Simple Summary
Soft-tissue sarcoma patients need regular check-ups after surgery to detect disease recurrence as early as possible. However, the current guidelines for follow-up are not based on strong evidence and do not consider individual patient or tumor characteristics. This has led to debates, especially due to concerns about cost, radiation frequency, and possible over-testing. The goal of this study was to see how often patients received the recommended follow-up visits and to identify which type of patients received more or fewer visits than advised. The results show that only 24% of patients received the advised three follow-up visits in the first year after surgery. More follow-up visits were observed in younger patients and those diagnosed with a high-grade tumor, suggesting that doctors incorporate the patient’s risk of recurrence in their decision on follow-up frequency. The results of this study can help to improve follow-up practices, taking the risk of disease recurrence into account while avoiding unnecessary costs and tests.
Abstract
Introduction: Follow-up (FU) in soft-tissue sarcoma (STS) patients is designed for early detection of disease recurrence. Current guidelines are not evidenced-based and not tailored to patient or tumor characteristics, so they remain debated, particularly given concerns about cost, radiation frequency, and over-testing. This study assesses the extent to which STS patients received guideline-concordant FU and to characterize which type of patients received more or fewer visits than advised. Methods: All STS patients surgically treated at the Leiden University Medical Center between 2000–2020 were included. For each patient, along with individual characteristics, all radiological examinations from FU start up to 5 years were included and compared to guidelines. Recurrence was defined as local/regional recurrence or metastasis. Results: A total of 394 patients was included, of whom 250 patients had a high-grade tumor (63.5%). Only 24% of patients received the advised three FU visits in the first year. More FU visits were observed in younger patients and those diagnosed with a high-grade tumor. Among patients with a recurrence, 10% received fewer visits than advised, while 28% of patients without a recurrence received more visits than advised. Conclusions: A minority of STS patients received guideline-concordant FU visits, suggesting that clinicians seem to incorporate recurrence risk in decisions on FU frequency.
1. Introduction
Soft-tissue sarcomas (STSs) are rare tumors, accounting for <1% of all tumors [1]. STSs are extremely heterogenous tumors with over 100 different histological subtypes and can affect people of all ages at any anatomical site [2,3]. Surgical excision is the standard treatment with or without adjuvant radiotherapy for local disease control [4]. Relapse following primary treatment occurs frequently, with 40–50% of STS patients developing either local or distant disease recurrence [5]. However, these percentages substantially differ per tumor grade, size, and subtype [6] and are highest in the first years after treatment [5,7,8]. Therefore, routine follow-up is designed to detect disease recurrence as early as possible because early treatment improves prognosis [4].
Long-term follow-up strategies have hardly been investigated in STS patients, and current guidelines are mostly based on expert opinions rather than on high-quality evidence [4]. Thus, the frequency and timing of follow-up and appropriate screening modalities continue to be the topic of debate, particularly as they are not tailored to the patient or tumor characteristics. Additionally, there are concerns about cost, radiation frequency, and possible over-testing [4,9,10]. Current ESMO-EURACAN (European Society for Medical Oncology- European Reference Network for rare adult solid cancers) and NCCN (National Comprehensive Cancer Network) consensus guidelines recommend follow-up visits every 3 to 6 months in the first three years, then twice a year up to the fifth year and annually thereafter, with NCCN guidelines distinguishing between low- and high-grade STS (Table 1) [4,11]. Survey data among musculoskeletal oncologists in the UK and orthopedic surgeons in the USA suggest high variation in current clinical practice regarding the number of follow-up visits [12,13]. However, it is unknown to what extent STS patients receive follow-up visits as recommended in guidelines and what type of patients receive more or fewer visits than advised, which may provide directions to improve care and limit overuse.

Table 1.
Frequency of follow-up visits per guideline.
Therefore, this study aimed to assess the extent to which STS patients after curative surgical treatment received guideline-concordant follow-up visits and to characterize which type of patients received more or fewer visits than advised. We distinguished between patients with and without an recurrence to identify possibilities for improvement of care, as fewer visits than advised among patients with a recurrence could point to possibilities to improve decisions on follow-up frequency for specific patient groups, and more visits than advised among patients without a recurrence could point to possible overuse.
2. Methods
2.1. Study Design and Patient Population
In this retrospective cohort study, all STS patients surgically treated at the Leiden University Medical Center (LUMC) between 1 January 2000 and 1 January 2020 were included. The LUMC is one of the largest tertiary referral center for patients with sarcoma in the Netherlands. Patients with a sarcoma in the abdomen or thorax or on the face were excluded because a different follow-up schedule applies to these patients. The study protocol was presented to the Medical Ethics Committee of the Leiden University Medical Center, which waived the need for ethical approval under the Dutch law (W22.013).
2.2. Definitions
Start of follow-up was defined as the last day of treatment when curative status was achieved, which was either the last day of post-operative radiotherapy (RT) or date of surgery, with all patients followed up for 5 years thereafter. This time period has been indicated because it is during the first 2–3 years that recurrences are most likely to occur [4]. If patients were treated surgically more than once, the last operation before reaching complete remission was used. The local guideline for follow-up was similar to ESMO and NCCN guidelines, namely three visits per year (every 4 months) in the first three years and two visits per year (every 6 months) in the fourth and fifth year (Table 1). A follow-up visit was defined as having a radiological examination (i.e., either a PET-CT, MRI, or chest X-ray) so that all radiological examinations in the follow-up for each patient were included. The radiological examinations that occurred within two weeks of surgery were considered postoperative imaging rather than a follow-up moment and were therefore excluded from the dataset. As more than one radiological examination can be performed during one follow-up moment, the dates can differ for each radiological examination, as it is logistically challenging to schedule these on one day. Therefore, if the difference between two radiological examinations was less than 21 days, it was considered one follow-up moment.
A recurrence was defined as a local recurrence (LR), regional recurrence, or distant metastasis (DM). The time to recurrence was defined as the time from follow-up start to the first recurrence. The time at risk was defined as the time from follow-up start until (1) the first recurrence or (2) death or (3) 1 January 2020 or (4) the end of the 5-year follow-up period. Every death (not only as a direct result of disease) was included.
2.3. Patient, Tumor and Treatment Characteristics
Routinely available patient, tumor, and treatment characteristics from our local cancer registry were used: age, sex, histological subtype, tumor grade, and tumor size. In addition, we retrospectively collected surgical margins (R0/R1–2) and treatment with (neo)adjuvant radiotherapy (RT) (yes/no) from the medical records using a pre-specified data form.
2.4. Statistical Analysis
Baseline characteristics were described using frequencies and percentages for categorical variables and mean (standard deviation) or median (interquartile range) for continuous variables, depending on the distribution. To indicate trends in follow-up over time, follow-up patterns were described for patients operated in three periods (2000–2005, 2006–2010, and 2011–2015) by time at risk and the extent to which each follow-up visit took place in the time window as advised in the guideline. Not all patients diagnosed between 2016–2020 completed the five-year follow-up and were therefore not visualized.
To characterize the type of patients receiving more or fewer follow-up visits, we compared all patients diagnosed from 2000 until 2020 receiving more visits with those receiving fewer visits than advised in the guideline on the abovementioned patient (age, sex), tumor (grade and size), and treatment characteristics (surgical margins, RT). This was carried out by year of follow-up using an independent sample t-test in case of continuous variables and a chi-square test (or Fisher’s exact test) in case of categorical variables. These analyses were repeated separately for patients with and without a recurrence.
All analyses were conducted using R software, version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria) [14]. A p-value < 0.05 was considered statistically significant in all analyses.
3. Results
3.1. Patient Characteristics, Follow-Up Visits, and Incidence of Recurrence
In total, 394 STS patients were included (Table 2). The median age was 60 years (IQR 46–71), and 51% was male. This group included 38 different diagnoses, including myxofibrosarcoma, liposarcoma, and leiomyosarcoma (combined 49% of the total patient population). The majority of patients had either a sarcoma in the lower extremities (69%), upper extremity (16%), or in the pelvis (8%), with a median tumor size of 7 cm (5–13). Overall, 250 patients had a high-grade tumor (63.5%) and 138 patients a low-grade tumor (35%), and for the remaining 6 patients (1.5%), the grade status was unknown. In most patients (n = 252), the tumor was removed with free surgical margins (R0, 63%). Fifty patients (13%) had more than one surgery before reaching complete remission. About one-quarter of patients (n = 102) were treated with radiotherapy, of whom 33 (32%) were treated pre-operatively and 69 (68%) post-operatively.

Table 2.
Patient characteristics and clinical-, pathological-, and treatment-related parameters (Leiden University Medical Center, N = 394).
Figure 1 shows the follow-up visits over the course of each patient’s time at risk (depicted on each row) for the three periods. The orange bars indicate the advised timing of follow-up based on the guidelines. It is shown that the frequency of imaging substantially increased over the years, with patients having surgery in 2011–2015 receiving more imaging, particularly in the first year after treatment. Most visits occurred outside the orange bars and thereby the time intervals advised in the guidelines.
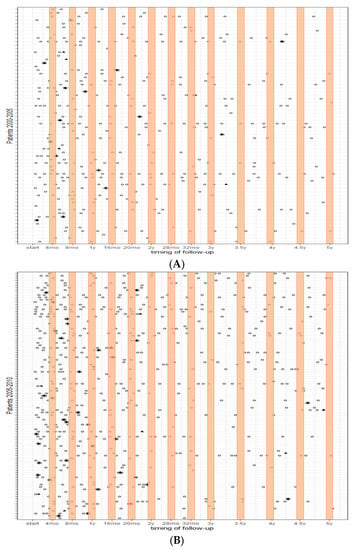
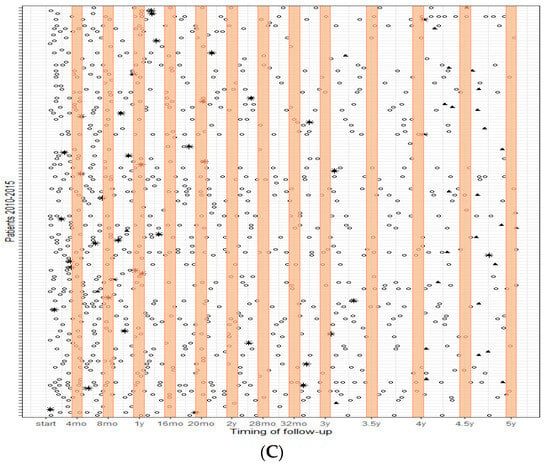
Figure 1.
Follow-up patterns over 5 years; (A) (top) patients who had surgery between 2000–2005, (B) patients who had surgery between 2005–2010, and (C) (bottom) patients who had surgery between 2010–2015. Each row represents each patient’s time at risk; the orange bars indicate the advised timing of follow-up based on the guidelines. ○ = surveillance imaging; * = recurrence; ⯅ = maximum time at risk.
A recurrence (distant or local) occurred in 110 patients (28%), with a median time to recurrence of 344 days (IQR 148–612). Moreover, 54% of recurrences occurred in the first year and 88% up until two years after surgery. Ninety-five patients (24%) died within the FU period, with a median of 663 days (IQR 318–1148) after surgery. The median age of a patient with recurrent disease was 64 years (IQR 46–74), and 52% was male. Among patient with a recurrence, 92 (84%) had a high-grade tumor compared with 16 patients with a low-grade tumor (14%). For two recurrences (2%), the grade status was unknown.
3.2. Type of Patients Receiving More or Fewer Follow-Up Visits
In the first year, 394 patients were at risk, with only 93 (24%) patients receiving the required three follow-up visits, while 185 (47%) patients had fewer visits, and 116 (29%) had more visits than advised in guidelines (Table 3). Patients having fewer visits than advised were significantly older than patients receiving more visits than advised (median age 62 vs. 54, p < 0.05) and were less often diagnosed with a high-grade tumor (43% vs. 87%, p < 0.05) or treated with RT (14% vs. 38%, p < 0.05). Surgical margins (p = 0.084) and tumor size (p = 0.221) did not significantly differ between patients receiving more and fewer visits than advised. Over the years, patients receiving more visits than advised were more likely to be patients with high-grade tumors compared with those receiving fewer visits, whereas the difference in age was smaller than in the first year. Similar patterns for free surgical margins and RT were observed in subsequent follow-up years but with fewer remaining patients at risk.

Table 3.
Observed n of follow-up compared to local guideline for soft-tissue sarcoma patients.
3.3. Type of Patients Receiving More or Fewer Follow-Up Visits in Relation to Recurrence
Among the 394 patients at risk, 62 patients (16%) experienced a recurrence in the first year after surgery (Table 4). Among the patients experiencing a recurrence, 28 (45%) received the number of follow-up visits advised in the guidelines, 28 (45%) patients received more, and 6 (10%) had fewer follow-up visits, which could point to possibilities for improving decision making on FU frequency for some patient groups. Patients with a recurrence receiving fewer visits than advised were older (72 vs. 55 p < 0.05) than patients receiving more visits than advised in the guidelines but did not differ in histological grade (p = 0.415) or any of the other characteristics in the first year of follow-up.

Table 4.
Type of STS patients with a recurrence receiving more or fewer visits than advised in guidelines.
In contrast, among patients without a recurrence, only 20% received the number of advised follow-up visits; 86 (28%) received more visits than advised, which may suggest possible over-testing; and 161 (51%) received fewer visits than advised in the guidelines (Table 5). Patients without a recurrence receiving more visits than advised were significantly younger than patients receiving fewer visits (53 vs. 61 years, p < 0.05), more often had a high-grade tumor (86% vs. 35%, p < 0.05), were treated with RT (42% vs. 14%, p < 0.05), and had free surgical margins (76% vs. 57%, p < 0.05). Tumor size (p = 0.37) did not significantly differ between patients receiving more and fewer follow-up visits than advised.

Table 5.
Type of STS patients without a recurrence receiving more or fewer visits than advised in guidelines.
4. Discussion
The present study has shown that the frequency of imaging substantially increased over time, particularly in the first year after surgery, and that only one-quarter of patients received the advised three FU visits, with similar patterns in subsequent FU years. Patients receiving fewer visits than advised in the guidelines in the first year were significantly older and less often diagnosed with a high-grade tumor or treated with RT than those receiving more visits than advised. Among patients with a recurrence, 10% received fewer visits than advised and were older but did not differ on other tumor or treatment characteristics. About one-quarter of patients without a recurrence received more visits than advised and generally were younger, more often diagnosed with a high-grade tumor, and treated with RT or with free surgical margins. These observations may point to physicians incorporating recurrence risk in decisions on FU frequency but could also indicate areas for improvement.
There may be several explanations why physicians deviate from guideline recommendation: (1) present guidelines are not tailored to patient, tumor, or treatment characteristics; (2) there may be fear and uncertainty from patients’ or physicians’ perspectives; and (3) the effectiveness of follow-up to influence overall survival is uncertain. As a first explanation, current ESMO and NCCN guidelines do not distinguish between tumor -and treatment-related risk of disease recurrence, even though the risk for local or metastatic disease recurrence is known to differ significantly between STS patients due to tumor and treatment factors [4,11,15,16,17,18]. Our results also reflect this, showing that among patients experiencing a recurrence, 84% had a high-grade tumor. Not including known risk factors for a recurrence in follow-up strategies in current guidelines may be why physicians do not follow guidelines. This is consistent with survey results from Gerrand (2007) and Greenberg (2016), showing that physicians determined follow-up strategies based on perceived recurrence risk rather than adhering to guidelines, mostly because guidelines were not evidence-based [12,13]. Overall, the wide variation in follow-up visits observed in this study points to follow-up visits occurring almost at random, which should create awareness among physicians. Furthermore, it highlights the need for high-quality evidence supporting the current FU frequency in guidelines that is tailored to recurrence risk by including known risk factors to create risk classes, which may improve attitudes and beliefs concerning the guidelines.
As a second explanation, patients may express psychological stress and fear of recurrence particularly in the first year after treatment, and more frequent visits can reassure patients [19,20,21]. Additionally, when physicians experience uncertainty about the right course of action, they may also prefer to “err on the side of caution” [22] and give patients more frequent follow-up visits just to be safe. In our study, physicians’ fear or uncertainty may have played a role particularly in younger patients with higher life expectancies, as we found that patients who had more follow-up visits than advised were significantly younger than patients receiving fewer visits. Particularly, the 28% patients without a recurrence receiving more visits than advised might indicate possible overuse. Thus, anxiety or uncertainty, whether it comes from a patient or physician, may influence follow-up strategies, and this is an important factor of overuse [23,24]. Future initiatives seeking to reduce the number of follow-up visits should include interventions to address such uncertainty.
As a third explanation, despite the excessive increase in surveillance imaging in recent years, overall survival of STS patients has not improved, adding to existing doubts about the effectiveness of follow-up for early detection of recurrent disease [5,25,26], which may be why physicians do not follow guidelines but could point to underuse. For example, the 10% of patients with a recurrence receiving fewer follow-up visits than advised might indicate such underuse.
To the best of our knowledge, this is the first study that empirically quantified follow-up patterns of STS patients. Current clinical practice is an essential first step to guide subsequent initiatives to improve quality of care and eliminate overuse. One of the limitations is that its retrospective design as policies for diagnosis, treatment, and follow-up of STS patients may have changed over the past 20 years, which could have influenced our results. However, similar and seemingly random patterns were shown for different periods in which patients received treatment, indicating that the problem remained even for patients treated more recently. Another limitation is that results are based on a cohort of patients from a single center, which limits generalizability to other settings. On the other hand, our results seem consistent with survey results from other settings [12,13,27]. Therefore, issues regarding attitudes and beliefs about follow-up guidelines and uncertainty among healthcare professionals are likely similar elsewhere, which is why we think our study adds to the existing literature on this topic.
The results from our study may have implications for clinical practice in reducing overuse from a patient (i.e., radiation exposure and psychological burden) as well as healthcare perspective (i.e., resources and cost). The amount of follow-up visits is increasingly under debate not just for sarcoma patients but also for other oncological diagnoses [20,28,29]. The current “one-size-fits-all” approach may not be well suited for the different risks of recurrence in the heterogenous sarcoma population and thereby result in overuse. Our study gives some suggestions for which patient groups may be targeted to further explore and reduce overuse. Furthermore, the use of prediction tools such as Sarculator and Personalised Sarcoma Care (PERSARC) make more individualized prediction of LR/DM-risk possible [15,17,18,30,31,32]. This may facilitate clinicians in developing risk-based follow-up schedules, which can result in less-frequent follow-up visits for low-risk patients.
5. Conclusions
A minority of soft-tissue sarcoma patients received the advised three follow-up visits in the first year after surgery, and clinicians seemed to incorporate recurrence risk in their decisions on follow-up frequency rather than the “one-size-fits-all” approach given in guidelines. In addition, a significant proportion of patients without a recurrence received more follow-up visits than advised. Clinicians may therefore need support in more accurately estimating recurrence risk, e.g., by using prediction models to facilitate risk-based follow-up schedules, which can reduce over-testing of patients and consequently the burden on healthcare and costs.
Author Contributions
Conceptualization, D.M.J.D., L.S.K. and M.A.J.v.d.S., methodology, P.J.M.-v.d.M.; formal analysis, L.S.K. and A.A.K.; investigation, L.S.K., A.A.K. and D.M.J.D.; data curation, D.M.J.D., L.S.K. and A.A.K.; writing—original draft preparation, A.A.K.; writing—review and editing, A.A.K., D.M.J.D., L.S.K., P.J.M.-v.d.M., M.A.J.v.d.S. and L.v.B.-V.; visualization, L.S.K. and A.A.K.; supervision, P.J.M.-v.d.M. All authors have read and agreed to the published version of the manuscript.
Funding
A.A.K. is supported by a grant from the Dutch Cancer Society (DCS)—KWF kankerbestrijding (grant number 12642). This funding source did not influence the study in any way, nor did it influence the writing of the manuscript.
Institutional Review Board Statement
The study protocol was presented to the Medical Ethics Committee of the Leiden University Medical Center, which waived the need for ethical approval under the Dutch law (W22.013).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics. Ca-A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Stiller, C.A.; Trama, A.; Serraino, D.; Rossi, S.; Navarro, C.; Chirlaque, M.D.; Casali, P.G.; Grp, R.W. Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur. J. Cancer 2013, 49, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Picci, P. Epidemiology of Soft Tissue Lesions. In Diagnosis of Musculoskeletal Tumors and Tumor-like Conditions: Clinical, Radiological and Histological Correlations—The Rizzoli Case Archive; Picci, P., Manfrini, M., Donati, D.M., Gambarotti, M., Righi, A., Vanel, D., Tos, A.P.D., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 15–18. [Google Scholar]
- Casali, P.G.; Abecassis, N.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brodowicz, T.; Martin-Broto, J.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, 51–67. [Google Scholar] [CrossRef]
- Whooley, B.P.; Mooney, M.M.; Gibbs, J.F.; Kraybill, W.G. Effective follow-up strategies in soft tissue sarcoma. Semin. Surg. Oncol. 1999, 17, 83–87. [Google Scholar] [CrossRef]
- Fletcher, C.D.M. WHO Classification of Tumours of Soft Tissue and Bone, 4th ed.; IARC Press: Lyon, France, 2013. [Google Scholar]
- Kane, J.M. Surveillance strategies for patients following surgical resection of soft tissue sarcomas. Curr. Opin. Oncol. 2004, 16, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Whooley, B.P.; Gibbs, J.F.; Mooney, M.M.; McGrath, B.E.; Kraybill, W.G. Primary extremity sarcoma: What is the appropriate follow-up? Ann. Surg. Oncol. 2000, 7, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.; Potter, M.; Damron, T.A. Image Intensive Soft Tissue Sarcoma Surveillance Uncovers Pathology Earlier Than Patient Complaints But with Frequent Initially Indeterminate Lesions. J. Surg. Oncol. 2016, 113, 818–822. [Google Scholar] [CrossRef]
- Goel, A.; Christy, M.E.L.; Virgo, K.S.; Kraybill, W.G.; Johnson, F.E. Costs of follow-up after potentially curative treatment for extremity soft-tissue sarcoma. Int. J. Oncol. 2004, 25, 429–435. [Google Scholar] [CrossRef] [PubMed]
- von Mehren, M.; Kane, J.M.; Agulnik, M.; Bui, M.M.; Carr-Ascher, J.; Choy, E.; Connelly, M.; Dry, S.; Ganjoo, K.N.; Gonzalez, R.J.; et al. Soft Tissue Sarcoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 815–833. [Google Scholar] [CrossRef]
- Greenberg, D.D.; Crawford, B. Surveillance Strategies for Sarcoma: Results of a Survey of Members of the Musculoskeletal Tumor Society. Sarcoma 2016, 2016, 8289509. [Google Scholar] [CrossRef]
- Gerrand, C.H.; Billingham, L.J.; Woll, P.J.; Grimer, R.J. Follow up after Primary Treatment of Soft Tissue Sarcoma: A Survey of Current Practice in the United Kingdom. Sarcoma 2007, 2007, 34128. [Google Scholar] [CrossRef]
- Team, R.D.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2009; Available online: http://www.R-project.org (accessed on 1 April 2023).
- Callegaro, D.; Miceli, R.; Bonvalot, S.; Ferguson, P.; Strauss, D.C.; Levy, A.; Griffin, A.; Hayes, A.J.; Stacchiotti, S.; Pechoux, C.L.; et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: A retrospective analysis. Lancet Oncol. 2016, 17, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Kattan, M.W.; Leung, D.H.Y.; Brennan, M.F. Postoperative nomogram for 12-year sarcoma-specific death. J. Clin. Oncol. 2002, 20, 791–796. [Google Scholar] [CrossRef]
- Smolle, M.A.; Sande, M.V.; Callegaro, D.; Wunder, J.; Hayes, A.; Leitner, L.; Bergovec, M.; Tunn, P.U.; van Praag, V.; Fiocco, M.; et al. Individualizing Follow-Up Strategies in High-Grade Soft Tissue Sarcoma with Flexible Parametric Competing Risk Regression Models. Cancers 2019, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- van Praag, V.M.; Rueten-Budde, A.J.; Jeys, L.M.; Laitinen, M.K.; Pollock, R.; Aston, W.; van der Hage, J.A.; Dijkstra, P.D.S.; Ferguson, P.C.; Griffin, A.M.; et al. A prediction model for treatment decisions in high-grade extremity soft-tissue sarcomas: Personalised sarcoma care (PERSARC). Eur. J. Cancer 2017, 83, 313–323. [Google Scholar] [CrossRef]
- Armes, J.; Crowe, M.; Colbourne, L.; Morgan, H.; Murrells, T.; Oakley, C.; Palmer, N.; Ream, E.; Young, A.; Richardson, A. Patients’ Supportive Care Needs Beyond the End of Cancer Treatment: A Prospective, Longitudinal Survey. J. Clin. Oncol. 2009, 27, 6172–6179. [Google Scholar] [CrossRef] [PubMed]
- Draeger, T.; Voelkel, V.; Schreuder, K.; Veltman, J.; Dassen, A.; Strobbe, L.; Heijmans, H.J.; Koelemij, R.; Groothuis-Oudshoorn, C.G.M.; Siesling, S. Adherence to the Dutch Breast Cancer Guidelines for Surveillance in Breast Cancer Survivors: Real-World Data from a Pooled Multicenter Analysis. Oncologist 2022, 27, E766–E773. [Google Scholar] [CrossRef]
- Damery, S.; Biswas, M.; Billingham, L.; Barton, P.; Al-Janabi, H.; Grimer, R. Patient preferences for clinical follow-up after primary treatment for soft tissue sarcoma: A cross-sectional survey and discrete choice experiment. Eur. J. Surg. Oncol. 2014, 40, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Tarrant, C.; Krockow, E.M. Antibiotic overuse: Managing uncertainty and mitigating against overtreatment. BMJ Qual. Saf. 2022, 31, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, C.D.; Rose, A.J.; Hartmann, C.W.; van Bodegom-Vos, L.; Graham, I.D.; Wood, S.J.; Majerczyk, B.R.; Good, C.B.; Pogach, L.M.; Ball, S.L.; et al. How the dual process model of human cognition can inform efforts to de-implement ineffective and harmful clinical practices: A preliminary model of unlearning and substitution. J. Eval. Clin. Pract. 2018, 24, 198–205. [Google Scholar] [CrossRef]
- van Bodegom-Vos, L.; de Mheen, P.M.-V. Reducing Low-Value Care: Uncertainty as Crucial Cross-Cutting Theme; Comment on “Key Factors That Promote Low-Value Care: Views of Experts from the United States, Canada, and the Netherlands”. Int. J. Health Policy Manag. 2022, 11, 1964. [Google Scholar] [CrossRef]
- Bozzo, A.; Baldawi, H.; Simchovich, G.; Ghert, M. Optimal surveillance strategies following curative surgery for extremity sarcoma: A systematic review of Randomized Control Trials. 2018. Available online: osf.io/7t64v (accessed on 10 September 2023).
- Brennan, M.F. Follow-up is valuable and effective: True, true and unrelated? Ann. Surg. Oncol. 2000, 7, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Acem, I.; Smit, M.M.; Verhoef, C.; van Houdt, W.J.; Haas, R.L.; van der Hage, J.A.; Grunhagen, D.J.; van de Sande, M.A.J. Management of Soft Tissue Sarcomas in Extremities: Variation in Treatment Recommendations and Surveillance according to Specialty and Continent. Ann. Surg. Oncol. 2021, 28, 7923–7936. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.J.; Evaniew, N.; McKay, P.; Ghert, M. Moving Forward Through Consensus: A Modified Delphi Approach to Determine the Top Research Priorities in Orthopaedic Oncology. Clin. Orthop. Relat. Res. 2017, 475, 3044–3055. [Google Scholar] [CrossRef]
- Grunfeld, E.; Hodgson, D.C.; Del Giudice, M.E.; Moineddin, R. Population-based longitudinal study of follow-up care for breast cancer survivors. J. Oncol. Pract. 2010, 6, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Rueten-Budde, A.J.; van Praag, V.M.; PERSARC studygroup; van de Sande, M.A.J.; Fiocco, M. Dynamic prediction of overall survival for patients with high-grade extremity soft tissue sarcoma. Surg. Oncol. 2018, 27, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Danieli, M.; Gronchi, A. Staging Systems and Nomograms for Soft Tissue Sarcoma. Curr. Oncol. 2023, 30, 3648–3671. [Google Scholar] [CrossRef] [PubMed]
- Acem, I.; van de Sande, M.A.J. Prediction tools for the personalized management of soft-tissue sarcomas of the extremity. Bone Jt. J. 2022, 104, 1011–1016. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).