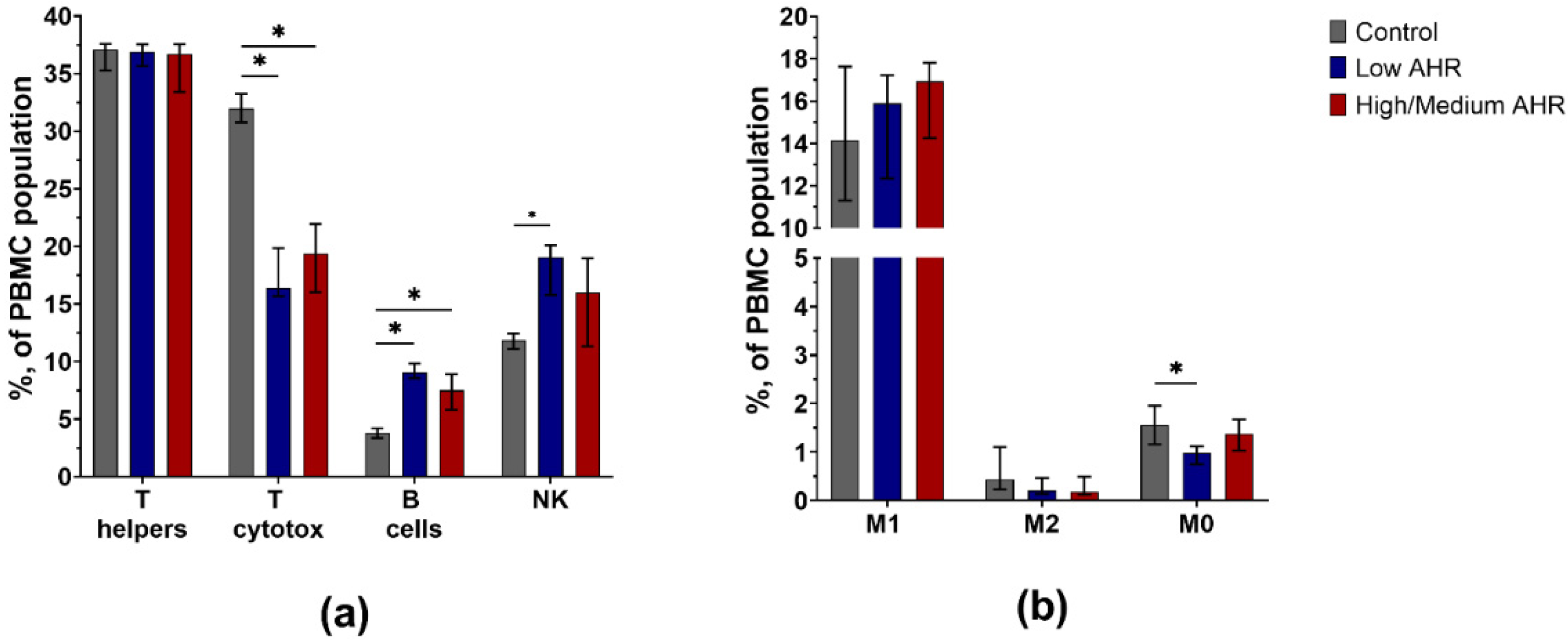
3.3. PBMC Population Composition in PDAC Patients’ Blood by AHR Level
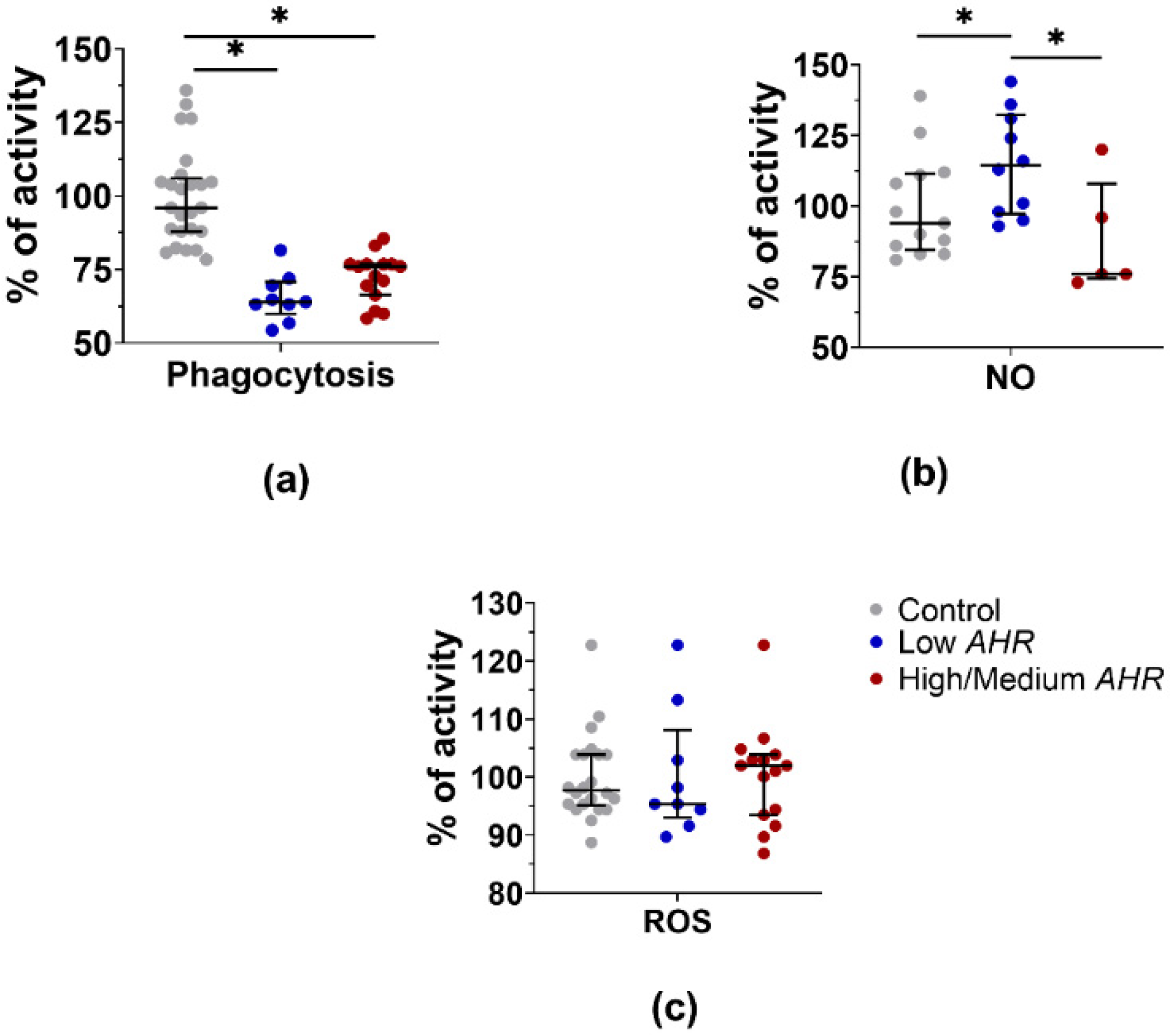
3.4. PBMC Functional Activity Dependence on AHR Level in PDAC Patients
3.5. KYN Level in PDAC Patients’ Serum Depending on AHR Level
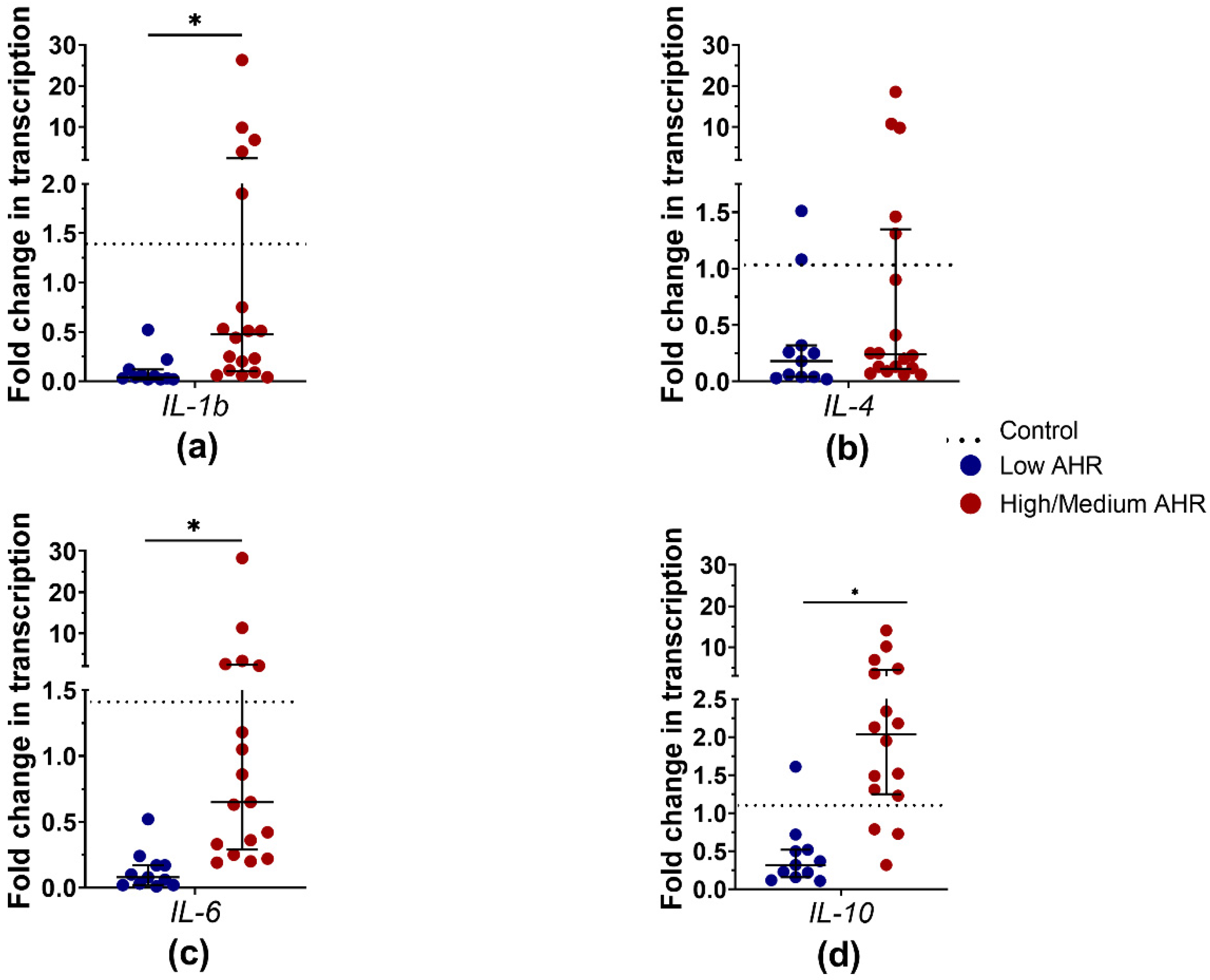
3.6. IL-1b, IL-4, IL-6 and IL-10 Differences in PDAC Patients’ Blood by AHR Level
4. Discussion
4.1. Low Expression of AHR in PDAC Patients Is Associated with Altered Immune Responses and Imbalances in Immune Checkpoint Molecules
4.2. PDAC Patients with Lower AHR Expression Exhibit Decreased PD1 and Significantly Decreased PD-L1 mRNA Expression, Indicating a Potential Dysregulation of the PD1/PD-L1 Pathway in Tumour Immunity
4.3. Low AHR Group Demonstrates Decreased Expression of T Cytotoxic Lymphocytes and M0 Type Monocytes, Suggesting a Potential Impairment in the Effector Arm of the Immune Response. B Lymphocytes and NK Cells Are More Abundant in the Low AHR Group, Indicating Potential Compensatory Mechanisms in the Immune System
4.4. Phagocytosis and ROS Production Are Decreased but NO Production Is Increased in the Low AHR Group, Reflecting Impaired Phagocytic and Oxidative Burst Activities of Immune Cells
4.5. Lack of Direct Connection between Kynurenine Level and AHR Expression in PDAC Patients’ Serum
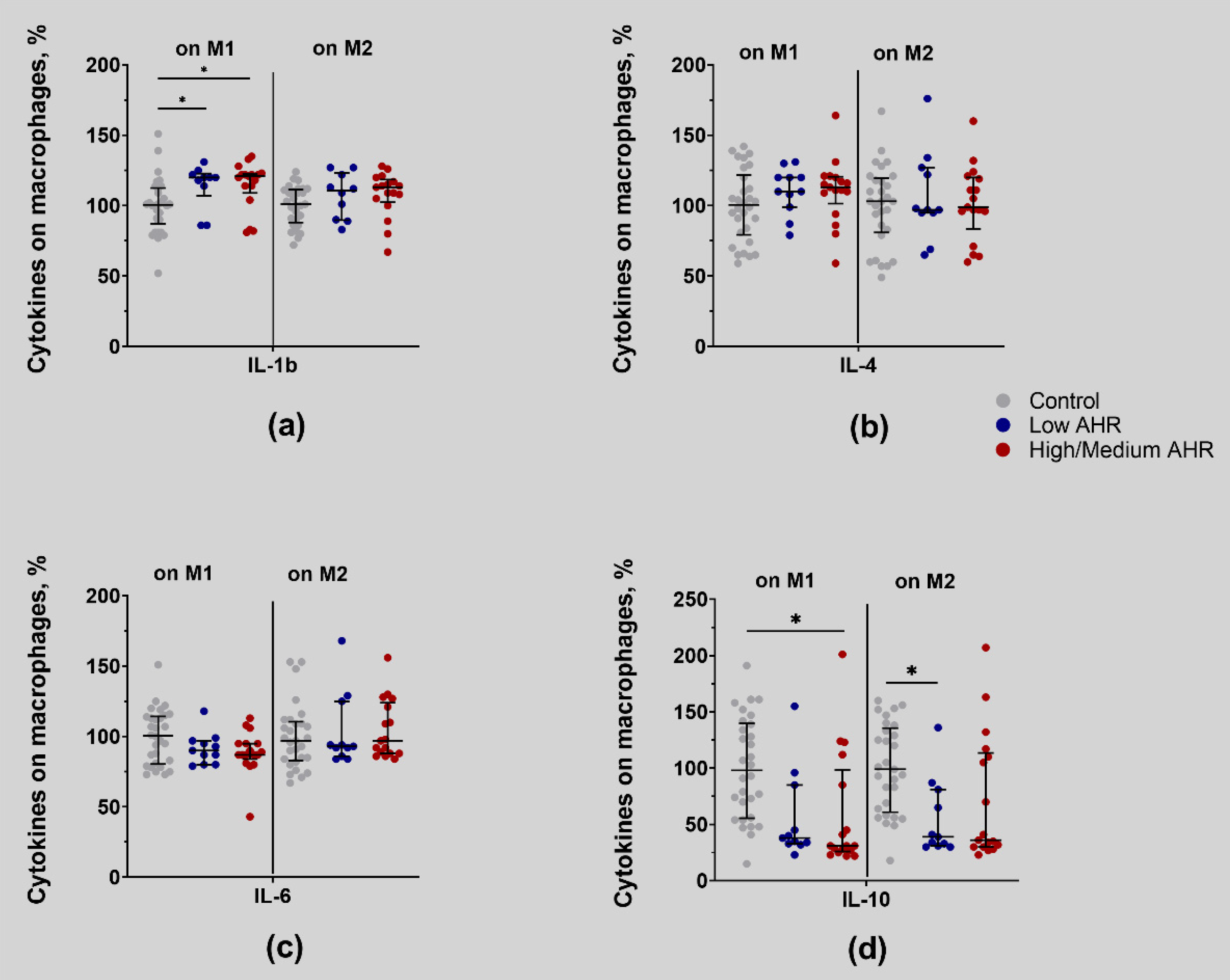
4.6. Low AHR Group Shows Significantly Reduced Expression of Pro-Inflammatory Cytokines, Such as IL-1b, IL-4, and IL-6, at the mRNA Level, Suggesting an Immunosuppressive Microenvironment. IL-4 Protein Levels Are Increased in the Serum of the Low AHR Group despite Lower IL-4 mRNA Expression, Indicating Post-Transcriptional Regulation of IL-4 Production
5. Conclusions
6. Study Limitations
7. Practical Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ushio, J.; Kanno, A.; Ikeda, E.; Ando, K.; Nagai, H.; Miwata, T.; Kawasaki, Y.; Tada, Y.; Yokoyama, K.; Numao, N.; et al. Pancreatic Ductal Adenocarcinoma: Epidemiology and Risk Factors. Diagnostics 2021, 11, 562. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-X.; Zhao, C.-F.; Chen, W.-B.; Liu, Q.-C.; Li, Q.-W.; Lin, Y.-Y.; Gao, F. Pancreatic Cancer: A Review of Epidemiology, Trend, and Risk Factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef]
- Kota, J.; Hancock, J.; Kwon, J.; Korc, M. Pancreatic Cancer: Stroma and Its Current and Emerging Targeted Therapies. Cancer Lett. 2017, 391, 38–49. [Google Scholar] [CrossRef]
- Mundry, C.S.; Eberle, K.C.; Singh, P.K.; Hollingsworth, M.A.; Mehla, K. Local and Systemic Immunosuppression in Pancreatic Cancer: Targeting the Stalwarts in Tumor’s Arsenal. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2020, 1874, 188387. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.A.; Balachandran, V.P. Scaling the Immune Incline in PDAC. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 453–454. [Google Scholar] [CrossRef]
- Castagnoli, L.; De Santis, F.; Volpari, T.; Vernieri, C.; Tagliabue, E.; Di Nicola, M.; Pupa, S.M. Cancer Stem Cells: Devil or Savior—Looking behind the Scenes of Immunotherapy Failure. Cells 2020, 9, 555. [Google Scholar] [CrossRef]
- Betsou, F.; Gaignaux, A.; Ammerlaan, W.; Norris, P.J.; Stone, M. Biospecimen Science of Blood for Peripheral Blood Mononuclear Cell (PBMC) Functional Applications. Curr. Pathobiol. Rep. 2019, 7, 17–27. [Google Scholar] [CrossRef]
- Amedei, A.; Niccolai, E.; Prisco, D. Pancreatic Cancer: Role of the Immune System in Cancer Progression and Vaccine-Based Immunotherapy. Hum. Vaccin. Immunother. 2014, 10, 3354–3368. [Google Scholar] [CrossRef]
- Janssen, J.J.E.; Lagerwaard, B.; Bunschoten, A.; Savelkoul, H.F.J.; van Neerven, R.J.J.; Keijer, J.; de Boer, V.C.J. Novel Standardized Method for Extracellular Flux Analysis of Oxidative and Glycolytic Metabolism in Peripheral Blood Mononuclear Cells. Sci. Rep. 2021, 11, 1662. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Bosch, N.; Vinaixa, J.; Navarro, P. Immune Evasion in Pancreatic Cancer: From Mechanisms to Therapy. Cancers 2018, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Melzer, M.K.; Arnold, F.; Stifter, K.; Zengerling, F.; Azoitei, N.; Seufferlein, T.; Bolenz, C.; Kleger, A. An Immunological Glance on Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2020, 21, 3345. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of Lupus-like Autoimmune Diseases by Disruption of the PD-1 Gene Encoding an ITIM Motif-Carrying Immunoreceptor. Immunity 1999, 11, 141–151. [Google Scholar] [CrossRef]
- Gordon, S.R.; Maute, R.L.; Dulken, B.W.; Hutter, G.; George, B.M.; McCracken, M.N.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 Expression by Tumour-Associated Macrophages Inhibits Phagocytosis and Tumour Immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef]
- Yin, C.; Han, Q.; Xu, D.; Zheng, B.; Zhao, X.; Zhang, J. SALL4-Mediated Upregulation of Exosomal MiR-146a-5p Drives T-Cell Exhaustion by M2 Tumor-Associated Macrophages in HCC. Oncoimmunology 2019, 8, e1601479. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as Tools and Targets in Cancer Therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Osipov, A.; Murphy, A.; Zheng, L. From Immune Checkpoints to Vaccines: The Past, Present and Future of Cancer Immunotherapy. Adv. Cancer Res. 2019, 143, 63–144. [Google Scholar]
- Ai, L.; Xu, A.; Xu, J. Roles of PD-1/PD-L1 Pathway: Signaling, Cancer, and Beyond. In Regulation of Cancer Immune Checkpoints; Advances in Experimental Medicine and Biology Book Series; Springer: Singapore, 2020; pp. 33–59. [Google Scholar]
- Wei, W.; Xu, B.; Wang, Y.; Wu, C.; Jiang, J.; Wu, C. Prognostic Significance of Circulating Soluble Programmed Death Ligand-1 in Patients with Solid Tumors. Medicine 2018, 97, e9617. [Google Scholar] [CrossRef]
- Nanda, R.; Chow, L.Q.M.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef]
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.-T.; Forero-Torres, A.; Boccia, R.; Lippman, M.E.; Somer, R.; Smakal, M.; et al. Avelumab, an Anti-PD-L1 Antibody, in Patients with Locally Advanced or Metastatic Breast Cancer: A Phase 1b JAVELIN Solid Tumor Study. Breast Cancer Res. Treat. 2018, 167, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Michl, P.; Krug, S. Overcoming Immune Evasion in Pancreatic Cancer: The Combination Matters. Gut 2018, 67, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Brincks, E.L.; Adams, J.; Wang, L.; Turner, B.; Marcinowicz, A.; Ke, J.; Essmann, M.; Mautino, L.M.; Van Allen, C.; Kumar, S.; et al. Indoximod Opposes the Immunosuppressive Effects Mediated by IDO and TDO via Modulation of AhR Function and Activation of MTORC1. Oncotarget 2020, 11, 2438–2461. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Dong, W.; Fang, Y.; Lv, J.; Zhang, T.; Fiskesund, R.; Xie, J.; Liu, J.; Yin, X.; et al. Tumor-Repopulating Cells Induce PD-1 Expression in CD8+ T Cells by Transferring Kynurenine and AhR Activation. Cancer Cell 2018, 33, 480–494.e7. [Google Scholar] [CrossRef]
- Sherr, D.H.; Monti, S. The Role of the Aryl Hydrocarbon Receptor in Normal and Malignant B Cell Development. Semin. Immunopathol. 2013, 35, 705–716. [Google Scholar] [CrossRef]
- Campesato, L.F.; Budhu, S.; Tchaicha, J.; Weng, C.-H.; Gigoux, M.; Cohen, I.J.; Redmond, D.; Mangarin, L.; Pourpe, S.; Liu, C.; et al. Blockade of the AHR Restricts a Treg-Macrophage Suppressive Axis Induced by L-Kynurenine. Nat. Commun. 2020, 11, 4011. [Google Scholar] [CrossRef]
- Yang, X.; Solomon, S.; Fraser, L.R.; Trombino, A.F.; Liu, D.; Sonenshein, G.E.; Hestermann, E.V.; Sherr, D.H. Constitutive Regulation OfCYP1B1 by the Aryl Hydrocarbon Receptor (AhR) in Pre-Malignant and Malignant Mammary Tissue. J. Cell Biochem. 2008, 104, 402–417. [Google Scholar] [CrossRef]
- Takenaka, M.C.; Gabriely, G.; Rothhammer, V.; Mascanfroni, I.D.; Wheeler, M.A.; Chao, C.-C.; Gutiérrez-Vázquez, C.; Kenison, J.; Tjon, E.C.; Barroso, A.; et al. Control of Tumor-Associated Macrophages and T Cells in Glioblastoma via AHR and CD39. Nat. Neurosci. 2019, 22, 729–740. [Google Scholar] [CrossRef]
- Hezaveh, K.; Shinde, R.S.; Klötgen, A.; Halaby, M.J.; Lamorte, S.; Ciudad, M.T.; Quevedo, R.; Neufeld, L.; Liu, Z.Q.; Jin, R.; et al. Tryptophan-Derived Microbial Metabolites Activate the Aryl Hydrocarbon Receptor in Tumor-Associated Macrophages to Suppress Anti-Tumor Immunity. Immunity 2022, 55, 324–340.e8. [Google Scholar] [CrossRef]
- Mucileanu, A.; Chira, R.; Mircea, P.A. PD-1/PD-L1 Expression in Pancreatic Cancer and Its Implication in Novel Therapies. Med. Pharm. Rep. 2021. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.-H.; Pauklin, S. Pancreatic Cancer Microenvironment and Cellular Composition: Current Understandings and Therapeutic Approaches. Cancers 2021, 13, 5028. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-Q.; Liu, P.; Mu, Z.-L.; Zhang, J.-Z. Aryl Hydrocarbon Receptor Expression in Serum, Peripheral Blood Mononuclear Cells, and Skin Lesions of Patients with Atopic Dermatitis and Its Correlation with Disease Severity. Chin. Med. J. 2020, 133, 148–153. [Google Scholar] [CrossRef]
- Zhao, R.; He, Q.; Sha, S.; Song, J.; Qin, J.; Liu, P.; Sun, Y.; Sun, L.; Hou, X.; Chen, L. Increased AHR Transcripts Correlate with Pro-Inflammatory T-Helper Lymphocytes Polarization in Both Metabolically Healthy Obesity and Type 2 Diabetic Patients. Front. Immunol. 2020, 11, 1644. [Google Scholar] [CrossRef]
- Recommended Standard Method for Isolating Mononuclear Cells. Available online: https://cdn.cytivalifesciences.com/api/public/content/digi-16156-pdf (accessed on 17 September 2023).
- Islam, M.A.; Pröll, M.; Hölker, M.; Tholen, E.; Tesfaye, D.; Looft, C.; Schellander, K.; Cinar, M.U. Alveolar Macrophage Phagocytic Activity Is Enhanced with LPS Priming, and Combined Stimulation of LPS and Lipoteichoic Acid Synergistically Induce pro-Inflammatory Cytokines in Pigs. Innate Immun. 2013, 19, 631–643. [Google Scholar] [CrossRef] [PubMed]
- pHrodoTM BioParticlesTM Conjugates for Phagocytosis and Phagocytosis Kit, for Flow Cytometry. Available online: https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets%2FLSG%2Fmanuals%2Fmp35361.pdf (accessed on 17 September 2023).
- Qiu, Y.; Yang, X.; Wang, L.; Gao, K.; Jiang, Z. L-Arginine Inhibited Inflammatory Response and Oxidative Stress Induced by Lipopolysaccharide via Arginase-1 Signaling in IPEC-J2 Cells. Int. J. Mol. Sci. 2019, 20, 1800. [Google Scholar] [CrossRef]
- Griess Reagent Kit, for Nitrite Quantitation. Available online: https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets%2FLSG%2Fmanuals%2Fmp07921.pdf (accessed on 17 September 2023).
- DCFDA/H2DCFDA—Cellular ROS Assay Kit (ab113851). Available online: https://www.abcam.com/products/assay-kits/dcfda--h2dcfda-cellular-ros-assay-kit-ab113851.html (accessed on 17 September 2023).
- Riemann, D.; Schütte, W.; Turzer, S.; Seliger, B.; Möller, M. High PD-L1/CD274 Expression of Monocytes and Blood Dendritic Cells Is a Risk Factor in Lung Cancer Patients Undergoing Treatment with PD1 Inhibitor Therapy. Cancers 2020, 12, 2966. [Google Scholar] [CrossRef]
- Kolluri, S.K.; Jin, U.-H.; Safe, S. Role of the Aryl Hydrocarbon Receptor in Carcinogenesis and Potential as an Anti-Cancer Drug Target. Arch. Toxicol. 2017, 91, 2497–2513. [Google Scholar] [CrossRef]
- Anderson, G. Tumour Microenvironment: Roles of the Aryl Hydrocarbon Receptor, O-GlcNAcylation, Acetyl-CoA and Melatonergic Pathway in Regulating Dynamic Metabolic Interactions across Cell Types—Tumour Microenvironment and Metabolism. Int. J. Mol. Sci. 2020, 22, 141. [Google Scholar] [CrossRef]
- Leclerc, D.; Staats Pires, A.C.; Guillemin, G.J.; Gilot, D. Detrimental Activation of AhR Pathway in Cancer: An Overview of Therapeutic Strategies. Curr. Opin. Immunol. 2021, 70, 15–26. [Google Scholar] [CrossRef]
- Stockinger, B.; Di Meglio, P.; Gialitakis, M.; Duarte, J.H. The Aryl Hydrocarbon Receptor: Multitasking in the Immune System. Annu. Rev. Immunol. 2014, 32, 403–432. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, S.; Hassanzadeh Nemati, A.; Fazli, H.R.; Beygi, S.; Moradzadeh, M.; Pourshams, A.; Mohamadkhani, A. An Increased Level of Aryl Hydrocarbon Receptor in Patients with Pancreatic Cancer. Middle East. J. Dig. Dis. 2018, 11, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cai, A.; Zheng, H.; Huang, H.; Sun, R.; Cui, X.; Ye, W.; Yao, Q.; Chen, R.; Kou, L. Carbidopa Suppresses Prostate Cancer via Aryl Hydrocarbon Receptor-Mediated Ubiquitination and Degradation of Androgen Receptor. Oncogenesis 2020, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Paris, A.; Tardif, N.; Galibert, M.-D.; Corre, S. AhR and Cancer: From Gene Profiling to Targeted Therapy. Int. J. Mol. Sci. 2021, 22, 752. [Google Scholar] [CrossRef]
- Díaz-Díaz, C.J.; Ronnekleiv-Kelly, S.M.; Nukaya, M.; Geiger, P.G.; Balbo, S.; Dator, R.; Megna, B.W.; Carney, P.R.; Bradfield, C.A.; Kennedy, G.D. The Aryl Hydrocarbon Receptor Is a Repressor of Inflammation-Associated Colorectal Tumorigenesis in Mouse. Ann. Surg. 2016, 264, 429–436. [Google Scholar] [CrossRef]
- Wang, G.-Z.; Zhang, L.; Zhao, X.-C.; Gao, S.-H.; Qu, L.-W.; Yu, H.; Fang, W.-F.; Zhou, Y.-C.; Liang, F.; Zhang, C.; et al. The Aryl Hydrocarbon Receptor Mediates Tobacco-Induced PD-L1 Expression and Is Associated with Response to Immunotherapy. Nat. Commun. 2019, 10, 1125. [Google Scholar] [CrossRef]
- Schenk, A.; Joisten, N.; Walzik, D.; Koliamitra, C.; Schoser, D.; Bloch, W.; Zimmer, P. Acute Exercise Impacts AhR and PD-1 Levels of CD8+ T-Cells—Exploratory Results from a Randomized Cross-over Trial Comparing Endurance versus Resistance Exercise. Eur. J. Appl. Physiol. 2021, 121, 637–644. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Hansen, F.J.; David, P.; Akram, M.; Knoedler, S.; Mittelstädt, A.; Merkel, S.; Podolska, M.J.; Swierzy, I.; Roßdeutsch, L.; Klösch, B.; et al. Circulating Monocytes Serve as Novel Prognostic Biomarker in Pancreatic Ductal Adenocarcinoma Patients. Cancers 2023, 15, 363. [Google Scholar] [CrossRef]
- Peng, Q.; Qiu, X.; Zhang, Z.; Zhang, S.; Zhang, Y.; Liang, Y.; Guo, J.; Peng, H.; Chen, M.; Fu, Y.-X.; et al. PD-L1 on Dendritic Cells Attenuates T Cell Activation and Regulates Response to Immune Checkpoint Blockade. Nat. Commun. 2020, 11, 4835. [Google Scholar] [CrossRef]
- Cho, I.; Lee, H.; Yoon, S.E.; Ryu, K.J.; Ko, Y.H.; Kim, W.S.; Kim, S.J. Serum Levels of Soluble Programmed Death-Ligand 1 (SPD-L1) in Patients with Primary Central Nervous System Diffuse Large B-Cell Lymphoma. BMC Cancer 2020, 20, 120. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Chen, H.; Wang, W.; Chen, X.; Geng, Q.; Xia, Z.; Lu, Y. Serum Levels of Soluble Programmed Death Ligand 1 Predict Treatment Response and Progression Free Survival in Multiple Myeloma. Oncotarget 2015, 6, 41228–41236. [Google Scholar] [CrossRef] [PubMed]
- Frigola, X.; Inman, B.A.; Lohse, C.M.; Krco, C.J.; Cheville, J.C.; Thompson, R.H.; Leibovich, B.; Blute, M.L.; Dong, H.; Kwon, E.D. Identification of a Soluble Form of B7-H1 That Retains Immunosuppressive Activity and Is Associated with Aggressive Renal Cell Carcinoma. Clin. Cancer Res. 2011, 17, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.-L.; Liu, L.; Qi, Z.-H.; Xu, H.-X.; Wang, W.-Q.; Wu, C.-T.; Zhang, S.-R.; Xu, J.-Z.; Ni, Q.-X.; Yu, X.-J. The Clinicopathological and Prognostic Significance of PD-L1 Expression in Pancreatic Cancer: A Meta-Analysis. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L. Does Vaccine-Primed Pancreatic Cancer Offer Better Candidates for Immune-Based Therapies? Immunotherapy 2014, 6, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Soares, K.C.; Rucki, A.A.; Wu, A.A.; Olino, K.; Xiao, Q.; Chai, Y.; Wamwea, A.; Bigelow, E.; Lutz, E.; Liu, L.; et al. PD-1/PD-L1 Blockade Together with Vaccine Therapy Facilitates Effector T-Cell Infiltration Into Pancreatic Tumors. J. Immunother. 2015, 38, 1–11. [Google Scholar] [CrossRef]
- Lu, C.; Paschall, A.V.; Shi, H.; Savage, N.; Waller, J.L.; Sabbatini, M.E.; Oberlies, N.H.; Pearce, C.; Liu, K. The MLL1-H3K4me3 Axis-Mediated PD-L1 Expression and Pancreatic Cancer Immune Evasion. J. Natl. Cancer Inst. 2017, 109, djw283. [Google Scholar] [CrossRef]
- Zhou, Q.; Tao, X.; Xia, S.; Guo, F.; Pan, C.; Xiang, H.; Shang, D. T Lymphocytes: A Promising Immunotherapeutic Target for Pancreatitis and Pancreatic Cancer? Front. Oncol. 2020, 10, 382. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Fu, T.; Jiang, Y.-Z.; Shao, Z.-M. Natural Killer Cells in Cancer Biology and Therapy. Mol. Cancer 2020, 19, 120. [Google Scholar] [CrossRef]
- Tan, R.; Nie, M.; Long, W. The Role of B Cells in Cancer Development. Front. Oncol. 2022, 12, 958756. [Google Scholar] [CrossRef]
- Wang, H.-C.; Hung, W.-C.; Chen, L.-T.; Pan, M.-R. From Friend to Enemy: Dissecting the Functional Alteration of Immunoregulatory Components during Pancreatic Tumorigenesis. Int. J. Mol. Sci. 2018, 19, 3584. [Google Scholar] [CrossRef]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The Tumour Microenvironment in Pancreatic Cancer—Clinical Challenges and Opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Xi, Q.; Zhang, J.; Yang, G.; Zhang, L.; Chen, Y.; Wang, C.; Zhang, Z.; Guo, X.; Zhao, J.; Xue, Z.; et al. Restoration of MiR-340 Controls Pancreatic Cancer Cell CD47 Expression to Promote Macrophage Phagocytosis and Enhance Antitumor Immunity. J. Immunother. Cancer 2020, 8, e000253. [Google Scholar] [CrossRef] [PubMed]
- Holl, E.K.; Frazier, V.N.; Landa, K.; Beasley, G.M.; Hwang, E.S.; Nair, S.K. Examining Peripheral and Tumor Cellular Immunome in Patients With Cancer. Front. Immunol. 2019, 10, 1767. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.M.; Hwang, S.; Seong, R.H. Transferrin Receptor Regulates Pancreatic Cancer Growth by Modulating Mitochondrial Respiration and ROS Generation. Biochem. Biophys. Res. Commun. 2016, 471, 373–379. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.M.S.; Cordon, B.; Ma, B.; Xie, J. Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications. Adv. Sci. 2023; early review. [Google Scholar] [CrossRef]
- Lindau, D.; Gielen, P.; Kroesen, M.; Wesseling, P.; Adema, G.J. The Immunosuppressive Tumour Network: Myeloid-Derived Suppressor Cells, Regulatory T Cells and Natural Killer T Cells. Immunology 2013, 138, 105–115. [Google Scholar] [CrossRef]
- Muller, A.J.; Mondal, A.; Dey, S.; Prendergast, G.C. IDO1 and Inflammatory Neovascularization: Bringing New Blood to Tumor-Promoting Inflammation. Front. Oncol. 2023, 13, 1125298. [Google Scholar] [CrossRef]
- Drehmer, D.; Mesquita Luiz, J.P.; Hernandez, C.A.S.; Alves-Filho, J.C.; Hussell, T.; Townsend, P.A.; Moncada, S. Nitric Oxide Favours Tumour-Promoting Inflammation through Mitochondria-Dependent and -Independent Actions on Macrophages. Redox Biol. 2022, 54, 102350. [Google Scholar] [CrossRef]
- Biswas, P.; Stuehr, D.J. Indoleamine Dioxygenase and Tryptophan Dioxygenase Activities Are Regulated through Control of Cell Heme Allocation by Nitric Oxide. J. Biol. Chem. 2023, 299, 104753. [Google Scholar] [CrossRef]
- Anu, R.I.; Shiu, K.-K.; Khan, K.H. The Immunomodulatory Role of IDO1-Kynurenine-NAD+ Pathway in Switching Cold Tumor Microenvironment in PDAC. Front. Oncol. 2023, 13, 1142838. [Google Scholar] [CrossRef]
- Mor, A.; Tankiewicz-Kwedlo, A.; Pawlak, D. Kynurenines as a Novel Target for the Treatment of Malignancies. Pharmaceuticals 2021, 14, 606. [Google Scholar] [CrossRef] [PubMed]
- Meireson, A.; Devos, M.; Brochez, L. IDO Expression in Cancer: Different Compartment, Different Functionality? Front. Immunol. 2020, 11, 531491. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Song, X.; Traub, B.; Luxenhofer, M.; Kornmann, M. Involvement of IL-4, IL-13 and Their Receptors in Pancreatic Cancer. Int. J. Mol. Sci. 2021, 22, 2998. [Google Scholar] [CrossRef] [PubMed]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta—A Friend or Foe in Malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.N.; Dotan, S.; Elkabets, M.; White, M.R.; Reich, E.; Carmi, Y.; Song, X.; Dvozkin, T.; Krelin, Y.; Voronov, E. The Involvement of IL-1 in Tumorigenesis, Tumor Invasiveness, Metastasis and Tumor-Host Interactions. Cancer Metastasis Rev. 2006, 25, 387–408. [Google Scholar] [CrossRef]
- Edderkaoui, M.; Xu, S.; Chheda, C.; Morvaridi, S.; Hu, R.W.; Grippo, P.J.; Mascariñas, E.; Principe, D.R.; Knudsen, B.; Xue, J.; et al. HDAC3 Mediates Smoking-Induced Pancreatic Cancer. Oncotarget 2016, 7, 7747–7760. [Google Scholar] [CrossRef]
- Farren, M.R.; Mace, T.A.; Geyer, S.; Mikhail, S.; Wu, C.; Ciombor, K.; Tahiri, S.; Ahn, D.; Noonan, A.M.; Villalona-Calero, M.; et al. Systemic Immune Activity Predicts Overall Survival in Treatment-Naïve Patients with Metastatic Pancreatic Cancer. Clin. Cancer Res. 2016, 22, 2565–2574. [Google Scholar] [CrossRef]
- Holmer, R.; Goumas, F.A.; Waetzig, G.H.; Rose-John, S.; Kalthoff, H. Interleukin-6: A Villain in the Drama of Pancreatic Cancer Development and Progression. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 371–380. [Google Scholar] [CrossRef]
- Feng, L.; Qi, Q.; Wang, P.; Chen, H.; Chen, Z.; Meng, Z.; Liu, L. Serum Levels of IL-6, IL-8, and IL-10 Are Indicators of Prognosis in Pancreatic Cancer. J. Int. Med. Res. 2018, 46, 5228–5236. [Google Scholar] [CrossRef]
- Li, H.; Zhai, N.; Wang, Z.; Song, H.; Yang, Y.; Cui, A.; Li, T.; Wang, G.; Niu, J.; Crispe, I.N.; et al. Regulatory NK Cells Mediated between Immunosuppressive Monocytes and Dysfunctional T Cells in Chronic HBV Infection. Gut 2018, 67, 2035–2044. [Google Scholar] [CrossRef]
- Sabat, R.; Grütz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginat, J. Biology of Interleukin-10. Cytokine Growth Factor Rev. 2010, 21, 331–344. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartkeviciene, A.; Jasukaitiene, A.; Zievyte, I.; Stukas, D.; Ivanauskiene, S.; Urboniene, D.; Maimets, T.; Jaudzems, K.; Vitkauskiene, A.; Matthews, J.; et al. Association between AHR Expression and Immune Dysregulation in Pancreatic Ductal Adenocarcinoma: Insights from Comprehensive Immune Profiling of Peripheral Blood Mononuclear Cells. Cancers 2023, 15, 4639. https://doi.org/10.3390/cancers15184639
Bartkeviciene A, Jasukaitiene A, Zievyte I, Stukas D, Ivanauskiene S, Urboniene D, Maimets T, Jaudzems K, Vitkauskiene A, Matthews J, et al. Association between AHR Expression and Immune Dysregulation in Pancreatic Ductal Adenocarcinoma: Insights from Comprehensive Immune Profiling of Peripheral Blood Mononuclear Cells. Cancers. 2023; 15(18):4639. https://doi.org/10.3390/cancers15184639
Chicago/Turabian StyleBartkeviciene, Arenida, Aldona Jasukaitiene, Inga Zievyte, Darius Stukas, Sandra Ivanauskiene, Daiva Urboniene, Toivo Maimets, Kristaps Jaudzems, Astra Vitkauskiene, Jason Matthews, and et al. 2023. "Association between AHR Expression and Immune Dysregulation in Pancreatic Ductal Adenocarcinoma: Insights from Comprehensive Immune Profiling of Peripheral Blood Mononuclear Cells" Cancers 15, no. 18: 4639. https://doi.org/10.3390/cancers15184639
APA StyleBartkeviciene, A., Jasukaitiene, A., Zievyte, I., Stukas, D., Ivanauskiene, S., Urboniene, D., Maimets, T., Jaudzems, K., Vitkauskiene, A., Matthews, J., Dambrauskas, Z., & Gulbinas, A. (2023). Association between AHR Expression and Immune Dysregulation in Pancreatic Ductal Adenocarcinoma: Insights from Comprehensive Immune Profiling of Peripheral Blood Mononuclear Cells. Cancers, 15(18), 4639. https://doi.org/10.3390/cancers15184639







